Abstract
Background
Pembrolizumab, an immune checkpoint inhibitor (ICI), is an IgG4 antibody that blocks interaction between programmed cell death protein 1 and programmed death-ligand 1. Myocarditis, an immune-related adverse event, has been reported in thymic epithelial tumours. Pembrolizumab has also been associated with development/exacerbation of myasthenia gravis (MG).
Case summary
A 70-year-old woman with metastatic thymic cancer presented to the hospital with shortness of breath, 21 days after initiation of pembrolizumab. She was diagnosed with ICI-related myocarditis and was subsequently intubated due to respiratory failure. A dual-chamber pacemaker was placed due to complete heart block with asystole. Her troponin levels were elevated, an electrocardiogram was suspicious for myocardial infarction, but coronary angiogram revealed normal coronary arteries and endomyocardial biopsy confirmed the presence of myocarditis. Treatment was started with high-dose intravenous methylprednisolone and cardiovascular status improved. However, the patient was unable to be weaned from mechanical ventilation and tested positive for acetylcholine receptor binding/blocking antibodies due to de novo MG. After 50 days of hospitalization, she was discharged home in stable condition. A computed tomography scan was performed 6 weeks after pembrolizumab; results showed significant decrease/resolution of all measurable sites of metastatic disease in the lungs.
Discussion
This is the first reported case of a patient developing single-agent pembrolizumab-induced myocarditis concomitant with new-onset MG after treatment for advanced thymic malignancy. Additional studies are needed to explore the association between myocarditis, MG, and ICI therapy.
Keywords: Myocarditis, Immune checkpoint inhibitors, Cancer, Myasthenia gravis, Thymic carcinoma, Case report
Learning points
Pembrolizumab is associated with an increased risk of cardiovascular and neuromuscular adverse effects, especially in patients with thymic epithelial tumours.
Endomyocardial biopsy that shows lymphocytic infiltrates with myonecrosis is considered gold standard for diagnosing immune checkpoint inhibitor (ICI)-related myocarditis.
The suggested first-line treatment for ICI-induced myocarditis includes high-dose methylprednisolone and a cessation of ICI therapy.
Primary specialties involved
Cardiology
Oncology
Neurology
Introduction
One critical function of T lymphocytes is the control of tumour cell growth and proliferation. This function is executed via the induction of tumour cell death, initiated through inter-ligand binding between the tumour cell and T lymphocyte. However, select tumour cells have developed mechanisms of resistance to this immune response by altering the activity of inhibitory T lymphocyte regulatory pathways, allowing cancer cells to proliferate with less restriction from these immune cells.1
Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are located on the surface of T lymphocytes, and normally play a role in peripheral tolerance and self-recognition. A class of oncologic agents, called immune checkpoint inhibitors (ICIs), are designed to block the interaction between CTLA-4, PD-1, and their cognate ligands.2 The inhibition of CTLA-4 and PD-1 increases T lymphocyte activation and decreases the effects of tumour cell-induced anergy.3 These ICIs have been therapeutically utilized for various cancer subtypes since the introduction of the CTLA-4 antibody, ipilimumab in 2011.1 Currently, there are several ICI therapies approved by the FDA for treatment of cancer, including ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, and cemiplimab.1
Pembrolizumab is a humanized IgG4 antibody that blocks the interaction between PD-1 and programmed death-ligand 1 (PD-L1) and is currently approved by the Food and Drug Administration for treatment of 11 distinct cancer subtypes, as well as advanced mismatch repair-deficient cancers.4 Pembrolizumab has also demonstrated meaningful clinical activity in patients with recurrent thymic carcinoma after previous chemotherapy.5 Immune-related adverse events (irAEs) are the primary toxicities associated with pembrolizumab use. Across all cancer subtypes, the most common, serious irAEs include hypothyroidism (8.5% of patients), hyperthyroidism (3.4% of patients), pneumonitis (3.4% of patients), and colitis (1.7%). Other rare irAEs, including myocarditis and neuromuscular adverse events, have also been reported.
