Abstract
Background
Giant coronary artery aneurysms (CAAs) are rare and have been reported in patients with connective tissue diseases, arteritides, and atherosclerosis. Given the rarity of the condition, multimodality imaging is essential for comprehensive evaluation of coronary aneurysms and determination of their haemodynamic significance.
Case summary
A 58-year-old Filipino female was evaluated for dyspnoea on exertion of one month. Chest computed tomography (CT) showed right coronary artery (RCA) aneurysms. Invasive coronary angiogram (ICA) confirmed two giant aneurysms of the RCA. Distal RCA could not be opacified due to contrast stagnation in the proximal aneurysms. Coronary CT angiography (CCTA) depicted an additional giant distal RCA aneurysm not visualized on ICA with intraluminal thrombosis. Contrast-enhanced cardiac magnetic resonance imaging (CMR) revealed delayed time to peak perfusion in the mid to apical inferior walls, on first-pass imaging, without myocardial scarring. Late gadolinium images revealed aneurysmal wall inflammation.
Discussion
This case highlights the anatomical findings of giant CAA and the application of multimodality imaging for their accurate characterization. While ICA confirmed the presence of the aneurysms, CCTA enabled the assessment of their full extent and depict intraluminal thrombosis. Contrast-enhanced CMR delineated aneurysm wall characteristics, with first-pass images demonstrating reduced inferior wall perfusion at rest, which was likely the cause of patient’s exertional symptoms. Management of giant coronary aneurysms involves surgical resection with bypass grafting.
Keywords: Coronary artery aneurysm, Case report, Multimodality imaging, CMR
Learning points
Understand the importance of multimodality imaging for accurate characterization of giant coronary aneurysms.
Understand the importance of characterizing haemodynamic significance of giant coronary aneurysms.
Recognize clinical presentation of giant coronary aneurysms, aetiologies, and their management.
Introduction
Giant coronary artery aneurysms are rare with a prevalence of 0.02–0.2%. Although the exact definition of giant coronary aneurysms is lacking, a diameter exceeding 50 mm has been proposed.1 Right coronary artery (RCA) is most commonly affected.2 Here, we describe a patient with RCA aneurysms and the use of multimodality imaging in characterizing their anatomical and haemodynamic features.
Timeline
| Time | Events |
| Day 1 | Patient presents with dyspnoea on exertion. Chest computed tomography (CT) reveals mediastinal lymphadenopathy and right coronary artery (RCA) aneurysms. |
| Day 2 | Invasive coronary angiogram confirms two large aneurysmal dilatations of the RCA. |
| Day 3 | Coronary CT angiography reveals giant distal RCA aneurysm, in addition to the proximal and mid-RCA aneurysms, with evidence of intraluminal thrombosis. |
| Day 4 | Contrast-enhanced cardiac magnetic resonance imaging is obtained and re-demonstrates RCA aneurysms and depicts findings of inferior wall ischaemia explaining patient’s symptoms. |
| Day 6 | Patient undergoes resection of the mid and distal RCA aneurysms with a single saphenous vein graft anastomosed to the right posterior descending coronary artery. |
Case presentation
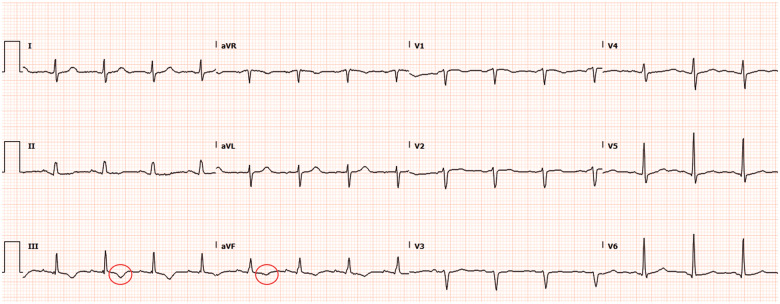
