Abstract
Background:
Potential adverse health effects of Asian dust exposure have been reported, but systematic reviews and quantitative syntheses are lacking.
Objective:
We reviewed epidemiologic studies that assessed the risk of mortality, hospital admissions, and symptoms/dysfunction associated with exposure to Asian dust.
Methods:
We performed a systematic search of PubMed and Web of Science to identify studies that reported the association between Asian dust exposure and human health outcomes. We conducted separate meta-analyses using a random-effects model for mortality and hospital admissions for a specific health outcome and assessed pooled estimates for each lag when at least three studies were available for a specific lag.
Results:
We identified 89 studies that met our inclusion criteria for the systematic review, and 21 studies were included in the meta-analysis. The pooled estimates (percentage changes) of mortality from circulatory and respiratory causes for Asian dust days vs. non-Asian dust days were 2.33% [95% confidence interval (CI): 0.76, 3.93] increase at lag 0 and 3.99% (95% CI: 0.08, 8.06) increase at lag 3, respectively. The increased risk for hospital admissions for respiratory disease, asthma, and pneumonia peaked at lag 3 by 8.85% (95% CI: 0.80, 17.55), 14.55% (95% CI: 6.74, 22.94), and 8.51% (95% CI: 2.89, 14.44), respectively. Seven of 12 studies reported reduced peak expiratory flow, and 16 of 21 studies reported increased respiratory symptoms associated with Asian dust exposure. There were substantial variations between the studies in definitions of Asian dust, study designs, model specifications, and confounder controls.
Discussion:
We found evidence of increased mortality and hospital admissions for circulatory and respiratory events. However, the number of studies included in the meta-analysis was not large and further evidences are merited to strengthen our conclusions. Standardized protocols for epidemiological studies would facilitate interstudy comparisons. https://doi.org/10.1289/EHP5312
Introduction
Asian dust is a seasonal meteorological phenomenon caused by dust storms that originate in the deserts of Mongolia and northern China and are carried eastward along mid-latitude westerlies to pass over China, Korea, and Japan. The dust travels thousands of kilometers and absorbs airborne pollutants from anthropogenic sources in industrial areas (Mori et al. 2003; Takemura et al. 2002). The coarse particles of desert dust are considered potentially toxic, and their constituents vary during long-range transport (Mori et al. 2003). Some studies have suggested that the health effects of Asian dust may vary by particle composition (Hiyoshi et al. 2005; Honda et al. 2014). Concerns have also been raised that the microorganisms in the dust may cause allergic reactions based on murine and in vitro studies and that dust events may increase the incidence of respiratory microbial-derived inflammation (Honda et al. 2017; Ichinose et al. 2006, 2008a).
The adverse effects induced by dust have been reported in Southern Europe, which is affected by Saharan dust (Perez et al. 2008; Stafoggia et al. 2016; Zauli Sajani et al. 2011). Multiple studies have reported the effect modification of dust events on the relationship between particulate matter (PM) exposure and mortality; the association of PM with mortality was stronger on dust days than on non-dust days (Jiménez et al. 2010; Mallone et al. 2011; Perez et al. 2008, 2012; Tobías et al. 2011). Other studies (Samoli et al. 2011a; Zauli Sajani et al. 2011) reported that dust events were independent risk factors for mortality, whereas the association of PM and mortality was similar on dust and non-dust days (Zauli Sajani et al. 2011) or was seen only on non-dust days (Samoli et al. 2011a). These discrepancies have also been reported in other studies of Southern European regions (Alessandrini et al. 2013; Middleton et al. 2008; Reyes et al. 2014; Samoli et al. 2011b). A study comparing the associations of desert- and non-desert–sourced in aerodynamic diameter () with mortality and hospital admissions reported that the health effects of desert-derived were of similar magnitude as those of non-desert sources (Stafoggia et al. 2016). The inconsistent findings in the Southern European studies were suggested to be due to different source areas and transport patterns of dust over the western and eastern sides of the Mediterranean (Stafoggia et al. 2016).
Epidemiological studies on the association between desert dust exposures and health outcomes have increased over the last decades. Previous review studies have reported the increased risk of respiratory and circulatory mortality after the dust exposures, but the findings are inconsistent across the studies and quantitative syntheses are lacking (de Longueville et al. 2013; Hashizume et al. 2010; Karanasiou et al. 2012; Zhang et al. 2016). We therefore conducted a systematic review and meta-analysis of epidemiologic studies on the health effects of exposure to Asian dust. To the best of knowledge, this is the first systematic review on the health effects of desert dust to perform a meta-analysis of suitable published studies.
Methods
Search Strategy
This study used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to report the results (Moher et al. 2009).
We performed a systematic search using the PubMed and Web of Science databases (1980 to August 2019) to identify studies that reported the association between exposure to Asian dust and human health outcomes. The search strategy included the following combinations of keywords: ((“asian dust” or “asian sand” or “Asian Desert Dust” or “Yellow Dust” or “Yellow Sand” or “Dust events” or “Desert dust” or “Dust storm”) or ((“sand dust” OR “dust event”) and (Taiwan OR Mongolia OR Korea OR Japan OR Macau OR “Hong Kong” OR China OR “Far East”))) and ((“Adverse effect” or Allergy or “Ambulatory Care” or Ambulatory or Asthma or Cardiac or Cardiopulmonary or Cardiovascular or Death or “Emergency Medical Services” or Epidemiology or “Health Risk” or Health or “Hospital admission” or Hospital or Hospitalization or “Human Experimentation” or Irritants or Morbidity or Mortality or Pulmonary or Respiratory or Symptom or “Air Pollutant” or “Air Pollutants” or “Air Pollution” or “Air Pollutions” or “Adverse effects” or Cardio or “Hospital admissions” or Pneumonia or Stroke or Symptoms)).
The literature search was restricted to articles published in English. The reference lists in the selected articles were searched manually.
Study Eligibility Criteria
We included epidemiological studies that examined the association between exposure to Asian dust and health outcomes. A Populations of interest, Exposures, Comparators, and Outcomes (PECO) statement (Morgan et al. 2018) was developed to identify epidemiological studies relevant to health effects of exposure to Asian dust. The population of interest was populations without any restrictions. Laboratory studies and animal experiments were excluded. Relevant exposures were Asian dust without any restrictions of the definition, measurement methods, length, or timing. Studies that examined the effects of PM were only included if PM was used in defining the Asian dust. Studies that reported other dust events (e.g., Saharan dust, local dust) were excluded. Comparators were nonexposed or lower-exposure individuals or the same individuals at different time points. Outcomes included mortality, hospital admissions or visits, ambulance transport, emergency room attendance, and clinician diagnoses (recorded or self-reported), symptoms, and dysfunction for any health outcomes. Studies with awareness or perception as the outcome were excluded. Conference abstracts, letters, and editorials were excluded.
Study Selection
Titles and abstracts of all papers identified by the electronic searches were screened by two independent reviewers (authors M.H. and Y.N.). The full text of articles that met the selection criteria was then assessed for inclusion eligibility in the systematic review. Disagreements were resolved by discussion between the two reviewers.
Data Extraction
We extracted information from studies that met the inclusion criteria using a standardized checklist. We collected data on study period, location, age group, health outcome, study design, exposure (Asian dust) definition, number of dust-event days, concentrations of , in aerodynamic diameter (), or other PM indicators such as suspended particulate matter (SPM) or in aerodynamic diameter () on event and non-event days, effect estimates and lag period, and confounders controlled in the model. Authors were contacted for information missing from the published reports. The health outcomes were divided into mortality, hospital admissions/visits, and symptoms/dysfunction.
Study Quality Assessment
We used the National Institute of Health (NIH) framework for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) to assess the quality of the studies meeting inclusion criteria. For mortality and hospital admission studies, we adapted the NIH framework in the absence of a validated quality assessment tool for time-series and case-crossover designs, which were commonly used in such studies. Specifically, we modified the selected questions related to exposure assessment [Questions (Q) 6 and 10], outcome assessment (Q11) and confounding control (Q14) for this purpose (see Table S1). For exposure assessment, we examined whether the lagged associations were examined (Q6), whether the study examined different levels of the exposure (Q8), whether the Asian dust event was clearly defined (Q9), and whether the multiple lagged associations were examined (Q10). For outcome assessment, we examined whether mortality or morbidity data were based on the International Classification of Diseases (ICD) (Q11). For confounding control, we assessed whether major potential confounders such as long-term trends, seasonality, and temperature were accounted for in assessing the exposure–outcome associations (Q14). We assigned a good, fair, or poor quality rating, following the NIH framework. The quality assessment was conducted independently by two reviewers (M.H. and Y.K.) and the results were reconciled until a consensus was reached.
Meta-Analysis
Before pooling the estimates, we standardized the extracted data by converting the various forms of reported estimates to a log relative risk (Asian dust days vs. non-dust days) and the corresponding standard error. If a 95% confidence interval (CI) was only available as variance estimates from the studies, we first converted the upper and lower limits to the absolute difference measures (i.e., taking the natural logarithm for relative measures) and divided the difference between upper and lower limits by 3.92 to obtain the standard error (Higgins and Green 2011). We used a random-effects model with a DerSimonian-Laird estimator to pool the estimates. We quantified the extent of heterogeneity with the statistic, representing the proportion of total variance in pooled estimates attributable to heterogeneity in the true effects. The Q statistic was used to address whether the heterogeneity was statistically significant ().
Pooling estimates by specific health outcomes, lag, and subgroups.
We performed meta-analysis for mortality and hospital admissions when at least three studies were available in order to ensure reliability of the pooled estimate (Borenstein et al. 2009) on a specific health outcome sharing ICD codes for a specific lag. Accordingly, mortality studies were further divided into three outcome categories (all-cause, circulatory, and respiratory deaths) and hospital admissions studies were into four categories (respiratory disease, asthma, pneumonia, and ischemic heart disease/acute myocardial infarction). Stratified analysis was performed by sex and age groups (nonelderly and elderly) for a specific health outcome when at least three estimates were available from different populations for a specific lag. The age cutoff of elderly was , as defined in the original study (see Excel Table S2). Meta-analysis was not performed for the symptoms or dysfunction studies because the study design, study subject, and statistical analysis methods varied considerably.
Selecting a representative from multiple effect estimates.
Some studies provided multiple effect estimates for the same health outcome by using different definitions of Asian dust, multiple models, or multiple study sites. If multiple definitions of Asian dust had been used in a given study, we included the estimates for one of the definitions that were most commonly used in other studies in the same city or country. If Asian dust had been classified according to its levels (e.g., moderate, heavy), we included the estimates for the higher level. Meta-analysis was not performed for estimates based on continuous exposure measures [i.e., nonspherical extinction coefficient of the light detection and ranging (LIDAR) method] because it was impossible to convert the continuous dust measure into a binary dust-day indicator based only on the information provided in the original studies. In addition, if multiple models (e.g., different sets of confounders) had been used in a study, we included the estimates from the main model as presented by the original authors. For multicity studies providing city-specific results, we included the estimates from each city separately if the cities were from different countries, but used pooled estimates reported if from the same country. The estimates and 95% CIs included in the meta-analysis for mortality and hospital admissions are shown in Excel Tables S1–S7.
Sensitivity analyses.
We repeated the meta-analysis for studies using only time-series or case-crossover study designs by excluding studies with other study designs. We also repeated analysis for hospital admissions by including five additional studies of which the outcome was hospital visits, emergency room visits, or ambulance transport. Moreover, we examined the robustness of pooled estimates by excluding some studies with largely overlapping periods in the same study location one by one (i.e., the leave-one-out approach).
We planned to address for publication bias by using funnel plots and Begg’s test if more than 10 studies were available, but we did not assess publication bias because of the small number of studies available on each outcome. All analyses were performed using R (version 3.5.1; R Development Core Team) and the metafor package.
Results
The searches of PubMed () and Web of Science () databases produced a total of 1,715 references (Figure 1). An additional article was identified through a manual search of the reference lists of the included articles and 179 duplicates were removed, leaving 1,537 references, of which 1,381 were excluded after reviewing the title or abstract. One hundred fifty-six studies underwent in-depth evaluation, of which 89 met the criteria for qualitative synthesis. Eleven of the studies measured mortality, 45 measured hospital admissions/visits, and 33 measured symptoms/dysfunction. Study characteristics are presented in Tables 1–3. The earliest study was published in 2002 (see Figure S1). For the meta-analysis, we did not consider all of the studies included in the qualitative review for the following reasons [33 described symptoms/dysfunctions, 15 had outcomes with fewer than three estimates, 9 estimated no quantitative risk specifically for Asian dust exposure, 7 described hospital visits/ambulance transport, 4 used continuous exposure variables rather than binary (Asian dust days vs non-dust days)], leaving 21 studies for inclusion in the meta-analysis.
Figure 1.
The literature search and screening results for studies reporting on the associations between Asian dust exposures and human health outcomes displayed as a PRISMA flow diagram (http://www.prisma-statement.org). Note: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RR, relative risk.
Table 1.
Summary of characteristics of mortality studies.
| Study | Period (y) | Location | Age | Outcome | Study design | Exposure definitiona | Days of event | Daily mean () | Multi-lags | Confounder control | Quality ratingb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event days | Non-event days | Seasonc | Trendd | DOW | Temp | Hum | PM | CO | |||||||||||||
| Kwon et al. 2002 | 1995–1998 | Seoul (Korea) | All | All cause, circulatory, and respiratory disease | Time-series | Not specified | 28 | 101.1 | 73.3 | Y | Y | Y | Y | Y | Y | N | N | N | N | N | F |
| Chen et al. 2004 | 1995–2000 | Taipei (Taiwan) | All | All cause, circulatory, and respiratory disease | Ad hoc approache | Particular enhancements in at a background monitoring station | 39 | 125.9 | 57.8 | Y | Y | Y | Y | N | N | N | N | N | N | N | F |
| Chan and Ng 2011 | 1994–2001 | Taipei (Taiwan) | All | All cause, circulatory, and respiratory disease | Case-crossover | Taiwan EPA | 380 | 85.7 44.4 () |
49.6 31.1 () |
Y | Y | Y | Y | Y | N | Y | Y | Y | N | N | G |
| Kashima et al. 2012 | 2005–2010 | 47 cities (Japan) | Elderly () | All cause, circulatory, and respiratory disease | Time-series | Dust exposure was modeled as a continuous variable using dust extinction coefficient by LIDAR | 42 | 44.3 (SPM) | 24.8 (SPM) | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | G |
| Kim et al. 2012 | 2003–2006 | Seoul (Korea) | All | All cause and circulatory disease | Time-series | KMA | 21 | 40.1 () | 41.1 () | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | G |
| Lee et al. 2013 | 2001–2009 | Seven cities (Korea) | All | All cause, circulatory, and respiratory disease | Time-series | KMA | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | G | |||
| Lee et al. 2014 | 2001–2009 | Seoul (Korea), Taipei (Taiwan), Kitakyushu (Japan) | All | All cause, circulatory, and respiratory disease | Time-series | KMA, JMA, Taiwan EPA | 107 (Seoul) 125 (Taipei) 38 (Kitakyushu) |
176.4 (Seoul) 71.4 (Taipei) 60.5 (Kitakyushu) |
65.8 (Seoul) 52.8 (Taipei) 30.8 (Kitakyushu) |
Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | G |
| Wang and Lin 2015 | 2000–2008 | Taipei (Taiwan) | All and Elderly () | All cause and circulatory disease | Time-series | Taiwan EPA | 132 | 82.7 | 48.7 (all days) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | G |
| Kashima et al. 2016 | 2005–2011 | Seoul (Korea), Nagasaki, Matsue, Osaka, Tokyo (Japan) | Elderly () | All cause, circulatory, and respiratory disease | Time-series | Dust exposure was modeled as a continuous variable using dust extinction coefficient by LIDAR | Not specified | Not specified | Not specified | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | G |
| Wong et al. 2017 | 2009–2010 | Hong Kong | All | All cause | Time-stratified spatial regression | Not specified | 8 | Not specified | Not specified | N | Y | Y | Y | Y | N | N | N | N | N | N | F |
| Ho et al. 2018 | 2006–2010 | Hong Kong | All | All cause, circulatory, and respiratory disease | Case-crossover | NASA Aerosol Robotic Network’s sunphotometer size distribution inversion data, the backward trajectories model of hybrid single-particle Lagrangian integrated trajectory, and meteorological reports | 10 | 33.3 () 44.5 () |
33.6 () 18.0 () |
Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | F |
Note: CO, carbon monoxide; DOW, day of week; EPA, Environmental Protection Agency; F, fair; G, good; Hum, humidity; JMA, Japan Meteorological Administration; KMA, Korea Meteorological Administration; LIDAR, light detection and ranging; N, no; , nitrogen dioxide/; , ozone/; P, poor; PM, particulate matter; , in aerodynamic diameter; , in aerodynamic diameter; , in aerodynamic diameter; SPM, suspended particulate matter; , sulfur dioxide; Temp, temperature; Trend, time trend; Y, yes.