Myocarditis, induced by pembrolizumab therapy, has been observed with a crude incidence rate between 0.06% and 2.4% for most cancers.6 However, the incidence of myocarditis has been reported to be much higher in two trials evaluating pembrolizumab in thymic epithelial tumours (TETs), at 5% and 9.1%, respectively.5,7 Pembrolizumab therapy has also been associated with neuromuscular adverse events, including development of de novo or acute exacerbation of myasthenia gravis (MG), neuropathy, and myopathy.8 The increased incidence of patients being treated with ICIs, combined with the potential morbidity/mortality of associated severe irAEs, necessitates a more thorough understanding of how to properly diagnose and treat these complications.
In this case report, we present a patient who developed myocarditis, complicated by complete atrioventricular heart block and concomitant de novo MG, 3 weeks following administration of one cycle of pembrolizumab therapy.
Timeline
| 10 years prior to presentation | Patient diagnosed with thymic carcinoma; treated with four cycles cisplatin/etoposide |
| 5 years prior to presentation | Presents with recurrent disease to bone and pleura; treated with sunitinib (discontinued after 1 year) |
| 1 year prior to presentation | Progressive disease of spine; undergoes decompressive laminectomy (levels T7–T8) |
| 16 days prior to presentation | New metastases discovered in bone and lung; treated with pembrolizumab (one cycle) |
| Upon first emergent presentation | Left lower lobe pulmonary embolism discovered; treated with enoxaparin (subcutaneous) |
| 2 days following first emergent presentation | Discharged to home |
| Upon second emergent presentation (5 days following first emergent presentation) | Presents with acute illness, right bundle branch block with elevated troponin, ST elevation in precordial leads, myocarditis suspected. Treated with methylprednisolone (IV); enoxaparin (subcutaneous); aspirin (oral) |
| Day 1 to Day 28 following second emergent presentation | Patient with complete heart block received dual-chamber pacemaker, coronary artery disease ruled out by negative cardiac catheterization, immune checkpoint inhibitor myocarditis confirmed by endomyocardial biopsy: pulse-dose methylprednisolone IV, followed by oral prednisone |
| Day 29 to Day 50 following second emergent presentation | Patient exhibits hypercapnia and respiratory failure; positive antibodies, physical findings significant for myasthenia gravis. Patient receives intubation [with eventual extubation to bilevel positive airway pressure (BiPAP)]; pyridostigmine; plasmapheresis; methylprednisolone (IV); and prednisone (oral) |
| Day 50 following second emergent presentation | Discharge to home with BiPAP treatment during sleep |
| 6 weeks following administration of pembrolizumab | Computed tomography results showed improvement of disease with significant decrease or resolution of all measurable sites of metastatic disease in the lungs |
Case presentation
Disease history
The presented patient is a 70-year-old woman with a history of Stage IIIA thymic carcinoma. She was originally diagnosed in September 2009 and was treated with cisplatin/etoposide chemotherapy (four cycles) and concurrent radiation, followed by surgical resection of the primary tumour which yielded clear margins. The pathology of the resected tumour demonstrated a thymic neoplasm favouring thymic carcinoma with squamoid features [World Health Organization histologic classification (WHO) Type C]. In July 2014, the patient developed recurrent disease with bone and pleural metastases. She declined systemic chemotherapy due to toxicity concerns, but accepted therapy with sunitinib, a tyrosine kinase inhibitor, which was discontinued, 1 year later, in July 2015, due to intolerance. She subsequently received palliative radiation therapy to areas of disease progression. In September 2018, the patient presented with progressive disease in the spine for which she underwent decompressive laminectomy at the level of T7–T8. The surgical pathology favoured metastatic thymoma (WHO Type B3), despite the original findings in 2009 favouring thymic carcinoma. She had no signs or symptoms of autoimmune disease and recovered normally from surgery. The patient received post-operative radiation therapy to the thoracic spine.