A 58-year-old Filipino female who has been residing in the USA for the past 3 years, and known to have long-standing history of hypertension and asthma, presented to the emergency room with 1 month of progressive dyspnoea on exertion. Review of systems was otherwise unremarkable. Cardiac exam showed normal heart sounds and respiratory exam revealed decreased air entry at the left lower lobe associated with fine crackles. Electrocardiogram showed sinus rhythm with T-wave inversion in leads 3 and AvF (Figure 1). Chest X-ray revealed hilar lymphadenopathy. Subsequently, contrast-enhanced computed tomography angiography (CTA) of the chest confirmed mediastinal lymphadenopathy and revealed left lower lobe atelectasis with traction bronchiectasis. In addition, chest CTA demonstrated giant RCA aneurysms (Figure 2, Supplementary material online, Movie S1). Echocardiogram demonstrated extrinsic compression of the right atrium by a complex mass with intrinsic blood flow consistent with the aforementioned finding of proximal RCA aneurysm (Supplementary material online, Movie S2). The patient underwent computed tomography (CT)-guided lymph node biopsy of the mediastinal lymphadenopathy that only showed a non-specific reactive process. Invasive coronary angiogram (ICA) confirmed two large aneurysmal dilatations of the RCA at the proximal and mid vessel segments, measuring 1.8 × 1.8 cm and 4.9 cm × 2.9 cm, respectively (Figure 3, Supplementary material online, Movie S3). Distal RCA, however, could not be opacified due to contrast stagnation in the proximal aneurysms. A focused cardiac CTA was then performed and revealed an additional distal RCA aneurysm, measuring 6 × 10 cm, with extensive luminal thrombosis (Figure 4). Contrast-enhanced cardiac magnetic resonance imaging (CMR) was obtained for further evaluation of the aneurysm characteristics and its effect on myocardial perfusion to guide therapeutic decision-making. First-pass perfusion CMR with time-signal-intensity analysis showed delayed time to peak perfusion in the mid to apical inferior walls consistent with resting hypoperfusion (Figure 5A and B). Late contrast-enhanced CMR showed no evidence of myocardial scarring, but depicted extensive luminal thrombosis of the aneurysms along with marked late enhancement of the RCA wall (Figure 5C and D), suggesting an inflammatory process. Serological work up for connective tissue diseases including antineutrophil cytoplasmic antibodies, rheumatoid, and systemic lupus erhythromatosis serologies were unremarkable. Complement levels and inflammatory markers were normal. Computed tomography angiography of the neck, chest, abdomen, and pelvis did not show evidence of vasculitis. Patient was referred to cardiothoracic surgery and subsequently underwent resection of the mid and distal right coronary aneurysms (Figure 6) with a single saphenous vein graft anastomosed to the right posterior descending artery. Pathology of the resected aneurysms revealed non-specific chronic inflammatory pattern. Patient’s post-operative course was uneventful. At 3 and 6 months of follow-up, patient reported no symptoms and there was no change in ventricular function.
Figure 1.
Electrocardiogram on admission revealing T-wave inversions in lead 3 and AvF.
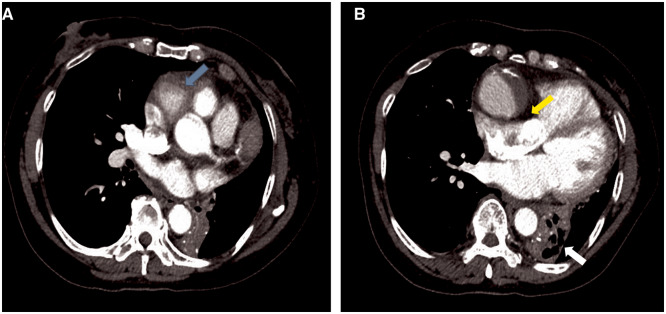
Figure 2.
Contrast-enhanced computed tomography of the chest, during the arterial phase in an axial orientation, depicting proximal aneurysmal dilatation of the right coronary artery (A, blue arrow) with evidence of right atrial compression (B, yellow arrow). There is an area of atelectasis within the left lower lobe with air bronchograms, with associated collapse of the left lower lobe (B, white arrow).
Figure 3.
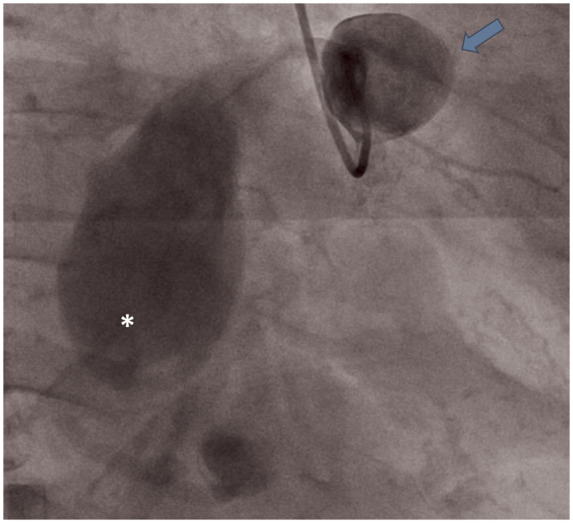
Invasive coronary angiography depicting giant saccular proximal (arrow) and giant fusiform mid (asterisk) right coronary artery aneurysm. Distal vessel segment is not well-opacified.
