Abstract
Background
Dabigatran is a direct competitive thrombin inhibitor approved for stroke prevention in non-valvular atrial fibrillation. At full-dose, dabigatran showed similar rates of bleedings and higher efficacy compared to warfarin.
Case summary
We report a case of acute ischaemic stroke in a patient treated with dabigatran 150 mg b.i.d. for atrial fibrillation. After an off-label treatment with idarucizumab, a humanized monoclonal antibody approved for dabigatran reversal, we performed a successful intravenous thrombolysis (IVT). Transoesophageal echocardiography showed a left atrial appendage (LAA) thrombus, despite full-dose dabigatran and an adequate therapy adherence.
Discussion
There are few cases of LAA thrombus during dabigatran treatment reported in literature till date. We analyse the possible pathogenetic mechanisms involved in dabigatran failure, including drug interactions and unexpected genetic variations interfering with dabigatran serum levels suggesting periodical assessment of direct Oral Anticoagulant levels. Furthermore, we confirm initial reports of safety and efficacy of intravenous thrombolysis after idarucizumab, in case of dabigatran failure.
Keywords: Case report, Atrial fibrillation, Dabigatran, Left atrial appendage thrombus, Intravenous thrombolysis, Idarucizumab
Learning points
Different mechanisms could lead to dabigatran failure in non-valvular atrial fibrillation, despite adequate dosing, good therapeutic adherence, and the absence of concomitant interacting drugs.
Off-label treatment with idarucizumab makes intravenous thrombolysis safe and effective in case of dabigatran failure.
Introduction
Atrial fibrillation (AF) is a well-known risk factor for stroke or systemic embolism, frequently associated with left atrial appendage (LAA) or left atrial (LA) thrombi. For decades, vitamin K antagonists (VKAs) have been the mainstay of stroke prevention in AF patients. Dabigatran etexilate is a direct, competitive, and reversible thrombin inhibitor, licensed for prevention of stroke/systemic embolism in non-valvular AF (NVAF). At 150 mg b.i.d., dabigatran showed similar rates of bleedings and higher efficacy compared to warfarin.1 Idarucizumab, a humanized monoclonal antibody fragment, reverses the anticoagulant effect of dabigatran within minutes and seems to be promising for rapid reversal of dabigatran effects, even before rt-PA.2,3 In case of dabigatran failure, a thorough investigation is warranted to identify the aetiological diagnosis of stroke and redefine the secondary prevention strategy. Here, we report the case of a dabigatran-treated patient presenting with acute stroke, who underwent idarucizumab followed by intravenous thrombolysis (IVT) and was subsequently diagnosed a large LAA thrombus. We reviewed the literature and tried to analyse the potential mechanisms underlying LAA or LA formation during dabigatran treatment.
Timeline
| Past history and medication at baseline | Non-valvular atrial fibrillation, hypertension, Type 2 diabetes mellitus, hypercolesterolaemia. Dabigatran 150 mg b.i.d., metformin, telmisartan, furosemide, atenolol, rosuvastatin, ezetimibe, and doxazosine. |
| Emergent assessment and treatment | Clinical features of total left anterior circulation syndrome (National Institute of Health Stroke Scale, NIHSS 21). Computed tomography scan shows distal left M1 occlusion. Off-label treatment with idarucizumab 5 g IV as a bolus, followed by IV rt-PA 0.9 mg/kg at 2 h from symptoms onset. |
| Aetiopathogenetic diagnostic work up | Transoesophageal echocardiography (TOE) reveals a left atrial appendage (LAA) thrombus. |
| Condition and treatment at discharge | No haemorrhagic complications after intravenous thrombolysis. At discharge, mild reduction in verbal fluency (NIHSS 2). Dabigatran is interrupted and warfarin is started. |
| 3 months follow-up | Independent patient (modified Rankin scale 1), with a good time-to-range. Complete resolution of her LAA thrombus at TOE. |
Case presentation
Patient information
A 73-year-old woman presented to our emergency department an hour after the acute onset of a total left anterior circulation syndrome, with right-sided hemiplegia, global aphasia, right-sided hemianopsia, forced left-sided eye deviation, and National Institute of Health Stroke Scale (NIHSS) score 21. Her past medical history, reported by her husband, was remarkable for diabetes, hypercholesterolaemia, hypertension, and permanent NVAF, treated with warfarin for many years. A year before, she was switched to dabigatran 150 mg b.i.d. for its more manageable profile. Last dabigatran intake was 10 h before stroke onset. The patient was also on metformin, telmisartan, furosemide, atenolol, rosuvastatin, ezetimibe, and doxazosin.
