Abstract
Background
Maternal obesity is associated with adverse pregnancy outcomes. Probiotic supplementation during pregnancy may have positive effects on blood glucose, gestational weight gain (GWG), and the risk of gestational diabetes mellitus [GDM and glycated hemoglobin (HbA1c)].
Objectives
This feasibility study involved a daily probiotic intervention in obese pregnant women from the early second trimester until delivery. The primary aim was to investigate the effect on GWG and maternal glucose homeostasis (GDM and HbA1c). Secondary aims were the effect on infant birth weight, maternal gut microbiota, and other pregnancy outcomes.
Methods
We carried out a randomized double-blinded placebo-controlled study in 50 obese pregnant women. Participants were randomly allocated (1:1) to multistrain probiotic (4 capsules of Vivomixx®; total of 450 billion CFU/d) or placebo at 14–20 weeks of gestation until delivery. Participants were followed with 2 predelivery visits at gestational week 27–30 and 36–37 and with 1 postdelivery visit. All visits included blood and fecal sampling. An oral-glucose-tolerance test was performed at inclusion and gestational week 27–30.
Results
Forty-nine participants completed the study. Thirty-eight participants took >80% of the capsules (n = 21), placebo (n = 17). There was no significant difference in GWG, GDM, HbA1c concentrations, and infant birth weight between groups. Fecal microbiota analyses showed an overall increase in α-diversity over time in the probiotic group only (P = 0.016).
Conclusions
Administration of probiotics during pregnancy is feasible in obese women and the women were willing to participate in additional study visits and collection of fecal samples during pregnancy. Multistrain probiotic can modulate the gut microbiota in obese women during pregnancy. A larger study population is needed to uncover pregnancy effects after probiotic supplementation. This trial was registered at clincaltrials.gov as NCT02508844.
Keywords: gestational diabetes mellitus, gestational weight gain, microbiota, obesity, pregnancy, probiotics
Introduction
The increasing prevalence of obesity among women of childbearing age and during pregnancy has turned into a global public health issue. In 2017, the prevalence of obesity (BMI ≥30 kg/m2) in Denmark was 15% in women aged 25–34 y. Obesity in pregnant women increased by 16.4% between 2004 and 2012 (1). Likewise, the prevalence of gestational diabetes mellitus (GDM) is increasing in Denmark and internationally (2, 3).
Accumulating evidence suggests that high prepregnancy BMI is associated with increased risk of developing maternal pregnancy-related complications such as hypertensive disorders, preeclampsia, GDM, and other adverse pregnancy outcomes (4) as well as increased risk of macrosomia, hypoglycemia, and excessive adiposity in the neonate (5, 6). Maternal gestational weight gain (GWG) is associated with the BMI of the offspring and the risk of obesity in adulthood (7). A GWG of maximum 5–9 kg for obese women is, therefore, recommended in Denmark according to the Institute of Medicine (IOM) guidelines (8).
Data suggest that the composition of the gut microbiota influences body weight, energy homeostasis, insulin resistance, and obesity-associated inflammation, all of which may play a role in the pathophysiology of obesity (9, 10). High bacterial diversity and richness is considered a defining factor of a healthy microbiota (11), and a decline in this diversity has been linked to obesity (12).
Probiotics are live microorganisms that, when consumed in adequate amounts, may provide health benefits to the host (13). Probiotics consist of 1 or more bacterial species, often including bifidobacteria and lactobacilli, which, during the period of administration, can modify gut microbiota (14). Probiotics are of particular interest because they are hypothesized to affect body weight, food intake, appetite, and the composition and metabolic functions of the gastrointestinal microbiota (15, 16).
Most lifestyle and dietary modification interventions used until now to prevent GDM and excessive GWG during pregnancy have resulted in limited effects (17, 18). A systematic review from 2013 reported beneficial effects of probiotics during pregnancy on maternal outcomes including reduced frequency of GDM, improved glycemic control, and a reduced risk of preeclampsia (19). Most studies, however, have only included normal-weight women.
The number of studies investigating the effect of probiotic supplementation in obese pregnant women on the prevention of GDM and excessive GWG are limited. A randomized controlled trial (RCT) showed no effect on either GWG, maternal fasting glucose, infant birthweight, or other metabolic variables after 4 wk of probiotic intervention (20). A recently published RCT including overweight and obese pregnant women showed a significant effect of probiotics in reducing excessive weight gain versus placebo when administrated from the second trimester until delivery (21). Fasting glucose measurements were, however, significantly higher in the women receiving probiotics. GDM also occurred more frequently in the probiotic group, but not significantly. They also mention that around 75% of a selected group of women in the probiotics group had abundance of the given probiotic species (BB-12) at 28 weeks of gestation confirmed by targeted PCR. This study has no systematic records of gut microbiota monitoring during probiotic intervention in either the obese mothers or their infants.
We hypothesized that probiotic intervention can modulate gut microbiota in obese pregnant women and thereby limit GWG and reduce the risk of adverse maternal and neonatal outcomes. The study was conducted as a feasibility study to clarify the feasibility of probiotic intervention including extra visits during pregnancy and collecting fecal samples of 50 obese pregnant women from the beginning of the second trimester until delivery. The primary aim was to investigate if probiotics can affect GWG and reduce impairment of glucose tolerance [GDM and glycated hemoglobin (HbA1c)]. Secondary aims were to investigate probiotic effects on maternal and infant perinatal health outcomes, namely infant birthweight as well as examine the impact of probiotic intervention on maternal gut microbiota diversity.
Methods
Participants
A randomized controlled study was carried out at Copenhagen University Hospital Hvidovre, Denmark, from February 2015 to January 2018 and included 50 obese pregnant women randomly assigned to treatment groups 1:1 to receive capsules containing Vivomixx® (Visbiome® in North America, DeSimone Formulation® in Asia) or placebo from gestational week 14–20 until delivery. The women and their newborns were followed until 9 mo after delivery. Participants were identified in connection with the initial nuchal translucency ultrasound scan performed in gestational week 12–14. The study protocol is published in detail elsewhere (22).
The inclusion criteria were as follows: nulliparous singleton pregnant women with BMI ≥30 and <35 kg/m2 aged older than 18 y; normal nuchal translucency ultrasound scan at gestational age 12–14 wk; able to read and speak Danish; a consent to complete an oral-glucose-tolerance test (OGTT) at gestational week 14–20.
