Abstract
HIV risk perception may influence the use of HIV prevention interventions. Using data from HIV-negative adults enrolled in a study of pre-exposure prophylaxis (PrEP) and antiretroviral therapy for HIV-serodiscordant couples in Kenya and Uganda, we examined associations between: 1) condom use and risk perception and 2) risk perception and PrEP adherence. Two-thirds of HIV-negative partners reported condomless sex with their HIV-positive partner or another partner in the month prior to study enrollment. Compared to those who reported no condomless sex, participants who reported condomless sex during the month prior to study visit had 5-fold higher odds of reporting “high risk” vs “no risk” perception (36.3 versus 10.9%: aOR=4.9, 95% CI: 3.4–6.9). Reporting condomless sex in the most recent sex act was associated with increased odds of perceiving some HIV risk (aOR for high risk=7.3, 95% CI 4.9–10.8; aOR for moderate risk=4.8, 95% CI 3.5–6.7; aOR for low risk=3.5, 95% CI 2.7–4.6). We found no significant association between risk perception and PrEP adherence. Sexual behavior aligned with perceived HIV risk, which can facilitate an HIV-negative individual’s decisions about PrEP use.
Keywords: HIV, risk perception, serodiscordant, condomless sex, PrEP
INTRODUCTION
Risk perception is an important factor in the uptake of HIV prevention interventions, but studies have found mixed results on HIV prevention behaviors that influence of risk perception [1]. Some factors that have been significantly associated with perception of high risk for HIV among people living in high-burdened settings in East and Southern Africa include single marital status, not knowing a partner’s HIV status, gender, having multiple partners, and being in an age-disparate partnership [2–5]. Substantial evidence supports the idea that sexual behavior also influences HIV risk perception – people reporting condomless sex or more frequent sex often have higher risk perception [2, 3, 5–9]. However, among serodiscordant couples, misconceptions about HIV serodiscordance have been associated with lower perception of risk and inconsistent or no condom use [10, 11]. Other barriers to condom use include male partners’ reluctance to use condoms, women’s difficulties in negotiating condom use, alcohol use, and the desire to have children [11]. These findings highlight the need to further investigate the association between risk perception and sexual behavior among serodiscordant couples and other at-risk populations.
In 2015, the World Health Organization (WHO) issued its first recommendation for pre-exposure prophylaxis (PrEP) to be used by people with substantial risk of acquiring HIV as a HIV prevention strategy [12]. By March 2019, an estimated 465,000–475,000 individuals were using PrEP globally, including 55 countries [13]. Adherence is strongly correlated with the level of protection afforded by PrEP[14], and challenges with adherence have been identified, especially among young women, limiting the individual benefit of PrEP for HIV prevention [15, 16]. In addition to factors such as pregnancy and breastfeeding status [17, 18], age <25 years [15, 17, 19, 20], being single [15], partner awareness and support [2, 21], social stigma [22], mobility patterns [23], and side effects [15, 20, 22, 24], multiple studies have found associations between risk perception and PrEP adherence, with individuals who report moderate to high HIV risk perception also having higher PrEP adherence [2, 21, 25–28].
Most studies to date which evaluated the association between sexual behavior and risk perception have been cross-sectional and unable to determine temporal relationships. Furthermore, the assessment of the association between perceived HIV risk and PrEP adherence is still not widely studied. In the current study, we used longitudinal data to prospectively assess the associations between sexual behavior and risk perception, as well as risk perception and PrEP adherence among high risk HIV-serodiscordant couples participating in an open-label evaluation of PrEP for HIV-negative partners during antiretroviral therapy (ART) initiation by HIV-positive partner with follow-up to 24 months.
METHODS
Study Population
Participants were HIV serodiscordant couples from the Partners Demonstration Project, an implementation science-driven evaluation of PrEP delivery integrated with ART in Kenya (Kisumu and Thika) and Uganda (Kabwohe and Kampala) between November 2012 and January 2015 [29, 30] – full eligibility criteria and study procedures have been reported elsewhere [31]. Following enrollment, participants attended visits one month after enrollment then 2 months later, then quarterly thereafter for a maximum of 24 months. At enrollment, HIV-negative partners were offered PrEP (as a daily regimen of oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF)), and PrEP discontinuation was encouraged once the partner living with HIV had used ART for at least 6 months if there was no indication of poor adherence, outside partners, or immediate plans for the woman to become pregnant. All HIV-positive partners were ART-naïve at enrollment and initiated ART according to national guidelines.
Data Collection
Demographic, clinical, and sexual behavior data were collected via self-report using standardized interviewer administered questionnaires. At enrollment and annually, the 16-item Hopkins Symptoms Checklist for Depression (HSCL-D) [32], the 4-item Rapid Alcohol Problems Screen (RAPS4) [33], and the 10-item Duke-UNC Functional Social Support Scale Screening [34] were used to screen for depression, alcohol dependence, and social support, respectively. PrEP adherence was monitored using medication event monitoring system (MEMS) caps, which electronically monitor the time and date of pill-bottle closings. Adherence was calculated during follow up for each study period with MEMS cap data as actual openings divided by the expected number of openings since the last visit – a value ≥80% was considered high adherence [35].