In April 2019, positron emission tomography–computed tomography (CT) imaging revealed new bone and lung metastases. Patient was extensively counselled regarding treatment options but decided against chemotherapy due to previous toxicities and associated poor quality of life. The original pathology was re-examined and determined to be most consistent with thymic carcinoma. The tumour tissue exhibited high PD-L1 expression with a tumour proportion score of 70%. After discussion of potential risks and benefits, immunotherapy was initiated with pembrolizumab (200 mg), with intravenous delivery every 3 weeks.
Complications and admission
Sixteen days after the first dose of pembrolizumab, the patient reported to the emergency department with exertional dyspnoea and was admitted to the hospital. Vital signs on admission indicated a heart rate (HR) of 100 b.p.m., blood pressure (BP) of 154/78 mmHg, respiratory rate (RR) of 22 rpm, and an oxygen saturation (O2Sat) of 92% (on room air); a physical exam had a non-contributory cardiovascular examination. A chest computerized tomography angiography revealed a left lower lobe pulmonary embolism (Figure 1). Electrocardiography on admission showed biphasic T waves in the septal leads. The reported troponin T levels peaked at 0.271 ng/L (normal: 0.0–0.29 ng/L) (not measured with high-sensitivity assay). Echocardiography revealed mild left ventricular (LV) hypertrophy with normal LV ejection fraction of 63%. The right ventricle was normal in size and function. Measured right ventricular systolic pressure was 50 mmHg. The patient was treated with therapeutic enoxaparin (80 mg, subcutaneous, every 24 h) and continued low-dose aspirin previously started at the emergency department (81 mg, per oral, once daily). The patient was discharged in stable condition 2 days later.
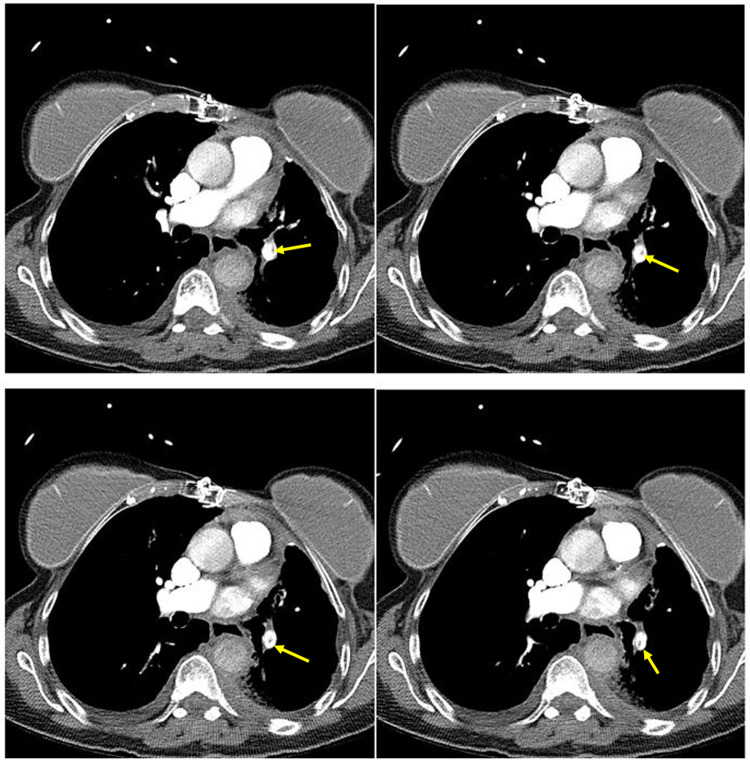
Figure 1.
Sequential computed tomography slices (3 mm) showing pulmonary embolism (indicated by yellow arrows) in left lower lobar pulmonary artery extending into a segmental branch.