The exclusion criteria were as follows: gestational age older than 20 wk at inclusion; pregestational diabetes or other serious diseases; multiple pregnancy; previous bariatric surgery; intake of probiotics within the last month before inclusion; ingestion of probiotics during the study intervention period other than the study provided probiotics; alcohol or drug abuse.
Study design
Participants were included at gestational week 14–20 (baseline) and were followed with clinical visits at gestational week 27–30, gestational week 36–37, and with the newborn 18–72 h after birth. Fasting blood samples and fecal samples were obtained at each visit and an OGTT was performed at inclusion and at gestational week 27–30.
Feasibility was defined as the possibility of including 50 participants who were willing to participate in the additional study visits during pregnancy and after delivery; if participants were able to deliver additional samples including fecal samples; and were able to consume the given study treatment in a minimum of 80%.
The study was approved by the Danish Data Protection Agency (AHH-2015-001), and permission for human experiments and recruitment of participants was obtained from the Scientific Ethics Committee for Copenhagen Regional Hospitals, Denmark (Permission no.: H-2-2014-076) version 2.1, 5 December, 2014. The study was performed in accordance with the Revised Declaration of Helsinki. The study was registered at www.clinicaltrials.gov as NCT02508844. All participants provided written informed consent to participate after verbal and written information was given. Participants were informed that they could withdraw from the study at any time.
Randomization
Participants were randomly assigned 1:1 to receive probiotic (Vivomixx®) capsules or placebo capsules and included by consecutive numbers. Randomization was done in blocks of 4. Both probiotic capsules and placebo capsules were identical in appearance and packaging. Investigators, participants, and outcome assessors were blinded to the allocation and intervention. The randomization key was revealed to the researchers only when all participants had completed the 9-mo follow-up and data analysis was complete.
Study treatment
Participants received 2 capsules of the probiotic mixture Vivomixx® or placebo twice daily (4 capsules of Vivomixx®; total of 450 billion CFU/d). Vivomixx® contains the following strains: Streptococcus thermophilus DSM 24,731, bifidobacteria (Bifidobacterium breve DSM 24,732, Bifidobacterium longum DSM 24,736, Bifidobacterium infantis DSM 24,737) and lactobacilli (Lactobacillus acidophilus DSM 24,735, Lactobacillus plantarum DSM 24,730, Lactobacillus paracasei DSM 24,733, Lactobacillus delbrueckii subsp. bulgaricus DSM 24,734), and is formulated in vegetable capsules. Placebo capsules contained microcrystalline cellulose, magnesium stearate, and silicon dioxide. The capsules were delivered in 2 batches due to limited durability. According to the instructions from the company, the capsules were stored in a temperature monitored refrigerator before delivery to the participant and the participant was instructed to store the capsules in their own refrigerator after delivery.
Pregnancy outcomes
GWG was defined as body weight at gestational week 36–37 minus self-reported prepregnancy body weight. Participants were weighed using the same scale at every study visit, wearing light clothes and no shoes. We decided to also calculate the intervention period weight gain (body weight at gestational week 36–37 minus weight at baseline), because self-reported prepregnancy body weight is an uncertain measurement.
A 2-h 3-time-point 75-g OGTT was carried out in gestational week 14–20 (baseline) and in gestational week 27–30. Diagnosis of GDM was defined according to the International Association of Diabetes in Pregnancy Study Group (IADPSG) criteria (23); the diagnosis was established if 1 or more glucose values were above the following values: 0 value ≥5.1 mmol/L; 60 min value ≥10.0 mmol/L; 120 min value ≥8.5 mmol/L. GDM in Danish routine pregnancy care was diagnosed using the Danish national guidelines by Danish Society of Obstetrics and Gynaecology (DSOG guidelines). According to these guidelines, GDM is diagnosed if the 2-h standard OGTT capillary blood glucose is 9 mmol/L or greater. OGTT is used in the analysis as AUC based on measures at all 3 time points. Participants diagnosed with GDM according to Danish guidelines continued their care in the multidisciplinary diabetic clinic but were not excluded from the study.
Data on pregnancy-related complications and mode of delivery were extracted from hospital files and validated using the DSOG guidelines; preeclampsia was diagnosed if participants had proteinuria (dipstick, >1 + protein) and persistently elevated blood pressure >140/90 mmHg on >1 occasion. Gestational hypertension was diagnosed using the same criteria but without proteinuria.
Neonatal outcomes
Neonatal outcomes included gestational age, birth weight, and birth length and were obtained from hospital records. Infants with a gestational age <38 wk were excluded from analyses involving anthropological measurements. A term-born neonate was considered large for gestational age (LGA) when weighing over 4000 g and small for gestational age (SGA) when weighing under 2500 g (24, 25). LGA/SGA was adjusted for infant sex and gestational age (26).
Fecal microbiota diversity analysis
Microbiota diversity analysis relied on sequencing of ribosomal small subunit (SSU rRNA) genes. Purified genomic DNA was submitted to PCR using a primer set targeting prokaryotes (1 primer pair). For prokaryotes, a modified version of the published universal prokaryotic primers 341F/806R (27) were used. Resulting PCR products were quantified using the Quant-ITTM dsDNA High Sensitive Assay Kit (Thermo Fisher Scientific) and pooled in equimolar amounts (PAL: Pooled Amplicon Library). Agencourt AMPure XP Beads (Beckman Coulter) were used to remove DNA fragments shorter than 300 bp and those longer than 1000 bp, and the purified DNA was sequenced on the Illumina MiSeq system in a 2 × 250 bp set up (Illumina Inc.). A maximum of 64 samples were sequenced in a single sequencing run (28, 29).
The sequence output was taxonomically mapped using BION, a newly developed k-mer-based mapping software. A k-mer length of 8 was used, with a step size of 4. Query sequences originating from prokaryotes were compared with the 340–807 bp region (rRNA gene positions from Escherichia coli) in RDP 11.04 (30).