Taiwan EPA: 1. Dust storm occurrence in China and Mongolia, 2. Hourly at either two background monitoring stations, and 3. Averaged hourly at three randomly selected stations among 16 stations in the Taipei metropolitan area. KMA: 1. Dust storm occurrence in China and Mongolia, and 2. visual observation. JMA: 1. Dust storm occurrence in China and Mongolia, and 2. visibility .
We used the adapted National Institute of Health (NIH) framework for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) to assess the quality of the studies (see Table S1). We assigned a good (G), fair (F), or poor (P) quality rating following the NIH framework.
If the referent selection was within 30 d of the event days, we regarded that the effects of season was controlled.
If the referent selection was bidirectional or time-stratified, we regarded that the effects of time trend was controlled (Janes et al. 2005).
Ad hoc approach: Comparison of the number of patients between event days and non-event days (7 d before and after each event day).
Table 2.
Summary of characteristics of hospitalization studies.
| Study | Period (y) | Location | Age | Outcome | Source | Study design | Exposure definitiona | Days of event | Daily mean () | Multi-lags | Confounder control | Quality ratingb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event days | Non-event days | Seasonc | Trendd | DOW | Temp | Hum | PM | CO | ||||||||||||||
| Yang et al. 2005a | 1996–2001 | Taipei (Taiwan) | All ages | Stroke | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Yang et al. 2005b | 1996–2001 | Taipei (Taiwan) | All ages | Asthma | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Chen and Yang 2005 | 1996–2001 | Taipei (Taiwan) | All ages | Cardiovascular diseases | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Yang 2006 | 1997–2001 | Taipei (Taiwan) | All ages | Conjunctivitis | Hospital visit | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 49 | 110.4 | 61.7 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Bennett et al. 2006 | 1997–1999 | Vancouver (Canada) | All ages | Cardiac, respiratory diseases | Hospital admission | Comparison of the number of patients between event days in 1998 and non-event days in the same period in 1997 | Gobi dust event in late April 1998 | 4 | 119–123 (hourly peak) | Not specified | N | N | N | N | N | N | N | N | N | N | N | P |
| Chang et al. 2006 | 1997–2001 | Taipei (Taiwan) | All ages | Allergic rhinitis | Hospital visit | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 49 | 110.4 | 61.7 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Meng and Lu 2007 | 1994–2003 | Minqin (China) | All ages | Cardiovascular, respiratory diseases | Hospital admission | Time-series | China Meteorological Administration | 413 | Not specified | Not specified | Y | Y | Y | Y | Y | Y | N | N | N | N | N | F |
| Lai and Cheng 2008 | 2000–2004 | Taipei (Taiwan) | All ages | Respiratory diseases | Hospital admission | Ad hoc approache | Taiwan EPA | 97 | 19.8–33.9 | Not specified | N | Y | Y | Y | N | N | N | N | N | N | N | F |
| Cheng et al. 2008 | 1996–2001 | Taipei (Taiwan) | All ages | Pneumonia | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Chiu et al. 2008 | 1996–2001 | Taipei (Taiwan) | All ages | COPD | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Chan et al. 2008 | 1995–2002 | Taipei (Taiwan) | All ages | Ischemic heart disease, cerebrovascular disease, COPD | Emergency hospital visit | Comparison of the difference between model-predicted patients (without Asian dust effects) and observed patients on Asian dust days | Taiwan EPA High-dust days: Low-dust days: |
85 (39 high-dust days and 46 low-dust days) | 112.7 (high-dust days) 61.1 (low-dust days) |
Not specified | N | Y | Y | Y | Y | N | Y | N | N | Y | N | F |
| Bell et al. 2008 | 1995–2002 | Taipei (Taiwan) | All ages | Asthma, pneumonia, ischemic heart disease, cerebrovascular disease | Hospital admission | Time-series | (a) in Taipei and (b) at the background monitoring station | 2.1% of days for (a), 1.6% of days for (b) | 49.1 (annual mean) 31.6 (, annual mean) |
Not specified | Y | Y | Y | Y | Yf | N | N | N | N | N | N | G |
| Yang et al. 2009 | 1996–2001 | Taipei (Taiwan) | All ages | Congestive heart failure | Hospital admission | Ad hoc approache | Hourly lasting for at least 3 h at the background monitoring station | 54 | 111.7 | 55.4 | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | F |
| Kanatani et al. 2010 | February–April 2005–2009 | Toyama (Japan) | Children (1–15 y old) | Asthma | Hospital admission | Case-crossover | Daily average dust extinction coefficient by the LIDAR method less than from the ground () | 6 | 66.3 (SPM) | 16.9 (SPM) | Y | Y | Y | Y | Y | Y | Yg | Y | Y | Y | N | G |
| Ueda et al. 2010 | 2001–2007 | Fukuoka (Japan) | Children () | Asthma | Emergency hospital admission | Case-crossover | JMA | 106 | 62.8 (SPM) | 34.3 (SPM) | N | Y | Y | Y | Y | Y | N | N | N | N | N | F |
| Ueda et al. 2012 | March–May 2003–2007 |
Nagasaki (Japan) | All ages | Circulatory, respiratory disease | Ambulance transport | Case-crossover | Daily average dust extinction coefficient by the LIDAR method at from the ground but (for heavy Asian dust) and 0.066–0.105/km (for moderate Asian dust) | 17 (heavy Asian dust) 31 (moderate Asian dust) | 57.7 (SPM, heavy dust days) 44.4 (SPM, moderate dust days) |
30.3 (SPM) | Y | Y | Y | Y | Y | Y | Yg | N | N | N | N | G |
| Kamouchi et al. 2012 | 1999–2010 | Fukuoka (Japan) | Adults () | Ischemic stroke | Hospital admission | Case-crossover | JMA | 137 | 59.6 (SPM) | 29.5 (SPM) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | G |
| Yu et al. 2012 | 1997–2007 | Taipei (Taiwan) | Children () | Respiratory diseases | Hospital visit | Spatiotemporal modeling | CCU database (1997–2000) Taiwan EPA (2001–2007) | 172 | 90.6 | 52.7 | Y | Y | Y | Y | Y | N | N | N | N | N | N | G |
| Kang et al. 2012 | 2000–2009 | Taipei (Taiwan) | All ages | Pneumonia | Hospital admission | Time-series | at three background monitoring stations | 135 | 121.7 | 49.2 | Y | N | Y | N | Y | N | N | Y | Y | N | Y | F |
| Tam et al. 2012a | 1998–2002 | Hong Kong (China) | Not specified | Circulatory diseases | Hospital admission | Case-crossover | • • • Predominant easterly wind profile • At least three times higher concentrations of dust storm tracer elements than annual averages |
5 | 134.3 59.9 () |
49.9 35.2 () |
Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | F |
| Tam et al. 2012b | 1998–2002 | Hong Kong (China) | Not specified | Respiratory diseases | Hospital admission | Case-crossover | • • • Predominant easterly wind profile • At least three times higher concentrations of dust storm tracer elements than annual averages |
5 | 134.3 59.9 () |
49.9 35.2 () |
Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | F |
| Chien et al. 2012 | 1997–2007 | Taipei (Taiwan) | Children () | Respiratory diseases | Hospital visit | Spatiotemporal modeling | CCU database (1997–2000) Taiwan EPA (2001–2007) |
172 | Not specified | Not specified | Y | N | Y | Y | Y | N | Y | N | N | N | N | G |
| Tao et al. 2012 | March–May 2001–2005 |
Lanzhou (China) |
Not specified | Respiratory diseases | Hospital admission | Time-series | Meteorological Bureau of Gansu Province | 49 | 536.1 | 190.6 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | F |
| Yu et al. 2013 | 1998–2007 | Taipei (Taiwan) | Children () | Respiratory diseases | Hospital visits | Spatiotemporal modeling | Taiwan EPA | 164 | 75.9 | 51.4 | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | F |
| Kang et al. 2013 | 2000–2009 | Taipei (Taiwan) | All ages | Stroke | Hospital admission | Time-series | at three background monitoring stations | 135 | 121.7 | 49.2 | Y | Y | Y | N | Y | N | N | Y | N | N | Y | F |
| Kashima et al. 2014 | 2006–2010 | Okayama (Japan) | Elderly () | All causes, cardiovascular, cerebrovascular, pulmonary diseases | Ambulance transport | Time-series | Asian dust was modeled as a continuous variable using the dust extinction coefficient by the LIDAR method | Not specified | 43.8 (SPM, moderate dust days) 67.3 (SPM, severe dust days) |
25.3 (SPM) | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | G |
| Chien et al. 2014 | 2002–2007 | Taipei (Taiwan) | Children () | Conjunctivitis | Hospital visit | Spatiotemporal modeling | Taiwan EPA | 90 | 81.1 | 53.3 | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | F |
| Wang et al. 2014 | 2000–2009 | Taiwan | All ages | Asthma | Hospital admission | Time-series | Taiwan EPA | Not specified | Not specified | Not specified | Y | Y | Y | N | Y | N | N | Y | Y | N | Y | F |
| Matsukawa et al. 2014 | 2003–2010 | Fukuoka (Japan) | Adults () | Acute myocardial infarction | Hospital admission | Case-crossover | JMA | 75 | 58.1 | 29.7 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | G |
| Nakamura et al. 2015 | 2005–2008 | Seven prefectures (Japan) | All ages | Out-of-hospital cardiac arrest | Utstein-style data | Case-crossover | 1. Daily maximum dust extinction coefficient by the LIDAR method 2. Daily maximum SPM 3. Correlation coefficient between hourly Asian dust extinction coefficient and hourly SPM |
average 28 (minimum 7, maximum 79) | Not specified | Not specified | Y | Y | Y | Y | Y | Y | Yg | Y | Y | Y | N | G |
| Teng et al. 2016 | 2000–2009 | Taiwan | All ages | Acute myocardial infarction | Hospital admission | Time-series | Taiwan EPA | 46 events | Not specified | Not specified | Y | Y | Y | N | Y | N | N | N | N | Y | Y | F |
| Lin et al. 2016 | 2000–2008 | Taipei (Taiwan) | All ages | All causes, circulatory, respiratory diseases | Emergency room visit | Time-series | Taiwan EPA | 132 | Not specified | Not specified | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | G |
| Wang et al. 2016 | 2005–2012 | Minqin (China) | All ages | Pulmonary tuberculosis | Hospital visit | 1. Seasonal periodicity analysis 2. Linear regression with three atmospheric variables (visibility, duration, and wind speed) |
China Meteorological Administration | Not specified | Not specified | Not specified | N | N | N | N | N | N | N | N | N | N | N | P |
| Y-S Park et al. 2016 | 2007–2013 | Seoul and Incheon (Korea) | All ages | Asthma | Hospital visit | Case-crossover | KMA | 7 | 448.6 (daily maximum) | 163.1 (daily maximum) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | F |
| Nakamura et al. 2016 | 2010–2013 | Nagasaki (Japan) | Children () | Asthma, respiratory disease | Emergency room visit | Case-crossover | 1. Daily maximum dust extinction coefficient by the LIDAR method 2. Daily maximum SPM 3. Correlation coefficient between hourly Asian dust extinction coefficient and hourly SPM |
47 | 53.1 (SPM) | 24.0 (SPM) | Y | Y | Y | Y | Y | Y | Yg | Y | Y | Y | N | G |
| Ma et al. 2016 | 2007–2011 | Lanzhou (China) | All ages | Respiratory diseases | Emergency room visit | Time-series, effect modification of , , and by Asian dust | Visibility | 32 | 324 | 146 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | F |
| Altindag et al. 2017 | 2003–2011 | All cities (Korea) | Birth | Birth weight, low birth weight, gestation, premature birth, fetal growth | Birth certificate | Linear regression model | KMA | Not specified | Not specified | Not specified | N | Y | Y | N | Y | Y | Y | N | N | N | N | F |
| Kojima et al. 2017 | 2010–2015 | Kumamoto (Japan) | All ages | Acute myocardial infarction | Hospital admission/visit | Case-crossover | JMA | 41 | 34.9 () | 20.5 () | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | G |
| Sakata et al. 2017 | 1989–2012 | Fukuoka (Japan) | All ages | Pollinosis | Hospital visit | Time-series | JMA | 238 | 58.9 (SPM) | 29.4 (SPM) | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | G |
| Liu et al. 2017 | 2006–2008 | Central Taiwan | All ages | All causes, circulatory, respiratory diseases | Emergency room visit | Case-crossover, effect of during Asian dust | Taiwan EPA | 16 | 133.0 61.9 () |
77.8 48.9 () |
Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | F |
| Kashima et al. 2017 | 2006–2010 | Okayama (Japan) | Elderly () | Circulatory, respiratory diseases | Emergency room visit | Case-crossover | Asian dust was modeled as a continuous variable using the dust extinction coefficient by the LIDAR method | 26 | 185.6 (converted from nonspherical extinction coefficient) | Not specified | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | G |
| Ma et al. 2017a | 2007–2011 | Lanzhou (China) | All ages | All causes, circulatory diseases | Hospital admission | Time-series, effect modification of , , and by Asian dust | Horizontal visibility | 32 | 324 | 146 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | F |
| Ma et al. 2017b | 1965–2005 | Gansu (China) | All ages | Measles | Not specified | Correlation, time-series | Meteorological Bureau of Gansu Province | Not specified | Not specified | Not specified | N | Y | Y | N | N | N | N | N | N | N | N | P |
| Chan et al. 2018 | 2000–2009 | Taiwan | All ages | Diabetes | Hospital admission | Time-series | Taiwan EPA | 55 | Not specified | Not specified | Y | Y | Y | N | Y | N | N | N | Y | Y | Y | F |
| Wang et al. 2018 | March 2016 | Inner Mongolia (China) | All ages | Cardiovascular, respiratory diseases | Hospital visit | Correlation | Not specified | 4 | Not specified | Not specified | N | N | N | N | N | N | N | N | N | N | N | F |
Note: CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; DOW, day of week; EPA, Environmental Protection Agency; F, fair; G, good; Hum, humidity; JMA, Japan Meteorological Administration; KMA, Korea Meteorological Administration; LIDAR, light detection and ranging; N, no; , nitrogen dioxide/; , ozone/; P, poor; PM, particulate matter; , in aerodynamic diameter; , in aerodynamic diameter; SPM, suspended particulate matter;, sulfur dioxide; Temp, temperature; Trend, time trend; Y, yes.