Statistical Analyses
Descriptive methods were used to summarize cohort characteristics. The primary outcome of interest was a self-reported perceived risk of HIV acquisition, which was measured quarterly by asking the following: “In general, what do you think is your risk of getting HIV from your partner?” Responses included: “high risk”, “moderate risk”, “low risk”, “no risk”, and “don’t know”. The key behavioral exposures assessed for an association with risk perception (collected quarterly) were: 1) any condomless sex with study or non-study partner in the past month and 2) condom use during the most recent sexual intercourse with a study partner. Any condomless sex was calculated based on the number of times the participant had sex in the past month; if the difference between this and the number of times the participant used a condom was >0, then the participant was categorized as having had condomless sex. If this difference was zero (i.e. 100% condom use) or if a participant reported no sex, then the participant was categorized as having had no condomless sex.
Generalized logistic regression was used to compare the odds of being in one category of HIV risk perception relative to perceiving no HIV risk dependent on condom use. Separate models for each measure of condom use were adjusted for time in study, age category, gender, whether married/cohabiting with study partner, social support index, years that their serodiscordant status was known, abuse (verbally, physically or emotionally) by study partner, alcohol dependence, probable depression, and PrEP adherence based on a priori knowledge of the association of each factor with sexual behavior and risk perception [2, 3, 5–9]. Based on commonly seen differences in the ways men and women report sexual behavior [3, 4], we conducted analyses stratified by gender. To evaluate the effect of risk perception on PrEP adherence, we repeated the adjusted models above with PrEP adherence as the outcome and risk perception as the exposure.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and significance level evaluated at an alpha level of 0.05.
Ethical statement
The study protocol was approved by the Human Subjects Division at the University of Washington (#STUDY00001674) and Ethics Review Committees overseeing each study site: Scientific Ethics Review Unit at the Kenya Medical Research Institute (SSC No. 2441), the Ethics Review Committee of Kenyatta National Hospital (P286/05/2012), and the AIDS Research Committee of the Uganda National Council of Science and Technology (ARC 135 and ARC126). All participants provided written informed consent.
RESULTS
Participant characteristics
A total of 908 seronegative participants were included in this study, 89% of individuals had 24 months of follow up and the median duration of PrEP use was 12 months [interquartile range (IQR): 6, 18]. At enrollment, the median age of the population was 30 years [IQR: 26, 36], and 8.6% of female participants and 13.6% of male participants had a partner living with HIV who was virally suppressed (<1,000 copies/mL, Table I). Participants reported knowing their serodiscordant status for a median of 3.0 months [IQR: 3.0, 9.1] and having cohabited with their study partner for a median of 2.8 years [IQR: 0.8, 7.0].
Table I.
Baseline characteristics of participants by gender
| Variable | Female (n = 317) n (%) or median [IQR] |
Male (n = 591) n (%) or median [IQR] |
|---|---|---|
| Age in years | ||
| ≤ 24 | 82 (25.9) | 99 (16.8) |
| 25–29 | 89 (28.1) | 177 (29.9) |
| 30–34 | 64 (20.2) | 117 (19.8) |
| 35–39 | 45 (14.2) | 75 (12.7) |
| ≥ 40 | 37 (11.7) | 123 (20.8) |
| Married or cohabiting with partner | 315 (99.4) | 575 (97.3) |
| Years serodiscordant status known | 0.1 [0.1, 0.6] | 0.1 [0.1, 0.2] |
| Years cohabiting with partner | 5.0 [1.5, 10.3] | 2.0 [0.6, 5.0] |
| Social support, mean scorea | 3.6 [3.2, 3.9] | 3.7 [3.2, 4.0] |
| Number of sex acts, past month | 4.0 [3.0, 8.0] | 6.0 [3.0, 12.0] |
| Number of condomless sex acts, past month | 1.0 [0.0, 4.0] | 2.0 [0.0, 6.0] |
| Reporting any partners outside of the study partner, past month | 4 (1.3) | 69 (11.7) |
| Abuse by study partner, last 3 monthsb | 1 (0.3) | 1 (0.2) |
| Alcohol dependence, last 1 yearc | 49 (15.5) | 133 (22.5) |
| Probable depression, last 1 yeard | 39 (12.3) | 51 (8.6) |
| HIV viral load (copies/ml) of the partner living with HIV | ||
| <1000 | 27 (8.6) | 79 (13.6) |
| < 10,000 | 60 (18.9) | 196 (33.2) |
| 10,000–49,999 | 74 (23.3) | 191 (32.3) |
| ≥ 50,000 | 183 (57.7) | 204 (34.5) |
| Circumcised (men only) | ||
| Circumcised | - | 393 (66.5) |
| Uncircumcised | - | 198 (33.5) |
| Effective contraception (women only)e | ||
| Yes | 110 (34.7) | - |
| No | 207 (65.3) | - |
| STI Symptomsf | 16 (5.1) | 7 (1.2) |
n = number; IQR = interquartile range;
Social support measured using the Duke-UNC Social Support Scale
Abuse = verbally, physically or economically;
Alcohol dependence screened using the Rapid Alcohol Problems Screen (RAPS4);
Depression screened using the Hopkins Symptoms Checklist for Depression (HSCL-D);
Effective contraception = oral, implant, IUD, surgical, injectable);
STI symptoms = genital ulcer disease, vaginitis, cervicitis, pelvic inflammatory disease, urethritis.