Statistical methods
All statistical analyses were performed in R 3.2.3 (R: A Language and Environment for Statistical Computing, 2015, R Foundation for Statistical Computing, Vienna, Austria) (31). Continuous variables are represented as mean values and SDs. Nonnormally distributed variables are represented as median values and IQRs. Continuous variables were compared using t-tests or, for nonnormally distributed data, Wilcoxon rank sum tests, normality distribution of clinical data was evaluated by QQ plots. Categorical variables were presented as frequencies and compared using the chi-squared test or Fisher's exact test, all tests comparing change over time points were done as paired tests. Adjustment for multiple testing was done using Bonferroni correction and a P value of <0.05 was considered statistically significant. The statistical analysis of microbiota was performed with the phyloseq and vegan packages, using ggplot2 and plotly for data visualization. Comparisons of α-diversity and relative abundance of specific operational taxonomic unit (OTU)s across sample times and between the probiotic and placebo groups were done using Mann–Whitney U tests and analysis of similarities was performed based on Bray–Curtis dissimilarity between samples. After manual inspection of the distribution of residuals via QQ plots linear models were fitted to rank of Shannon diversities when analyzing α-diversity and to log + 1 transformed data when analyzing relative abundance of individual OTUs. A univariate analysis was performed to identify genera with a general increasing or decreasing trend over time. Seventy-nine genera were found in ≥5 samples, and for each of these a linear model was fitted to predict the log-transformed relative abundance based on the first 3 sample times (excluding postbirth samples) and group.
Both intention-to-treat (ITT) and per protocol (PP) analyses were performed, ITT analyses was the primary analyses. ITT analyses included all study participants with measured outcomes. PP analyses included all participants with a compliance of >80% capsule intake and excluded all participants diagnosed with GDM according to Danish national guidelines, these were excluded to avoid possible bias related to the additional consultations with health care professionals, including dietician counseling, offered to these participants.
Results
Study population and feasibility
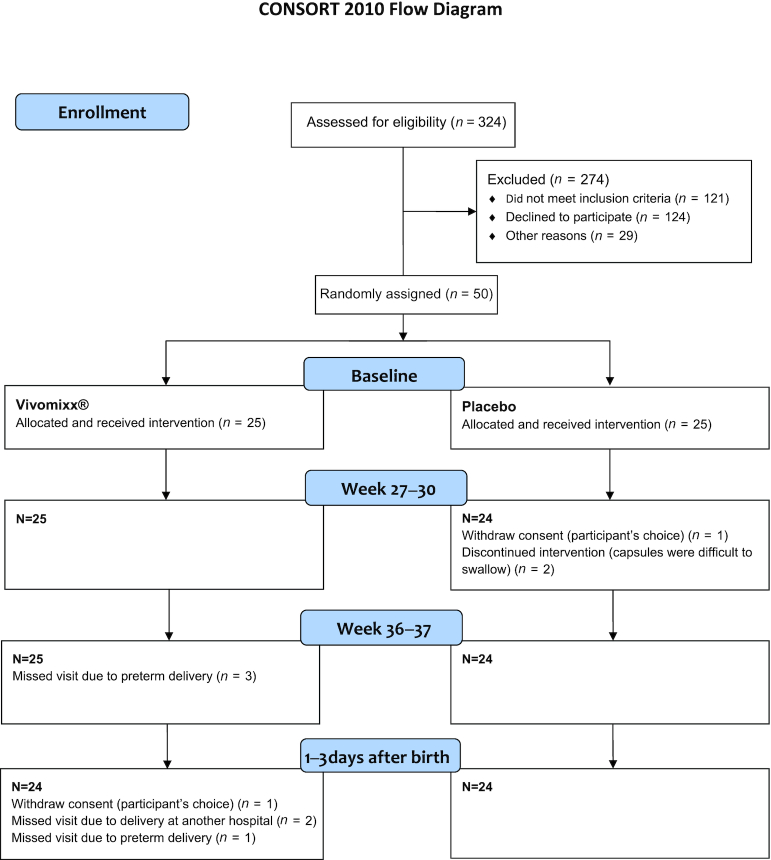
Fifty of 324 women from the screening cohort were included and randomly assigned to Vivomixx® or placebo administration (124 declined participation, 29 were participating in other research projects, and 121 met other exclusion criteria). Of the 50 nulliparous included women, 1 withdrew consent during pregnancy and 1 after delivery (Figure 1). In total, 49 participants (25 Vivomixx® and 24 placebo) were included in ITT analyses and 37 (20 Vivomixx® and 17 placebo) were included in PP analyses. No significant differences between Vivomixx® and placebo participants were found regarding baseline characteristics (Table 1).
FIGURE 1.
CONSORT flow diagram of inclusion and study visits for participants randomly assigned to probiotic and placebo group. Forty-nine participants completed the study until delivery. Participants with missed visits due to preterm delivery or discontinued capsule intervention were not excluded from following study visits.
TABLE 1.
Baseline characteristics for probiotic and placebo participants
| Probiotic (n = 25) | Placebo (n = 25) | P value | |
|---|---|---|---|
| Prepregnancy BMI, kg/m2 (mean ± SD) | 31.7 ± 1.8 | 32.1 ± 1.3 | 0.32 |
| Age, y (mean ± SD) | 30.7 ± 4.5 | 30.7 ± 4.7 | 0.99 |
| Gestational age at baseline, wk (mean ± SD) | 15.5 ± 1.5 | 15.1 ± 1.4 | 0.36 |
| High-risk disposition to GDM*, n (%) | 7 (28) | 11 (44) | 0.38 |
| No. of women reporting smoking during pregnancy | 0 | 1 | 1.00 |
| No. of women reporting smoking at conception | 4 | 2 | 0.67 |
GDM, gestational diabetes mellitus. *Family history of diabetes, polycystic ovary syndrome, or glycosuria. Between group differences was done using 2-sample t-test or Fishers exact test.
Both the probiotic and placebo capsules were widely accepted by the participants. Thirty-eight participants had a capsule intake >80% (21 probiotics and 17 placebo). Two participants from the placebo group reported that capsules were difficult to swallow and stopped taking them. Both continued in the study but were excluded from the PP analysis. No other side effects were reported.
Infant and pregnancy outcomes
GWG at gestational week 36–37 was comparable in the 2 treatment groups, (P = 0.82) (Table 2, Figure 2). Four (16%) women in the probiotic group and 2 (8%) in the placebo group were diagnosed with GDM (IADPSG criteria) in gestational week 27–30 when analyzed in an ITT analysis (Table 3). In a PP analysis, the corresponding numbers were 4 (19%) and 1 (5.9%). The difference in OGTT values when examining the change in AUC at baseline and gestational week 27–30 was not significantly different in the 2 groups; probiotic: 39.19 (–100.00: 127.03) compared with placebo 35.14 (–110.81: 186.49), (P = 0.693). HbA1c measurements during the intervention period were not significantly different between the 2 treatment groups, (P = 0.90) (data not shown). A GWG within the recommended interval of 5–9 kg was achieved in only 6 of 46 women (13%). GWG exceeded the IOM guidelines in 38 of 46 women (83%) (Table 4).