Three days following discharge, the patient was readmitted to the hospital, presenting with dyspnoea, orthopnoea, and weakness. Vital signs on readmission indicated an HR of 87 b.p.m., BP of 158/93 mmHg, RR of 32 rpm, and O2Sat of 95% (on room air); a physical exam was within normal limits. However, her troponin T during this presentation had increased to 10.50 ng/L (normal: 0.0–0.29 ng/L). In addition, the patient developed severe respiratory failure and was intubated and placed on supportive mechanical ventilation. Electrocardiography revealed a new right bundle branch block, with T-wave inversions in I and aVL, poor R-wave progression, ST elevation in precordial leads, and Q waves in anterolateral leads suggestive of anterior myocardial infarction (Figure 2). Limited echocardiography showed preserved LV ejection fraction and probable anterior wall hypokinesis with compensatory hyperdynamic posterior wall. Lab studies indicated mild peak troponin T levels of 10.50 ng/L (not measured with high-sensitivity assay), creatine kinase levels of 1667 U/L, and creatine kinase muscle/brain levels of 107 U/L. Myocarditis was suspected, likely secondary to the ICI therapy. The patient received methylprednisolone (1 g per day, intravenously, for 3 days), and subsequently switched to oral prednisone, and enoxaparin (80 mg, subcutaneous, every 24 h) was continued.
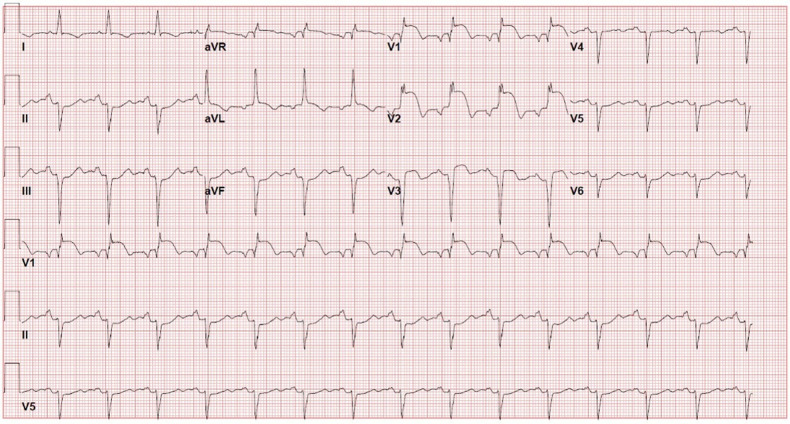
Figure 2.
Electrocardiography, from second hospital admission, revealed a new right bundle branch block, with T-wave inversions in I and aVL, poor R-wave progression, ST elevation in precordial leads, and Q waves in anterolateral leads, suggestive of anterior myocardial infarction.
Subsequently, the patient was found to be in complete heart block with ventricular escape rhythm between 20 and 30 b.p.m. and severe respiratory failure. The patient was placed on intravenous isoproterenol drip and underwent coronary angiography, right/left heart catheterization, and urgent temporary pacemaker placement. The coronary angiography revealed normal coronary artery anatomy (Figure 3).
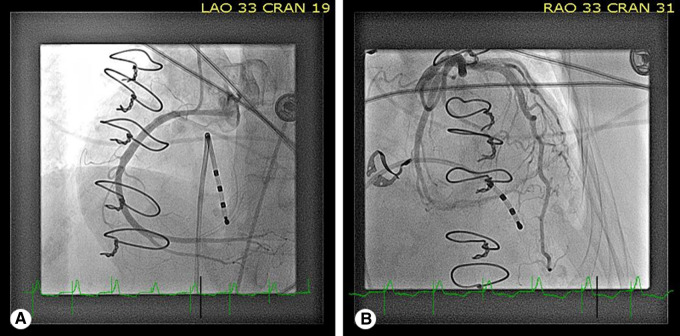
Figure 3.
Coronary angiography revealed normal coronary artery anatomy, as seen in left anterior oblique—cranial (A) and right anterior oblique—cranial (B) views.
A right heart catheterization showed pulmonary artery pressure of 24/16 mmHg (mean: 18 mmHg), and pulmonary capillary wedge pressure of 12 mmHg. At that time, an endomyocardial biopsy (EMB) was performed, and the patient was treated with pulse-dose steroids using methylprednisolone (1 g per day, intravenously, for 3 days). A dual-chamber pacemaker was subsequently placed due to intermittent complete heart block with asystole. The EMB revealed mild myocyte hypertrophy with diameters in the 30–40 µm range. Lipofuscin granules were observed in the perinuclear region of myocytes. Abundant lymphocytosis, consistent with myocarditis, was reported. Three of the seven tissue samples exhibited the presence lymphocytes and macrophages. No giant cells or eosinophils were present. Trichrome staining indicated early interstitial fibrosis and endocardial fibrosis in areas of inflammatory infiltration. There was no evidence of endocardial fibroelastosis, glycogen storage, or iron depletion. Amyloid was also not present (Figure 4).
