Abstract
Background/objective
The COVID-19 epidemic is particularly serious in older adults. The symptomatology and epidemic profile remain little known in this population, especially in disabled oldest-old people with chronic diseases living in nursing homes. The objective of the present study was to comprehensively describe symptoms and chronological aspects of the diffusion of the SARS-CoV-2 virus in a nursing home, among both residents and caregivers.
Design
Five-week retrospective cohort study.
Setting
A middle-sized nursing home in Maine-et-Loire, west of France.
Participants
Eighty-seven frail older residents (87.9 ± 7.2years; 71 % female) and 92 staff members (38.3 ± 11.7years; 89 % female) were included.
Measurements
Mass screening for SARS-CoV-2 was performed in both residents and staff. Attack rate, mortality rate, and symptoms among residents and staff infected with SARS-CoV-2 were recorded.
Results
The attack rate of COVID-19 was 47 % in residents (case fatality rate, 27 %), and 24 % in staff. Epidemic curves revealed that the epidemic started in residents before spreading to caregivers. Residents exhibited both general and respiratory signs (59 % hyperthermia, 49 % cough, 42 % polypnea) together with geriatric syndromes (15 % falls, 10 % altered consciousness). The classification tree revealed 100 % COVID-19 probability in the following groups: i) residents younger than 90 with dyspnea and falls; ii) residents older than 90 with anorexia; iii) residents older than 90 without anorexia but with altered consciousness. Finally, 41 % of staff members diagnosed with COVID-19 were asymptomatic.
Conclusions
The pauci-symptomatic expression of COVID-19 in older residents, together with the high prevalence of asymptomatic forms in caregivers, justifies mass screening in nursing homes, possibly prioritizing residents with suggestive combinations of clinical signs including dyspnea, falls, anorexia and/or altered consciousness.
Keywords: COVID-19, SARS-CoV-2, Prognosis, Symptoms, Older adults, Nursing home
1. Introduction
Since December 2019, the SARS-CoV-2 virus is propagating worldwide from China. As of 28 June 2020, the COVID-19 pandemic has infected almost 10 000 000 people in 194 countries around the world, leaving 498 900 dead, mostly in frail older adults aged 70 years and over. First epidemiological data confirm that older adults are more prone to develop severe and lethal forms of COVID-19 [1]. Clinical data regarding older patients’ symptomatology are yet limited [2], and the first report on oldest-old people (i.e., aged 80 years and over) with major neurocognitive disorders revealed clinical peculiarities that make difficult to diagnose COVID-19 during the first days of the infection [3]. What is more, previous studies described individuals for whom tests had been performed following a clinical suspicion, which could have biased the prevalence of some symptoms [3]. Thus, specific attention should be paid to older people in nursing homes for whom mass screening had been organized. Whilst residents of nursing homes seem seriously affected by the SARS-CoV-2 infection [4], clinical and epidemiological data remain yet fragmented thus far [5,6]. The objective of the present study was to clarify symptoms and chronological aspects of the propagation of the SARS-CoV-2 in a nursing home, both in residents and staff members.
2. Methods
2.1. Design and settings
The study consisted in a five-week retrospective observational cohort study in a middle-sized nursing home in Maine-et-Loire, West of France, having performed COVID-19 mass screening of residents (n = 87) and staff members (n = 92).
The nursing home is dedicated to patients with major neurocognitive disorders combined with behavioral disturbances. The facility includes 86 apartments (with one couple living in a single apartment), along with communal dining, library, and activity areas. The nursing home is divided into five units: one day care unit, one open unit, and three secured closed units. The open unit is dedicated to patients with mild major neurocognitive disorders but poor autonomy. Closed units are dedicated to patients with severe behavioral disturbances including wandering.
All 87 residents were allowed to move around the building until 16 March 2020, while social distancing and other preventive measures were implemented. Residents were isolated in their rooms with no communal meals or group activities. No visitors, including families, were allowed in the nursing home since 10 March 2020. Walks in the garden were organized for the residents one by one, in the presence of one staff member. Residents could receive and update their families by phone or video. Mail and packages were stored 24 h before being delivered to residents. Enhanced hygiene measures were implemented, including cleaning and disinfection of frequently touched surfaces, permanent face masks, and additional hand hygiene stations for staff members.
In total, 92 individuals were working as staff members during the study. The teams change three times a day, i.e. in the morning, in the afternoon and at night.
The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). No participant objected to the use of anonymized clinical and biological data for research purposes. The study was approved by an independent institutional review board under the number 2020/51. The study protocol was declared to the National Commission for Information Technology and civil Liberties (CNIL) under number ar20-0053v0.