TABLE 2.
Total GWG, intervention period weight gain, and birth weight of infants born to term by women in the probiotic and placebo groups
| ITT | PP | |||||
|---|---|---|---|---|---|---|
| Probiotic n = 20 | Placebo n = 23 | P value | Probiotic n = 15 | Placebo n = 16 | P value | |
| Total GWG, kg (mean ± SD) | 12.7 ± 5.3 | 13.1 ± 5.8 | 0.82 | 11.9 ± 4.9 | 13.0 ± 4.2 | 0.46 |
| Intervention period weight gain, kg (mean ± SD) | 10.2 ± 3.4 | 10.0 ± 4.2 | 0.87 | 9.9 ± 3.4 | 10.8 ± 3.4 | 0.44 |
| Infant birth weight of term-born infants, g (mean ± SD) | 3608 ± 475 | 3640 ± 454 | 0.82 | 3554 ± 487 | 3658 ± 428 | 0.52 |
GWG, gestational weight gain; ITT, intention-to-treat; PP, per protocol. Total GWG = weight in week 36–37 minus prepregnancy weight. Intervention period weight gain = weight in gestational week 36–37 minus weight in gestational week 16–20 (baseline). Between group differences was done using a 2-sample t-test.
FIGURE 2.
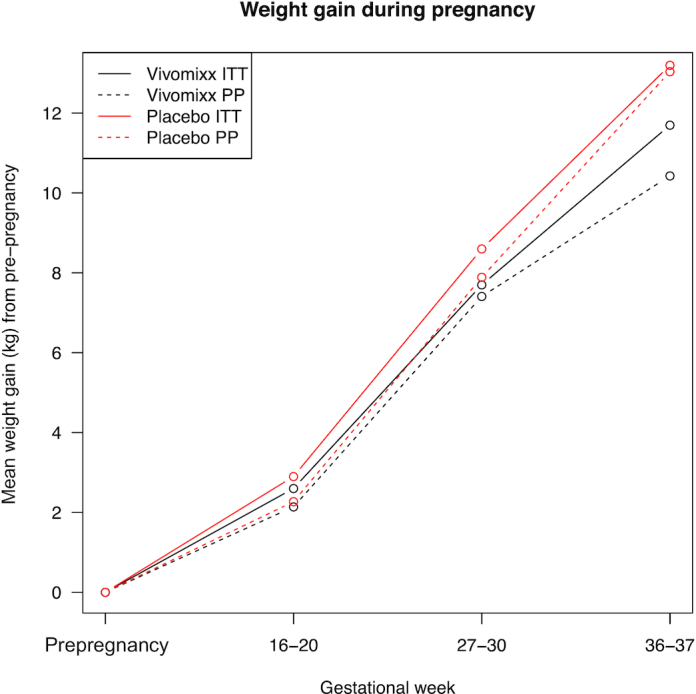
Gestational weight gain in probiotic and placebo group. GWG, gestational weight gain; ITT, intention-to-treat; PP, per protocol.
TABLE 3.
Glucose values (mmol/L) in fasting state, and 60 and 120 min into OGTT at week 14–20 and at week 27–30 in the probiotic and placebo group. Furthermore, the number of women with a diagnosis of GDM (IADPSG criteria) as well as AUC values for OGTT in the Vivomixx® and placebo group are given
| OGTT glucose values, | Probiotic (n = 25) | Placebo (n = 25) | Probiotic (n = 25) | Placebo (n = 24) | ||
|---|---|---|---|---|---|---|
| mmol/L [median (IQL)] | Week (14–20) | Week (14–20) | P value | Week 27–30 | Week 27–30 | P value |
| 0 | 4.3 (4.1: 4.6) | 4.4 (4.1: 4.6) | 0.54 | 4.3 (3.9: 4.9) | 4.3 (4.0: 4.5) | 0.85 |
| 60 | 6.9 (6.2: 8.0) | 6.8 (6.3: 8.4) | 0.63 | 7.3 (6.5: 8.4) | 7.3 (6.8: 8.7) | 0.32 |
| 120 | 6.2 (5.4: 6.8) | 5.9 (5.4: 6.9) | 0.41 | 6.3 (5.9: 7.1) | 6.0 (5.3: 6.9) | 0.41 |
| GDM diagnosis, n (%) | 2 (8%) | 2 (8%) | 1.00 | 4 (16%) | 2 (8%) | 0.67 |
| OGTT AUC values, mmol/L,[mean (IQL)] | 732.4 (662.2: 810.8) | 723.0 (661.5: 820.3) | 0.83 | 752.7 (674.3: 834.5) | 743.24 (690.5: 855.4) | 0.59 |
AUC = area under the curve; GDM = gestational diabetes mellitus; IADPSG= International Association of Diabetes and Pregnancy Study Groups; OGTT= oral glucose tolerance test; SD= standard deviation; IQL = Interquartile limits. All values are derived from an ITT analysis. Between group differences was done using two-sample t-test or Fishers exact test.
TABLE 4.
Other pregnancy outcomes in the probiotic and placebo group
| Probiotic (n = 25) | Placebo (n = 24) | |
|---|---|---|
| GWG <5 kg, n (%) | 2 (9 %) | 1 (4 %) |
| GWG 5–9 kg, n (%) | 2 (9 %) | 4 (17 %) |
| GWG >9 kg, n (%) | 18 (82 %) | 19 (79 %) |
| Hypertension, n (%) | 6 (24 %) | 5 (21 %) |
| Preeclampsia, n (%) | 3 (12 %) | 3 (12 %) |
| Induction of labor, n (%) | 14 (56 %) | 12 (50 %) |
| Cesarean section, n (%) | 11 (44 %) | 5 (21 %) |
| Gestational age at birth, d ± SD | 274 ± 19 | 280 ± 11 |
| Preterm delivery, n (%) | ||
| Gestational age 28–34 wk | 1 (4 %) | 0 (0 %) |
| Gestational age 34–37 wk | 3 (12 %) | 0 (0 %) |
| Birth weight of all infants, g (mean ± SD) | 3414 ± 676 | 3640 ± 454 |
| Birth weight of PP all infants, g (mean ± SD) | 3320 ± 703 | 3658 ± 428) |
| >4 kg at term, n (%) | 4 (16 %) | 7 (29 %) |
| Small for gestational age, n (%) | 1 (4 %) | 0 (0 %) |
| Large for gestational age, n (%) | 1 (4 %) | 4 (17 %) |
| Male gender, n (%) | 13 (52 %) | 13 (54 %) |
| Antibiotics during pregnancy, n (%) | 6 (24 %) | 5 (21 %) |
| Antibiotics at birth*, n (%) | 7 (28 %) | 12 (50 %) |
GWG, gestational weight gain; PP, per protocol.*Antibiotic treatment at birth was given in cases of premature rupture of the membranes >18 h in accordance with Danish guidelines.