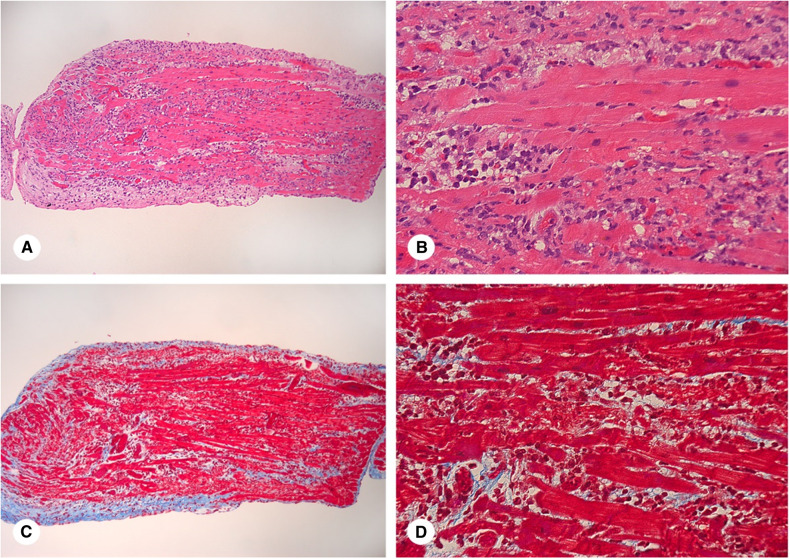
Figure 4.
Endomyocardial biopsy: A and C. Light micrograph of a biopsy sample of myocardium. The endocardium surrounds the entire biopsy piece (blue fibrous tissue in C). The normal endocardium is seen in the right lower corner of the piece. The remainder of the endocardium is much thicker and shows mononuclear cells as well as spindle shaped cells (fibroblasts) indicating a repair process. The myocardium shows abundant inflammatory infiltrate as well as areas of interstitial fibrosis (blue stain in C). (A. Haematoxylin and eosin stain. X100, C. Trichrome stain. X100). B and D. Higher magnification shows the inflammatory infiltrates (predominantly lymphocytes and macrophages with a minor component of neutrophils and eosinophils) which encroach and separate the myocyte bundles. The interstitial fibrosis is more subtle but present (blue in D), indicating a repair process ongoing concomitantly with the inflammatory process. (B. Haematoxylin and eosin stain. X500, D. Trichrome stain. X500).
Although the patient had been intubated emergently upon admission to the floors due to severe bradycardia and respiratory failure, she was unable to be weaned from mechanical ventilation due to persistent hypercapnia. After neurology consultation, testing for muscle-specific tyrosine kinase (MuSK) and acetylcholine receptor (AChR) antibodies was performed, which were positive for AChR binding and blocking antibodies. The patient was determined to have de novo MG given the lack of previous signs or symptoms, although an exacerbation of subclinical MG could not be excluded. Pyridostigmine (60 mg, every 6 h) was initiated and the patient received five plasmapheresis sessions. Methylprednisolone (1 gr per day, intravenously, for 3 days) followed by oral prednisone (1 mg/kg per day) was administered. The use of intravenous immunoglobulin (IVIG) was avoided in this patient, due to her recent thromboembolic event. Because IVIG increases blood viscosity in vitro and in vivo, it should generally be avoided in high-risk patients due to potential for cardiovascular and cerebrovascular thromboembolism. On Day 13 of hospitalization, the patient was extubated to non-invasive ventilation via bilevel positive airway pressure (BiPAP) treatment with successful weaning to nasal cannula oxygen.