Trends in risk perception and sexual behavior
During follow-up, men tended to report perception of no risk more frequently than women (38% vs 24% of observations, Π2 = 87.2, p<0.001) (Figure I). The frequency of condomless sex declined from 66% at enrollment to 31% at the first month of follow up and then fluctuated between 31% and 37% from month 3 to month 24 (Figure II). There was evidence of a linear increase in the proportion of reporting condomless sex over time (Π2 = 102.8, p<0.001), which was driven by changes between enrollment and the first month of follow up.
Figure I.
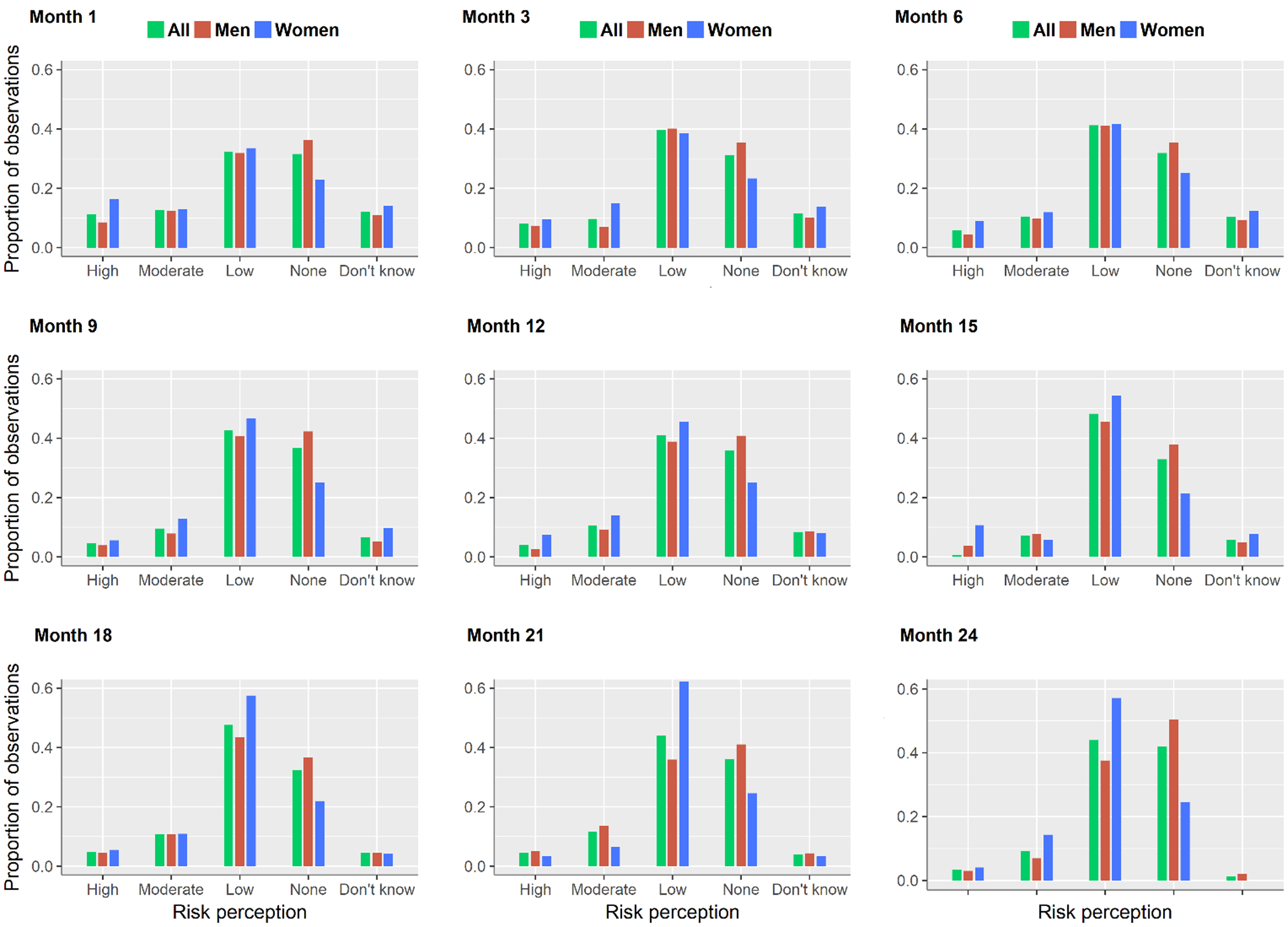
Frequency of risk perception by month: overall and by gender
Figure II.