Taiwan EPA: 1. Dust storm occurrence in China and Mongolia, 2. Hourly at either of two background monitoring stations, and 3. Averaged hourly at three randomly selected stations of 16 in the Taipei metropolitan area. Chinese Culture University database: 1. Visibility less than for 24 h in any of three neighboring First GARP Global Experiment-type ground stations in East Asia, and 2. concentrations observed by at least one of the air quality monitoring stations located in Wanli, Guanyin, Danshui, and Yilan. KMA (Korea Meteorological Administration): 1. Dust storm occurrence in China and Mongolia, and 2. Visual observation. JMA (Japan Meteorological Administration): 1. Dust storm occurrence in China and Mongolia, and 2. Visibility . China Meteorological Administration: Unknown definition of Asian dust.
We used the adapted National Institute of Health (NIH) framework for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) to assess the quality of the studies (see Table S1). We assigned a good (G), fair (F), or poor (P) quality rating following the NIH framework.
If the referent selection was within 30 d of the event days, we regarded the effects of season as controlled.
If the referent selection was bidirectional or time-stratified, we regarded the effects of time trend as controlled (Janes et al. 2005).
Ad hoc approach: Comparison of the number of patients between event days and non-event days (7 d before and after of each event day).
Extinction coefficients for spherical particles.
Apparent temperature.
Table 3.
Summary of characteristics of symptom and dysfunction studies.
| Study | Period (y) | Location | Subject | Outcome | Study design/statistical analysis | Exposure definition | Days of event | Daily mean () | Summary results | Confounder control | Quality ratinga | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event days | Non-event days | |||||||||||
| Park et al. 2005 | March–June 2002 | Incheon (Korea) | 64 asthmatic adults (16–75 y old) | Changes in PEF, respiratory symptoms | PEF, respiratory symptoms, and daily activities were recorded twice daily. Generalized estimating equations were used to analyze the relationship between respiratory symptoms, pulmonary function, and air pollution levels. | Reduced visibility and meteorological experts’ judgment | 14 | 188.5 | 60.0 | An increase in concentrations was associated with increases in PEF variability of , more nighttime symptoms, and a decrease in the mean PEF. | Time trend | P |
| Yoo et al. 2008 | March–May 2004 | Seoul (Korea) | 52 asthmatic children | Changes in PEF, respiratory symptoms | Respiratory symptoms and PEF were recorded twice daily; participants underwent methacholine bronchial challenge tests. Average levels of respiratory symptoms and pulmonary function parameters on the Asian dust days and the following 2 d were compared with those of the control days using the Kruskal-Wallis test. | KMA | 5 | Not specified | Not specified | The prevalence of acute respiratory symptoms and signs was significantly higher during Asian dust days. Reduction in morning and evening PEF and increases in PEF diurnal variability and bronchodilator response were significant during Asian dust days. | Not specified | P |
| Hong et al. 2010 | May–June 2007 | Seoul (Korea) | 110 school children (9 y old) | Changes in PEF | PEF was measured three times a day and daily mean PEF was used for analysis. Linear mixed-effects model was used to estimate particulates or metal effects on daily PEF. | Not specified | Not specified | Not specified | and concentrations were not significantly associated with PEF except in asthmatics. Most of the metals bound to the particulates were associated with a decrease in children’s PEF. | Age, sex, height, weight, asthma history, passive smoking, daily mean temperature, mean relative humidity, air pressure, time trend | F | |
| Mu et al. 2011 | May–2008 | Ulaanbaatar, Gobi Desert (Mongolia) | 36 urban and 87 desert area residents (all ages) | Eye and respiratory symptoms | The prevalence of subjective eye and respiratory symptoms was compared between 36 urban and 87 desert area residents after a dust storm. | A dust storm on 26–27 May 2008 | Not specified | Not specified | Not specified | The prevalence of tearing but not respiratory symptoms were significantly higher in the desert area residents than in the urban area residents. | Age, sex, and smoking | P |
| Watanabe et al. 2011a | February–March, December 2009 | Yonago (Japan) | 145 asthmatic adults () | Changes in PEF, respiratory symptoms | PEF was measured three times a day. Mean morning PEF was compared between Asian dust days and non-dust days. | JMA/MOE | 11 | 65.3 | 27.8 | There was no significant difference in mean morning PEF and respiratory symptoms between Asian dust days and non-dust days. | Temperature, atmospheric pressure, pollen, , , | F |
| Watanabe et al. 2011b | April–May 2007 | Yonago (Japan) | 98 asthmatic adults () | Changes in PEF, respiratory symptoms | Telephone survey: Aggravation of respiratory symptoms (cough, sputum, dyspnea, wheezing) 3 d after the Asian dust event. Mean morning PEF was compared between Asian dust days and non-dust days. | JMA/MOE | 10 | 101.2 | 40.5 | 22% reported worsening lower respiratory symptoms during Asian dust events and significant reduction of the lowest PEF over a week expressed as a percentage of the highest PEF (Min%Max) during 6 d after the dust event. | Not specified | F |
| Otani et al. 2011 | February 2009 | Yonago (Japan) | 54 healthy volunteers | Nasopharyngeal, ocular, respiratory, and dermal symptoms | Symptom scores collected by questionnaire were compared between Asian dust days and non-Asian dust days. | JMA | 6 | 33.0 (SPM) | 15.6 (SPM) | The total symptom score on Asian dust days was significantly higher than on non-Asian dust days. The dermal symptom scores were positively correlated with levels of SPM. | Not specified | P |
| Otani et al. 2012 | March 2010 | Yonago (Japan) | 62 healthy volunteers | Dermal symptoms and allergic reactions to heavy metals | Allergic reactions to heavy metals were examined by patch test and compared between 9 participants with dermal symptoms and 11 participants without dermal symptoms on Asian dust days. | JMA | 1 | 151 (SPM) | Not specified | Reactions to iron, aluminum, and nickel were higher in participants with dermal symptoms on Asian dust days. | Not specified | P |
| Watanabe et al. 2012 | March 2007–2010 | Tottori (Japan) | 46 asthma patients | Respiratory, ocular, and dermal symptoms | Telephone survey: respiratory, ocular, and dermal symptoms on the Asian dust days compared with a week prior. | JMA/MOE | 8 | 32.0–151.0 (SPM) | Not specified | The number of patients who reported exacerbation of symptoms varied between dust events. Only two patients consistently reported symptom exacerbation. | Not specified | P |
| Onishi et al. 2012 | February–March 2009 | Yonago (Japan) | 54 healthy volunteers | Nasopharyngeal, ocular, respiratory, and dermal symptoms | Symptom scores recorded by questionnaire were compared between before and after Asian dust days and stratified by dust component | SYNOP report of WMO and JMA | 9 | Non-mineral dust aerosols: Type 1: 28.3–56.1; Type 2: 24.3–38.3; Type 3: 9.1 | Not specified | Nasal and ocular symptoms scores increased after exposure to Type 1 events. Nasal symptom scores decreased after exposure to Type 3 events. | Not specified | F |
| Otani et al. 2014 | 2012 | Tottori (Japan) | 25 healthy volunteers | Nasal, pharyngeal, ocular, respiratory, and dermal symptoms | Symptom scores recorded by questionnaire were correlated with serum IgE levels measured after the Asian dust event. | JMA | 3 | 34.3 (SPM) | 13.6 (SPM) | There was a positive association between nasal symptom scores and two microbial-specific IgE levels. | Not specified | P |
| Ogi et al. 2014 | February–March 2009 | Fukui (Japan) | 41 patients with nasal and ocular allergy symptoms () | Nasopharyngeal and ocular symptoms, medication use | Symptom scores recorded by diary were compared between Asian dust days and non-Asian dust days. | Visibility | Not specified | Not specified | Not specified | Scores for nasal and ocular symptoms increased after an Asian dust event both pre- and post-Japanese cedar pollen season. | Not specified | P |
| Mimura et al. 2014 | March 2011 | Tokyo (Japan) | 10 allergic rhinoconjunctivitis patients, 3 atopic keratoconjunctivitis patients, 10 healthy controls | Skin pick tests positive | Skin pick tests were performed with untreated Asian dust, Asian dust extract, heat-sterilized Asian dust, silicon dioxide, and phosphate-buffered saline. | Not specified | Not specified | Not specified | Not specified | Positive skin patch tests for untreated Asian dust, Asian dust extract, and heat-sterilized Asian dust were higher in the conjunctivitis groups than in the control group. | Not specified | P |
| Higashi et al. 2014a | 2011 | Kanazawa (Japan) | 86 adult asthma patients | Cough | Incidence of cough symptoms recorded by diary was regressed with graded categories of Asian dust days. | Five-grade categories of Asian dust days created by dust extinction coefficient (LIDAR) | Not specified | Not specified | Not specified | A dose-response relationship between Asian dust concentrations and daily cough incidence was observed. | Sex, age, body mass index, temperature, rain, seasonality, spherical particles (LIDAR), and | G |
| Higashi et al. 2014b | 2011 | Kanazawa (Japan) | 86 adult patients with chronic cough | Respiratory, nasal, and ophthalmic allergic symptoms | Incidence of symptoms recorded by diary was compared between Asian dust days and non-dust days. | Four consecutive days when the dust extinction coefficient at above the ground | 15 | 68.4–125.1 (TSP) | 17.5 (TSP) | More patients experienced coughing and itchy eyes during Asian dust periods. | Not specified | F |
| Watanabe et al. 2014 | February–May 2011 | Tottori (Japan) | 231 adult asthma patients | Changes in PEF, respiratory symptoms | Daily PEF and respiratory symptom scores recorded by diary were compared between Asian dust days and non-Asian dust days. | JMA | 3 | 64.0–109.0 (SPM) | 17.2–28.8 (SPM) | Upper and lower respiratory tract symptom scores were higher on Asian dust days. There was no significant association between daily PEF and Asian dust exposure. | Not specified | P |
| Onishi et al. 2015 | February 2009 | Yonago (Japan) | 54 healthy volunteers | Nasopharyngeal, ocular, respiratory, and dermal symptoms | Symptom scores recorded by questionnaire were regressed with air pollutants and ambient heavy metals. | JMA | 6 | 35.8 (SPM) | 16.8 (SPM) | The dermal symptom score was positively associated with levels of SPM and nickel. Heavy metal levels were significantly higher on Asian dust days. | Not specified | P |
| Watanabe et al. 2015a | March–May 2012 | Yonago (Japan) | 33 adult asthma patients | Changes in PEF and fractional exhaled nitric oxide (FeNO) | Daily records of morning PEF and FeNO were compared between Asian dust days and non-Asian dust days using linear regression. | JMA | 2 | Not specified | Not specified | No significant association of PEF and FeNO with Asian dust exposure. | Not specified | P |
| Watanabe et al. 2015b | 2012–2013 | Matsue (Japan) | 399 schoolchildren (8–9 y old) | Changes in PEF | Daily records of morning PEF were compared between Asian dust days and non-Asian dust days using linear mixed models. | JMA and LIDAR method | 7 | 17.2–37.8 () | 10.3 in 2012 () 17.5 in 2013 () |
PEF decreased after Asian dust exposure from Day 0 to Day 3. | Age, sex, height, weight, allergy history, air pollutants (SPM, , , , ) and weather conditions (temperature, humidity, and atmospheric pressure) | F |
| Watanabe et al. 2015c | March–May 2013 | Yonago (Japan) | 137 asthma patients | Changes in PEF, respiratory symptoms | Daily PEF and respiratory symptom scores recorded by diary were compared between Asian dust days and non-Asian dust days using linear mixed models. | Hourly dust extinction coefficient (LIDAR) | 8 | Not specified | Not specified | Symptom scores were higher on Asian dust days. There was no significant association between PEF and Asian dust exposure. | Age, sex, smoking, allergy history, treatments, pulmonary function, air pollutants (, , ), and weather conditions (temperature, humidity, and atmospheric pressure) | F |
| Aili and Oanh 2015 | February–May 2013 | Burgur (China) | 810 residents (all ages) | Symptoms (cough, expectoration, shortness of breath, heavy chest, dry throat, dry eyes, tears, runny nose, sneezing, depressed mood) | Severity of symptoms recorded by questionnaire was compared between suspended dust days, blowing dust days, sand storm days, and non-dust days. | Suspended dust: visibility , blowing dust: visibility , sand storm: wind velocity and visibility | Suspended dust: 26; blowing dust: 8; sand storm: 3 | 1,073 (TSP, suspended dust); 1,379 (TSP, blowing dust); 2,522 (TSP, sand storm) | Not specified | Air pollutants that increased during the dust event were correlated with respiratory symptoms and ear, nose, and throat symptoms. | Not specified | P |
| Wang et al. 2015 | 2011 | Minqin (China) | 728 farmers () | Respiratory diseases and symptoms | Prevalence of respiratory symptoms measured by questionnaire was compared between randomly selected farmers living in exposed towns (near the desert) and control town. | China Meteorological Administration | Not specified | Not specified | Not specified | The odds ratios of chronic rhinitis, chronic bronchitis, and chronic cough were 3.1, 2.5, and 1.8, respectively. | Not specified | P |
| Watanabe et al. 2016a | April–May 2012 | Shimane (Japan) | 399 schoolchildren | Changes in PEF | The association between daily records of morning PEF and dust extinction coefficient was analyzed using linear mixed models. | Asian dust was modeled as a continuous variable using the dust extinction coefficient (LIDAR) | Not specified | Not specified | Not specified | Increase in sand dust particles was associated with a decrease in PEF. | Sex, height, weight, allergy history, air pollutants (SPM, , , , ), and weather conditions (temperature, humidity, and atmospheric pressure) | F |
| Watanabe et al. 2016b | March–May 2012 | Tottori (Japan) | 231 adult asthma patients | Changes in PEF | The association between daily records of morning PEF and Asian dust exposures was analyzed using linear mixed models. | Daily average dust extinction coefficient (LIDAR) at a altitude | 6 | Not specified | Not specified | Daily PEF was significantly lower on Asian dust days. | Age, sex, smoking, allergic rhinitis, treatments, weather conditions (temperature, humidity, and atmospheric pressure) and air pollutants (, , , spherical particles, SPM, and ) | F |
| Watanabe et al. 2016c | 2012 | Tottori (Japan) | 231 adult asthma patients | Changes in PEF | The association between daily records of morning PEF and Asian dust exposures was analyzed using linear mixed models. | JMA | 2 | Not specified | Not specified | PEF decreased after exposure to heavy Asian dust in patients with asthma and in patients with asthma and COPD. | Age, sex, smoking, treatment, ACT score, and weather conditions (temperature, humidity, and atmospheric pressure) | F |
| Kanatani et al. 2016 | 2011, 2013 | Kyoto, Toyama, Tottori (Japan) | 3,327 pregnant women | Allergic symptoms (allergy-control score) | Symptom scores were collected by questionnaire and compared between Asian dust and non-Asian dust days. | Daily average dust extinction coefficient (LIDAR) at a 135-m altitude | 27 | 34.2 (SPM); 27.6 () | 11.6 (SPM); 11.8 () | Pregnant women had an increased risk of allergic symptoms on high desert-dust days. The increase was mostly driven by sensitivity to Japanese cedar pollen. | Pollen, , , , spherical particles, air pressure, pressure change from the previous day, mean temperature, temperature change from the previous day, temperature change within the day, minimum temperature, humidity | G |