Discharge
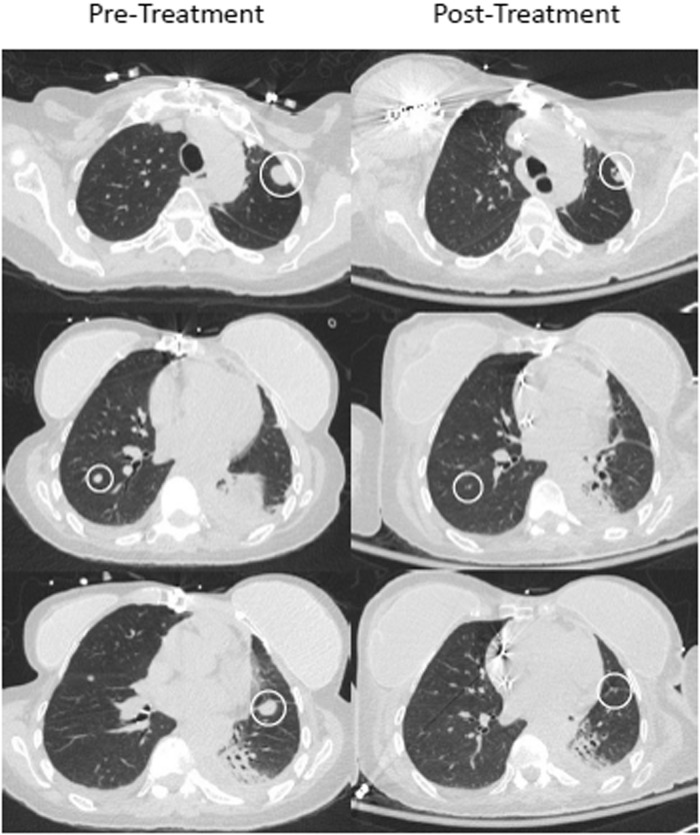
After 50 days of hospitalization, the patient’s respiratory status progressively improved, and she was discharged home in stable condition with home BiPAP treatment during sleep. Prior to discharge, a chest CT, without contrast, was performed approximately 6 weeks after pembrolizumab treatment to evaluate the disease status. The CT results showed improvement with significant decrease or resolution of all measurable sites of metastatic disease in the lungs (Figure 5).
Figure 5.
Pre-treatment (left) and post-treatment (right) computed tomography imaging of the chest, 6 weeks after single dose of pembrolizumab.
Discussion
Myocarditis is an inflammatory disease of the myocardium that is often a result of infection, autoimmune diseases, or adverse reactions to pharmaceuticals.9 Patients treated with ICI therapy may present with non-specific cardiovascular symptoms resulting from associated myocarditis. Common symptoms include fatigue, myalgia, chest pain, dyspnoea, orthopnoea, leg swelling, palpitations, lightheadedness/dizziness, syncope, and change in mental status.10 According to the Dallas Criteria, lymphocytic infiltrates with myonecrosis seen on EMB is the gold standard for diagnosing ICI-related myocarditis. However, cardiac magnetic resonance imaging (MRI) is often used as a diagnostic tool due to its high degree of sensitivity, minimal invasiveness, and specific imaging technique.9,10
A multicentre registry with data collected from 2013 to 2017 reported an ICI-associated myocarditis crude incidence rate of 1.14%, with more recent reports indicating rates up to 2.4%. However, this autoimmune phenomenon is likely underdiagnosed due to the broad range of symptoms, lack of awareness, and challenges of diagnosis.10–12 Proposed diagnostic algorithms may help the clinician to stratify the probability of ICI-associated myocarditis into three tiers: possible, probable, or definitive as determined by the clinical presentation, biomarkers, MRI features, and EMB. The risk factors for ICI-associated myocarditis are not well-defined, but patients with pre-existing cardiac disease, diabetes mellitus, and/or obstructive sleep apnoea appear to be at greater risk.11
There is a lack of significant data with respect to the most appropriate course of treatment for ICI-associated myocarditis. Currently, the suggested first-line treatment includes high-dose methylprednisolone and a cessation of ICI therapy.10 In addition, temporary pacemaker insertion is indicated in patients with symptomatic atrioventricular block II or III.9 Fulminant myocarditis is associated with a 42% fatality rate, underscoring the importance of monitoring, detection, and early intervention.13
Interestingly, the prevalence of ICI-associated myocarditis is dramatically increased in patients with TETs compared to other types of cancers.14 Case reports have already described the use of nivolumab in TET, including at least one report of fatal myocarditis and rhabdomyolysis in a patient with Type B3 thymoma.15 In addition, in a phase II trial, investigating the use of pembrolizumab for treating advanced thymic carcinoma, myocarditis occurred in 5% of patients. In the same study, 15% of patients developed high-grade [National Cancer Institute-Terminology