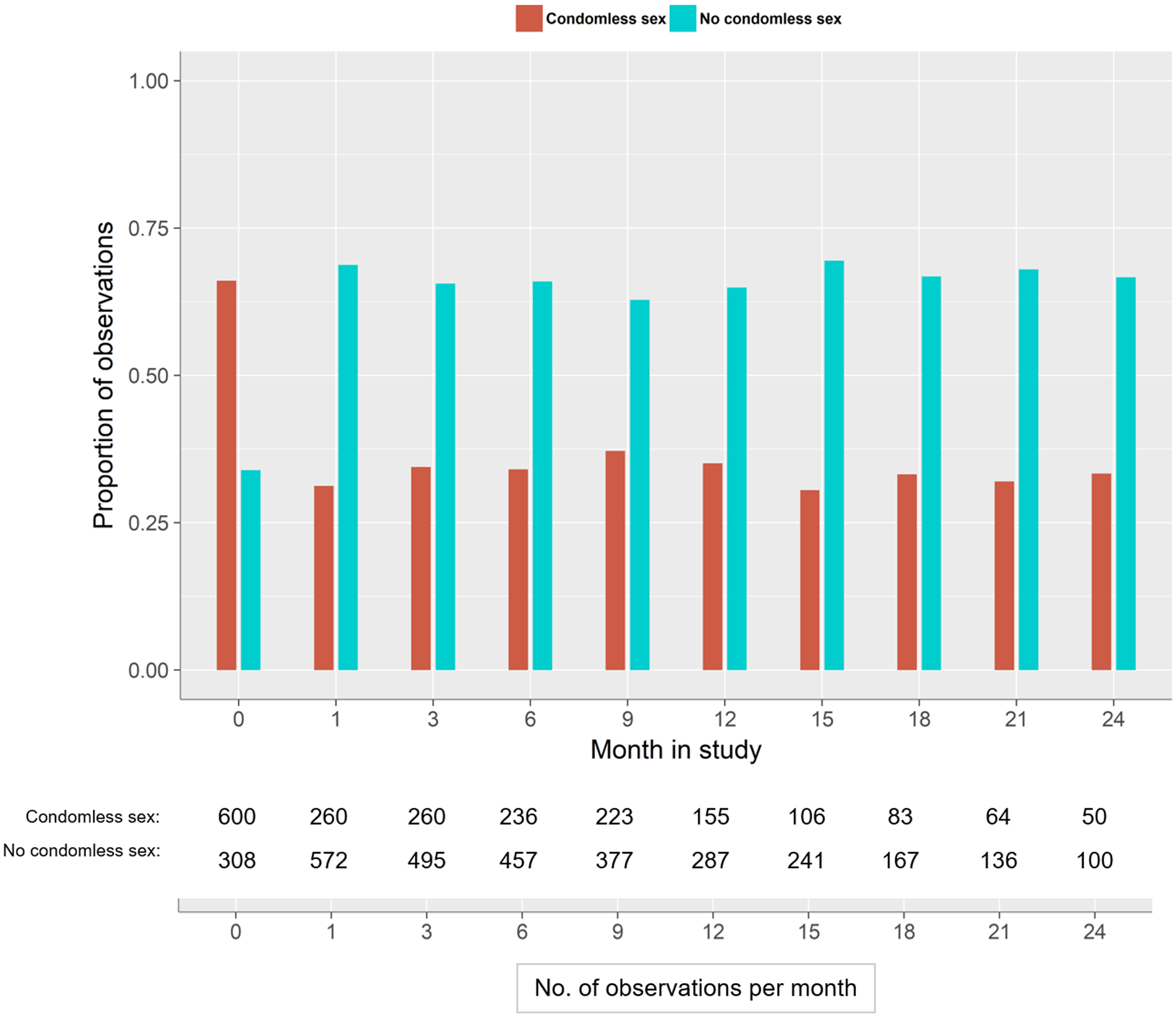
Distribution of observations over time by condomless sex
Association between sexual behavior and risk perception
Individuals who reported any condomless sex had almost five-fold higher odds of reporting themselves as “high risk” for HIV acquisition than those who reported no condomless sex (adjusted odds ratio [aOR]=4.7; 95% CI: 3.4–6.9, Table II). Correspondingly, condomless sex was associated with ~4-fold higher odds of reporting “moderate risk” (aOR=4.4; 95% CI: 3.3–5.9) and ~3-fold higher odds of reporting “low risk” (aOR=2.9; 95% CI: 2.3–3.6). Not using a condom during the most recent sex with study partner was significantly associated with nearly three to seven-fold increase in odds of perceiving some risk of HIV acquisition (aOR for high risk=7.3, 95% CI 4.9–10.8; aOR for moderate risk=4.8, 95% CI 3.5–6.7; aOR for low risk=3.5, 95% CI 2.7–4.6).