2.2. Data collection
Residents’ clinical signs and results of the reverse-transcriptase–polymerase-chain-reaction (RT-PCR) tests were retrospectively obtained by reviewing medical records of the nursing home from 17 March 2020. Staff members’ clinical signs and results of the RT-PCR tests were retrospectively obtained from the nursing home coordinating doctor. Data were censored at the time of data cutoff, which occurred on 26 April 2020.
Infection with COVID-19 was defined for both residents and staff members as a positive result on RT-PCR tests of a specimen collected with nasopharyngeal swab in accordance with the World Health Organization standards [7]. Five residents had a test in hospital due to suggestive symptoms between 6 March 2020 and 24 March 2020, before tests were made available outside of the hospital. Other residents and staff members had a test as part of the mass screening on 2 April 2020 (5 staff members), 6 April (2 residents and 3 staff members), 8 April (18 residents and 6 staff members), 9 April (13 residents and 10 staff members), 14 April (18 residents and 12 staff members), 15 April (18 staff members), 16 April (16 residents and 14 staff members), 21 April (7 staff members) and 23 April 2020 (7 staff members). For patients who died without RT-PCR test, the cause of death indicated on the death certificate (i.e. related or not to COVID-19) was collected.
The following measures were collected for each resident: demographic (age, gender, residence unit), vaccinal status regarding influenza virus for the current year, clinical signs, date and result of the RT-PCR test, hospitalization (all-cause or specifically due to COVID-19), and mortality (all-cause or specifically due to COVID-19). Finally, medical history of residents was extracted for the whole population using the PATHOS data [8]. The mean weighted PATHOS is used in nursing homes to describe the care profile of residents. In fact 49 pertinent pathological statuses (but not the whole ICD 10) are described, including major neurocognitive disorders or abdominal pain for instance. Each pathological status is qualified by one of the 12 possible care profiles according to the clinical context (e.g., profile T2: requiring multiweekly medical supervision and 24 -h nursing care).
The following measures were collected for each staff member: date and result of the RT-PCR test, date of first COVID-19 symptoms if applicable, and position in the nursing home by distinguishing between caregivers (i.e. physician, nurses, assistant nurses, animator, physiotherapist, occupational therapist, psychomotrician), non-caregivers who had contact with residents (i.e. restaurant, laundry and housekeepers), and non-caregivers who had no contact with residents (administrative functions).
2.3. Statistical analyses
The participants’ characteristics were summarized using mean and standard deviation (SD), median and 95 % confidence interval (95CI), or frequency and percentage, as appropriate. Normality of data was assessed using Kolmogorov-Smirnov test.
First, the case fatality rate (i.e., the number of deaths from COVID-19 divided by the total number of people diagnosed with COVID-19 during the study), the attack rate (i.e., the number of new cases during the study divided by the number of residents), and the person-time incidence rate per 100 person-days (i.e., as the number of new cases during the study divided by the sum of persons exposed each day during the study [considering that each new case was no longer exposed after COVID-19 diagnosis] divided by 100) were calculated. Each positive case was excluded from people exposed either on the date of the first symptom, or on the date of the positive RT-PCR test for asymptomatic people, or on the date of work disruption for staff members when the two previous conditions were not met. For those with positive RT-PCR test but no symptoms, the date of the screening test was used to calculate the incidence rate.
Second, participants were divided into three groups according to the COVID-19 status, i.e. the “COVID-19 group” for those with a positive RT-PCR test or death attributed to COVID-19, the “non-COVID-19 group” for those with a negative RT-PCR test or death not attributed to COVID-19, and the “non-tested group” for the survivors with no RT-PCR test. Comparisons were performed using Chi square test or exact Fisher test for qualitative variables, and Student t test or Mann-Whitney U test for quantitative variables, as appropriate.
Third, a classification tree (Chi-square Automatic Interaction Detector, CHAID) was performed using all available residents’ data at once [9]. The CHAID analysis is an algorithm used for discovering relationships between a categorical response variable (i.e., COVID-19 here) and other categorical predictor variables (i.e., all clinical variables collected as part of the study). It splits a parent group into two subgroups (“nodes”) within which covariates are homogenous and between which outcome is distinct. The CHAID analysis is useful when looking for patterns in datasets with lots of categorical variables and is a convenient way of summarizing the data as the relationships can be easily visualized. Here, we forced the use of age 90 years (median) as the first split, and the CHAID analysis was adjusted for age. The probability of COVID-19 (relative risk with 95CI) was calculated for each end node using the end node with the lowest prevalence of COVID-19 as a reference (node 7) [10].