| Ko et al. 2016 | March–May 2013 | Fukuoka (Japan) | 45 patients with acute conjunctivitis | Acute conjunctivitis | Clinical symptoms were recorded and scored for patients newly diagnosed with acute conjunctivitis. The clinical scores were compared between patients with higher and lower silicon/aluminum-rich compounds (components of Asian dust). | JMA | 2 | Not specified | Not specified | Clinical conjunctivitis scores were higher in patients on Asian dust days. | Not specified | P |
| Majbauddin et al. 2016 | March 2013 | Yonago (Japan) | 42 healthy volunteers (mean age 33.6 y old) | Nasal, ocular, respiratory, and skin symptoms | Symptom scores collected by web-based survey application were compared between Asian dust days and non-Asian dust days. | JMA | 4 | 52.3 (SPM) 40.9 () |
19.6 (SPM) 17.1 () |
Ocular, nasal, and skin symptom scores were significantly higher on Asian dust days than on non-Asian dust days. | Not specified | P |
| Watanabe et al. 2017 | February 2015 | Matsue (Japan) | 345 elementary school students (10–12 y old) | Skin symptoms | Daily records of skin symptoms were regressed with air pollutants and Asian dust indicator measured by LIDAR | Daily median dust extinction coefficient (LIDAR) at a altitude | Not specified | Not specified | Not specified | Dust extinction coefficient was not associated with skin symptoms. | Sex, height, weight, asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and food allergies and meteorological variables (temperature, humidity, and atmospheric pressure) | F |
| Onishi et al. 2018 | October–November 2011 | Yonago (Japan) | 29 healthy volunteers (mean age 39.3 y old) | Nasal, ocular, respiratory and skin symptoms, fever, headache | Symptom scores recorded by diary questionnaire were regressed with air pollutants (dust, , BC, OC). | Dust concentrations by the numerical aerosol simulation models | Not specified | Not specified | Not specified | A significant linear association of dust concentrations with respiratory symptoms was observed. | Age, sex, temperature, humidity, and atmospheric pressure | F |
| Li et al. 2018 | 2010–2012 | North of Qinling Mountail-Huaihe River Line (China) | 2,693 children (30–180 months old) | Cognitive function | The association between prenatal exposure to dust and children’s cognitive function was examined using data from a nationally representative panel study. | Visibility , observation of the storm at three or more neighboring meteorological stations | 6 d (median exposure during the entire prenatal period) | Not specified | Not specified | Prenatal exposure to dust in the seventh gestational month was significantly associated with reduced mathematics test scores and word test scores, additional months to begin speaking in sentences and to begin counting. | Children’s, parents’, and household characteristics and cooking fuel | G |
| Nakao et al. 2018 | 2013–2015 | Hwaseong (Korea) | 75 COPD patients (40–79 y old) | Respiratory symptoms and health-related quality of life | Panel study: patients with and without COPD filled out the symptom questionnaire and were followed up. | Criteria by National Institute of Environmental Research | Not specified | Not specified | Not specified | There was no evidence for the association between dust events and respiratory symptoms. | Age, sex, body mass index, smoking, COPD severity, use of air conditioner, time spent outdoors | F |
| Nakao et al. 2019 | 2010–2015 | Kumamoto/Niigata (Japan) | 2,287 healthy adults (40–79 y old) | Respiratory symptoms and health-related quality of life | Panel study: participants filled out the symptom questionnaire and were followed up. | Not specified | 39 (Kumamoto); 12 (Niigata) | Not specified | Not specified | Increased number of dust exposures was associated with cough in Kumamoto and with allergic symptoms in both areas. | Years of survey, age, sex, body mass index, smoking, working status | F |
Note: BC, black carbon; COPD, chronic obstructive pulmonary disease; F, fair; G, good; JMA, Japan Meteorological Agency; KMA, Korea Meteorological Agency; LIDAR, Light detection and ranging; MOE, Ministry of Environment; , nitrogen dioxide; OC, organic carbon; , ozone; P, poor; PEF, peak expiratory flow; PM, particulate matter; , in aerodynamic diameter; , in aerodynamic diameter; , sulfur dioxide; , sulfate; SPM, suspended particulate matter; TSP, total suspended particulates; WMO, World Meteorological Organization.
We used the National Institute of Health (NIH) framework for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) to assess the quality of the studies (see Table S1). We assigned a good (G), fair (F), or poor (P) quality rating following the NIH framework.
Study Quality Assessment
Study quality varied substantially between the outcome categories (Tables 1–3). Overall, we rated quality more highly in mortality studies followed by hospital admission and symptoms/dysfunction studies. Seven (63.6%) studies were rated good in mortality studies, and 14 (31.1%) and 3 (9.1%) studies were rated good in hospital admission and symptom/dysfunction studies, respectively. Sixteen (48.5%) studies were rated poor in symptom/dysfunction studies, and 3 (6.7%) and 0 studies were rated poor in hospital admission and mortality studies, respectively. The high proportion of poor quality in symptom/dysfunction studies was mainly because multiple studies in this category did not control for confounders or did not describe confounder control, did not report how information bias was controlled when the outcomes were self-reported symptoms or self-measured PEF, or were of a cross-sectional design that is prone to causal inferences between exposures and outcomes. The lower-rated quality in hospital admission studies compared with mortality studies is partly because more hospital admission studies were conducted in the early 2000s, before the standard time-series or case-crossover designs were widely used in this field.
Mortality
All 11 studies assessed all-cause mortality (Table 1). Nine studies assessed mortality from circulatory causes (Chan and Ng 2011; Chen et al. 2004; Ho et al. 2018; Kashima et al. 2012, 2016; Kim et al. 2012; Lee et al. 2013, 2014; Wang and Lin 2015), 7 assessed mortality from respiratory causes (Chan and Ng 2011; Chen et al. 2004; Ho et al. 2018; Kashima et al. 2012, 2016; Lee et al. 2013, 2014), and 1 assessed mortality from both causes combined (Kwon et al. 2002). Seven studies used a time-series design (Kashima et al. 2012, 2016; Kim et al. 2012; Kwon et al. 2002; Lee et al. 2013, 2014; Wang and Lin 2015), 2 used a case-crossover design (Chan and Ng 2011; Ho et al. 2018), and 1 compared the number of deaths between dust days and control days using Poisson regression (Chen et al. 2004). For exposure definition, five studies used a local meteorological authority’s definition of Asian dust (Chan and Ng 2011; Kim et al. 2012; Lee et al. 2013, 2014; Wang and Lin 2015), and 2 used the LIDAR as an indicator of Asian dust with the nonspherical extinction coefficient as a continuous variable (Kashima et al. 2012, 2016). Ten studies assessed multiple lag associations (Table 1).
Figure 2 displays the individual and pooled estimates of the associations between Asian dust exposure at lag 0 and all-cause, circulatory, and respiratory mortality. The pooled estimate (percentage change) of circulatory mortality for Asian dust days vs. non-Asian dust days at lag 0 was 2.33% (95% CI: 0.76, 3.93) (, , , ). There was no evidence of a pooled association between all-cause mortality and Asian dust exposure at any lag up to lag 7 (Figure 3; see also Excel Table S1). There was a 3.99% (95% CI: 0.08, 8.06) increase in the pooled estimate of mortality from respiratory causes at lag 3 (, , , ). There was little evidence of heterogeneity between the estimates for all three outcomes at all lags. The association between Asian dust and all-cause mortality did not differ between age groups (see Figure S2, Excel Table S2).
Figure 2.
Forest plot of the meta-analysis for the association of all-cause, circulatory, and respiratory mortality with Asian dust days vs. non-Asian dust days at lag 0. Solid squares represent point estimates (percentage changes) of the individual studies, and the whiskers represent the 95% CIs. Arrowheads indicate where the CI extends outside the range allocated. Diamonds represent the pooled random-effects estimates, with the width indicating the 95% CIs. The vertical dotted line represents a percentage change of 0. Point estimates and 95% CIs for Chen et al. 2004 and Kim et al. 2012 were recalculated by the authors based on the information in the paper. Note: CI, confidence interval; df, degrees of freedom; RE, random effects.
Figure 3.
Random-effects pooled estimates (percentage changes) of mortality for Asian dust days vs. non-Asian dust days, stratified by outcome and lag time. Solid squares represent point estimates (percentage changes) of the individual studies, and the whiskers represent the 95% CIs. Arrowheads indicate where the CI extends outside the range allocated. The vertical dotted line represents a percentage change of 0. Note: CI, confidence interval.
We repeated the analysis by limiting the studies to those with time-series and case-crossover designs by excluding one study (Chen et al. 2004) for all three categories of mortality outcomes. The study selection did not affect the main results and interpretation, but the pooled estimate for mortality from respiratory causes at lag 3 increased slightly to 4.22% (95% CI: 0.28, 8.32) (see Figure S3, Excel Table S3). Sensitivity analysis by excluding studies with largely overlapping periods in the same study location (the leave-one-out approach) showed that the pooled estimates were robust (see Figure S4, Excel Table S1).
Hospital Admissions
Studies examining hospital admissions/visits listed multiple diseases and conditions as the cause (Table 2). Fourteen studies used a time-series design, 13 used a case-crossover design, 11 simply compared the number of deaths between dust days and control days, 4 used spatiotemporal modeling (Chien et al. 2012, 2014; Yu et al. 2012, 2013), 2 used seasonal periodicity analysis and/or linear regression (Altindag et al. 2017; Wang et al. 2016), and 1 used correlation analysis (Wang et al. 2018). In terms of exposure definition, 20 studies used a local meteorological authority’s definition of Asian dust, and 17 studies set their own definition using PM of a certain diameter, with or without other indicators such as wind profile or LIDAR. Four studies relied solely on LIDAR as a continuous variable (Kashima et al. 2014) or with a certain threshold for the nonspherical extinction coefficient (Kanatani et al. 2010; Kashima et al. 2014; Ueda et al. 2012), 2 studies used visibility (Ma et al. 2016, 2017a), and 1 study did not report the definition (Wang et al. 2018). Most of the studies published before 2010, with the exception of two (Bell et al. 2008; Meng and Lu 2007), simply compared the number of patients between event days and non-event days (7 d before and after each event day) rather than using time-series or case-crossover analysis.
Figure 4 shows the associations between Asian dust exposure at lag 3 [the lag with the most associations in the pooled analysis (Figure 5)] and hospital admissions for respiratory disease, asthma, pneumonia, and ischemic heart disease/acute myocardial infarction. The meta-analysis was not applied to other hospitalization causes because there were fewer than three estimates. The lag pattern of the meta-analysis showed evidence of a positive association for respiratory diseases (lag 3), asthma (lags 1 and 3), and pneumonia (lags 1 and 3) (Figure 5; see also Excel Table S4). The increased risk for respiratory diseases (8.85%), asthma (14.55%), and pneumonia (8.51%) peaked at lag 3. There was little evidence of an association with ischemic heart disease/acute myocardial infarction. There was little evidence of heterogeneity between the estimates for respiratory disease (lag 3) and asthma (lags 1 and 3) when there was an evidence of the pooled association (). The association between Asian dust exposure and hospital admissions for respiratory distress did not differ by sex (see Figure S5, Excel Table S5).
Figure 4.
Forest plot of the meta-analysis for the association of hospital admissions for respiratory disease, asthma, pneumonia, and ischemic heart disease/acute myocardial infarction with Asian dust days vs. non-Asian dust days at lag 3. Solid squares represent point estimates (percentage changes) of the individual studies, and the whiskers represent the 95% CIs. Arrowheads indicate where the CI extends outside the range allocated. Diamonds represent the pooled random-effects estimates, with the width indicating the 95% CIs. The vertical dotted line represents a percentage change of 0. Note: CI, confidence interval; df, degrees of freedom; RE, random effects.
Figure 5.
Random-effects pooled estimates (percentage changes) hospital admissions for Asian dust days vs. non-Asian dust days, stratified by outcome and lag time. Solid squares represent point estimates (percentage changes) of the individual studies, and the whiskers represent the 95% CIs. Arrowheads indicate where the CI extends outside the range allocated. The vertical dotted line represents a percentage change of 0. Note: CI, confidence interval.
We repeated the analysis using only time-series and case-crossover studies by excluding Lai and Cheng 2008 from respiratory disease category, Yang et al. 2005b from asthma category and Cheng et al. 2008 from pneumonia category. The study selection did not affect the main results and interpretation (see Figure S6, Excel Table S6). The addition of two studies of hospital visits and emergency room visits (Nakamura et al. 2016; J Park et al. 2016) made the pooled estimates for asthma 1.4–1.8 times higher than the original (lag 1–3), whereas the pooled estimate for respiratory disease at lag 0 became significantly protective after adding five studies of hospital visits, emergency room visits, or ambulance transport (Chien et al. 2012; Lin et al. 2016; Nakamura et al. 2016; Ueda et al. 2012; Yu et al. 2012) (see Figure S7, Excel Table S7). The addition of one study of hospital visits for ischemic heart disease (Chan et al. 2008) at lag 0 did not change the interpretation (see Figure S7, Excel Table S7). Sensitivity analysis by excluding studies with largely overlapping periods in the same study location (the leave-one-out approach) showed that the pooled estimates were robust (see Figure S8, Excel Table S4).
Symptoms/Dysfunction
The most common outcome was respiratory symptoms and peak expiratory flow (PEF) rate, followed by eye, nasopharyngeal, and skin symptoms (Table 3). Daily PEF rates and symptoms (of asthmatic patients or healthy volunteers) were typically recorded in diaries and compared between dust and non-dust days. Of the 12 studies that examined the association between Asian dust exposure and PEF, 8 (66.7%) studies (Hong et al. 2010; Park et al. 2005; Watanabe et al. 2011b, 2015b, 2016a, 2016b, 2016c; Yoo et al. 2008) reported reduced PEF following exposure, although 1 (Watanabe et al. 2011b) reported reduced PEF only among individuals with existing lower respiratory symptoms. Four (33.3%) studies reported no evidence of an association with PEF (Watanabe et al. 2011a, 2014, 2015a, 2015c). Among the 21 studies that examined the association between Asian dust exposure and respiratory symptoms, 16 (76.2%) reported increased respiratory symptoms associated with Asian dust exposures (Table 3). Most studies that examined eye, nasopharyngeal, skin, and allergy symptoms reported increased risk of these symptoms during Asian dust events.
