Abstract
Background
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital disorder resulting in ischaemia and myocardial infarction which can act as a potential substrate for life-threatening arrhythmias and sudden cardiac death.
Case summary
A 19-year-old man was admitted to the hospital after successful resuscitation from an out-of-hospital cardiac arrest (OHCA) due to ventricular fibrillation occurring during jogging. In the diagnostic work-up of the OHCA, computed tomography identified an ALCAPA. The patient was referred to our tertiary hospital for surgical correction. Direct reimplantation of the left coronary artery in the aorta was performed. During follow-up, 24-h electrocardiogram revealed short episodes of non-sustained ventricular tachycardia (VT). The magnetic resonance imaging at initial admission showed focal wall thinning and transmural late gadolinium enhancement consistent with a previous anterolateral myocardial infarction. Therefore, the aetiology of the OHCA could be due to a scar-related mechanism and not necessarily due to a reversible cause and an implantable cardioverter-defibrillator (ICD) was considered indicated. Given the young age and the lower complication rates, a subcutaneous device was preferred over a transvenous ICD. However, as a subcutaneous ICD (S-ICD) lacks the possibility of anti-tachycardia pacing, programmed electrical stimulation (PES) was performed to test for inducibility of monomorphic, re-entrant VT. After a negative PES, an S-ICD was implanted.
Discussion
ALCAPA is a potential cause of OHCA in young patients. Some of these patients keep an irreversible substrate for ventricular arrhythmias despite full surgical revascularization and might be candidates for (subcutaneous) ICD implantation.
Keywords: Anomalous left coronary artery from the pulmonary artery (ALCAPA), Ventricular arrhythmia, Sudden cardiac death, Subcutaneous implantable cardioverter-defibrillator, Case report
Learning points
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a rare cause of life-threatening arrhythmias and sudden cardiac death in the adult population and surgical correction should be pursued.
Adult ALCAPA patients should undergo thorough multidisciplinary evaluation to assess the risk of (recurrent) ventricular arrhythmias despite full revascularization.
Patients with previous transmural myocardial infarction due to ALCAPA have a potential irreversible substrate for ventricular tachyarrhythmias.
Introduction
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital disorder, occurring in approximately 1 in 300 000 live births.1 The condition was first described in 1911 by the Russian pathologist Alexei Ivanovitch Abrikosov.2 However, it was not well-known until 1933 when a clinical case of a 3-month-old boy who died due to ALCAPA was published by William Franklin Bland, Paul Dudley White, and Joseph Garland. Untreated ALCAPA results in 90% mortality during the first year of life.2
Pulmonary artery pressure, pulmonary vascular resistance, and oxygen content gradually decrease hours–days after birth. In ALCAPA, these changes cause a decrease in antegrade flow and oxygen content in the left coronary artery (LCA) resulting in myocardial ischaemia which may progress to myocardial infarction, left ventricular (LV) dilatation, heart failure, and functional mitral regurgitation. In this situation, sudden cardiac death can occur, either due to primary ventricular fibrillation (VF) caused by (reversible) ischaemia or due to scar-related ventricular tachycardia (VT) arising from irreversible damaged myocardium degenerating into VF.3
Timeline
| 2018 | ||
| May | Out-of-hospital cardiac arrest due to ventricular fibrillation (VF) | |
| Computed tomography: anomalous left coronary artery from the pulmonary artery, transthoracic echocardiography: moderate left ventricular function, anterolateral hypokinesia | ||
| Magnetic resonance imaging: ejection fraction (EF) 42%, late enhancement anterolateral | ||
| June | Surgery: reimplantation left coronary artery in aorta, start bisoprolol | |
| August | 24-h electrocardiogram: 5× non-sustained ventricular tachycardia (VT) | |
| Magnetic resonance imaging: EF 47%, focal transmural anterolateral infarction | ||
| November | Discussion electrophysiology team:
|
|
| 2019 | ||
| January | Patient consent for electrophysiological study and ICD | |
| May | Programmed electrical stimulation: only fast non-sustained polymorphic VT inducible, no ablation | |
| subcutaneous ICD implantation | ||
| November | Outpatient clinic: no ventricular tachyarrhythmias under bisoprolol | |
Blue indicates local hospital and orange indicates tertiary care hospital.
Case presentation
A 19-year-old man was presented to a local hospital after successful resuscitation following an out-of-hospital cardiac arrest (OHCA). He was treated for asthma as a child and experienced mild exertional dyspnoea for the past 2 years. Bystander basic life support was initiated directly after the patient collapsed during jogging. Emergency services arrived 10 min later and the first registered heart rhythm was VF. He was defibrillated three times resulting in return of spontaneous circulation. He was intubated and physical examination showed stable haemodynamic parameters (blood pressure 116/78 mmHg, heart rate: 93 b.p.m.) upon arrival at the hospital. There were no cardiac murmurs upon auscultation. His 12-lead electrocardiogram (ECG) showed negative T waves in the lateral leads, but no conduction abnormalities and no signs of acute transmural ischaemia. Based on the above findings, no emergency angiography was performed.