Two-sided P-values were considered as significant if <0.05. All statistics were performed using SPSS (v23.0; IBM corp, Chicago, IL).
3. Results
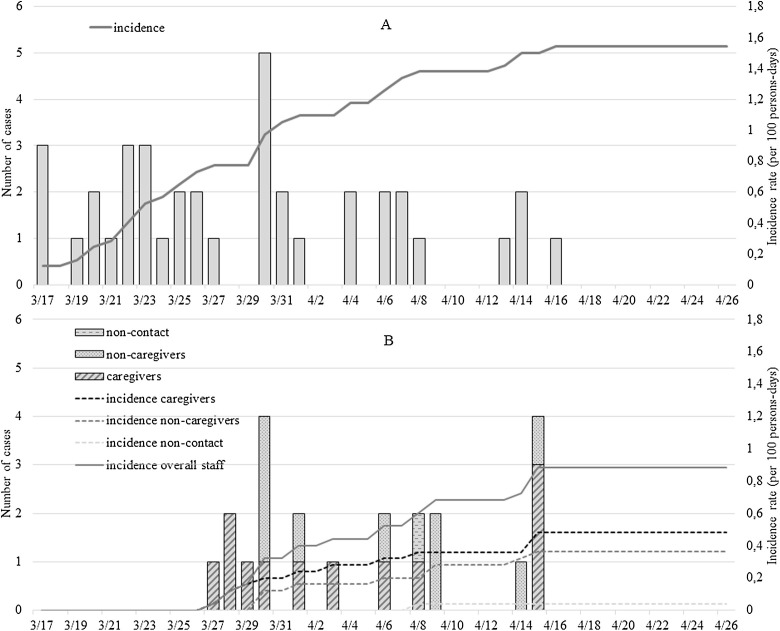
A total of 87 residents (mean ± SD age, 87.9 ± 7.2 years; 71 % female) and 92 staff members (mean, 38.3 ± 11.7 years; 89 % female) were included in the study. As illustrated in Fig. 1, the first confirmed COVID-19 cases were reported on 17 and 27 March 2020 for residents and staff members, respectively. Seventy-seven residents were tested for COVID-19 between 6 March and 16 April 2020 (10 deaths before testing), and 84 (91.3 %) staff members between 2 and 23 April 2020. The attack rate was 47 % in residents, and 24 % in staff members. The incidence rate was 1.54 per 100 persons-days among residents, and 0.88 persons-days among staff members (respectively, 0.48 among caregivers, 0.36 among non-caregivers with contact to residents, and 0.04 among non-caregivers without contact) ( Fig. 1). The case fatality rate was 27 % among residents. The all-cause mortality rate was 15 % among residents, compared 3% on average during the same period in the preceding five years. No staff members died during the study period. Epidemic curves revealed that the epidemic started in residents, and then spread to caregivers, non-caregivers and finally to staff members with no contact to the residents (Fig. 1).
Fig. 1.
Epidemic curve (A: residents, B: staff members).
Demographic characteristics and medical history of residents are detailed in Table 1. Residents exhibited 13 chronic pathological statuses on average. Among all residents, 41 had a confirmed infection with SARS-CoV-2 (mean, 89.0 ± 6.9 years; 66 % female).
Table 1.
Characteristics of nursing home residents (N = 87).
| Total N = 87 |
||
|---|---|---|
| n (%) | 95 % CI | |
| Age (years), mean ± SD | 87.9 ± 7.2 | 86.4–89.4 |
| Female gender | 62 (71) | – |
| Medical history | ||
| Major neurocognitive disorders | 85 (98) | 92.0–99.4 |
| Chronic behavioral disturbances | 70 (81) | 70.9–87.4 |
| Chronic renal failure | 55 (63) | 52.7–72.6 |
| Hypertension | 57 (66) | 55.1–74.7 |
| Chronic heart rhythm dysfunction | 23 (26) | 18.3–36.6 |
| Coronaropathy | 14 (16) | 9.8–25.2 |
| Chronic heart failure | 12 (14) | 8.1–22.6 |
| Chronic pulmonary disease | 17 (20) | 12.6–29.1 |
| Diabetes mellitus | 9 (10) | 5.5–18.5 |
| Cancer | 0 (0) | 0 – 0 |
| Seasonal flu shotsa | 69 (79) | 69.6–86.5 |
| Hospitalization | 14 (16) | 9.8–25.2 |
| All-cause mortality b | 11 (13) | 7.2–21.2 |
CI: confidence interval.
10 missing data.
1 patient COVID-19 negative still hospitalized at censure of data.