Discussion
In the present study, we reviewed epidemiologic studies that assessed the risk of mortality, hospital admissions, and symptoms/dysfunction associated with exposure to Asian dust. The results of the meta-analyses indicate an exposure to Asian dust was associated with an immediate increased risk of mortality from circulatory causes (lag 0) and a slower increased risk of mortality from respiratory causes (lag 3). Risk of hospital admissions for asthma or pneumonia also increased after exposure. There was little evidence of heterogeneity between the estimates for both mortality and hospital admissions when there was evidence of increased risk.
There were substantial variations between the studies in dust concentrations on Asian dust days. Mean concentrations on Asian dust days varied by location, from in Kitakyushu, Japan, to in Seoul, South Korea (Lee et al. 2014). This could be partly explained by the differing definitions of Asian dust used in the studies. Among the 56 mortality and hospital admission studies, 25 studies used a local meteorological authority’s definition of Asian dust, and 18 studies set their own definition using PM of a certain diameter, with or without other indicators such as wind profile and LIDAR. For example, Tam et al. (2012a, 2012b) defined Asian dust days as when the following four conditions were met: a) air pollution ; b) ; c) predominant easterly wind profile; and d) at least three times higher concentrations of dust storm tracer elements than annual averages. Bell et al. (2008) used several definitions, such as days with in the city (Taipei) and days with at a background monitoring location.
Nevertheless, the average concentrations varied by location (from in Incheon to in Seoul, Korea) even when using the same definition (Lee et al. 2013). A study tracking the mass concentration of aerosol along its transport route reported that the concentration decreased by one order of magnitude as the dust was transported from inland China to Japan (Mori et al. 2003). Dust concentrations on Asian dust days also appear to vary over time: In Taipei, Taiwan, concentrations on Asian dust days were reported as and in the late 1990s (Chan and Ng 2011; Chen et al. 2004) and decreased to and in the 2000s (Lee et al. 2014; Wang and Lin 2015).
Eight mortality/hospitalization studies used LIDAR as an indicator of Asian dust with a certain threshold (Kanatani et al. 2010; Nakamura et al. 2015, 2016; Ueda et al. 2012) or as a continuous variable (Kashima et al. 2012, 2014, 2016, 2017) for the nonspherical extinction coefficient. LIDAR is an optical remote sensing technology that can distinguish nonspherical mineral dust particles from spherical nonmineral dust particles at certain heights (typically from the ground for epidemiological studies) (Shimizu et al. 2004, 2011; Sugimoto et al. 2003). The effect of Asian dust days can differ substantially by altitude and the cutoff values of the extinction coefficient (Ueda et al. 2014); interstudy comparability would therefore require a standardized definition of Asian dust.
Variations in particle size and chemical and biological constituents have been reported (Cha et al. 2016; Ichinose et al. 2008b; Mori et al. 2003; Zhang et al. 2003). Particle size was altered during long-range transport from inland China to Japan (Mori et al. 2003; Zhang et al. 2003). Measurements of mass size distributions of aerosols have shown that 64% of total mass was attributable to coarse particles ( aerodynamic diameter), lower than the percentage of coarse particles reported for aerosols sampled in Beijing, China (93%) (Mori et al. 2003). The addition of sea salt during transport over the sea increased particle size, and between 60% and 91% of dust aerosols in southwestern Japan were reported to be mixed with sea salt and sulfate, whereas dust collected in China did not (Zhang et al. 2003). Previous studies have provided evidence for the effects of PM, especially , on systemic inflammation and heart rate variability (U.S. EPA 2009). Asian dust contains both coarse and fine particles (Mori et al. 2003), which may contribute to the effects on cardiovascular and respiratory mortality.
The main constituents of Asian dust are silica and alumina (Ichinose et al. 2008b), and crystalline silica and aluminum have been suggested to cause cytokine-mediated inflammatory responses in the murine lung (Eisenbarth et al. 2008; Ichinose et al. 2008b). Dust can mix with anthropogenic substances during long-range transport, changing its chemical composition by, for example, the addition of nitrates (Mori et al. 2003). Aerosols in the dust provide surfaces for chemical and physical reactions and serve as carriers of anthropogenic substances (Sun et al. 2005). Sulfate also accumulates on the surface of dust particles during transport from China to Japan; the formation of sulfate and nitrate on the surface of dust particles has been well documented by laboratory simulations, model calculations, and individual particle analysis (Dentener et al. 1996; Iwasaka et al. 1988; Okada et al. 1990; Song and Carmichael 2001; Sun et al. 2005; Underwood et al. 2001; Zhang and Iwasaka 1999). Sulfate in Asian dust has been shown to potentiate allergic reactions in mice (Hiyoshi et al. 2005).
Asian dust particles transported at a lower altitude may contain higher levels of anthropogenic chemicals, consequently exerting more severe health effects. Higher levels of metals were found in Asian dust when the air masses passed through heavily industrial areas (Onishi et al. 2012). Ueda et al. (2012) found increases in ambulance dispatches on Asian dust days when air masses passed through industrial areas in China at an altitude of . The toxicity of Asian dust may, therefore, depend in part on its metal contents, but the sources of pollutants in Asian dust are not well understood, and the modifying effects of transport pathways on pollutant composition are complex.
Another important characteristic of Asian dust involves microorganisms such as bacteria, fungi, and viruses. Microorganisms in the dust can affect the immune system of individuals sensitive to those agents, and lipopolysaccharide and in the microorganisms are known to provoke immune responses (Willart and Lambrecht 2009). Asian dust with microbial contents was shown to enhance allergic lung inflammation in mice (Ichinose et al. 2006, 2008b), and inhalation of dust sand containing caused eosinophil infiltration in the murine airway (Ichinose et al. 2006). Heated desert sand, from which microorganisms had been removed, had less effect on allergic lung inflammation (Ichinose et al. 2008a). Studies have reported that atmospheric bacterial abundance can increase 10–100 times during Asian dust events (Cha et al. 2016; Hara and Zhang 2012), but aerosol bacterial communities near a dust source were more affected by wind activity (J Park et al. 2016).
Some of the studies we reviewed simply compared health events on dust and non-dust (control) days, a procedure that may not fully account for time trends and seasonality as well as typical time-series and case-crossover analyses. Our sensitivity analyses, however, showed that limiting the data to the time-series regression and case-crossover studies did not affect the main results and interpretation. Still, differences in time-series studies such as the degrees of freedom used per year for the time variable, the covariates included in the analyses, and the lag and degrees of freedom used to control for weather variables imply that the nature of residual confounding may differ between studies.
Despite these potential sources of heterogeneity, we found, in general, little evidence of heterogeneity between the estimates for both mortality and hospital admissions. This may be due to the meta-analyses being conducted in most cases by pooling the estimates of fewer than 10 studies and that some study-specific estimates were accompanied by large uncertainty (i.e., large CIs or standard error values). In such cases, the pooling may be influenced largely by a few study-specific estimates with small uncertainty, and the statistics to quantify the heterogeneity should be interpreted with caution. (Higgins and Green 2011)
A standardized protocol for epidemiological studies would facilitate comparisons between studies. For example, one study (Stafoggia et al. 2016) employed a so-called EU reference method whereby multiple tools were used to establish dust events, including monitoring stations selected for regional background investigation of PM, back-trajectory calculations using a hybrid single-particle Lagrangian integrated trajectory model, remote sensing data (aerosol maps), satellite imagery, and meteorological charts to understand the transport mechanism, and chemical composition analysis of PM (Pey et al. 2013). By applying official EU methodologies (Escudero et al. 2007), it was possible to further estimate the separate, independent contribution of dust to the short-term health effects.
There are some limitations to our study. First, chemical and biological components of Asian dust may interact and affect the risks. Studies investigating the effects of particle composition on health outcomes are scarce, and this association warrants further research. The components of Asian dust may vary from those of other dusts, thus the findings may not be applicable to other dust events. Second, the studies included in our meta-analysis compared risks between Asian dust and non-dust days, but the dust concentrations and duration of the events varied. Future studies should consider this when formulating exposure assessments. Distinguishing between local and long-range-transported dust is also worthwhile. Third, we did not conduct a meta-analysis for outcomes with fewer than three estimates; there may have been other effects. In addition, meta-analysis for the long-term effects was not done due to the lack of qualified studies. Fourth, we did not assess the quality of the overall body of evidence using the National Toxicology Program Office of Health Assessment and Translation framework or any other quality scale in the absence of a validated quality assessment tool for time-series and case-crossover designs that were commonly used for mortality and hospitalization studies. Finally, the estimates of the meta-analysis in this study are from a total of 21 studies and, at maximum, only 9 estimates for specific categories of the diseases: Future evidences are merited to strengthen our overall conclusion.
Conclusion
Overall, the existing evidence indicates a positive association between Asian dust exposure and mortality and hospital admissions for circulatory and respiratory events. However, the number of studies included in the meta-analysis was not large and further evidence is merited to strengthen our conclusions. Furthermore, a variety of definitions of Asian dust, study designs, confounder controls, and model specifications have been applied in the original studies. Standardized protocols for epidemiological studies are needed and should be taken into consideration the composition of the dust and a consistent definition of Asian dust.
Supplementary Material
Acknowledgments
We thank Y. Otani and S. Hirakura for supporting the literature search. This work was supported by JSPS KAKENHI (grant 19H03900); by the Global Research Lab (K21004000001-10A0500-00710); by the framework of an international cooperation program (2015K2A2A4000119) of the National Research Foundation, the Ministry of Science, Information and Communication Technologies in South Korea; and by the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan.
References
- Aili A, Oanh NTK. 2015. Effects of dust storm on public health in desert fringe area: case study of northeast edge of Taklimakan Desert, China. Atmos Pollut Res 6(5):805–814, 10.5094/APR.2015.089. [DOI] [Google Scholar]
- Alessandrini ER, Stafoggia M, Faustini A, Gobbi GP, Forastiere F. 2013. Saharan dust and the association between particulate matter and daily hospitalisations in Rome, Italy. Occup Environ Med 70(6):432–434, PMID: 23503419, 10.1136/oemed-2012-101182. [DOI] [PubMed] [Google Scholar]
- Altindag DT, Baek D, Mocan N. 2017. Chinese yellow dust and Korean infant health. Soc Sci Med 186:78–86, PMID: 28599141, 10.1016/j.socscimed.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Bell ML, Levy JK, Lin Z. 2008. The effect of sandstorms and air pollution on cause-specific hospital admissions in Taipei, Taiwan. Occup Environ Med 65(2):104–111, PMID: 17626134, 10.1136/oem.2006.031500. [DOI] [PubMed] [Google Scholar]
- Bennett CM, McKendry IG, Kelly S, Denike K, Koch T. 2006. Impact of the 1998 Gobi dust event on hospital admissions in the Lower Fraser Valley, British Columbia. Sci Total Environ 366(2–3):918–925, PMID: 16483637, 10.1016/j.scitotenv.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. 2009. Introduction to Meta-Analysis. Chichester, West Sussex, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Cha S, Lee D, Jang JH, Lim S, Yang D, Seo T. 2016. Alterations in the airborne bacterial community during Asian dust events occurring between February and March 2015 in South Korea. Sci Rep 6:37271, PMID: 27849049, 10.1038/srep37271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C-C, Chuang K-J, Chen W-J, Chang W-T, Lee C-T, Peng C-M. 2008. Increasing cardiopulmonary emergency visits by long-range transported Asian dust storms in Taiwan. Environ Res 106(3):393–400, PMID: 17959168, 10.1016/j.envres.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Chan C-C, Ng H-C. 2011. A case-crossover analysis of Asian dust storms and mortality in the downwind areas using 14-year data in Taipei. Sci Total Environ 410–411:47–52, PMID: 21995878, 10.1016/j.scitotenv.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Chan Y-S, Teng JC-Y, Liu T-C, Peng Y-I. 2018. Asian dust storms and diabetes hospitalization: a nationwide population-based study. Air Quality Atmos Health 11:1243–1250, 10.1007/s11869-018-0623-z. [DOI] [Google Scholar]
- Chang C-C, Lee IM, Tsai S-S, Yang C-Y. 2006. Correlation of Asian dust storm events with daily clinic visits for allergic rhinitis in Taipei, Taiwan. J Toxicol Environ Health A 69(3–4):229–235, PMID: 16263693, 10.1080/15287390500227415. [DOI] [PubMed] [Google Scholar]