Diagnostic assessment
The electrocardiogram showed AF with a ventricular response of 80 b.p.m. Coagulation studies revealed activated partial thromboplastin time (aPTT) ratio 1.6, prothrombin time (PT) ratio 1.24. An ecarin clotting time (ECT) could not be performed. The brain computed tomography angio showed an occlusion of left middle cerebral artery (Figure 1), according to her clinical deficits.
Figure 1.
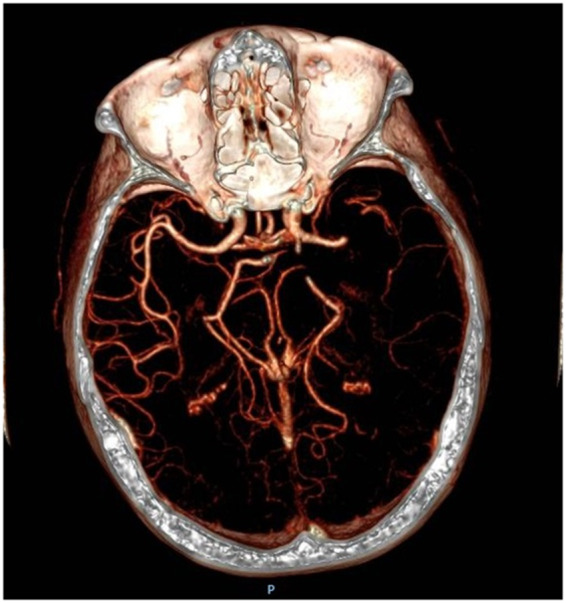
Computed tomography angio showing left middle cerebral artery occlusion.
Interventions
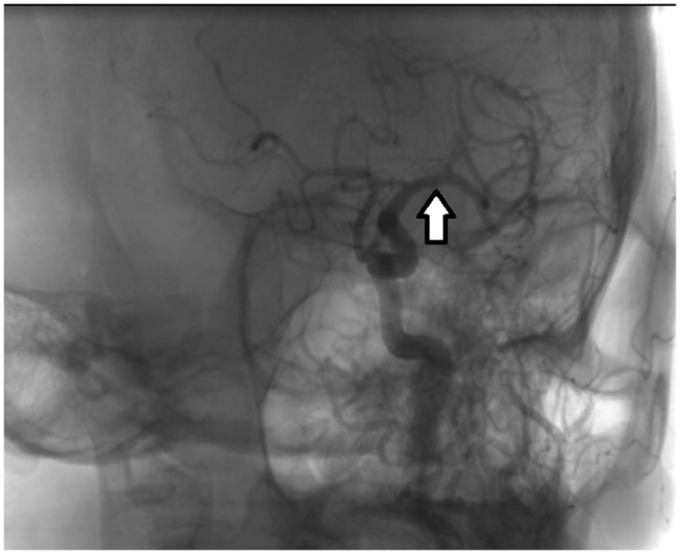
She was off-label treated with idarucizumab 5 g IV as a bolus, followed by IV rt-PA 0.9 mg/kg at 2 h from symptoms onset and directly transferred to cath lab for mechanical thrombectomy. The diagnostic angiography revealed complete middle cerebral artery recanalization by the end of IVT (Figure 2).
Figure 2.
Digital subtraction angiography showing complete recanalization of left middle cerebral artery, by the end of intravenous thrombolysis with rt-PA.
The patient experienced no haemorrhagic complications. The neurological conditions progressively improved, with the exception of mild reduction in verbal fluency, NIHSS score 2. She underwent a thorough cardiological investigation and a large thrombus in the LAA was demonstrated at the transoesophageal echocardiography (Figure 3). The patient refused LAA closure and warfarin was started. At 3 months, she was independent with a modified Rankin scale 1, with a good interantional normalized ratio (INR) time-to-range. A follow-up echocardiography showed the complete resolution of her LAA thrombus.