As illustrated in Table 2 , the most frequent symptoms retrieved in COVID-19 residents were thermal changes (n = 28, 68 %); 59 % of patients having hyperthermia (i.e., temperature >38 °C) and 10 % hypothermia (i.e., temperature <36 °C). Dyspnea was retrieved in 61 % (n = 25) of COVID-19, with 46 % of low pulse oximetry (n = 19) and 42 % of polypnea (n = 17). Twenty (49 %) COVID-19 residents presented with cough (n = 20), 14 (34 %) with marked asthenia, and 5 (12 %) with diarrhea. It is noticeable that the comparison with non-COVID-19 residents showed multiple symptomatic differences, in particular a greater number of simultaneous clinical signs (Table 2). Finally, 3 COVID-19 residents (7%) were totally asymptomatic and finally diagnosed through mass screening. All of them had major neurocognitive disorders related to alcohol use. One had hypertension and mild chronic renal failure, another one had hypertension and moderate chronic renal failure, and the last one had polyvascular disease with brain and heart lesions.
Table 2.
Clinical signs retrieved in nursing home residents (N = 87).
| Residents without COVID-19 (n = 46) | Residents diagnosed with COVID-19 (n = 41) | P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 87.1 ± 7.3 | 88.8 ± 7.0 | 0.27 |
| Female gender | 36 (76) | 26 (63) | 0.16 |
| Seasonal flu shotsa | 37 (80) | 32 (78) | 0.64 |
| Hospitalization | 3 (7) | 11 (27) | 0.017 |
| All-cause mortality | 2 (4) | 11 (27) | 0.005 |
| Number of symptoms, median [95CI] | 2.0 [1.5‒2.6] | 4.6 [3.8‒5.4] | <0.001 |
| Asymptomatic | 14 (30) | 3 (7) | – |
| 1 symptom | 8 (17) | 1 (2) | – |
| 2 symptoms | 5 (11) | 3 (7) | – |
| 3 symptoms | 7 (15) | 6 (15) | – |
| 4 symptoms | 6 (13) | 7 (17) | – |
| 5 symptoms | 5 (11) | 7 (17) | – |
| ≥6 symptoms | 1 (2) | 14 (34) | – |
| Duration of symptoms (days), median [95CI]b | 10.3 [6.3‒14.3] | 12.6 [9.8‒15.4] | 0.34 |
| General signs | |||
| Asthenia | 8 (17) | 14 (34) | 0.09 |
| Anorexia | 0 (0) | 7 (17) | 0.004 |
| Myalgia - arthralgia | 3 (6) | 3 (7) | >0.99 |
| Low blood pressure | 0 (0) | 2 (5) | 0.22 |
| Temperature changes | 16 (35) | 28 (68) | 0.003 |
| Normothermia | 30 (65) | 13 (32) | 0.003 |
| Hypothermia < 36 °C | 7 (15) | 4 (10) | 0.53 |
| Hyperthermia > 38 °C | 9 (20) | 24 (59) | <0.001 |
| Respiratory signs | |||
| No respiratory signs | 22 (48) | 8 (20) | 0.007 |
| Cough | 15 (33) | 20 (49) | 0.14 |
| Dyspnea | 13 (28) | 25 (61) | 0.003 |
| Polypnea | 6 (13) | 17 (42) | 0.003 |
| Between 23 and 29/minute | 3 (7) | 8 (20) | 0.11 |
| ≥30/minute | 3 (7) | 9 (22) | 0.06 |
| Pulse oximetry under 90 % | 9 (20) | 19 (46) | 0.020 |
| Geriatric syndromes | |||
| Delirium (over- or hypoactive) | 3 (7) | 3 (7) | >0.99 |
| Fall | 1 (2) | 6 (15) | 0.048 |
| Altered consciousness | 0 (0) | 4 (10) | 0.045 |
| ENT signs | |||
| Rhinitis | 5 (11) | 8 (20) | 0.37 |
| Odynophagia | 2 (4) | 2 (5) | >0.99 |
| Anosmia | 0 (0) | 1 (2) | 0.47 |
| Conjunctivitis | 2 (4) | 1 (2) | >0.99 |
| Gastrointestinal signs | |||
| Diarrhea | 2 (4) | 5 (12) | 0.25 |
| Nausea | 1 (2) | 2 (5) | 0.60 |
| Vomiting | 1 (2) | 1 (2) | >0.99 |
| Other signs c | 3 (7) | 9 (22) | 0.06 |
Data presented as n (%) where applicable; for qualitative variable, all tests were performed using exact Fisher test; CI: confidence interval; ENT: ear, nose, and throat.
4 missing data for COVID-19+ patients and 6 missing data for COVID-19‒.