Criteria for Adverse Events (NCI-CTCAE) Grade 3–4] irAEs overall.5 In another phase II trial, exploring the use of pembrolizumab in patients with recurrent or relapsed TETs, 9.1% of thymoma patients developed myocarditis. No patients in the thymic carcinoma group developed myocarditis, but 15% developed other high-grade irAEs such as neuromuscular disease.7 Based on results from limited clinical studies, the incidence of myocarditis and other irAEs in patients with TETs who are treated with pembrolizumab appears to be higher than patients undergoing similar treatment for other cancer types.
In this case, the patient developed MG concomitantly with myocarditis. Thymic epithelial tumours, more commonly thymomas, are associated with autoimmune phenomena, particularly MG.16 Interestingly, myocardial changes have also been reported in patients with MG, with rates up to 37%.17 The use of avelumab, an anti-PD-L1 ICI, was reported in a small series (eight patients) of TET with a high incidence of autoimmune phenomenon (37%), including myositis but no myocarditis reported.18 Case reports have also described the use of nivolumab in TET, including at least one report of fatal myocarditis and rhabdomyolysis in a patient with Type B3 thymoma.15 Aarli et al.19 reported that the heart may be an autoimmune target of MG, based on the interactions of anti-RYR antibodies and cardiac ryanodine receptors, as well as the production of antibody-specific to a voltage-gated potassium channel protein, Kv1.4. In another study specific to nivolumab-induced MG, 33% of patients developed myocarditis concurrently with MG.20 Additional literature suggests that the mechanism associated with the development of MG in patients with TETs may be a result of immature, TET-derived thymocytes that have escaped self-tolerance and become auto-reactive.16 Immune checkpoint inhibitor therapy further activates T lymphocytes, exacerbating the autoimmune reactivity of the cells and most likely resulting in the increased rates of irAEs compared to other cancers.7,20 Further research is needed to explore the intricacies of these mechanisms.
In 2015, there were nearly 600 000 patients eligible to receive ICI therapy.21 With the immune modulation industry projected to experience a compound annual growth rate of 14.6%, between 2018 and 2026, many more patients may be treated with ICIs, who may subsequently be at-risk of exhibiting life-threatening irAEs.22 This case report, combined with results from the aforementioned phase II studies, should raise awareness of irAEs in patients with TETs. Patients presenting with heart failure symptoms who are being treated with ICIs should raise clinical suspicion of therapy-related myocarditis.
To our knowledge, this is the first reported case of a patient developing single-agent pembrolizumab-induced myocarditis, concomitant with de novo MG, after treatment for advanced thymic malignancy. While the patient had radiographic improvement in her metastatic disease, she developed life-threatening autoimmune complications that preclude further ICI therapy. Additional studies are needed to mechanistically explore and understand the association between myocarditis, MG, and ICI therapy to allow for earlier diagnoses, more efficacious treatment, and better long-term prognosis.