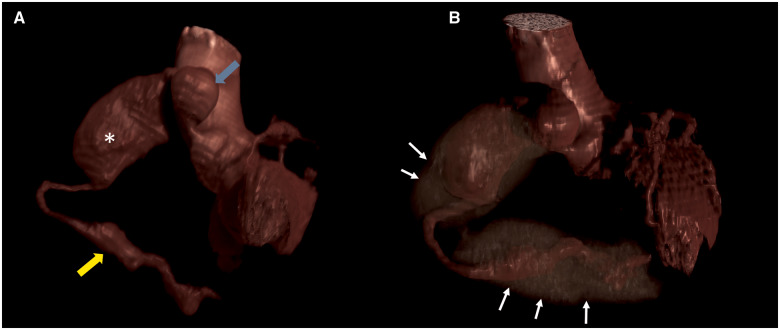
Figure 4.
Three-dimensional volume rendered imaging of cardiac computed tomography angiography depicting proximal (blue arrow), mid (asterisk), and distal (yellow arrow) coronary aneurysms (A). Extensive intraluminal thrombosis is identified by white arrows in B.
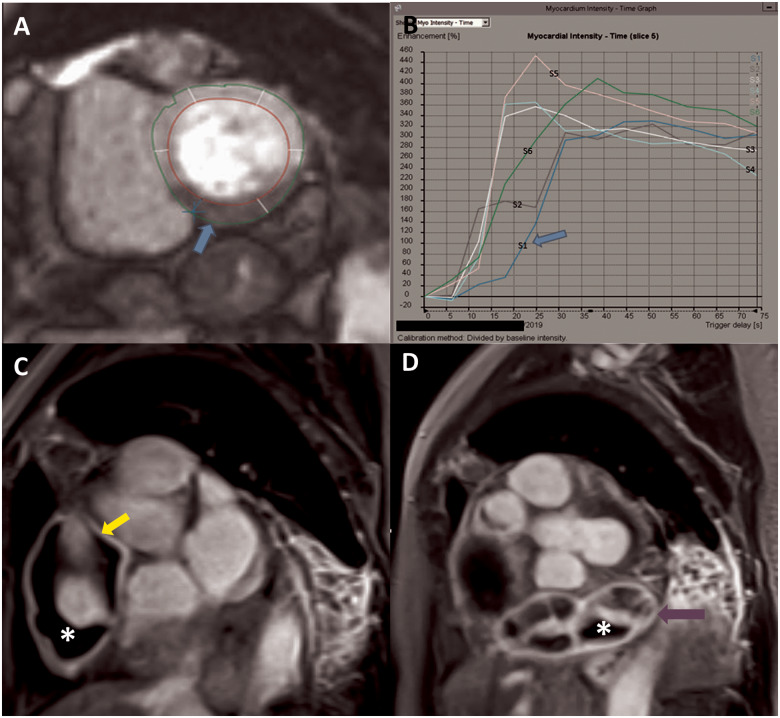
Figure 5.
Gadolinium-enhanced cardiac magnetic resonance depicting resting hypoperfusion of the inferior wall on first-pass perfusion imaging (A), with reduced time to peak myocardial intensity on time signal analysis (B). Late gadolinium-enhanced cardiac magnetic resonance showing giant aneurysm in the mid right coronary artery with evidence of hyperenhancement of the aneurysm wall (yellow arrow) indicative of inflammation, with intraluminal thrombosis (asterisk) (C). Distal coronary artery giant aneurysm previously not visualized on coronary angiography with wall hyperenhancement (purple arrow) and thrombosis (asterisk) (D).
Figure 6.
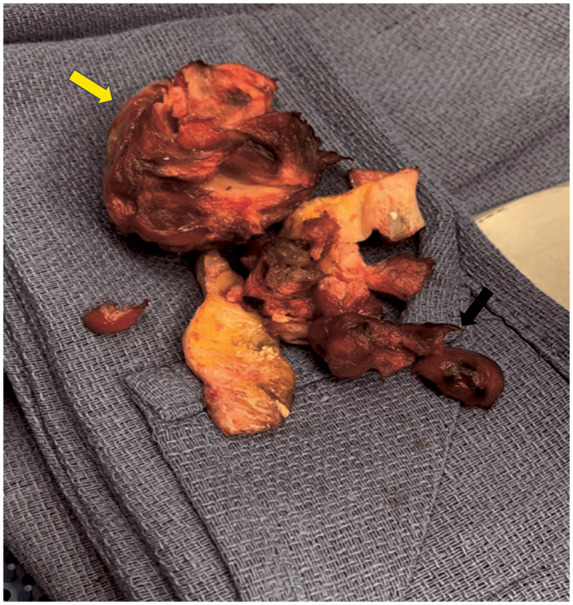
Surgical specimen of the resected mid segment (yellow arrow) and distal segment (black arrow) giant coronary artery aneurysms.