1 missing data for each group related to an hospitalization.
Other signs include dizziness (n = 3), headache (n = 2), facial erythrosis (n = 2), pallor, erythematous rash, marble skin, chest pain, crying (n = 1 for each sign).
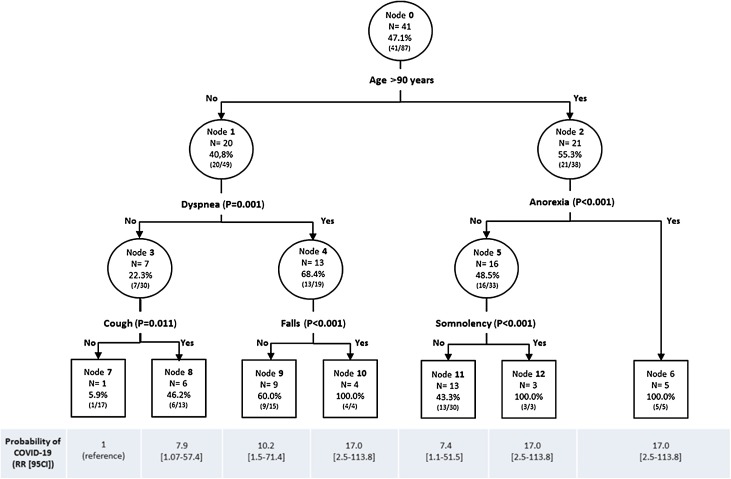
The classification tree identified 7 end groups among residents for the prediction of COVID-19 (Fig. 2 ). The first split was dyspnea below 90 years of age, and anorexia above 90 years. Among individuals younger than 90 and without dyspnea, those who reported no cough formed the end node with the lowest prevalence of COVID-19 (6%; node 7). Compared to this reference node, the probability of COVID-19 was 7.4-fold higher among those older than 90 without anorexia and without altered consciousness (43 % COVID-19), 7.9-fold higher among those younger than 90 without dyspnea but with cough (46 % COVID-19), and 10.2-fold higher among those younger than 90 with dyspnea but no falls (60 % COVID-19). Finally, we found a 100 % COVID-19 probability in the following groups: i) those younger than 90 with dyspnea and falls; ii) those older than 90 with anorexia; iii) those older than 90 without anorexia but with altered consciousness.
Fig. 2.
Classification tree for the prediction of COVID-19 (i.e., RT-PCR positive test result) among residents (N = 87).
For each node: node number; N of residents with COVID-19 within node; proportion of residents with COVID-19 within node (N with COVID-19 / N node). RR: relative risk; 95CI: 95 % confidence interval.
Table 3 presents the characteristics of the staff members. In total, 22 had a confirmed infection with SARS-CoV-2 (mean, 38.1 ± 11.3 years; 96 % female). Caregivers represented 64 % (n = 14) of infected staff members, non-caregivers with contact to residents 32 % (n = 7), and staff members without contact to residents 5% (n = 1). Most frequent symptoms were fever (32 %, n = 7), general signs (i.e. asthenia, anorexia, myalgia) (27 %, n = 6), cough (23 %, n = 5), and ENT signs (18 %, n = 4). Nine staff members were asymptomatic and identified through mass screening.
Table 3.
Characteristics of staff members (N = 92).
| Staff members without COVID-19 (n = 62) | Staff members with COVID-19 (n = 22) | Non-tested staff members (n = 8) | P-value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 39.4 ± 12.1 | 38.1 ± 11.3 | 30.3 ± 5.4 | 0.09 |
| Female gender | 54 (87) | 21 (96) | 7 (88) | 0.56 |
| Positions (in %) | ||||
| Caregivers | 43 (69–72) | 14 (64‒23) | 3 (38‒5) | 0.19 |
| Non-caregivers with contact | 11 (18–55) | 7 (32–35) | 2 (25‒10) | 0.29 |
| Non-caregivers with no contact | 8 (13–67) | 1 (5–8) | 3 (38‒25) | 0.08 |
| Hospitalizationa | 1 (2) | 1 (5) | 0 (0) | 0.55 |
| Symptomsa | ||||
| Asymptomatic | 46 (74) | 9 (41) | 7 (88) | 0.007 |
| General signs | 9 (15) | 6 (27) | 0 (0) | 0.22 |
| Hyperthermia | 8 (13) | 7 (32) | 0 (0) | 0.06 |
| Pulmonary signs | 3 (5) | 1 (5) | 0 (0) | 1.00 |
| Cough | 13 (21) | 5 (23) | 0 (0) | 0.49 |
| ENT signs | 2 (3) | 4 (18) | 0 (0) | 0.06 |
| Other signsb | 7 (11) | 0 (0) | 0 (0) | 0.33 |
Data presented as n (%) where applicable; except for age, all tests were performed using exact Fisher test.