The birth weight of term-born infants was comparable in the 2 treatment groups, (P = 0.82) in the ITT analysis. In the PP analysis mean ± SD birth weight of term-born infants were 3554 ± 487 compared with 3658 ± 428 in the probiotic and placebo groups, (P = 0.52). (Table 2). Four infants were born prematurely, all from mothers in the probiotic group (Table 4). Other pregnancy outcomes are shown in Table 4.
Microbiota results
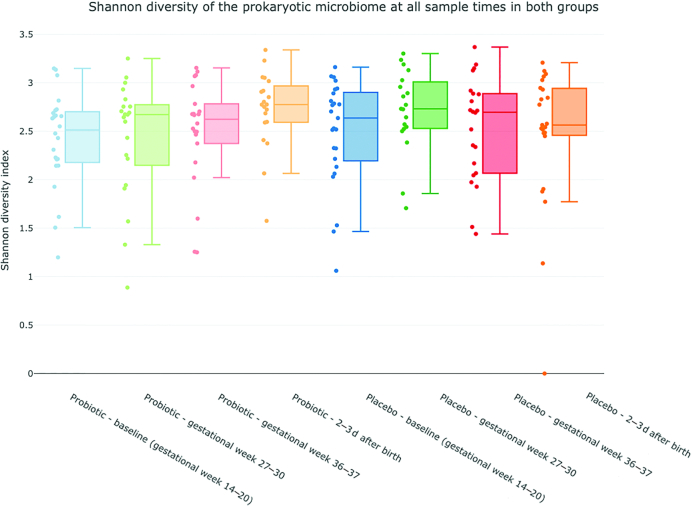
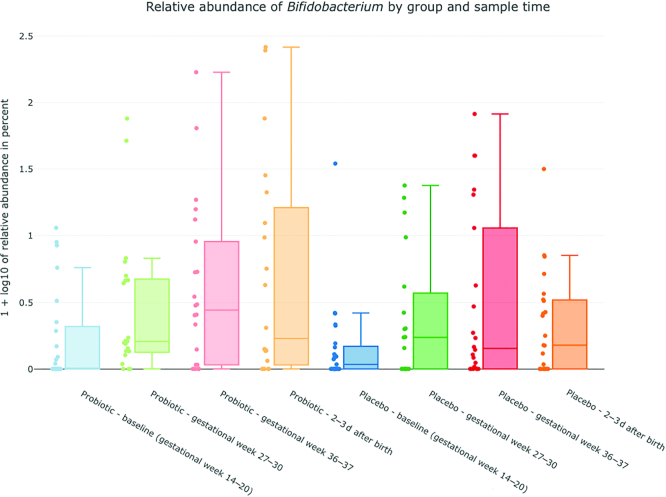
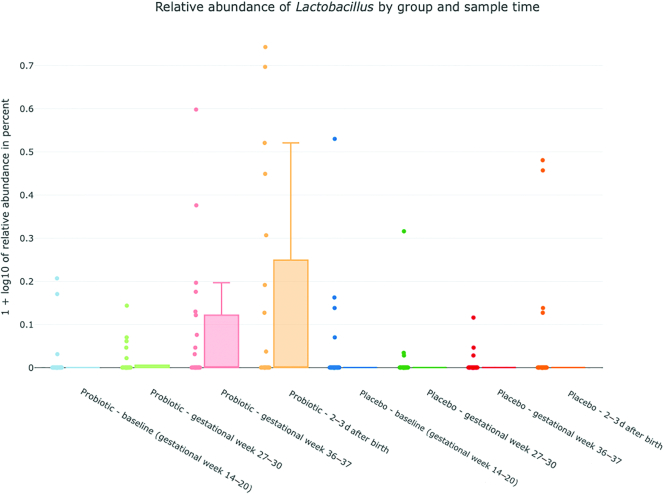
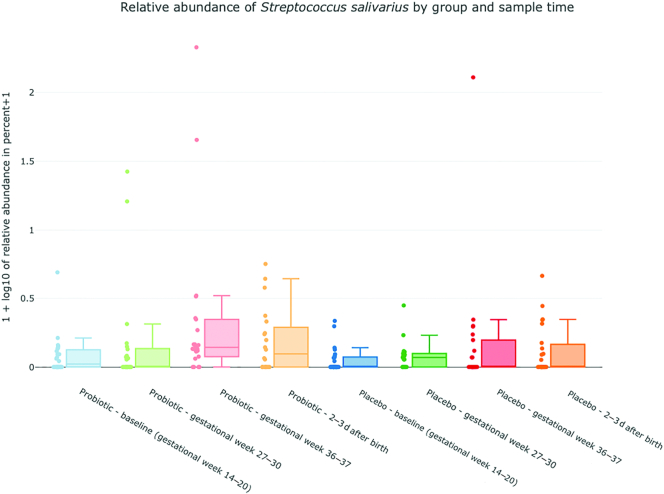
The α-diversity analysis of fecal specimens, showed an increasing diversity in the probiotic group, with a significant difference between the baseline and after birth samples obtained, (P = 0.016) (Figure 3). No statistically significant differences were observed between any sample times in the placebo group. Bifidobacteria, lactobacilli, and S. salivarius were only found in a fraction of the samples from probiotic-treated women, but their relative abundance increased during the intervention period (Figure 4). In samples from probiotic-treated women the relative abundance of Bifidobacterium was significantly lower in the baseline sample obtained compared with gestational week 27–30 (P = 0.021), gestational week 36–37 (P = 0.015), and after birth (P = 0.020) samples. The relative abundance of Lactobacillus was lower in baseline samples compared with gestational week 36–37 and after birth samples (P = 0.03 and P = 0.02), and the relative abundance of S. salivarius was found to be significantly higher in the gestational week 36–37 sample compared with baseline and gestational week 27–30 samples (P = 0.0045 and P = 0.015). Although Bifidobacteriumdoes nominally increase over time in the placebo group there was no significant difference between sample times in the placebo group for either Bifidobacterium, Lactobacillus or S. salivarius.