Lead author biography

Dr Diego Sadler is the Section Head of Cardio-Oncology at the Heart and Vascular Center of Cleveland Clinic Florida. He is also Chairman of the Cardio-Oncology Committee of the Florida Chapter of the American College of Cardiology (FCACC), a member of the Board of Directors of the FCACC, and a member of the Advocacy Work Group at the Cardio-Oncology Council for the American College of Cardiology. Dr Sadler is Board Certified in Internal Medicine, Cardiovascular Diseases, and Nuclear Cardiology and is actively involved in clinical, educational, and research activities in cardio-oncology with main focus in improving access to advanced care in this rapidly growing new sub-specialty.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Hargadon KM, Johnson CE, Williams CJ.. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- 2. Seidel JA, Otsuka A, Kabashima K.. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diesendruck Y, Benhar I.. Novel immune check point inhibiting antibodies in cancer therapy—opportunities and challenges. Drug Resist Updates 2017;30:39–47. [DOI] [PubMed] [Google Scholar]
- 4. KEYTRUDA (pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck & Co., INC; 2014. –2019. [Google Scholar]
- 5. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, Subramaniam DS, Liu SV, Kaplan IM, McCutcheon JN.. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tajiri K, Ieda M.. Cardiac complications in immune checkpoint inhibition therapy. Front Cardiovasc Med 2019;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho J, Kim HS, Ku BM, Choi Y-L, Cristescu R, Han J, Sun J-M, Lee S-H, Ahn JS, Park K.. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol 2019;37:2162–2170. [DOI] [PubMed] [Google Scholar]
- 8. Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D.. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology 2019;92:663–674. [DOI] [PubMed] [Google Scholar]
- 9. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M.. Update on myocarditis. J Am Coll Cardiol 2012;59:779–792. [DOI] [PubMed] [Google Scholar]
- 10. Ganatra S, Neilan TG.. Immune checkpoint inhibitor-associated myocarditis. Oncologist 2018;23:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG.. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balanescu D, Donisan T, Palaskas NL, Iliescu C.. Emerging concerns in cardio-oncology: immune checkpoint inhibitor cardiotoxicity. American College of Cardiology. 24 June 2019; cited December 2019. https://www.acc.org/latest-in-cardiology/articles/2019/06/21/08/45/emerging-concerns-in-cardio-oncology#.XbiPA3WTwOw.email. [Google Scholar]
- 13. Varricchi G, Marone G, Mercurio V, Galdiero MR, Bonaduce D, Tocchetti CG.. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr Med Chem 2018;25:1327–1339. [DOI] [PubMed] [Google Scholar]
- 14. De Velasco G, Je Y, Bosse D, Awad MM, Ott PA, Moreira RB, Schutz F, Bellmunt J, Sonpavde GP, Hodi FS, Choueiri TK.. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Q, Huang DS, Zhang LW, Li YQ, Wang HW, Liu HB.. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 2018;56:667–671. [DOI] [PubMed] [Google Scholar]
- 16. Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y.. Thymoma and autoimmunity. Cell Mol Immunol 2011;8:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukasawa Y, Sasaki K, Natsume M, Nakashima M, Ota S, Watanabe K, Takahashi Y, Kondo F, Kozuma K, Seki N.. Nivolumab-induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol 2017;10:809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajan A, Heery C, Mammen A, Pittaluga S, Lepone L, Donahue R, Grenga I, Schlom J, Gulley J, Hassan R.. OA18. 03 safety and clinical activity of Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced thymic epithelial tumors (TETs). J Thorac Oncol 2017;12:S314–S315. [Google Scholar]
- 19. Aarli JA. Herzmyasthenie: myasthenia of the heart. Arch Neurol 2009;66:1322–1323. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, Uhara H, Hasegawa Y, Inomata S, Otani Y, Yokota K, Hirose T, Tanaka R, Suzuki N, Matsui M.. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–1134. [DOI] [PubMed] [Google Scholar]
- 21. Andrews A. Treating with checkpoint inhibitors-figure $1 million per patient. Am Health Drug Benefits 2015;8:9. [PMC free article] [PubMed] [Google Scholar]
- 22.Research PM. Immune Checkpoint Inhibitors Expanding at a Booming CAGR of 14.6% During 2018-2026—Persistence Market Research 5 September 2018; cited December 2019: https://www.globenewswire.com/news-release/2018/09/05/1565767/0/en/Immune-Checkpoint-Inhibitors-Expanding-at-a-booming-CAGR-of-14-6-during-2018-2026-Persistence-Market-Research.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.