Four missing data related to sudden working cessation (two non-tested staff members, and two in the group of COVID-19 positive staff members).
Headache, dizziness and/or gastrointestinal signs.
4. Discussion
The present report of COVID-19 mass screening in a nursing home showed a high prevalence of asymptomatic infected staff members, and confirmed that older residents exhibit few and mainly nonspecific symptoms. We were nevertheless able to clarify the symptomatology of COVID-19 residents, and to specify three different clinical profiles of residents with 100 % infection within a nursing home affected by the SARS-CoV-2.
This monocentric observational study contributes to emerging understanding of the presentation and trajectory of COVID-19 in nursing-home residents. This case series showed that frail older adults exhibit relatively few symptoms, and notably less often fever, cough [11] and ENT signs [12] than younger adults. In this sense, our results are consistent with the few previous studies on symptoms met in older adults infected with COVID-19 [2,3]. It is also consistent with the clinical presentation of other viral infections in older adults such as influenza [13]. Here, the residents exhibited both general and respiratory signs (59 % hyperthermia, 49 % cough, 42 % polypnea) together with gastro-intestinal signs (12 % diarrhea) and geriatric syndromes (15 % falls, 10 % altered consciousness). Surprisingly, delirium was less frequent compared to one previous report in adults aged 70 and over (7% here versus 26.7 % previously) [3]. This may be explained by the characteristics of the present sample, which involved mainly frail older residents with major neurocognitive disorders and behavioral disturbances. Delirium is commonly under-recognized when superimposed to major neurocognitive disorders, especially during the severe stages of the disease since a clear distinction between symptoms attributable to delirium or to underlying dementia proves difficult [14].
We also found that the COVID-19 disease was asymptomatic in 8% of the residents. This result is in accordance with recent results reporting 5% of asymptomatic patients (3/57) in a nursing facility [15]. It suggests that COVID-19 may have either non-expressive forms, or nonspecific symptoms that have gone unnoticed, or symptoms not expressed by older adults with advanced cognitive disorders. Moreover, positive RT-PCR for SARS-CoV-2 was found in the absence of any symptom in 41 % of the staff members; a large group likely involving both asymptomatic and presymptomatic individuals [16]. These findings encourage systematic screening in nursing homes of all residents and staff members, starting with caregivers.
Our classification tree is thought to be an interesting tool to assist clinicians in prioritizing tests and in rapid decision-making for older residents. Three clinical profiles should particularly draw the clinicians’ attention as they were associated with 100 % COVID-19 probability in our study. These are residents i) younger than 90 with dyspnea and falls, ii) older than 90 with anorexia, and iii) older than 90 without anorexia but with altered consciousness. Thus, even if these results need to be confirmed by further and preferentially prospective analyses, it seems reasonable, in a nursing home affected by COVID-19 epidemic, to quickly isolate residents with one of these combinations of symptoms, and to test them as a priority to make the diagnosis of COVID-19.
We found a case fatality rate of 27 %, consistent with previous findings ranging between 26 % and 33.7 % in similar populations [15,17]. Such high lethality rate in older patients should be considered in light of the worldwide COVID-19 lethality rate for all ages, which is around 7% [18]. The over-mortality in older patients was early reported by Wang et al. [19] and by the Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [20]. It is likely explained by the higher prevalence of severe infections in older patients compared to younger ones, due to the higher prevalence of multimorbidity leading to cascading decompensation in this population [21,22]. Consistently, the studied residents exhibited 13 chronic pathological statuses on average here.
Finally, we found that the attack rate was 47 % among the residents in our study, which was close to the 43.5 % rate previously established in a call center in South Korea [23], but twice as high as the attack rate among the staff members. This differential was possibly explained by a presymptomatic phase among staff members [16] as they were affected by SARS-CoV-2 later than the residents. In our study, it is noticeable that the epidemic started in the nursing home among residents (Fig. 1) and then spread to staff. Since residents were unable to leave the nursing home, this suggests that the SARS-CoV-2 was likely imported into the nursing home by a family member before containment and before visiting bans. Thus, the isolation measures from the outside imposed by some governments appear justified to limit and slow down the spread of the virus in nursing homes. However, such isolation of residents also raises questions about the quality of life of those with short life expectancy. Many initiatives are proposed in nursing homes to keep social life during this period, i.e. animations in corridors, music playing, individual walks, individual activities in the garden, sports coach, singing activities, or dematerialized communications with relatives; all alternative solutions, the degree of satisfaction of which needs to be evaluated (NCT04333849).