Figure 3.
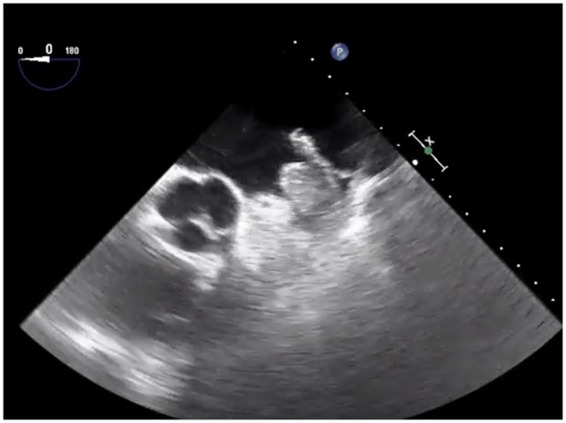
Transoesophageal echocardiography showing a large left atrial appendage thrombus.
Discussion
Since its approval in 2010, dabigatran has been an effective alternative to VKAs, reducing the primary outcome of stroke/systemic embolism by 34% compared to warfarin in the RE-LY trial.1 However, during dabigatran treatment, LAA or LA thrombi formation were reported. Five out of 10 patients were on low-dose dabigatran, at the time of diagnosis.4–8 Two out of 10 patients were on full-dose dabigatran. One of them was simultaneously taking phenytoin.9 In another case, an LAA thrombus persisted despite warfarin and full-dose dabigatran.10 One out of 10 patient was on dabigatran, despite valvular AF.11 Of the last two patients, one was also affected by lung cancer.12
No certain mechanisms for dabigatran failure have been identified, but several theories have been proposed.4,8 Firstly, inadequate dosing seems to be the most common cause for dabigatran failure. Secondly, single level downstream inhibition of thrombin may lead to compensatory increase in upstream clotting factors. Thirdly, all the available thrombin may not be inhibited. Fourthly, absence of monitoring may hamper assessment of therapeutic adherence and anticoagulation status. Moreover, due to its short half-life, dabigatran may possibly cause an hourly variation of anticoagulation level. Finally, a genetic susceptibility may be present, involving the ABCB1 and CES-1 genes. The former encodes for P-glycoprotein (P-gp), one of the drug transporters in the gastrointestinal and urinary tracts. Particular ABCB1 haplotypes may result in 5% lower trough concentration of dabigatran. CES-1 gene encodes for the liver carboxylesterase 1 enzyme and it is responsible for the biotransformation of dabigatran etexilate into its active metabolite. Patients carrying particular alleles showed up to 28% decrease in adjusted peak concentration. Interestingly, the frequency of these genetic haplotypes may be present in up to one-third of overall population.13,14
Knowledge regarding drugs interacting with dabigatran is expanding.15 The main mechanism involved is at P-gp level. Competitive inhibitors of P-gp result in increased dabigatran plasma levels, through gastrointestinal re-secretion. Among them, verapamil, quinidine, dronedarone, and amiodarone are frequently used for AF. Significant reduction of dabigatran plasma levels is known for P-gp inducers, such as rifampicin, dexamethasone, as well as several antiepileptic and anticancer drugs. The concomitant use of proton pump inhibitors and H2-blockers leads to a small reduction in bioavailability of dabigatran, apparently without clinically relevant effects.
In dabigatran-treated patients, aPTT only provides a qualitative assessment of anticoagulant activity: aPTT in the normal range in fact only rules out supra-therapeutic dabigatran levels. For a quantitative assessment, diluted thrombin time (dTT) and ECT are recommended.15 The limitation of our case report is that we were not able to quantitatively measure dabigatran anticoagulant activity due to the absence of dTT and ECT in our laboratory.
Conclusion
In our case, we confirmed initial reports of safety of IVT after idarucizumab, in case of dabigatran failure. In our case, IVT treatment resulted in complete arterial recanalization. We also demonstrated the presence of an LAA thrombus despite adequate dosing, good therapeutic adherence, and the absence of concomitant interacting drugs. We suggest that individual variations in anticoagulation status may possibly account for drug failure. Thus, periodical assessment of dabigatran anticoagulant levels, by dTT or ECT assessment, may thus be warranted.