He was treated with target temperature management at the intensive care unit and recovered well neurologically. Diagnostic work-up of the OHCA was initiated. Family history was unremarkable for sudden cardiac death or congenital anomalies. Cardiac markers were only mildly elevated, consistent with resuscitation but not acute ischaemia (troponin T 161 ng/L, reference value: <14 ng/L; CK 445 U/L, reference value: <171 U/L). Transthoracic echocardiography revealed a dilated left ventricle with moderately reduced systolic function. Cardiac computed tomography angiography revealed the presence of an ALCAPA, a compensatory dilated right coronary artery (RCA), and backflow of contrast through the collaterals to the LCA and into the pulmonary artery (Figure 1). Cardiac magnetic resonance imaging (MRI) showed a dilated left ventricle with an ejection fraction of 42%. There was anterolateral wall thinning with akinesia and transmural late gadolinium enhancement compatible with a previous anterolateral myocardial infarction (Figure 2). Patient underwent successful surgical reimplantation of the LCA from the pulmonary artery into the aorta under cardiopulmonary bypass and reconstruction of the pulmonary artery with a pericardial patch. Post-operative recovery was unremarkable and he was discharged home on bisoprolol 2.5 mg once daily.
Figure 1.
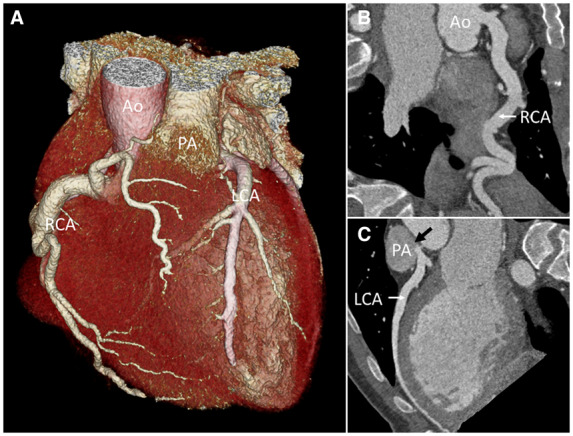
Multislice computed tomography coronary angiography. (A) Three-dimensional image of the heart, great vessels, and coronary arteries with an enlarged right coronary artery originating from the aorta and a left coronary artery originating from the pulmonic artery. (B) Curved planar reconstruction of right coronary artery from aorta. (C) Curved planar reconstruction of left coronary artery from pulmonic artery with contrast backflow into the pulmonic artery (black arrow). Ao, aorta; LCA, left coronary artery; PA, pulmonic artery; RCA, right coronary artery.
Figure 2.
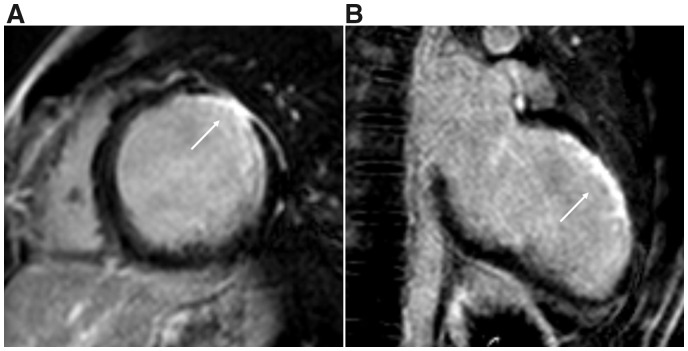
Magnetic resonance imaging with late gadolinium enhancement. (A) Midventricular short-axis view with transmural late gadolinium enhancement anterolateral (white arrow). (B) Two chamber view with late gadolinium enhancement anterior (white arrow).
Patient was followed at the outpatient clinic 2 months after surgery. A 24-h ambulatory ECG showed five episodes of non-sustained VT. An MRI was repeated and showed an improved ejection fraction of 47% with signs of a previous anterolateral myocardial infarction. Patient was referred to our tertiary centre to evaluate the indication for an implantable cardioverter-defibrillator (ICD). The MRI during his first admission, performed 13 days after the OHCA, already showed anterolateral wall thinning with transmural late gadolinium enhancement which was compatible with a previous myocardial infarction before the OHCA.4 Accordingly, the documented VF could be due to a scar-related re-entrant VT degenerating into VF and not necessarily due to ischaemia. Therefore, an ICD was indicated for secondary prevention.5
Given the young age of the patient and absence of documented sustained monomorphic VT, a subcutaneous ICD (S-ICD) was preferred over a transvenous system given higher rate of lifetime complication rates associated with the latter one.6,7 A major drawback of an S-ICD system is the lack of anti-tachycardia pacing (ATP) modalities. It was thus decided to perform a programmed electrical stimulation (PES) to test for induction of a sustained monomorphic VT due to a scar-related re-entry mechanism. A re-entrant VT could be either targeted by catheter ablation followed by S-ICD implantation or could justify the implantation of a transvenous system with the capability of ATP. The patient consented with the proposed course of action. During the PES only fast non-sustained polymorphic VTs were inducible and no ablation was performed. Subsequently, an S-ICD was implanted (Figure 3). The patient is currently symptom free and no clinically relevant ventricular tachyarrhythmias have been documented 6 months after ICD implantation.