Table II.
Adjusted associations between sexual behavior and perceived risk of getting HIV
| Na (%) Any condomless sex |
Na (%) No condomless sex |
Adjusted OR (95% CI) Any condomless sexb |
Na (%) No condom use in last sex with study partner |
Na (%) Condom use in last sex with study partner |
Adjusted OR (95% CI) No condom use during most recent sex with study partnerb |
|
|---|---|---|---|---|---|---|
| Risk perception: Overall | ||||||
| High | 143 (36.3) | 143 (10.9) | 4.7 (3.4, 6.9) | 107 (46.9) | 129 (11.8) | 7.3 (4.9, 10.8) |
| Moderate | 210 (45.6) | 233 (16.6) | 4.4 (3.3, 5.9) | 152 (55.7) | 254 (20.8) | 4.8 (3.5, 6.7) |
| Low | 667 (72.7) | 1058 (47.4) | 2.9 (2.3, 3.6) | 472 (79.6) | 1033 (51.6) | 3.5 (2.7, 4.6) |
| Don’t know | 164 (39.5) | 211 (15.2) | 3.7 (2.7, 5.0) | 116 (48.9) | 195 (16.8) | 4.9 (3.4, 7.0) |
| None | 251 (c) | 1174 (c) | - | 121 (c) | 968 (c) | - |
| Risk perception: Women | ||||||
| High | 66 (62.3) | 68 (18.7) | 7.2 (3.9, 13.2) | 51 (68.0) | 63 (21.1) | 8.2 (3.9, 17.3) |
| Moderate | 80 (66.7) | 94 (24.2) | 6.5 (3.7, 11.5) | 58 (70.7) | 106 (31.0) | 5.3 (2.8, 10.3) |
| Low | 223 (84.8) | 391 (23.6) | 3.8 (2.4, 6.1) | 162 (87.1) | 368 (60.9) | 3.8 (2.1, 6.9) |
| Don’t know | 64 (61.5) | 85 (22.4) | 5.4 (3.0, 9.7) | 52 (68.4) | 79 (25.1) | 6.3 (3.2, 12.3) |
| None | 40 (c) | 295 (c) | - | 24 (c) | 236 (c) | - |
| Risk perception: Men | ||||||
| High | 77 (26.7) | 75 (7.9) | 4.3 (2.7, 6.9) | 56 (36.6) | 66 (8.3) | 7.1 (4.3, 11.7) |
| Moderate | 130 (38.1) | 139 (13.7) | 4.0 (2.9, 5.7) | 94 (49.2) | 148 (16.8) | 4.9 (3.3, 7.2) |
| Low | 444 (67.8) | 667 (43.1) | 2.8 (2.1, 3.6) | 310 (76.2) | 665 (47.6) | 3.5 (2.6, 4.8) |
| Don’t know | 100 (32.2) | 126 (12.5) | 3.3 (2.2, 4.8) | 64 (39.8) | 116 (13.7) | 4.3 (2.8, 6.8) |
| None | 211 (c) | 879 (c) | - | 97 (c) | 732 (c) | - |
N = number of observations over the duration of the study
OR (95% CI) adjusted for time, age, gender (only in overall model), married/cohabiting, mean social support, years serodiscordant status known, abuse by study partner, alcohol dependence, probable depression and PrEP adherence; all p-values < 0.001.
percent varies with comparison, e.g., for “high” vs. “none” risk perception, the OR compares 63.7% of observations with any condomless sex versus 89.1% with no condomless sex.
Although there was no interaction between sexual behavior and risk perception (p>0.05), in gender-stratified models, we observed a stronger association of condomless sex and perceiving HIV risk among women than men (e.g. aOR=7.2, 95% CI 3.9–13.2 in women versus aOR=4.3, 95% CI 2.7–6.9 in men for the association of high risk perception and reporting condomless sex). In all models with any condomless sex as the exposure, the effect estimates of associations comparing the “don’t know” versus “no” risk perception category fell between those of moderate and low risk perception categories.
Association between risk perception and PrEP adherence
Among all HIV negative partners enrolled in the Partners Demonstration project, 97% initiated PrEP. Tenofovir was detected in 81% of plasma samples and 71% of individuals had high adherence by MEMS caps data [36]. Overall, compared to those who reported a risk perception of none, those who reported high, moderate, and low risk perception had 8% lower odds, 12% higher odds and 17% higher odds, respectively, of having high PrEP adherence, but these associations were not statistically significant (Table III). We found similar results in separate models for women and men.
Table III.