- Chen Y-S, Sheen P-C, Chen E-R, Liu Y-K, Wu T-N, Yang C-Y. 2004. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ Res 95(2):151–155, PMID: 15147920, 10.1016/j.envres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Chen Y-S, Yang C-Y. 2005. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J Toxicol Environ Health A 68(17–18):1457–1464, PMID: 16076758, 10.1080/15287390590967388. [DOI] [PubMed] [Google Scholar]
- Cheng M-F, Ho S-C, Chiu H-F, Wu T-N, Chen P-S, Yang C-Y. 2008. Consequences of exposure to Asian dust storm events on daily pneumonia hospital admissions in Taipei, Taiwan. J Toxicol Environ Health A 71(19):1295–1299, PMID: 18686199, 10.1080/15287390802114808. [DOI] [PubMed] [Google Scholar]
- Chien L-C, Lien Y-J, Yang C-H, Yu H-L. 2014. Acute increase of children’s conjunctivitis clinic visits by Asian dust storms exposure—a spatiotemporal study in Taipei, Taiwan. PLoS One 9(10):e109175, PMID: 25347189, 10.1371/journal.pone.0109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien L-C, Yang C-H, Yu H-L. 2012. Estimated effects of Asian dust storms on spatiotemporal distributions of clinic visits for respiratory diseases in Taipei children (Taiwan). Environ Health Perspect 120(8):1215–1220, PMID: 22538266, 10.1289/ehp.1104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H-F, Tiao M-M, Ho S-C, Kuo H-W, Wu T-N, Yang C-Y. 2008. Effects of Asian dust storm events on hospital admissions for chronic obstructive pulmonary disease in Taipei, Taiwan. Inhal Toxicol 20(9):777–781, PMID: 18645716, 10.1080/08958370802005308. [DOI] [PubMed] [Google Scholar]
- de Longueville F, Ozer P, Doumbia S, Henry S. 2013. Desert dust impacts on human health: an alarming worldwide reality and a need for studies in West Africa. Int J Biometeorol 57(1):1–19, PMID: 22552909, 10.1007/s00484-012-0541-y. [DOI] [PubMed] [Google Scholar]
- Dentener FJ, Carmichael GR, Zhang Y, Lelieveld J, Crutzen PJ. 1996. Role of mineral aerosol as a reactive surface in the global troposphere. J Geophys Res 101(D17):22869–22889, 10.1029/96JD01818. [DOI] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453(7198):1122–1126, PMID: 18496530, 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero M, Querol X, Pey J, Alastuey A, Pérez N, Ferreira F, et al. 2007. A methodology for the quantification of the net African dust load in air quality monitoring networks. Atmos Environ 41(26):5516–5524, 10.1016/j.atmosenv.2007.04.047. [DOI] [Google Scholar]
- Hara K, Zhang D. 2012. Bacterial abundance and viability in long-range transported dust. Atmos Environ 47:20–25, 10.1016/j.atmosenv.2011.11.050. [DOI] [Google Scholar]
- Hashizume M, Ueda K, Nishiwaki Y, Michikawa T, Onozuka D. 2010. Health effects of Asian dust events: a review of the literature. [In Japanese.] Nihon Eiseigaku Zasshi 65(3):413–421, PMID: 20508385, 10.1265/jjh.65.413. [DOI] [PubMed] [Google Scholar]
- Higashi T, Kambayashi Y, Ohkura N, Fujimura M, Nakai S, Honda Y, et al. 2014a. Effects of Asian dust on daily cough occurrence in patients with chronic cough: a panel study. Atmos Environ 92:506–513, 10.1016/j.atmosenv.2014.04.034. [DOI] [Google Scholar]
- Higashi T, Kambayashi Y, Ohkura N, Fujimura M, Nakanishi S, Yoshizaki T, et al. 2014b. Exacerbation of daily cough and allergic symptoms in adult patients with chronic cough by Asian dust: a hospital-based study in Kanazawa. Atmos Environ 97:537–543, 10.1016/j.atmosenv.2014.01.041. [DOI] [Google Scholar]
- Higgins J, Green S (eds.). 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Oxford, Oxfordshire, UK: Cochrane Collaboration; http://handbook-5-1.cochrane.org/ [accessed 10 June 2020]. [Google Scholar]
- Hiyoshi K, Ichinose T, Sadakane K, Takano H, Nishikawa M, Mori I, et al. 2005. Asian sand dust enhances ovalbumin-induced eosinophil recruitment in the alveoli and airway of mice. Environ Res 99(3):361–368, PMID: 16307978, 10.1016/j.envres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Ho HC, Wong MS, Yang L, Chan T-C, Bilal M. 2018. Influences of socioeconomic vulnerability and intra-urban air pollution exposure on short-term mortality during extreme dust events. Environ Pollut 235:155–162, PMID: 29288928, 10.1016/j.envpol.2017.12.047. [DOI] [PubMed] [Google Scholar]
- Honda A, Matsuda Y, Murayama R, Tsuji K, Nishikawa M, Koike E, et al. 2014. Effects of Asian sand dust particles on the respiratory and immune system. J Appl Toxicol 34(3):250–257, PMID: 23576315, 10.1002/jat.2871. [DOI] [PubMed] [Google Scholar]
- Honda A, Sawahara T, Hayashi T, Tsuji K, Fukushima W, Oishi M, et al. 2017. Biological factor related to Asian sand dust particles contributes to the exacerbation of asthma. J Appl Toxicol 37(5):583–590, PMID: 27714829, 10.1002/jat.3395. [DOI] [PubMed] [Google Scholar]
- Hong Y-C, Pan X-C, Kim S-Y, Park K, Park E-J, Jin X, et al. 2010. Asian dust storm and pulmonary function of school children in Seoul. Sci Total Environ 408(4):754–759, PMID: 19939437, 10.1016/j.scitotenv.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Sadakane K, Takano H, Yanagisawa R, Nishikawa M, Mori I, et al. 2006. Enhancement of mite allergen-induced eosinophil infiltration in the murine airway and local cytokine/chemokine expression by Asian sand dust. J Toxicol Environ Health A 69(16):1571–1585, PMID: 16854786, 10.1080/15287390500470833. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Yoshida S, Hiyoshi K, Sadakane K, Takano H, Nishikawa M, et al. 2008a. The effects of microbial materials adhered to Asian sand dust on allergic lung inflammation. Arch Environ Contam Toxicol 55(3):348–357, PMID: 18227959, 10.1007/s00244-007-9128-8. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Yoshida S, Sadakane K, Takano H, Yanagisawa R, Inoue K, et al. 2008b. Effects of Asian sand dust, Arizona sand dust, amorphous silica and aluminum oxide on allergic inflammation in the murine lung. Inhal Toxicol 20(7):685–694, PMID: 18464056, 10.1080/08958370801935133. [DOI] [PubMed] [Google Scholar]
- Iwasaka Y, Yamato M, Imasu R, Ono A. 1988. Transport of Asian dust (KOSA) particles; importance of weak KOSA events on the geochemical cycle of soil particles. Tellus B 40B(5):494–503, 10.3402/tellusb.v40i5.16017. [DOI] [Google Scholar]
- Janes H, Sheppard L, Lumley T. 2005. Case–crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 16(6):717–726, PMID: 16222160, 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Linares C, Martínez D, Díaz J. 2010. Role of Saharan dust in the relationship between particulate matter and short-term daily mortality among the elderly in Madrid (Spain). Sci Total Environ 408(23):5729–5736, PMID: 20855107, 10.1016/j.scitotenv.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Ueda K, Ago T, Nitta H, Kitazono T, Fukuoka Stroke Registry Investigators. 2012. Relationship between Asian dust and ischemic stroke: a time-stratified case-crossover study. Stroke 43(11):3085–3087, PMID: 22968465, 10.1161/STROKEAHA.112.672501. [DOI] [PubMed] [Google Scholar]
- Kanatani KT, Hamazaki K, Inadera H, Sugimoto N, Shimizu A, Noma H, et al. 2016. Effect of desert dust exposure on allergic symptoms: a natural experiment in Japan. Ann Allergy Asthma Immunol 116(5):425–430.e7, PMID: 26976782, 10.1016/j.anai.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, Ramsdell JW, et al. 2010. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am J Respir Crit Care Med 182(12):1475–1481, PMID: 20656941, 10.1164/rccm.201002-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-H, Keller JJ, Chen C-S, Lin H-C. 2012. Asian dust storm events are associated with an acute increase in pneumonia hospitalization. Ann Epidemiol 22(4):257–263, PMID: 22391266, 10.1016/j.annepidem.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Kang J-H, Liu T-C, Keller J, Lin H-C. 2013. Asian dust storm events are associated with an acute increase in stroke hospitalisation. J Epidemiol Community Health 67(2):125–131, PMID: 22826296, 10.1136/jech-2011-200794. [DOI] [PubMed] [Google Scholar]
- Karanasiou A, Moreno N, Moreno T, Viana M, de Leeuw F, Querol X. 2012. Health effects from Sahara dust episodes in Europe: literature review and research gaps. Environ Int 47:107–114, PMID: 22796892, 10.1016/j.envint.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Kashima S, Yorifuji T, Bae S, Honda Y, Lim Y, Hong Y. 2016. Asian dust effect on cause-specific mortality in five cities across South Korea and Japan. Atmos Environ 128:20–27, 10.1016/j.atmosenv.2015.12.063. [DOI] [Google Scholar]
- Kashima S, Yorifuji T, Suzuki E. 2014. Asian dust and daily emergency ambulance calls among elderly people in Japan: an analysis of its double role as a direct cause and as an effect modifier. J Occup Environ Med 56(12):1277–1283, PMID: 25479297, 10.1097/JOM.0000000000000334. [DOI] [PubMed] [Google Scholar]
- Kashima S, Yorifuji T, Suzuki E. 2017. Are people with a history of disease more susceptible to a short-term exposure to Asian dust?: a case-crossover study among the elderly in Japan. Epidemiology 28(suppl 1):S60–S66, PMID: 29028677, 10.1097/EDE.0000000000000700. [DOI] [PubMed] [Google Scholar]
- Kashima S, Yorifuji T, Tsuda T, Eboshida A. 2012. Asian dust and daily all-cause or cause-specific mortality in Western Japan. Occup Environ Med 69(12):908–915, PMID: 23085558, 10.1136/oemed-2012-100797. [DOI] [PubMed] [Google Scholar]
- Kim H-S, Kim D-S, Kim H, Yi S-M. 2012. Relationship between mortality and fine particles during Asian dust, smog–Asian dust, and smog days in Korea. Int J Environ Health Res 22(6):518–530, PMID: 22428926, 10.1080/09603123.2012.667796. [DOI] [PubMed] [Google Scholar]
- Ko R, Hayashi M, Hayashi H, Hayashi K, Kato H, Kurata Y, et al. 2016. Correlation between acute conjunctivitis and Asian dust on ocular surfaces. J Toxicol Environ Health A 79(8):367–375, PMID: 27142484, 10.1080/15287394.2016.1162248. [DOI] [PubMed] [Google Scholar]
- Kojima S, Michikawa T, Ueda K, Sakamoto T, Matsui K, Kojima T, et al. 2017. Asian dust exposure triggers acute myocardial infarction. Eur Heart J 38(43):3202–3208, PMID: 29020374, 10.1093/eurheartj/ehx509. [DOI] [PubMed] [Google Scholar]
- Kwon H-J, Cho S-H, Chun Y, Lagarde F, Pershagen G. 2002. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ Res 90(1):1–5, PMID: 12359184, 10.1006/enrs.2002.4377. [DOI] [PubMed] [Google Scholar]
- Lai L-W, Cheng W-L. 2008. The impact of air quality on respiratory admissions during Asian dust storm periods. Int J Environ Health Res 18(6):429–450, PMID: 19031147, 10.1080/09603120802272227. [DOI] [PubMed] [Google Scholar]
- Lee H, Honda Y, Lim Y-H, Guo YL, Hashizume M, Kim H. 2014. Effect of Asian dust storms on mortality in three Asian cities. Atmos Environ 89:309–317, 10.1016/j.atmosenv.2014.02.048. [DOI] [Google Scholar]
- Lee H, Kim H, Honda Y, Lim Y-H, Yi S. 2013. Effect of Asian dust storms on daily mortality in seven metropolitan cities of Korea. Atmos Environ 79:510–517, 10.1016/j.atmosenv.2013.06.046. [DOI] [Google Scholar]
- Li Z, Chen L, Li M, Cohen J. 2018. Prenatal exposure to sand and dust storms and children’s cognitive function in China: a quasi-experimental study. Lancet Planet Health 2(5):e214–e222, PMID: 29709285, 10.1016/S2542-5196(18)30068-8. [DOI] [PubMed] [Google Scholar]
- Lin Y-K, Chen C-F, Yeh H-C, Wang Y-C. 2016. Emergency room visits associated with particulate concentration and Asian dust storms in metropolitan Taipei. J Expo Sci Environ Epidemiol 26(2):189–196, PMID: 26531803, 10.1038/jes.2015.70. [DOI] [PubMed] [Google Scholar]
- Liu S-T, Liao C-Y, Kuo C-Y, Kuo H-W. 2017. The effects of PM2.5 from Asian dust storms on emergency room visits for cardiovascular and respiratory diseases. Int J Environ Res Public Health 14(4):428, PMID: 28420157, 10.3390/ijerph14040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Xiao B, Liu C, Zhao Y, Zheng X. 2016. Association between ambient air pollution and emergency room visits for respiratory diseases in spring dust storm season in Lanzhou, China. Int J Environ Res Public Health 13(6):613, PMID: 27338430, 10.3390/ijerph13060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang H, Zhao Y, Zhou J, Yang S, Zheng X, et al. 2017a. Short-term effects of air pollution on daily hospital admissions for cardiovascular diseases in western China. Environ Sci Pollut Res Int 24(16):14071–14079, PMID: 28409432, 10.1007/s11356-017-8971-z. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhou J, Yang S, Zhao Y, Zheng X. 2017b. Assessment for the impact of dust events on measles incidence in western China. Atmos Environ 157:1–9, 10.1016/j.atmosenv.2017.03.010. [DOI] [Google Scholar]
- Majbauddin A, Onishi K, Otani S, Kurosaki Y, Kurozawa Y. 2016. Association between Asian dust-borne air pollutants and daily symptoms on healthy subjects: a web-based pilot study in Yonago, Japan. J Environ Public Health 2016:8280423, PMID: 28053609, 10.1155/2016/8280423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallone S, Stafoggia M, Faustini A, Gobbi GP, Marconi A, Forastiere F. 2011. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ Health Perspect 119(10):1409–1414, PMID: 21970945, 10.1289/ehp.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa R, Michikawa T, Ueda K, Nitta H, Kawasaki T, Tashiro H, et al. 2014. Desert dust is a risk factor for the incidence of acute myocardial infarction in Western Japan. Circ Cardiovasc Qual Outcomes 7(5):743–748, PMID: 25074374, 10.1161/CIRCOUTCOMES.114.000921. [DOI] [PubMed] [Google Scholar]
- Meng Z, Lu B. 2007. Dust events as a risk factor for daily hospitalization for respiratory and cardiovascular diseases in Minqin, China. Atmos Environ 41(33):7048–7058, 10.1016/j.atmosenv.2007.05.006. [DOI] [Google Scholar]