Figure 3.
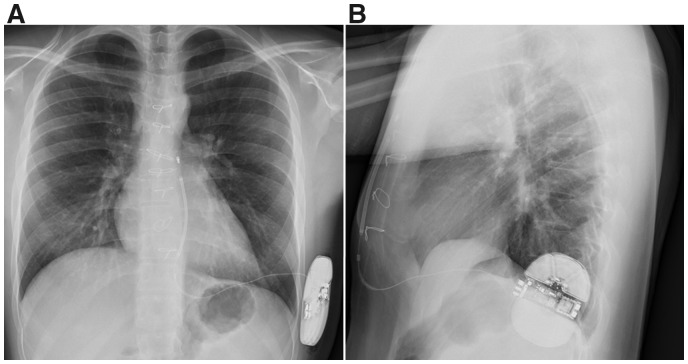
Conventional chest X-ray after subcutaneous implantable cardioverter-defibrillator implantation left laterally with subcutaneous parasternal shock lead. (A) Postero-anterior image. (B) lateral image.
Discussion
In ALCAPA syndrome, the neonatal decrease in the pulmonary arterial pressure and the decrease in antegrade flow and oxygen content in the LCA results in myocardial ischaemia. The extent of the collateral circulation from the oxygen-rich RCA is critical and of prognostic importance. Patients who are diagnosed with ALCAPA qualify for surgery to prevent further ischaemia, LV dilatation, and deterioration of LV function. Previously, the LCA was ligated at its take off from the pulmonary artery, however, this approach was associated with late sudden cardiac death and has therefore been abandoned and replaced by combined ligation and subsequent saphenous venous grafting.8 The Takeuchi procedure creating an aortapulmonary window and an intrapulmonary tunnel to redirect blood from the aorta into the LCA has become less popular due to baffle related complications and supravalvular pulmonary stenosis.8,9 Currently, direct reimplantation of the LCA into the aorta (as was performed in our patient) is the preferred technique.8 Left ventricular ejection fraction usually improves significantly after surgery. This was also the case in our patient and can be attributed to having alleviated ischaemia leading to functional recovery of hibernating myocardium. However, more subtle parameters of LV dysfunction such as global longitudinal strain may remain impaired.10,11 Magnetic resonance imaging is able to visualize myocardial scarring by late gadolinium enhancement imaging,4 which is generally not or only discretely present in ALCAPA patients who underwent successful surgical repair.12
In a case report of two young-adult patients with ALCAPA presenting with OHCA who underwent surgical reimplantation, there was no recurrence of ventricular tachyarrhythmias during follow-up of respectively 10.5 and 4.5 years.13 However, large scale studies in this population are missing.
Although ischaemia is resolved by reimplantation of the LCA into the aorta, patients with previous myocardial infarction keep a potential substrate for ventricular tachyarrhythmias.3 In these patients, ICD therapy should be considered and is indicated for secondary prevention in OHCA survivors.3,5,14 Implantable cardioverter-defibrillators are, however, associated with long-term complications such as lead failure, inappropriate therapy and infections, especially in young and active patients.7
A promising alternative for young patients requiring ICD therapy is S-ICD implantation. Although associated with lower complication rates, long-term experience with S-ICD in young adults is still limited.6,15 A drawback of S-ICDs is the inability to terminate re-entrant-VT by painless ATP. Therefore, our patient was thoroughly discussed in our electrophysiology team and after carefully weighing pros and cons and after performing PES in which no monomorphic VT approachable by catheter ablation or treatable by ATP could be induced, it was decided to implant an S-ICD for secondary prevention.
In conclusion, ALCAPA is a potential cause of OHCA in young patients. Depending on the initial clinical presentation and the extend of the myocardial scar, some of these patients keep a substrate for ventricular arrhythmias despite full surgical revascularization. These patients should be thoroughly evaluated to assess their individual risk and the potential substrate for recurrent life-threatening arrhythmias.