Adjusted associations between perceived risk of getting HIV and ≥80% PrEP adherence
| Risk perception | Overall |
Women | Men | |||
|---|---|---|---|---|---|---|
| Na (%) with adherence to PrEPc | Adjusted OR (95% CI) | Na (%) with adherence to PrEPc | Adjusted OR (95% CI) | Na (%) with adherence to PrEPc | Adjusted OR (95% CI) | |
| Highb | 40 (83.3) | 0.9 (0.7, 1.3) | 20 (90.9) | 0.8 (0.4, 1.3) | 20 (76.9) | 1.0 (0.7, 1.6) |
| Moderateb | 69 (92.0) | 1.1 (0.8, 1.5) | 34 (97.1) | 1.2 (0.7, 2.0) | 35 (87.5) | 1.1(0.8, 1.5) |
| Lowb | 213 (87.7) | 1.2 (1.0, 1.4) | 88 (88.9) | 1.0 (0.7, 1.5) | 125 (86.8) | 1.3 (1.0, 1.6) |
| Don’t knowb | 49 (84.5) | 0.9 (0.7, 1.2) | 30 (96.8) | 0.9 (0.5, 1.8) | 19 (70.4) | 0.8 (0.6, 1.2) |
| None | 210 (89.0) | Reference | 58 (89.2) | Reference | 152 (88.9) | Reference |
N = number of observations over the duration of the study
OR (95% CI) adjusted for any condomless sex, age, gender (only in overall model), married/cohabiting, mean social support, years serodiscordant status was known, abuse by study partner, alcohol dependence and probable depression.
Adherence to PrEP = observed divided by expected number of MEMS cap openings ≥80%
DISCUSSION
In a PrEP demonstration study in Kenya and Uganda, we evaluated the effect of 1) sexual behavior on HIV risk perception and 2) HIV risk perception on PrEP adherence among HIV-negative participants. Reporting condomless sex (either in general or at the last sex act specifically) was associated with having greater HIV risk perception, yet HIV risk perception was not associated with PrEP adherence. Condom use was also associated with the likelihood of reporting moderate/great risk perception of HIV in a recent study in South Africa [5]. In our study, men reported having no perceived risk for HIV more frequently than women. These results are consistent with studies in Zambia and Mozambique where men were more likely to have multiple sex partners and to report lower risk perception than women [3, 6], and condom use at last sex was more prevalent among men and women whose perceived risk aligned with past and current sexual behavior [4].
Contrary to results from a quantitative study that found a positive association between risk perception (small/moderate/high vs. none) and good adherence (OR: 2.0; 95% CI: 1.1–3.5) [2], we did not find that the level of risk perception was associated with PrEP adherence. In addition to being in mutually disclosed serodiscordant partnerships, the HIV-negative individuals in this study received considerable PrEP counseling [37], likely greater than that received by those who attend public health facilities. This increased awareness of HIV risk possibly contributed to high PrEP adherence regardless of what risk perception was reported during visits and may explain our null findings.
Although our study and others show evidence that having condomless sex is associated with greater odds of perceiving that one has risk for HIV, measuring risk perception remains a challenge. Presently, three studies have investigated the accuracy and validity of HIV risk perception scales and individual items. The first evaluated the “perceived risk of HIV infection scale”, which measures perceived risk using likelihood estimates, intuitive feelings about risk, and the salience of the risk of HIV infection [38]. In the second study, risk perception scales were developed from items measuring perceived risk and perceived vulnerability [39]. The third study assessed risk using four questions: two about general perceived risk, and two about partner-specific perceived risk [40]. The scales developed in these studies demonstrated good reliability and validity. However, their use in other settings is still limited since they were conducted in specific settings and populations in Ethiopia [39] and the US [38, 40].
Most studies, including ours, have evaluated risk perception using a single question about the likelihood of getting HIV at a past or future time and a Likert scale with 4–5 response options [2–5, 41, 42]. Some have grouped scale responses to formulate a binary variable (“high vs low” or “some vs none”) [3, 5, 41], which limits assessment of the effect of different levels of risk perception. Another limitation to a one-question approach for assessing risk perception is the inability to evaluate drivers of risk perception such as partner’s or own sexual behavior and whether participants understand the HIV risk posed by that behavior. This “imperfect” measurement of risk perception may also explain our inability to detect a significant association with PrEP adherence. Some studies have used qualitative methods to assess risk perception in greater detail [8]. One strength of our study is that we assessed risk perception quarterly over a 24-month period, creating frequent opportunities for individuals to evaluate their personal HIV risk and sexual behavior.
The observed alignment of sexual behavior and risk perception in our study suggests that some individuals understand how certain behaviors influence their risk of HIV infection. While this finding is encouraging, studies that have indicated a misalignment between risk perception and actual risk, particularly among men [3, 4] and young women [5], highlight the need to assess the alignment of true HIV risk (or exposure) and risk perception. This relationship is particularly important in the context of PrEP, because if measured more comprehensively, risk perception, among other factors, could influence uptake and adherence to PrEP. Identification of times when risk perception is misaligned with PrEP adherence would present opportunities to potentially increase adherence to PrEP through HIV counseling or to promote alternative HIV prevention strategies [43].