Lead author biography

Paolo Candelaresi is a stroke neurologist at AORN Cardarelli, in Naples. His main interest is acute reperfusion therapy in stroke. He is a researcher in the Cochrane Neurological Field.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2. Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI.. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511–520. [DOI] [PubMed] [Google Scholar]
- 3. Giannandrea D, Caponi C, Mengoni A, Romoli M, Marando C, Gallina A, Marsili E, Sacchini E, Mastrocola S, Padiglioni C, Mazzoli T, Cenciarelli S, Ricci S.. Intravenous thrombolysis in stroke after dabigatran reversal with idarucizumab: case series and systematic review. J Neurol Neurosurg Psychiatry 2019;90:619–623. [DOI] [PubMed] [Google Scholar]
- 4. Koyama T, Otsuka Y, Kawahara M, Imoto Y, Nakamura K, Kodama S, Noguchi H.. A left atrial appendage thrombus that developed during prophylactic low‐dose dabigatran treatment resolved after switching to apixaban. Clin Case Rep 2017;5:711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bachvarova V, Bucci C, Ho N, Yazdan-Ashoori P, Morgan C.. Massive left atrial thrombi during dabigatran therapy for nonvalvular atrial fibrillation. CASE (Phila) 2017;1:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tabata E, Yasaka M, Wakugawa Y, Komori M, Mori K, Tsurusaki Y, Kokuba K, Sambongi Y, Maeda K, Okada Y.. Increase in the size of an intracardiac thrombus during Dabigatran therapy (110 mg b.i.d.) in an acute cardioembolic stroke patient. Cerebrovasc Dis Extra 2013;3:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janssen AM, van de Kerkhof D, Szabó B, Durian MF, van der Voort PH.. Left atrial thrombus under dabigatran in a patient with nonvalvular atrial fibrillation. Neth J Med 2016;74:313–315. [PubMed] [Google Scholar]
- 8. Shah P, Mithawala P, Konlian D, Soyombo A, Bikkina M.. Thrombus formation in left atrium on dabigatran therapy. Case Rep Cardiol 2015;2015:518982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hager N, Bolt J, Albers L, Wojcik W, Duffy P, Semchuk W.. Development of left atrial thrombus after coadministration of dabigatran etexilate and phenytoin. Can J Cardiol 2017;33:554.e13–554.e14. [DOI] [PubMed] [Google Scholar]
- 10. Miwa Y, Minamishima T, Sato T, Sakata K, Yoshino H, Soejima K.. Resolution of a warfarin and dabigatran-resistant left atrial appendage thrombus with apixaban. J Arrhythm 2016;32:233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luis SA1, Poon K, Luis C, Shukla A, Bett N, Hamilton-Craig C.. Massive left atrial thrombus in a patient with rheumatic mitral stenosis and atrial fibrillation while anticoagulated with dabigatran. Circ Cardiovasc Imaging 2013;6:491–492. [DOI] [PubMed] [Google Scholar]
- 12. Sharma S, Singh S, Sandhu R, Monterroso M, Bhambi N, Sharma R.. Case report series of left atrial thrombus formation in patients on dabigatran therapy. Am J Ther 2014;21:e71–e74. [DOI] [PubMed] [Google Scholar]
- 13. Paré G, Eriksson N, Lehr T, Connolly S, Eikelboom J, Ezekowitz MD, Axelsson T, Haertter S, Oldgren J, Reilly P, Siegbahn A, Syvanen AC, Wadelius C, Wadelius M, Zimdahl-Gelling H, Yusuf S, Wallentin L.. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 2013;127:1404–1412. [DOI] [PubMed] [Google Scholar]
- 14. Dimatteo C, D'Andrea G, Vecchione G, Paoletti O, Cappucci F, Tiscia GL, Buono M, Grandone E, Testa S, Margaglione M.. Pharmacogenetics of dabigatran etexilate interindividual variability. Thromb Res 2016;144:1–5. [DOI] [PubMed] [Google Scholar]
- 15. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.