FIGURE 3.
Change in α-diversity over time in the probiotic and placebo group. Blue = baseline sample, gestational week 14–20; green = gestational week 27–30; red = gestational week 36–37; orange= 2–3 d after birth. Central line is median, box range extend to 25th and 75th percentile, whiskers extend to the value present in the data set that is furthest from the mean but within 1.5 IQR of the 25th/75th percentile.
FIGURE 4.
Relative abundance of Bifidobacterium (4a), Lactobacillus (4b) and Streptococcus(4c) salivarius (y-axis) in the probiotic and placebo group. x-axis: blue = probiotic/placebo baseline, gestational week 14–20; green = probiotic/placebo gestational week 27–30; red = probiotic/placebo gestational week 36–37; blue = probiotic/placebo 2–3 d after birth. y-axis: plotted values are log10(percent abundance +1).
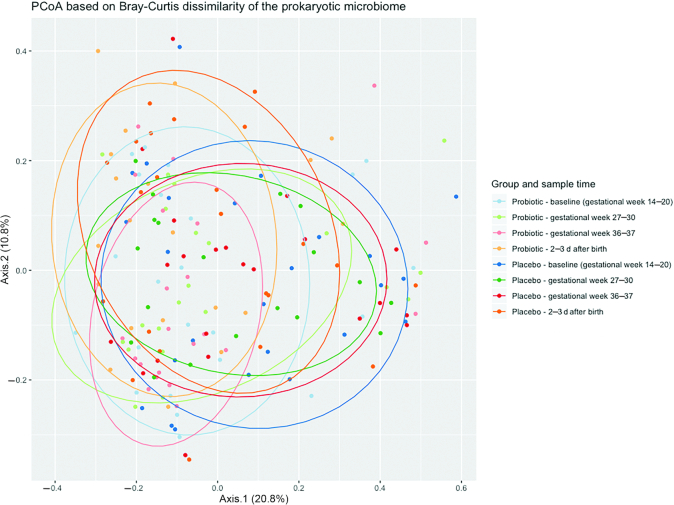
Samples grouped by probiotic and placebo groups and sample times are difficult to distinguish from a simple principal coordinate analysis (PCoA) plot, however, an analysis of similarities did reveal that Bray–Curtis dissimilarity between baseline samples and samples from gestational week 27–30, gestational week 36–37, and after birth were greater in the probiotic group than in the placebo group (P = 3.72e−6, P = 1.26e−4, and P = 0.0014, respectively) (Figure 5).
FIGURE 5.
Principal coordinate analysis on the prokaryotic microbiome in the probiotic and placebo group during the study. Blue = baseline, gestational week 14–20; green = gestational week 27–30; red = gestational week 36–37; orange = 2–3 d after birth.
Dissimilarity analysis indicated that the microbial composition correlated more closely with the concentration of probiotics than it did with group and sample time. This is, in itself, not surprising since the probiotics are themselves part of the microbial composition, however, the results were similar when excluding the 3 genera Bifidobacterium, Lactobacillus, and Streptococcus from the analysis. In addition, multiple individual genera were found to correlate significantly with an increased concentration of probiotics when performing a simple correlation test on log-transformed relative abundances. Supplementary Table 1 lists all genera with P values <0.05 after multiple testing correction.
Key findings from fitting linear models to predict log-transformed relative abundances based on group and sample time are listed in Supplementary Table 2. T-values, P values, and multiple-testing corrected P values are listed for the 6 genera that had a noncorrected P value <0.05 in either group. The relative abundances of Bifidobacterium, Lactobacillus, Streptococcus, Adlercreutzia, and Collinsella were found to increase over time in the probiotic group, whereas Lachnospira was found to decrease over time, although only the trend for Bifidobacterium was significant after multiple testing correction.
Discussion
In this randomized double-blinded placebo-controlled study we examined the use of the probiotic supplement Vivomixx® in 50 obese pregnant women. To the best of our knowledge, this study is the first to evaluate the effect of daily multispecies probiotic supplementation on pregnancy outcomes and gut microbiota in pregnant obese women with a long intervention period from gestational week 14–20 until delivery. Forty-nine participants completed the study until delivery, indicating that the study design and intervention is appropriate for further testing. We have shown that Vivomixx® is safe and well tolerated in pregnant women. Drop-outs were not due to the capsule intervention. Only 2 participants in the placebo group stopped taking the capsules due to discomfort when swallowing them. This problem may be solved by using the powdered version of the product, which is also available on the market.
The dietary supplement in this study, Vivomixx®, was chosen for the beneficial effects reported in earlier studies. Vivomixx® consists of 8 strains of freeze-dried probiotic bacteria (previously named VSL#3) and has shown promising results in human trials. A study in 60 overweight (BMI >25) but otherwise healthy adults showed improved insulin sensitivity (P <0.01), decreased C-reactive protein (CRP), (P <0.05), and a favorable effect on the gut microbiota after 6 wk of treatment (32). A randomized study in 48 obese children with nonalcoholic steatohepatitis reported a significantly reduced BMI and an increased glucagon-like peptide 1 (GLP-1) secretion after 4 mo of treatment compared with placebo (P <0.001) (33). A small interventional study in pregnant women reported that VSL#3 administrated during the last trimester of pregnancy was associated with modulation of the vaginal microbiota and cytokine secretion (34).
Our data on gut microbiota showed a slight increase in α-diversity during the intervention period in the probiotic group. This was not seen in the placebo group. Likewise, an increased abundance of lactobacilli, bifidobacteria, and S. salivarius was found in samples from the probiotic group during the intervention period, indicating successful administration and compliance. The same was not seen in samples from the placebo group. Equally, PCoA indicated that the probiotic did result in differences compared with the placebo group.