Results from our study highlight the potential role for HIV risk perception to influence use of PrEP and other prevention strategies. As discussed in recommendations for programmatic success of global PrEP roll-out [44], it is essential for PrEP implementers and providers to conduct holistic sexual health assessments, such as high quality measurement of risk perception and the evaluation of actual HIV risk and the salience of HIV risk, to guide conversations about PrEP as a HIV prevention option for clients. As PrEP becomes more available, there is an opportunity for its delivery to incorporate counseling for HIV risk perception, sexual behavior, and PrEP adherence, which integrates the complexities and dynamics of a client’s life. Understanding how these factors are linked, through research, can aid in developing and improving guidelines and programmatic tools available to providers.
Acknowledgements
We thank the couples who participated in this study for their motivation and dedication, and the referral partners, community advisory groups, institutions, and communities that supported this work.
Partners Demonstration Project Team
Coordinating Center (University of Washington) and collaborating investigators (Harvard Medical School, Johns Hopkins University, Massachusetts General Hospital): Jared Baeten (protocol chair), Connie Celum (protocol co-chair), Renee Heffron (project director), Deborah Donnell (statistician), Ruanne Barnabas, Jessica Haberer, Harald Haugen, Craig Hendrix, Lara Kidoguchi, Mark Marzinke, Susan Morrison, Jennifer Morton, Norma Ware, Monique Wyatt
Project sites
Kabwohe, Uganda (Kabwohe Clinical Research Centre): Stephen Asiimwe, Edna Tindimwebwa
Kampala, Uganda (Makerere University): Elly Katabira, Nulu Bulya
Kisumu, Kenya (Kenya Medical Research Institute): Elizabeth Bukusi, Josephine Odoyo
Thika, Kenya (Kenya Medical Research Institute, University of Washington): Nelly Rwamba Mugo, Kenneth Ngure
Data management: DF/Net Research
Data availability
Data are available upon request to the authors’ research center by icrc@uw.edu.
Funding information
The Partners Demonstration Project was funded by the Bill & Melinda Gates Foundation (OPP1056051), the National Institute of Mental Health of the US National Institutes of Health (R01MH095507) and the United States Agency for International Development (AID-OAA-A-12-00023). This work is made possible by the generous support of the American people through USAID; the contents are the responsibility of the authors and do not necessarily reflect the views of USAID, NIH, or the United States Government.
Footnotes
Competing interests
Gilead Sciences donated the PrEP medication but had no role in data collection or analysis. The authors disclosed no competing interests.
References
- 1.Warren EA, Paterson P, Schulz WS, et al. Risk perception and the influence on uptake and use of biomedical prevention interventions for HIV in sub-Saharan Africa: A systematic literature review. PloS one. 2018;13(6):e0198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corneli A, Wang M, Agot K, Ahmed K, Lombaard J, Van Damme L. Perception of HIV risk and adherence to a daily, investigational pill for HIV prevention in FEM-PrEP. Journal of acquired immune deficiency syndromes (1999). 2014;67(5):555–63. [DOI] [PubMed] [Google Scholar]
- 3.Do M, Meekers D. Multiple sex partners and perceived risk of HIV infection in Zambia: attitudinal determinants and gender differences. AIDS care. 2009;21(10):1211–21. [DOI] [PubMed] [Google Scholar]
- 4.Prata N, Morris L, Mazive E, Vahidnia F, Stehr M. Relationship between HIV risk perception and condom use: Evidence from a population-based survey in Mozambique. International family planning perspectives. 2006;32(4):192–200. [DOI] [PubMed] [Google Scholar]
- 5.Maughan-Brown B, Venkataramani AS. Accuracy and determinants of perceived HIV risk among young women in South Africa. BMC public health. 2017;18(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akwara PA, Madise NJ, Hinde A. Perception of risk of HIV/AIDS and sexual behaviour in Kenya. Journal of biosocial science. 2003;35(3):385–411. [DOI] [PubMed] [Google Scholar]
- 7.Maharaj P, Cleland J. Risk perception and condom use among married or cohabiting couples in KwaZulu-Natal, South Africa. International family planning perspectives. 2005;31(1):24–9. [DOI] [PubMed] [Google Scholar]
- 8.Nkomazana N, Maharaj P. Perception of risk of HIV infections and sexual behaviour of the sexually active university students in Zimbabwe. SAHARA-J: Journal of Social Aspects of HIV/AIDS. 2014;11(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omungo PA. Sexual Relationships, Risk Perception and Condom Use at the University of Nairobi. International Journal of Health Science. 2008;1(3):80–7. [Google Scholar]
- 10.Bunnell RE, Nassozi J, Marum E, et al. Living with discordance: knowledge, challenges, and prevention strategies of HIV-discordant couples in Uganda. AIDS care. 2005;17(8):999–1012. [DOI] [PubMed] [Google Scholar]
- 11.Ngure K, Mugo N, Celum C, et al. A qualitative study of barriers to consistent condom use among HIV-1 serodiscordant couples in Kenya. AIDS care. 2012;24(4):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO expands recommendation on oral pre-exposure prophylaxis of HIV infection (PrEP). Available from: http://www.who.int/hiv/pub/prep/policy-brief-prep-2015/en/. Accessed June 11 2018.