- Middleton N, Yiallouros P, Kleanthous S, Kolokotroni O, Schwartz J, Dockery DW, et al. 2008. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: the effect of short-term changes in air pollution and dust storms. Environ Health 7:39, PMID: 18647382, 10.1186/1476-069X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Fujishima H, Noma H, Kamei Y, Goto M, et al. 2014. Sensitization to Asian dust and allergic rhinoconjunctivitis. Environ Res 132:220–225, PMID: 24815334, 10.1016/j.envres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Collaborators. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535, PMID: 19622551, 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Whaley P, Thayer KA, Schünemann HJ. 2018. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 121(pt 1):1027–1031, PMID: 30166065, 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Nishikawa M, Tanimura T, Quan H. 2003. Change in size distribution and chemical composition of kosa (Asian dust) aerosol during long-range transport. Atmos Environ 37(30):4253–4263, 10.1016/S1352-2310(03)00535-1. [DOI] [Google Scholar]
- Mu H, Battsetseg B, Ito TY, Otani S, Onishi K, Kurozawa Y. 2011. Health effects of dust storms: subjective eye and respiratory system symptoms in inhabitants in Mongolia. J Environ Health 73(8):18–20, PMID: 21488467. [PubMed] [Google Scholar]
- Nakamura T, Hashizume M, Ueda K, Kubo T, Shimizu A, Okamura T, et al. 2015. The relationship between Asian dust events and out-of-hospital cardiac arrests in Japan. J Epidemiol 25(4):289–296, PMID: 25797600, 10.2188/jea.JE20140179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hashizume M, Ueda K, Shimizu A, Takeuchi A, Kubo T, et al. 2016. Asian dust and pediatric emergency department visits due to bronchial asthma and respiratory diseases in Nagasaki, Japan. J Epidemiol 26(11):593–601, PMID: 27180931, 10.2188/jea.JE20150309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Ishihara Y, Kim C-H, Hyun I-G. 2018. The impact of air pollution, including Asian sand dust, on respiratory symptoms and health-related quality of life in outpatients with chronic respiratory disease in Korea: a panel study. J Prev Med Public Health 51(3):130–139, PMID: 29886708, 10.3961/jpmph.18.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Yamauchi K, Mitsuma S, Omori H, Ishihara Y. 2019. Relationships between perceived health status and ambient air quality parameters in healthy Japanese: a panel study. BMC Public Health 19(1):620, PMID: 31117980, 10.1186/s12889-019-6934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogi K, Takabayashi T, Sakashita M, Susuki D, Yamada T, Manabe Y, et al. 2014. Effect of Asian sand dust on Japanese cedar pollinosis. Auris Nasus Larynx 41(6):518–522, PMID: 24928063, 10.1016/j.anl.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Okada K, Naruse H, Tanaka T, Nemoto O, Iwasaka Y, Wu P-M, et al. 1990. X-ray spectrometry of individual Asian dust-storm particles over the Japanese islands and the North Pacific Ocean. Atmos Environ A Gen Top 24(6):1369–1378, 10.1016/0960-1686(90)90043-M. [DOI] [Google Scholar]
- Onishi K, Kurosaki Y, Otani S, Yoshida A, Sugimoto N, Kurozawa Y. 2012. Atmospheric transport route determines components of Asian dust and health effects in Japan. Atmos Environ 49:94–102, 10.1016/j.atmosenv.2011.12.018. [DOI] [Google Scholar]
- Onishi K, Otani S, Yoshida A, Mu H, Kurozawa Y. 2015. Adverse health effects of Asian dust particles and heavy metals in Japan. Asia Pac J Public Health 27(2):NP1719–NP1726, PMID: 22865718, 10.1177/1010539511428667. [DOI] [PubMed] [Google Scholar]
- Onishi K, Sekiyama TT, Nojima M, Kurosaki Y, Fujitani Y, Otani S, et al. 2018. Prediction of health effects of cross-border atmospheric pollutants using an aerosol forecast model. Environ Int 117:48–56, PMID: 29727752, 10.1016/j.envint.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Otani S, Onishi K, Mu H, Hosoda T, Kurozawa Y, Ikeguchi M. 2014. Associations between subjective symptoms and serum immunoglobulin E levels during Asian dust events. Int J Environ Res Public Health 11(8):7636–7641, PMID: 25075882, 10.3390/ijerph110807636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Onishi K, Mu H, Kurozawa Y. 2011. The effect of Asian dust events on the daily symptoms in Yonago, Japan: a pilot study on healthy subjects. Arch Environ Occup Health 66(1):43–46, PMID: 21337185, 10.1080/19338244.2010.506499. [DOI] [PubMed] [Google Scholar]
- Otani S, Onishi K, Mu H, Yokoyama Y, Hosoda T, Okamoto M, et al. 2012. The relationship between skin symptoms and allergic reactions to Asian dust. Int J Environ Res Public Health 9(12):4606–4614, PMID: 23222253, 10.3390/ijerph9124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Ichijo T, Nasu M, Yamaguchi N. 2016. Investigation of bacterial effects of Asian dust events through comparison with seasonal variability in outdoor airborne bacterial community. Sci Rep 6:35706, PMID: 27761018, 10.1038/srep35706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, et al. 2005. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology 10(4):470–476, PMID: 16135170, 10.1111/j.1440-1843.2005.00728.x. [DOI] [PubMed] [Google Scholar]
- Park Y-S, Kim J-H, Jang H-J, Tae Y-H, Lim DH. 2016. The effect of Asian dust on asthma by socioeconomic status using national health insurance claims data in Korea. Inhal Toxicol 28(1):1–6, PMID: 26785149, 10.3109/08958378.2015.1123331. [DOI] [PubMed] [Google Scholar]
- Perez L, Tobías A, Querol X, Künzli N, Pey J, Alastuey A, et al. 2008. Coarse particles from Saharan dust and daily mortality. Epidemiology 19(6):800–807, PMID: 18938653, 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- Perez L, Tobías A, Querol X, Pey J, Alastuey A, Díaz J, et al. 2012. Saharan dust, particulate matter and cause-specific mortality: a case–crossover study in Barcelona (Spain). Environ Int 48:150–155, PMID: 22935765, 10.1016/j.envint.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Pey J, Querol X, Alastuey A, Forastiere F, Stafoggia M. 2013. African dust outbreaks over the Mediterranean Basin during 2001–2011: PM10 concentrations, phenomenology and trends, and its relation with synoptic and mesoscale meteorology. Atmos Chem Phys 13:1395–1410, 10.5194/acp-13-1395-2013. [DOI] [Google Scholar]
- Reyes M, Díaz J, Tobias A, Montero JC, Linares C. 2014. Impact of Saharan dust particles on hospital admissions in Madrid (Spain). Int J Environ Health Res 24(1):63–72, PMID: 23544440, 10.1080/09603123.2013.782604. [DOI] [PubMed] [Google Scholar]
- Sakata S, Konishi S, Ng CFS, Kishikawa R, Watanabe C. 2017. Association of Asian dust with daily medical consultations for pollinosis in Fukuoka City, Japan. Environ Health Prev Med 22(1):25, PMID: 29165121, 10.1186/s12199-017-0623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Kougea E, Kassomenos P, Analitis A, Katsouyanni K. 2011a. Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci Total Environ 409(11):2049–2054, PMID: 21421257, 10.1016/j.scitotenv.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. 2011b. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res 111(3):418–424, PMID: 21296347, 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Sugimoto N, Matsui I, Arao K, Uno I, Murayama T, et al. 2004. Continuous observations of Asian dust and other aerosols by polarization lidars in China and Japan during ACE-Asia. J Geophys Res 109(D19):D19S17, 10.1029/2002JD003253. [DOI] [Google Scholar]
- Shimizu A, Sugimoto N, Matsui I, Mori I, Nishikawa M, Kido M. 2011. Relationship between lidar-derived dust extinction coefficients and mass concentrations in Japan. SOLA 7A(Special Ed):1–4, 10.2151/sola.7A-001. [DOI] [Google Scholar]
- Song CH, Carmichael GR. 2001. A three-dimensional modeling investigation of the evolution processes of dust and sea-salt particles in east Asia. J Geophys Res 106(D16):18131–18154, 10.1029/2000JD900352. [DOI] [Google Scholar]
- Stafoggia M, Zauli-Sajani S, Pey J, Samoli E, Alessandrini E, Basagaña X, et al. 2016. Desert dust outbreaks in Southern Europe: contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ Health Perspect 124(4):413–419, PMID: 26219103, 10.1289/ehp.1409164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Uno I, Nishikawa M, Shimizu A, Matsui I, Dong X, et al. 2003. Record heavy Asian dust in Beijing in 2002: observations and model analysis of recent events. Geophys Res Lett 30(12):1640, 10.1029/2002GL016349. [DOI] [Google Scholar]
- Sun Y, Zhuang G, Wang Y, Zhao X, Li J, Wang Z, et al. 2005. Chemical composition of dust storms in Beijing and implications for the mixing of mineral aerosol with pollution aerosol on the pathway. J Geophys Res 110(D24):D24209, 10.1029/2005JD006054. [DOI] [Google Scholar]
- Takemura T, Uno I, Nakajima T, Higurashi A, Sano I. 2002. Modeling study of long-range transport of Asian dust and anthropogenic aerosols from East Asia. Geophys Res Lett 29(24):2158, 10.1029/2002GL016251. [DOI] [Google Scholar]
- Tam WWS, Wong TW, Wong AHS. 2012a. Effect of dust storm events on daily emergency admissions for cardiovascular diseases. Circ J 76(3):655–660, PMID: 22251752, 10.1253/circj.cj-11-0894. [DOI] [PubMed] [Google Scholar]
- Tam WWS, Wong TW, Wong AHS, Hui DSC. 2012b. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology 17(1):143–148, PMID: 22092966, 10.1111/j.1440-1843.2011.02056.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, An X, Sun Z, Hou Q, Wang Y. 2012. Association between dust weather and number of admissions for patients with respiratory diseases in spring in Lanzhou. Sci Total Environ 423:8–11, PMID: 22386996, 10.1016/j.scitotenv.2012.01.064. [DOI] [PubMed] [Google Scholar]
- Teng CJ-Y, Chan Y-S, Peng Y-I, Liu T-C. 2016. Influence of Asian dust storms on daily acute myocardial infarction hospital admissions. Public Health Nurs 33(2):118–128, PMID: 26058799, 10.1111/phn.12209. [DOI] [PubMed] [Google Scholar]
- Tobías A, Pérez L, Díaz J, Linares C, Pey J, Alastruey A, et al. 2011. Short-term effects of particulate matter on total mortality during Saharan dust outbreaks: a case-crossover analysis in Madrid (Spain). Sci Total Environ 412–413:386–389, PMID: 22055453, 10.1016/j.scitotenv.2011.10.027. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2009. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009). EPA/600/R-08/139F. Washington, DC: U.S. EPA. [Google Scholar]
- Ueda K, Nitta H, Odajima H. 2010. The effects of weather, air pollutants, and Asian dust on hospitalization for asthma in Fukuoka. Environ Health Prev Med 15(6):350–357, PMID: 21432566, 10.1007/s12199-010-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Shimizu A, Inoue K. 2014. Exposure assessment by light detection and ranging for Asian dust aerosol in epidemiological studies to investigate its health effects. [In Japanese.] Earozoru Kenkyu 29:7, 10.11203/jar.29.s230. [DOI] [Google Scholar]
- Ueda K, Shimizu A, Nitta H, Inoue K. 2012. Long-range transported Asian dust and emergency ambulance dispatches. Inhal Toxicol 24(12):858–867, PMID: 23033999, 10.3109/08958378.2012.724729. [DOI] [PubMed] [Google Scholar]
- Underwood GM, Li P, Al-Abadleh H, Grassian VH. 2001. A Knudsen cell study of the heterogeneous reactivity of nitric acid on oxide and mineral dust particles. J Phys Chem A 105(27):6609–6620, 10.1021/jp002223h. [DOI] [Google Scholar]
- Wang C-H, Chen C-S, Lin C-L. 2014. The threat of Asian dust storms on asthma patients: a population-based study in Taiwan. Glob Public Health 9(9):1040–1052, PMID: 25186129, 10.1080/17441692.2014.951871. [DOI] [PubMed] [Google Scholar]
- Wang H, Lv S, Diao Z, Wang B, Zhang H, Yu C. 2018. Study on sandstorm PM10 exposure assessment in the large-scale region: a case study in Inner Mongolia. Environ Sci Pollut Res Int 25(17):17144–17155, PMID: 29644617, 10.1007/s11356-018-1841-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Li S, Wang S, Shang K. 2015. Effects of long-term dust exposure on human respiratory system health in Minqin County, China. Arch Environ Occup Health 70(4):225–231, PMID: 24460854, 10.1080/19338244.2013.872077. [DOI] [PubMed] [Google Scholar]
- Wang Y-C, Lin Y-K. 2015. Mortality associated with particulate concentration and Asian dust storms in Metropolitan Taipei. Atmos Environ 117:32–40, 10.1016/j.atmosenv.2015.06.055. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang R, Ming J, Liu G, Chen T, Liu X, et al. 2016. Effects of dust storm events on weekly clinic visits related to pulmonary tuberculosis disease in Minqin, China. Atmos Environ 127:205–212, 10.1016/j.atmosenv.2015.12.041. [DOI] [Google Scholar]
- Watanabe M, Igishi T, Burioka N, Yamasaki A, Kurai J, Takeuchi H, et al. 2011a. Pollen augments the influence of desert dust on symptoms of adult asthma patients. Allergol Int 60(4):517–524, PMID: 22113159, 10.2332/allergolint.10-OA-0298. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kurai J, Igishi T, Yamasaki A, Burioka N, Takeuchi H, et al. 2012. Influence of Asian desert dust on lower respiratory tract symptoms in patients with asthma over 4 years. Yonago Acta Med 55(2):41–48, PMID: 24031138. [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kurai J, Sano H, Shimizu E. 2015a. Effect of exposure to an Asian dust storm on fractional exhaled nitric oxide in adult asthma patients in Western Japan. J Med Invest 62(3–4):233–237, PMID: 26399354, 10.2152/jmi.62.233. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kurai J, Tomita K, Sano H, Abe S, Saito R, et al. 2014. Effects on asthma and induction of interleukin-8 caused by Asian dust particles collected in Western Japan. J Asthma 51(6):595–602, PMID: 24628524, 10.3109/02770903.2014.903965. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Kato K, Sano H, Tatsukawa T, et al. 2016a. Association between pulmonary function and daily levels of sand dust particles assessed by light detection and ranging in schoolchildren in Western Japan: a panel study. Allergol Int 65(1):56–61, PMID: 26666494, 10.1016/j.alit.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Sano H, Iwata K, Hantan D, et al. 2017. Association of short-term exposure to ambient fine particulate matter with skin symptoms in schoolchildren: a panel study in a rural area of Western Japan. Int J Environ Res Public Health 14(3):299, PMID: 28335405, 10.3390/ijerph14030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Sano H, Mikami M, Yamamoto H, et al. 2016b. Effect of Asian dust on pulmonary function in adult asthma patients in Western Japan: a panel study. Allergol Int 65(2):147–152, PMID: 26666479, 10.1016/j.alit.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Sano H, Saito R, Abe S, et al. 2015b. Decreased pulmonary function in school children in Western Japan after exposures to Asian desert dusts and its association with interleukin-8. Biomed Res Int 2015:583293, PMID: 26060816, 10.1155/2015/583293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Sano H, Ueda Y, Mikami M, et al. 2016c. Differences in the effects of Asian dust on pulmonary function between adult patients with asthma and those with asthma–chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis 11:183–190, PMID: 26869784, 10.2147/COPD.S97460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Shimizu A, Sano H, Kato K, et al. 2015c. Association of sand dust particles with pulmonary function and respiratory symptoms in adult patients with asthma in Western Japan using light detection and ranging: a panel study. Int J Environ Res Public Health 12(10):13038–13052, PMID: 26501307, 10.3390/ijerph121013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yamasaki A, Burioka N, Kurai J, Yoneda K, Yoshida A, et al. 2011b. Correlation between Asian dust storms and worsening asthma in Western Japan. Allergol Int 60(3):267–275, PMID: 21364309, 10.2332/allergolint.10-OA-0239. [DOI] [PubMed] [Google Scholar]