A PP analysis did show a lower GWG within the intervention period in the probiotic group compared with the placebo group, this difference did, however, not reach statistical significance. Callaway et al. did likewise, and in support of our data, show that probiotic intervention had a positive effect on excessive weight gain during pregnancy, where excessive weight gain was seen in 32.5% (55/169) of probiotic-treated women versus 46.0% (81/176) of placebo-treated women (P = 0.01) (21). An effect of probiotics on body weight has also been described in nonpregnant overweight and obese women (35, 36). In a previous RCT, 6 mo of treatment with L. rhamnosus CGMCC1.3724 (LPR) resulted in significant weight loss and reductions in fat mass and circulating leptin concentrations in obese women (35). In an RCT in 87 adults with a BMI of 24–31, a 12-wk intake of probiotic milk (L. gasseri SBT2055 [LG2055]) showed a significant effect on weight loss (P <0.001), with significant reductions in abdominal, visceral, and subcutaneous fat depots compared with placebo treatment (P <0.01) (36).
There are several hypotheses about the effects of probiotics in preventing GDM, including modulation of glucose tolerance through balancing gut microbiota, normalizing increased intestinal permeability, and lowering systemic and local low-grade inflammation (37). We found no effect of Vivomixx® on the glucose tolerance of obese women during pregnancy.
The first RCT assessing the efficacy of a probiotic and dietary intervention in reducing the risk of GDM in normal-weight and overweight pregnant women, conducted by Luoto et al. showed a significantly reduced rate of GDM (13%) in women receiving both dietary counseling and probiotics [L. rhamnosus GG (ATCC 53,103) and B. lactis (BB-12)], (P = 0.003) (38). Dietary counseling alone had no effect on the rate of GDM (36%) compared with no intervention (34%). Other studies have shown inconclusive results. Lindsay et al. conducted an RCT probiotic intervention in 138 obese pregnant women with conflicting results – no difference in the incidence of GDM was found between the probiotic (L. salivarius UCC118) and placebo group (20). They reported no effect on either the metabolic profile or pregnancy outcomes of their participants. Treatment was, however, only given for 4 wk (20). In the study by Callaway et al. reporting a reduction in GWG after probiotic treatment, no probiotic effect was shown on GDM, which occurred in 18.4% (38/207) of probiotic-treated women versus 12.3% (25/204) of placebo-treated women, (P = 0.10) (21). Other probiotic interventions including pregnant women diagnosed with GDM are also inconclusive (39, 40). Two RCTs including healthy pregnant women have shown that probiotic intervention could maintain serum insulin concentrations (41) and had significant beneficial effects on markers of insulin metabolism (42).
Comparisons between studies is challenging. All the aforementioned studies were conducted with varying probiotic products containing different species and strains. The use of single- versus multistrain probiotic products can also be discussed. Multistrain probiotics have been suggested to have improved functionality over single-strain cultures. A newly published study by Forssten et al. reported that probiotics do not have an antagonistic effect on each other's survival when used in a multistrain product compared with a single-strain product in a simulated colonic environment (43). In addition, the duration and time of the probiotic intervention during pregnancy varies widely among the studies. Presumably, a longer intervention period would increase the beneficial effects on gut microbiota and metabolism. Furthermore, the effectiveness of probiotic supplements might differ based on the background microbiota compositions of the participants or the prepregnancy health status of the women including normal compared with overweight or obese weight class. As an example, Luoto et al. (38) and Callaway et al. (21) used the same probiotic strains, but with very different outcomes on the occurrence of GDM. In addition, studies on the gut microbiota in pregnant women have shown that pregnancy in itself influences the gut microbiota composition, which changes from the first to the third trimester in parallel with weight gain (44, 45). Finally, the reviewed studies were conducted in widely different countries, all with different disease prevalence of e.g. GDM.
The strengths of this study lie in its design (double-blind placebo-controlled study) and the high concentration multistrain probiotic formulation chosen (8 strains, 450 billion CFU/d). Furthermore, the same clinical staff took care of all study visits and participants during the entirety of the study. This ensured continuity, minimized drop-out, and eliminated interobserver variations in the handling of measurements. A limitation of the study is the low number of study participants, where our sample size was, unfortunately, too small to detect any significant differences in clinical outcomes. Likewise, the use of prepregnancy weight values that were self-reported by the study participants are a limitation. Therefore, we have also conducted analysis on intervention period weight gain. Also, an increased risk of making type I errors due to the testing of multiple outcomes are a limitation of the statistical analysis. Further, we did not perform additional tests on the probiotic capsules regarding variability or between-batch variation.
In conclusion, in this randomized double-blinded placebo-controlled study, we found that intervention with this specific probiotic formulation from gestational week 14–20 until delivery is feasible in obese pregnant women and the women were willing to participate in additional study visits and collection of fecal samples during pregnancy.
No significant difference was seen in GWG and the occurrence of GDM or HbA1c measurements between the 2 groups.
A multistrain probiotic can modulate the gut microbiota in obese women during pregnancy and microbiota profiling showed a slight increase in α-diversity in women in the probiotic group during the intervention period. Also, an increased abundance of lactobacilli, bifidobacteria and S. salivarius was found, which indicate successful administration. A larger study population is needed to uncover pregnancy effects after probiotic supplementation.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to express our gratitude to all the women and their infants who participated in the study and to all midwives and other hospital staff who accommodated the study into their daily work functions. We also wish to thank the clinical microbiology laboratory staff for help with handling the fecal samples.
The authors’ contributions were as follows—SIH, LN, DC, KAK, AEP, and AMP: designed the research project; SIH and VEK: conducted the clinical research; SIH, VEK, and BL: provided essential materials (database and entered data); TK, TBJ, LOA, HCM, CRS, and HVN: analyzed data and performed statistical analysis; SIH: wrote the manuscript and coauthors supervised the manuscript; and all authors read and approved the final manuscript.
Notes
The study was financed by grants from the following private foundations: Jeppe Juhls og hustru Ovita Juhls Mindelegat, Else og Mogens Wedell-Wedellborgs Fond, Aase og Ejnar Danielsens Fond, Knud og Edith Eriksens Mindefond, Toyota-Fonden Denmark, and Next Gen Pharma India Pvt. Ltd. The study was cofinanced by the Faculty of Health and Medical Sciences, University of Copenhagen. All funding sources had no role in the study design, data collection, interpretation of analyses, writing of the manuscript, or decision to submit the publication. Both the probiotic and placebo capsules as well as half a year's salary to the study have been donated to Copenhagen University Hospital Hvidovre by Next Gen Pharma India Pvt. Ltd., National Capital Region, India.
Author disclosures: The authors report no conflicts of interest.