Discussion
Coronary artery aneurysms occur in approximately 0.9–4.9% of patients undergoing coronary angiography yet giant aneurysms are rather rare.2 Aetiologies encompass a myriad of pathophysiological processes from atherosclerosis and post percutaneous coronary interventions to arteriopathies, including Takayasu arteritis, Kawasaki disease, and connective tissue diseases such as scleroderma, lupus, Marfan, and Ehlers–Danlos syndromes.3 Imaging evaluation involves delineation of the location, size, shape, number, and distribution of the aneurysms, along with presence of wall calcifications and luminal thrombosis. Assessment of the extent of the aneurysms and manifestation of the associated complications such as myocardial perfusion abnormalities, fistula formation, and extrinsic mass compression is critical for operative planning.4 This case highlights the importance of utilizing multimodality cardiovascular imaging for anatomical and haemodynamic assessment of coronary aneurysms.
While ICA confirmed the presence of the RCA aneurysms, it could not assess the distal coronary segment. In addition, ICA does not reliably differentiate between true and false aneurysms without the use of intravascular ultrasound.1,5,6 On the other hand, coronary CT angiography (CCTA) can define the vessel layers more reliably, depict intraluminal thrombus formation, and delineate the origin, the termination and the course of the aneurysms. Contrast-enhanced CMR, similarly to CCTA, enabled the assessment of the full extent of the aneurysms and the presence of wall inflammation and intraluminal thrombosis which could not be visualized on ICA. In addition, intraluminal flow stagnation was noted to result in resting perfusion abnormality on gadolinium-enhanced first-pass CMR. Since patient’s asthma was well controlled and lower lobe atelectasis persisted on post-operative chest CT despite improvement of patient’s symptoms, inferior wall ischaemia secondary to flow stagnation in the RCA aneurysms was likely the cause of patient’s presentation. Serological work up for connective tissue diseases was unremarkable, CT angiography did not suggest any evidence of systemic vasculitis, and pathology of the resected aneurysms revealed non-specific chronic inflammatory pattern. Management of giant coronary aneurysms has not been clearly defined. Limited data exists in support of antiplatelet and/or antithrombotic therapy but surgical correction is generally accepted as the preferred treatment considering the risk of rupture and myocardial infarction from distal embolization of intra-aneurysmal thrombi.3,6,7
Conclusion
Most patients with giant coronary aneurysms are asymptomatic but some may present with non-specific complaints, including dyspnoea on exertion as an angina equivalent, similar to our case. Intracoronary flow stagnation from giant aneurysms can result in resting perfusion abnormalities and induce ischaemia with exertion. Multimodality imaging is essential for accurate diagnosis of coronary aneurysms and to inform therapeutic decision-making.
Lead author biography

Dr Kameel Kassab received his medical training at the American University of Beirut Medical Center. He completed his residency in internal medicine at Indiana University. Currently, he is a general cardiology fellow at Cook County hospital in Chicago.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to thank the patient for allowing us to share her case. The authors would like to thank the cardiothoracic surgical team at Cook County Health for their surgical expertise.
Slide sets: A fully edited slide set detailing this vcase and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Crawley PD, Mahlow WJ, Huntsinger DR, Afiniwala S, Wortham DC.. Giant coronary artery aneurysms: review and update. Tex Heart Inst J 2014;41:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, Mudd JG, Gosselin AJ.. Aneurysmal coronary artery disease. Circulation 1983;67:134–138. [DOI] [PubMed] [Google Scholar]
- 3. Keyser A, Hilker MK, Husser O, Diez C, Schmid C.. Giant coronary aneurysms exceeding 5 cm in size. Interact Cardiovasc Thorac Surg 2012;15:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Díaz-Zamudio M, Bacilio-Pérez U, Herrera-Zarza MC, Meave-González A, Alexanderson-Rosas E, Zambrana-Balta GF, Kimura-Hayama ET.. Coronary artery aneurysms and ectasia: role of coronary CT angiography. Radiographics 2009;29:1939–1954. [DOI] [PubMed] [Google Scholar]
- 5. Aqel RA, Zoghbi GJ, Iskandrian A.. Spontaneous coronary artery dissection, aneurysms, and pseudoaneurysms: a review. Echocardiography 2004;21:175–182. [DOI] [PubMed] [Google Scholar]
- 6. Pahlavan PS, Niroomand F.. Coronary artery aneurysm: a review. Clin Cardiol 2006;29:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen P, O’gara PT.. Coronary artery aneurysms: a review of the natural history, pathophysiology, and management. Cardiol Rev 2008;16:301–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.