Lead author biography

Madelien V. Regeer (1987) is currently a cardiologist in training at the Leiden University Medical Centre, the Netherlands. She defended her PhD thesis on ‘Imaging techniques in aortic valve and root surgery’ in 2017. Madelien has a specific interest in the field of multimodality imaging and the complementary role of various imaging techniques in clinical diagnostics and care. She recently completed a congenital heart disease traineeship at the Leiden University Medical Centre, during which the work described in this case report was performed.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Funding
The authors were funded by the general funding of the Department of Cardiology of the Leiden University Medical Center (to M.V.R., K.Z., and A.D.E.) and the Medical Centre Leeuwarden (to O.B.), the Netherlands.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: All procedures performed involving the human participant were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The patient provided consent for publication.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Keith JD. The anomalous origin of the left coronary artery from the pulmonary artery. Br Heart J 1959;21:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazurak M, Kusa J.. The radiologist’s tragedy, or Bland-White-Garland syndrome (BWGS). On the 80(th) anniversary of the first clinical description of ALCAPA (anomalous left coronary artery from the pulmonary artery). Kardiochir Torakochirurgia Pol 2014;11:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kristensen T, Kofoed KF, Helqvist S, Helvind M, Søndergaard L.. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) presenting with ventricular fibrillation in an adult: a case report. J Cardiothorac Surg 2008;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, Klocke FJ, Kim RJ, Judd RM.. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol 2004;43:2124–2131. [DOI] [PubMed] [Google Scholar]
- 5. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliot PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, van Veldhuizen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Eur Heart J 2015;36:2793–2867.26320108 [Google Scholar]
- 6. Bettin M, Larbig R, Rath B, Fischer A, Frommeyer G, Reinke F, Köbe J, Eckardt L.. Long-term experience with the subcutaneous implantable cardioverter-defibrillator in teenagers and young adults. JACC Clin Electrophysiol 2017;3:1499–1506. [DOI] [PubMed] [Google Scholar]
- 7. Lewandowski M, Syska P, Kowalik I, Maciąg A, Sterliński M, Ateńska-Pawłowska J, Szwed H.. Fifteen years’ experience of implantable cardioverter defibrillator in children and young adults: Mortality and complications study. Pediatr Int 2018;60:923–930. [DOI] [PubMed] [Google Scholar]
- 8. Patil S, Shah M, Patel B, Garg L, Jacobs L, Islam N, Martinez M.. Incidental finding of the anomalous origin of left main coronary artery from pulmonary artery in an adult presenting with arrhythmia-induced myocardial ischemia. Case Rep Cardiol 2018;2018:6485831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang C, Hua Z, Liu J, Yan F, Wang X, Li S.. A reoperation with coronary reimplantation after Takeuchi repair of Bland-White-Garland syndrome. Ann Thorac Surg 2019;108:e381–e382. [DOI] [PubMed] [Google Scholar]
- 10. Di Salvo G, Eyskens B, Claus P, D'hooge J, Bijnens B, Suys B, De Wolf D, Gewillig M, Sutherland GR, Mertens L.. Late post-repair ventricular function in patients with origin of the left main coronary artery from the pulmonary trunk. Am J Cardiol 2004;93:506–508. [DOI] [PubMed] [Google Scholar]
- 11. Di Salvo G, Siblini G, Issa Z, Mohammed H, Abu Hazeem A, Pergola V, Muhanna N, Al Qweai N, Galzerano D, Fadel B, Fayyadh M, Joufan M, Halees Z, Bulbul Z.. Left ventricular mechanics in patients with abnormal origin of the left main coronary artery from the pulmonary trunk late after successful repair. Cardiology 2017;136:71–76. [DOI] [PubMed] [Google Scholar]
- 12. Fratz S, Hager A, Schreiber C, Schwaiger M, Hess J, Stern HC.. Long-term myocardial scarring after operation for anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg 2011;92:1761–1765. [DOI] [PubMed] [Google Scholar]
- 13. Parizek P, Haman L, Harrer J, Tauchman M, Rozsival V, Varvarovsky I, Pleskot M, Mestan M, Stasek J.. Bland-White-Garland syndrome in adults: sudden cardiac death as a first symptom and long-term follow-up after successful resuscitation and surgery. Europace 2010;12:1338–1340. [DOI] [PubMed] [Google Scholar]
- 14. Dilaveris P, Koutagiar I, Alexopoulos N, Tsiachris D, Gatzoulis K.. Secondary prevention of sudden cardiac death in a 65 year untreated ALCAPA patient. Int J Cardiol 2014;176:e73–e74. [DOI] [PubMed] [Google Scholar]
- 15. Quast ABE, Brouwer TF, Kooiman KM, van Dessel P, Blom NA, Wilde AAM, Knops RE.. Comparison of complications and shocks in paediatric and young transvenous and subcutaneous implantable cardioverter-defibrillator patients. Neth Heart J 2018;26:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.