- 13.PrEPWatch: An initiative of AIDS Vaccine Advocacy Coalition. Global PrEP Use Landscape as of April 2019. Available from: https://www.prepwatch.org/resource/global-prep-tracker/. Accessed June 21 2019.
- 14.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Current opinion in HIV and AIDS. 2016;11(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware NC, Wyatt MA, Haberer JE, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. Journal of acquired immune deficiency syndromes (1999). 2012;59(5):463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet infectious diseases. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Vanichseni S, Suntharasamai P, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. Aids. 2015;29(7):819–24. [DOI] [PubMed] [Google Scholar]
- 21.Corneli A, Perry B, Agot K, Ahmed K, Malamatsho F, Van Damme L. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PloS one. 2015;10(4):e0125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Elst EM, Mbogua J, Operario D, et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS and behavior. 2013;17(6):2162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PloS one. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. [DOI] [PubMed] [Google Scholar]
- 25.Corneli AL, McKenna K, Headley J, et al. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. Journal of the International AIDS Society. 2014;17(3 Suppl 2):19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberer JE, Kidoguchi L, Heffron R, et al. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. Journal of the International AIDS Society. 2017;20(1):21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haberer JE, Ngure K, Muwonge T, et al. Brief Report: Context Matters: PrEP Adherence is Associated With Sexual Behavior Among HIV Serodiscordant Couples in East Africa. Journal of acquired immune deficiency syndromes (1999). 2017;76(5):488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyra M, Brown ER, Haberer JE, et al. Patterns of Oral PrEP Adherence and HIV Risk Among Eastern African Women in HIV Serodiscordant Partnerships. AIDS and behavior. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeten JM, Heffron R, Kidoguchi L, et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1-Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa [version 1; referees: 2 approved]. Gates Open Research. 2017;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irungu EM, Heffron R, Mugo N, et al. Use of a risk scoring tool to identify higher-risk HIV-1 serodiscordant couples for an antiretroviral-based HIV-1 prevention intervention. BMC Infectious Diseases. 2016;16(1):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaros C, Haberer JE, Boum Y 2nd, et al. The factor structure and presentation of depression among HIV-positive adults in Uganda. AIDS and behavior. 2015;19(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherpitel CJ, Ye Y, Bond J, et al. Cross-national performance of the RAPS4/RAPS4-QF for tolerance and heavy drinking: data from 13 countries. Journal of studies on alcohol. 2005;66(3):428–32. [DOI] [PubMed] [Google Scholar]
- 34.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Medical care. 1988;26(7):709–23. [DOI] [PubMed] [Google Scholar]
- 35.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10(9):e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates Open Res. 2017;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton JF, Celum C, Njoroge J, et al. Counseling Framework for HIV-Serodiscordant Couples on the Integrated Use of Antiretroviral Therapy and Pre-exposure Prophylaxis for HIV Prevention. Journal of acquired immune deficiency syndromes (1999). 2017;74 Suppl 1(Suppl 1):S15–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS and behavior. 2012;16(4):1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley H, Tsui A, Hindin M, Kidanu A, Gillespie D. Developing scales to measure perceived HIV risk and vulnerability among Ethiopian women testing for HIV. AIDS care. 2011;23(8):1043–52. [DOI] [PubMed] [Google Scholar]
- 40.Vargas SE, Fava JL, Severy L, et al. Psychometric Properties and Validity of a Multi-dimensional Risk Perception Scale Developed in the Context of a Microbicide Acceptability Study. Archives of sexual behavior. 2016;45(2):415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringer EM, Sinkala M, Kumwenda R, et al. Personal risk perception, HIV knowledge and risk avoidance behavior, and their relationships to actual HIV serostatus in an urban African obstetric population. Journal of acquired immune deficiency syndromes (1999). 2004;35(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenkorang EY, Rajulton F, Maticka-Tyndale E. Perceived Risks of HIV/AIDS and First Sexual Intercourse among Youth in Cape Town, South Africa. AIDS and behavior. 2009;13(2):234–45. [DOI] [PubMed] [Google Scholar]
- 43.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS (London, England). 2015;29(11):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivet Amico K, Bekker LG. Global PrEP roll-out: recommendations for programmatic success. The lancet HIV. 2019;6(2):e137–e40. [DOI] [PubMed] [Google Scholar]