Our study has some limitations. First, it is an observational study conducted on a relatively limited sample of older adults living in a single nursing home and who may be not fully representative of the general population of residents as they all suffered from major neurocognitive disorders. Second, even if data were collected each day during the epidemic in a standardized manner, recall and reporting bias in retrospective studies cannot be ruled out especially regarding the symptoms of staff members. Third, the diagnosis of COVID-19 was based either on death certificate or RT-PCR test, although the first one assumes a high clinical probability but no biological confirmation of COVID-19, and the second one suffers from a relatively low sensitivity of 72 % with high risk of false negatives [24].
5. Conclusion
In conclusion, the pauci-symptomatic expression of COVID-19 in older residents, together with the high prevalence of asymptomatic forms in caregivers, justifies conducting mass screening in nursing homes, possibly prioritizing residents with suggestive combinations of clinical signs including dyspnea, falls, anorexia and/or altered consciousness. Moreover, the finding of an initial contamination likely brought by non-professional visitors encourages isolation measures in nursing homes to break the contamination chain.
Contributors
Guillaume Sacco contributed to study concept and design, analysis and interpretation of data, and drafting of the manuscript.
Gonzague Foucault contributed to study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support.
Olivier Briere contributed to critical revision of the manuscript for important intellectual content.
Cédric Annweiler contributed to study concept and design, analysis and interpretation of data, drafting of the manuscript, and study supervision, and has full access to all of the data in the study, takes responsibility for the data, the analyses and interpretation and has the right to publish any and all data, separate and apart from the attitudes of the sponsors.
All authors have read and approved the manuscript.
Conflict of interest
All authors state that they have no conflicts of interest with this paper. The authors have no relevant personal financial interest in this manuscript.
Funding
GS is supported by a postdoctoral grant from the Research Center on Autonomy and Longevity, University Hospital of Angers, France (2019–2020).
The sponsors had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Ethics
The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). No participant objected to the use of anonymized clinical and biological data for research purposes. The study was approved by an independent institutional review board under the number 2020/51. The study protocol was declared to the National Commission for Information Technology and civil Liberties (CNIL) under number ar20-0053v0.
Data sharing and collaboration
There are no linked research data sets for this paper. Data will be made available on request.
Provenance and peer review
This article was not commissioned. Peer review was directed by Leon Flicker independently of Cédric Annweiler, an author and Maturitas editor, who was blinded to the process.
Acknowledgments
The authors have listed everyone who contributed significantly to the work in the Acknowledgments section. Permission has been obtained from all persons named in the Acknowledgments section.
- Melinda Beaudenon, MSc, Romain Simon, MSc, and Jennifer Gautier, MSc, from the Research Center on Autonomy and Longevity, University Hospital of Angers, France, for daily assistance. There was no compensation for this contribution.
References
- 1.Deng Y., Liu W., Liu K., Fang Y.-Y., Shang J., Zhou L., Wang K., Leng F., Wei S., Chen L., Liu H.-G. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin. Med. J. (Engl.) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacco G., Berrut G., Guérin O., Annweiler C. Symptoms of COVID-19 among older adults: systematic review of biomedical literature. Gériatr. Psychol. Neuropsychiatr. Vieil. 2020;18(2):135–140. doi: 10.1684/pnv.2020.0863. [published online ahead of print, 2020 May 28] [DOI] [PubMed] [Google Scholar]