Data described in the manuscript will be made available upon request.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: DSOG, Danish Society of Obstetrics and Gynaecology; GDM, gestational diabetes mellitus; GWG, gestational weight gain; HbA1c, glycated hemoglobin; IADPSG, International Association of Diabetes in Pregnancy Study Group; IOM, Institute of Medicine; ITT, intention-to-treat; LGA, large for gestational age; OGTT, oral-glucose-tolerance test; OTU, operational taxonomic unit; PCoA, principal coordinate analysis; PP, per protocol; RCT, randomized controlled trial; SGA, small for gestational age.
Contributor Information
Sofie Ingdam Halkjær, Gastrounit, Medical Division, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
Victoria Elizabeth de Knegt, Department of Pediatrics, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
Bobby Lo, Gastrounit, Medical Division, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
Lisbeth Nilas, Department of Obstetrics and Gynaecology, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
Dina Cortes, Department of Pediatrics, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Anders Elm Pedersen, Department of Dentistry, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Hengameh Chloé Mirsepasi-Lauridsen, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark.
Lee O'Brien Andersen, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark.
Henrik Vedel Nielsen, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark.
Christen Rune Stensvold, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark.
Thor Bech Johannesen, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark.
Thomas Kallemose, Clinical Research Centre, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
Karen Angeliki Krogfelt, Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark; Department of Science and Environment, Roskilde University, Roskilde, Denmark.
Andreas Munk Petersen, Email: andreas.munk.petersen@regionh.dk, Gastrounit, Medical Division, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Clinical Microbiology, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark.
References
- 1. Iversen DS, Kesmodel US, Ovesen PG. Associations between parity and maternal BMI in a population-based cohort study. Acta Obstet Gynecol Scand. 2018;97:694–700. [DOI] [PubMed] [Google Scholar]
- 2. Ovesen PG, Fuglsang J, Andersen MB, Wolff C, Petersen OB, McIntyre HD. Temporal trends in gestational diabetes prevalence, treatment, and outcomes at Aarhus University Hospital, Skejby, between 2004 and 2016. J Diabetes Res. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–146. [DOI] [PubMed] [Google Scholar]
- 4. Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118:305–12. [DOI] [PubMed] [Google Scholar]
- 5. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DR et al.. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig DS, Currie J.. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TIA. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes. 2009;34:67–74. [DOI] [PubMed] [Google Scholar]
- 8. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. [Internet] Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009; [cited 25 July, 2018]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK32813/. [PubMed] [Google Scholar]
- 9. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab. 2016;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–22. [DOI] [PubMed] [Google Scholar]
- 12. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S et al.. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 13. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al.. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 14. Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–46. [DOI] [PubMed] [Google Scholar]
- 15. Sanchez M, Panahi S, Tremblay A. Childhood obesity: a role for gut microbiota?. Int J Environ Res Public Health. 2014;12:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang Y, Cai Y. The development of probiotics therapy to obesity: a therapy that has gained considerable momentum. Hormones. 2018;17:141–51. [DOI] [PubMed] [Google Scholar]
- 17. Morisset A-S, St-Yves A, Veillette J, Weisnagel SJ, Tchernof A, Robitaille J. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26:17–25. [DOI] [PubMed] [Google Scholar]
- 18. Egan AM, Simmons D.. Lessons learned from lifestyle prevention trials in gestational diabetes mellitus. Diabet Med. 2019;36:142–50. [DOI] [PubMed] [Google Scholar]
- 19. Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. J Matern Fetal Neonatal Med. 2013;26(8):772–8. [DOI] [PubMed] [Google Scholar]
- 20. Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, Brennan L, McAuliffe FM. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr. 2014;99:1432–9. [DOI] [PubMed] [Google Scholar]
- 21. Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, Tobin JM, Wilkinson S, Kothari A, Morrison M et al.. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. 2019;42:364–71. [DOI] [PubMed] [Google Scholar]
- 22. Halkjaer SI, Nilas L, Carlsen EM, Cortes D, Halldórsson TI, Olsen SF, Pedersen AE, Krogfelt KA, Petersen AM. Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial. Trials. 2016;17:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, de Leiva A, Hod M et al.. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawrence EJ. Part 1: a matter of size: evaluating the growth-restricted neonate. Adv Neonatal Care. 2006;6:313–22. [DOI] [PubMed] [Google Scholar]
- 25. Lawrence EJ. A matter of size: Part 2. evaluating the large-for-gestational-age neonate. Adv Neonatal Care. 2007;7:187–97. [DOI] [PubMed] [Google Scholar]
- 26. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–8. [DOI] [PubMed] [Google Scholar]
- 27. Yu Y, Lee C, Kim J, Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng. 2005;89:670–9. [DOI] [PubMed] [Google Scholar]
- 28. Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Riis PT, Larsen N, Andersen LO, Nielsen HV, Miller IM et al.. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krogsgaard LR, Andersen LO'B, Johannesen TB, Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin Transl Gastroenterol. 2018;9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R: The R Project for Statistical Computing. [Internet] [cited 25 Oct, 2018]. Available from: https://www.r-project.org/. [Google Scholar]
- 32. Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC, Candela M, Turroni S, Brigidi P. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C et al.. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111:1507–19. [DOI] [PubMed] [Google Scholar]
- 36. Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43. [DOI] [PubMed] [Google Scholar]
- 37. Isolauri E, Rautava S, Collado MC, Salminen S. Role of probiotics in reducing the risk of gestational diabetes. Diabetes Obes Metab. 2015;17:713–9. [DOI] [PubMed] [Google Scholar]
- 38. Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792–9. [DOI] [PubMed] [Google Scholar]
- 39. Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, Coffey M, Foley ME, Hatunic M, Shanahan F et al.. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gynecol. 2015;212:496.e1–11. [DOI] [PubMed] [Google Scholar]
- 40. Kijmanawat A, Panburana P, Reutrakul S, Tangshewinsirikul C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. J Diabetes Investig. 2019;10:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr. 2013;67:71–4. [DOI] [PubMed] [Google Scholar]
- 42. Jamilian M, Bahmani F, Vahedpoor Z, Salmani A, Tajabadi-Ebrahimi M, Jafari P, Hashemi Dizaji S, Asemi Z. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2016;19:687–682. [PubMed] [Google Scholar]
- 43. Forssten SD, Ouwehand AC.. Simulating colonic survival of probiotics in single-strain products compared to multi-strain products. Microb Ecol Health Dis. 2017;28:1378061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R et al.. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother's weight on infant's microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92:1023–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.