- Willart MAM, Lambrecht BN. 2009. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 39(1):12–19, PMID: 19016800, 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- Wong MS, Ho HC, Yang L, Shi W, Yang J, Chan T-C. 2017. Spatial variability of excess mortality during prolonged dust events in a high-density city: a time-stratified spatial regression approach. Int J Health Geogr 16(1):26, PMID: 28738805, 10.1186/s12942-017-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Y. 2006. Effects of Asian dust storm events on daily clinical visits for conjunctivitis in Taipei, Taiwan. J Toxicol Environ Health A 69(18):1673–1680, PMID: 16864418, 10.1080/15287390600630096. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Chen Y-S, Chiu H-F, Goggins WB. 2005a. Effects of Asian dust storm events on daily stroke admissions in Taipei. Environ Res 99(1):79–84, PMID: 16053931, 10.1016/j.envres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Cheng M-H, Chen C-C. 2009. Effects of Asian dust storm events on hospital admissions for congestive heart failure in Taipei, Taiwan. J Toxicol Environ Health A 72(5):324–328, PMID: 19184748, 10.1080/15287390802529880. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Tsai S-S, Chang C-C, Ho S-C. 2005b. Effects of Asian dust storm events on daily admissions for asthma in Taipei, Taiwan. Inhal Toxicol 17(14):817–821, PMID: 16282159, 10.1080/08958370500241254. [DOI] [PubMed] [Google Scholar]
- Yoo Y, Choung JT, Yu J, Kim DK, Koh YY. 2008. Acute effects of Asian dust events on respiratory symptoms and peak expiratory flow in children with mild asthma. J Korean Med Sci 23(1):66–71, PMID: 18303201, 10.3346/jkms.2008.23.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H-L, Chien L-C, Yang C-H. 2012. Asian dust storm elevates children’s respiratory health risks: a spatiotemporal analysis of children’s clinic visits across Taipei (Taiwan). PLoS One 7(7):e41317, PMID: 22848461, 10.1371/journal.pone.0041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H-L, Yang C-H, Chien L-C. 2013. Spatial vulnerability under extreme events: a case of Asian dust storm’s effects on children’s respiratory health. Environ Int 54:35–44, PMID: 23403144, 10.1016/j.envint.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Zauli Sajani S, Miglio R, Bonasoni P, Cristofanelli P, Marinoni A, Sartini C, et al. 2011. Saharan dust and daily mortality in Emilia-Romagna (Italy). Occup Environ Med 68(6):446–451, PMID: 21172793, 10.1136/oem.2010.058156. [DOI] [PubMed] [Google Scholar]
- Zhang D, Iwasaka Y. 1999. Nitrate and sulfate in individual Asian dust-storm particles in Beijing, China in Spring of 1995 and 1996. Atmos Environ 33(19):3213–3223, 10.1016/S1352-2310(99)00116-8. [DOI] [Google Scholar]
- Zhang D, Iwasaka Y, Shi G, Zang J, Matsuki A, Trochkine D. 2003. Mixture state and size of Asian dust particles collected at southwestern Japan in spring 2000. J Geophys Res 108(D24):4760, 10.1029/2003JD003869. [DOI] [Google Scholar]
- Zhang X, Zhao L, Tong DQ, Wu G, Dan M, Teng B. 2016. A systematic review of global desert dust and associated human health effects. Atmosphere 7(12):158, 10.3390/atmos7120158. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



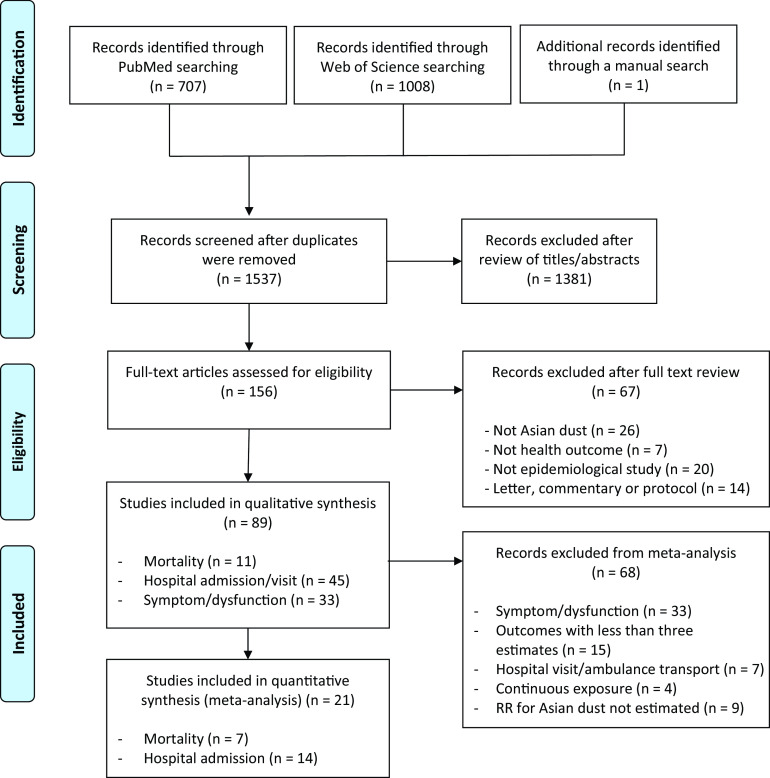
![Figure 2 is tabular representation, having 3 columns, namely, authors and year, study region, and percent change (95 percent CI), comprising three forest plots. From bottom to top, the first forest plot plots respiratory (lag 0), including RE model for subgroup (Q equals 9.84, df equals 5, p equals 0.08; I squared equals 49.2 percent), Lee et al. 2014 at Kitakyushu (Japan), Lee et al. 2014 at Taipei (Taiwan), Lee et al. 2014 at Seoul (Korea), Lee et al. 2013 at 7 cities pooled (Korea), Chan et al. 2011 at Taipei (Taiwan), and Chen et al. 2004 at Taipei (Taiwan; y-axis) across percent change, ranging from negative 20 to 20 in increments of 5 (x-axis) for percent change (95 percent CI), including negative 2.58 [negative 7.27, 2.34], 5.83 [negative 10.40, 25.02], negative 10.62 [negative 17.29, negative 3.41], negative 3.38 [negative 11.54, 5.53], 2.43 [negative 3.30, 8.50], negative 1.20 [negative 5.90, 3.80], and negative 16.15 [negative 39.49, 7.19]. Second forest plot plots circulatory (lag 0), including RE model for subgroup (Q equals 3.88, df equals 7, p equals 0.79; I squared equals 0.0 percent), Wang et al. 2015 at Taipei (Taiwan), Lee et al. 2014 at Kitakyushu (Japan), Lee et al. 2014 at Taipei (Taiwan), Lee et al. 2014 at Seoul (Korea), Lee et al. 2013 at 7 cities pooled (Korea), Kim et al. 2012 at Seoul (Korea), Chan et al. 2011 at Taipei (Taiwan), and Chen et al. 2004 at Taipei (Taiwan; y-axis) across percent change, ranging from negative 20 to 20 in increments of 5 (x-axis) for percent change (95 percent CI), including 2.33 [0.76, 3.93], 4.00 [negative 1.00, 9.00], 4.90 [negative 7.08, 18.41], negative 0.22 [negative 4.91, 4.71], 2.91 [0.10, 5.80], 1.54 [negative 2.55, 5.80], 291 [0.10, 5.80], negative 3.85 [negative 13.08, 5.39], 3.00 [0.00, 6.00], and negative 0.14 [negative 13.09, negative 12.81]. Third forest plot plots all cause (lag 0), including RE model for subgroup (Q equals 14.31, df equals 8, p equals 0.07; I squared equals 44.1 percent), Wang et al. 2015 at Taipei (Taiwan), Lee et al. 2014 at Kitakyushu (Japan), Lee et al. 2014 at Taipei (Taiwan), Lee et al. 2014 at Seoul (Korea), Lee et al. 2013 at 7 cities pooled (Korea), Kim et al. 2012 at Seoul (Korea), Chan et al. 2011 at Taipei (Taiwan), Chen et al. 2004 at Taipei (Taiwan), and Kwon et al. 2002 in Seoul (Korea; y-axis) across percent change, ranging from negative 20 to 20 in increments of 5 (x-axis) for percent change (95 percent CI), including 1.09 [negative 0.14, 2.34], 3.00 [0.00, 5.00], 3.47 [negative 3.28, 10.70], negative 2.77 [negative 5.13, negative 0.36], 1.73 [negative 0.47, 3.98], 1.46 [0.00, 3.00], 0.95 [negative 5.33, 7.23], 1.90 [0.30, 3.50], 0.48 [negative 6.09, 7.05], and negative 0.30 [negative 4.20, 3.70].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e19/7319773/13832bf0ee1d/ehp5312_f2.jpg)
![Figure 3 is a tabular representation, having 4 columns, namely, categories, number of estimates, I squared, and Percent change (95 percent CI), comprising three forest plots. From bottom to top, the first forest plot plots the following respiratory with number of estimates: Lag 7 with 3, Lag 6 with 3, Lag 5 with 3, Lag 4 with 3, Lag 3 with 5, Lag 2 with 5, Lag 1 with 6, and Lag 0 with 6 (y-axis) across percent change, ranging from negative 10 to 10 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, 2.65 [negative 2.69, 8.29]; 0.0, 4.86 [negative 0.56, 10.57]; 0.0, 0.54 [negative 4.72, 6.10]; 0.0, negative 1.61 [negative 6.78, 3.85]; 0.0, 3.99 [0.08, 8.06]; 55.0, 2.67 [negative 3.90, 9.69]; 44.3, negative 1.71 [negative 6.12, 2.91]; and 49.2, negative 2.58 [negative 7.27, 2.34]. Second forest plot plots the following circulatory with number of estimates: Lag 7 with 3, Lag 6 with 3, Lag 5 with 3, Lag 4 with 3, Lag 3 with 5, Lag 2 with 6, Lag 1 with 7, and Lag 0 with 8 (y-axis) across percent change, ranging from negative 10 to 10 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, negative 0.51 [negative 3.48, 2.55]; 55.1, 3.32 [negative 1.77, 8.67]; 0.0, 2.30 [negative 0.71, 5.41]; 0.0, 0.75 [negative 2.22, 3.82]; 11.5, 0.14 [negative 2.11, 2.45]; 26.2, 0.96 [negative 1.96, 3.96]; 0.8, 1.42 [negative 0.47, 3.34]; and 0.0, 2.33 [0.76, 3.93]. Third forest plot plots the following all cause with number of estimates: Lag 7 with 3, Lag 6 with 3, Lag 5 with 3, Lag 4 with 3, Lag 3 with 6, Lag 2 with 7, Lag 1 with 8, and Lag 0 with 9 (y-axis) across percent change, ranging from negative 10 to 10 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, negative 0.12 [negative 1.69, 1.47]; 0.0, 0.40 [negative 1.17, 2.00]; 0.0, negative 0.10 [negative 1.66, 1.49]; 0.0, 0.21 [negative 1.35, 1.80]; 52.4, 0.24 [negative 1.54, 2.04]; 38.1, 1.01 [negative 0.43, 2.47]; 32.6, 0.07 [negative 1.19, 1.34]; and 44.1, 1.09 [negative 0.14, 2.34].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e19/7319773/7a73999bb9b4/ehp5312_f3.jpg)
![Figure 4 is a tabular representation, having 3 columns, namely, authors and year, study population, and percent change (95 percent CI), comprising four forest plots. From bottom to top, the first forest plot plots Ischemic heart disease or acute myocardial infraction (Lag 3), including RE model for subgroup (Q equals 4.25, df equals 3, p equals 0.22; I squared equals 29.5 percent), Teng et al. 2015 for total, Bell et al. 2008 for total, Meng and Lu, 2007 for female, Meng and Lu, 2007 for male (y-axis) across percent change, ranging from negative 60 to 60 in increments of 20 (x-axis) for percent change [95 percent CI], including 5.09 [negative 3.94, 14.97], 8.18 [1.73, 14.64], 12.22 [negative 3.81, 30.93], negative 14.00 [negative 33.00, 11.00], and negative 7.00 [negative 31.00, 24.00]. Second forest plot plots Pneumonia (Lag 3), including RE model for subgroup (Q equals 5.76, df equals 4, p equals 0.22; I squared equals 30.5 percent), Kang et al. 2012 for total, Cheng et al. 2008 for Total, Bell et al. 2008 for total, Meng and Lu, 2007 for Female, and Meng and Lu, 2007 for Male (y-axis) across percent change, ranging from negative 60 to 60 in increments of 20 (x-axis) for percent change [95 percent CI], including 8.51 [2.89, 14.44], 14.03 [6.05, 22.61], 3.70 [negative 0.70, 8.40], 12.97 [negative 2.14, 30.40], 16.00 [negative 14.00, 57.00], and 8.00 [negative 8.00, 28.00]. Third forest plot plots Asthma (Lag 3), including RE model for subgroup (Q equals 1.17, df equals 3, p equals 0.76; I squared equals 0.0 percent), Park et al. 2016 for total, Wang et al. 2014 for total, Bell et al. 2008 for Total, and Yang et al. 2005 for total (y-axis) across percent change, ranging from negative 60 to 60 in increments of 20 (x-axis) for percent change [95 percent CI], including 14.55 [6.74, 22.94], 15.00 [negative 3.00, 37.00], 12.16 [2.52, 21.81], 25.04 [4.62, 49.45], and 8.00 [negative 3.00, 776.00]. Forth forest plot plots respiratory disease (Lag 3), including RE model for subgroup (Q equals 3.31, df equals 4, p equals 0.51; I squared equals 0.0 percent), Tao et al. 2012 for total, Lai and Cheng, 2008 for female, Lai and Cheng, 2008 for male, Meng and Lu, 2007 for female, and Meng and Lu, 2007 for male (y-axis) across percent change, ranging from negative 60 to 60 in increments of 20 (x-axis) for percent change [95 percent CI], including 8.85 [0.80, 17.55], negative 0.10 [negative 12.20, 12.00], 7.00 [negative 69.00, 272.00], 6.00 [negative 73.00, 317.00], 18.00 [0.00, 41.00], and 14.00 [1.00, 29.00].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e19/7319773/ba5d975b19a9/ehp5312_f4.jpg)
![Figure 5 is a tabular representation, having 4 columns, namely, categories, number of estimates, I squared, and Percent change (95 percent CI), comprising four forest plots. From bottom to top, the first forest plot plots the following Ischemic heart disease or acute myocardial infraction with number of estimates: Lag 6 with 3, Lag 5 with 3, Lag 4 with 4, Lag 3 with 4, Lag 2 with 4, Lag 1 with 5, and Lag 0 with 4 (y-axis) across percent change, ranging from negative 25 to 25 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, negative 3.12 [negative 8.08, 2.11]; 0.0, negative 0.92 [negative 7.11, 5.69]; 65.3, 6.00 [negative 10.03, 24.90]; 29.5, 5.09 [negative 3.94, 14.97]; 60.2, 8.77 [negative 4.27, 23.58]; 53.7, 2.78 [negative 8.92, 15.98]; and 0.0, negative 0.95 [negative 4.69, 2.94]. Second forest plot plots the following Pneumonia with number of estimates: Lag 6 with 3, Lag 5 with 3, Lag 4 with 3, Lag 3 with 5, Lag 2 with 5, Lag 1 with 5, and Lag 0 at 5 (y-axis) across percent change, ranging from negative 25 to 25 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 39.5, 4.19 [negative 6.66, 16.30]; 0.0, 6.14 [negative1.26, 14.09]; 32.5, 4.61 [negative 6.72, 17.32]; 30.5, 8.51 [2.89, 14.44]; 55.7, 7.33 [negative 0.57, 15.86]; 30.8, 7.56 [1.83, 13.61]; and 18.7, 3.73 [negative 0.92, 8.59]. Third forest plot plots the following Asthma with number of estimates: Lag 3 with 4, Lag 2 with 4, Lag 1 with 4, and Lag 0 with 6 (y-axis) across percent change, ranging from negative 25 to 25 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, 14.55 [6.74, 22.94]; 30.2, 7.18 [negative 4.18, 19.88]; 0.0, 8.09 [0.86, 15.84]; and 20.9, 3.68 [negative 5.34, 13.57]. Forth forest plot plots the following respiratory disease with number of estimates: Lag 6 with 3, Lag 5 with 3, Lag 4 with 3, Lag 3 with 5, Lag 2 with 5, Lag 1 with 5, and Lag 0 with 5 (y-axis) across percent change, ranging from negative 25 to 25 in increments of 5 (x-axis) for I squared and percent change [95 percent CI], including 0.0, 5.35 [negative 2.59, 13.93]; 37.5, negative 3.84 [negative 13.42, 6.80]; 79.6, 4.26 [negative 12.63, 24.43]; 0.0, 8.85 [0.80, 17.55]; 0.0, negative 4.76 [negative 12.54, 3.72]; 0.0, 3.29 [negative 4.78, 12.05]; and 5.5, negative 0.71 [negative 10.11, 9.68].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e19/7319773/60d4b33abc38/ehp5312_f5.jpg)