- 3.Annweiler C., Sacco G., Salles N., Aquino J.-P., Gautier J., Berrut G., Guérin O. National French survey of symptoms in people aged 70 and over diagnosed with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa792. [published online ahead of print, 2020 Jun 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMichael T.M. COVID-19 in a long-term care facility—King County, Washington, February 27–March 9, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roxby A.C., Greninger A.L., Hatfield K.M., Lynch J.B., Dellit T.H., James A., Taylor J., Page L.C., Kimball A., Arons M., Schieve L.A., Munanga A., Stone N., Jernigan J.A., Reddy S.C., Lewis J., Cohen S.A., Jerome K.R., Duchin J.S., Neme S. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older adults - Seattle, Washington, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:416–418. doi: 10.15585/mmwr.mm6914e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K., Bardossy A.C., Oakley L.P., Tanwar S., Chisty Z., Bell J.M., Methner M., Harney J., Jacobs J.R., Carlson C.M., McLaughlin H.P., Stone N., Clark S., Brostrom-Smith C., Page L.C., Kay M., Lewis J., Russell D., Hiatt B., Gant J., Duchin J.S., Clark T.A., Honein M.A., Reddy S.C., Jernigan J.A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le modèle « PATHOS » Guide d’utilisation . 2017. Caisse nationale de solidarité pour l’autonomie, 2017.https://www.cnsa.fr/documentation/modele_pathos_2017.pdf [Google Scholar]
- 9.Magidson J. In: Adv. Methods Mark. Res. Bagozzi R.P., editor. Blackwell; Oxford: 1994. The CHAID approach to segmentation modeling: chi-squared automatic interaction detection; pp. 118–159. [Google Scholar]
- 10.Katz D., Baptista J., Azen S.P., Pike M.C. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–474. doi: 10.2307/2530610. [DOI] [Google Scholar]
- 11.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I., Esfahani M.A., Civile V.T., Marusic A., Jeroncic A., Carvas Junior N., Pericic T.P., Zakarija-Grkovic I., Meirelles Guimarães S.M., Luigi Bragazzi N., Bjorklund M., Sofi-Mahmudi A., Altujjar M., Tian M., Arcani D.M.C., O’Mathúna D.P., Marcolino M.S. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J. Clin. Med. 2020;9 doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechien J.R., Chiesa-Estomba C.M., Siati D.R.D., Horoi M., Bon S.D.L., Rodriguez A., Dequanter D., Blecic S., Afia F.E., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., Filippis C.D., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czaja C.A., Miller L., Alden N., Wald H.L., Cummings C.N., Rolfes M.A., Anderson E.J., Bennett N.M., Billing L.M., Chai S.J., Eckel S., Mansmann R., McMahon M., Monroe M.L., Muse A., Risk I., Schaffner W., Thomas A.R., Yousey-Hindes K., Garg S., Herlihy R.K. Age-related differences in hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza—U.S. influenza hospitalization surveillance network (FluSurv-NET) Open Forum Infect. Dis. 2019;6 doi: 10.1093/ofid/ofz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morandi A., Bellelli G. Delirium superimposed on dementia. Eur. Geriatr. Med. 2020;11:53–62. doi: 10.1007/s41999-019-00261-6. [DOI] [PubMed] [Google Scholar]
- 15.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., Taylor J., Spicer K., Bardossy A.C., Oakley L.P., Tanwar S., Dyal J.W., Harney J., Chisty Z., Bell J.M., Methner M., Paul P., Carlson C.M., McLaughlin H.P., Thornburg N., Tong S., Tamin A., Tao Y., Uehara A., Harcourt J., Clark S., Brostrom-Smith C., Page L.C., Kay M., Lewis J., Montgomery P., Stone N.D., Clark T.A., Honein M.A., Duchin J.S., Jernigan J.A. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N. Engl. J. Med. 2020;382(22):2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., Lewis J., Baer A., Kawakami V., Lukoff M.D., Ferro J., Brostrom-Smith C., Rea T.D., Sayre M.R., Riedo F.X., Russell D., Hiatt B., Montgomery P., Rao A.K., Chow E.J., Tobolowsky F., Hughes M.J., Bardossy A.C., Oakley L.P., Jacobs J.R., Stone N.D., Reddy S.C., Jernigan J.A., Honein M.A., Clark T.A., Duchin J.S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [published online ahead of print, 2020 Mar 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du R.-H., Liu L.-M., Yin W., Wang W., Guan L.-L., Yuan M.-L., Li Y.-L., Hu Y., Li X.-Y., Sun B., Peng P., Shi H.-Z. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann. Am. Thorac. Soc. 2020 doi: 10.1513/AnnalsATS.202003-225OC. [published online ahead of print, 2020 Apr 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S.Y., Kim Y.-M., Yi S., Lee S., Na B.-J., Kim C.B., Kim J.-I., Kim H.S., Kim Y.B., Park Y., Huh I.S., Kim H.K., Yoon H.J., Jang H., Kim K., Chang Y., Kim I., Lee H., Gwack J., Kim S.S., Kim M., Kweon S., Choe Y.J., Park O., Park Y.J., Jeong E.K. Coronavirus disease outbreak in call center, South Korea. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., Yang M., Shen C., Wang F., Yuan J., Li J., Zhang M., Wang Z., Xing L., Wei J., Peng L., Wong G., Zheng H., Liao M., Feng K., Li J., Yang Q., Zhao J., Zhang Z., Liu L., Liu Y. 2020. Evaluating the Accuracy of Different Respiratory Specimens in the Laboratory Diagnosis and Monitoring the Viral Shedding of 2019-nCoV Infections. MedRxiv. [DOI] [Google Scholar]