Abstract
The success of antiretroviral therapy (ART) has led to both extended life expectancy and improved quality of life among people living with HIV (PLWH). To maximize the efficacy of first line ART regimens in low- and middle-income countries (LMIC), we need culturally-relevant interventions that empower participants to reduce barriers to long-term uninterrupted adherence. The Chetana adherence intervention trial was designed in collaboration with local community groups as a comprehensive wellness program for adherence-challenged PLWH and included peer-led adherence support, yoga, nutrition, information about local resources, and individual counseling using motivational interviewing techniques. Intervention arm participants were almost twice as likely to be virally suppressed at their 12-month follow-up visit (AOR = 1.98; 95% CI [1.2, 3.23]) as were participants in the active control arm. They were also about twice as likely as control arm participants to self-report ≥95% adherence (AOR = 1.86, 95% CI [1.09, 3.15]), and as having eliminated individual adherence barriers (AOR = 2.33, 95% CI [1.51, 3.62]) and clinic attendance barriers (AOR = 2.01, 95% CI [1.20, 3.38]) These low-cost strategies can be implemented by local NGOs, making it both scalable and sustainable in this and similar settings.
Keywords: HIV, ART adherence trial, viral suppression, India, PLWH, LMIC, cultural relevance
INTRODUCTION:
The global antiretroviral therapy (ART) scale-up has been one of the greatest public health success stories in the fight against AIDS, resulting in decreased mortality rates and improved quality of life, in both high income (1) and low and middle income countries (LMIC) (2). Although universal access has not yet been achieved, UNAIDS estimated in 2018 that 22 million of the approximately 37 million people living with HIV worldwide were receiving ART (3). In India, approximately 49% of HIV-infected individuals are estimated to be on therapy, under its newly established test-and-treat protocol. In order for ART-roll out programs to be successful in LMIC, major public health challenges remain, including the need to improve medication adherence rates and develop better low-cost monitoring strategies for adherence and treatment outcomes. The success of ART has led to both extended life expectancy and an improved quality of life among PLWH. A recent analysis (4) of viral load data among PLWH in the US showed that viral suppression rates increased dramatically between 1997 and 2015 among PLWH under clinical care. Achieving long term HIV viral suppression leads both to optimization of health among people living with HIV (PLWH) and the reduction of risk of transmission to their partners, and is thus a crucial component of the attainment of the UNAIDS 90-90-90 goals. Great progress has been made on ART coverage around the world (3), including in LMIC, but much work remains to be done to ensure consistent medication supply, training of clinic staff in prescription and treatment monitoring as well as in reducing barriers to consistent adherence.
To achieve and maintain optimal adherence, it is crucial that we understand the factors associated with local adherence patterns (5–8). In a review of global adherence barriers and facilitators (6), multiple barriers to adherence were identified in both resource rich and resource limited settings, including forgetting, sleeping, substance use, fear of disclosure, family and work responsibilities, medication access, complicated regimens, and decreased quality of life (6). LMIC-specific adherence barriers are likely to be structural in nature, such as transportation problems and having an irregular drug supply (6, 9–11). Studies in LMIC settings have identified additional adherence barriers including fear of stigma (9, 11–14), shame and depression (15–18), food insecurity (19), younger age (20–22), associated cost (23), and medication side effects (24). Consistent with the global literature, Indian patients who are sub-optimally adherent often cite ART medication side effects (25, 26), psychological distress (27–30), lack of a daily routine, and alcohol use (27, 29, 31) as common barriers.
Research also demonstrates that adherence rates often decline with length of time on ART, both in India (5, 25, 29, 32) and globally (15, 20, 33, 34), even when ART is provided at no cost. Regular clinic attendance appears to be associated with long term stable adherence rates(35) and short term treatment interruptions are often due to social and structural factors, such as financial issues, pharmacy stock-outs, and transportation problems, which are exacerbated during monsoon season in the Indian context (31, 36). Our India-based research shows that fear of HIV stigma (14, 37, 38) is often the underlying factor that is associated with these barriers, due to fear of HIV status disclosure. This fear necessitates avoidant coping behaviors, such as consuming one’s pills in private, and having to invent reasons for clinic visits and visits to the pharmacy. All of these behaviors make consistent pill consumption and prescription refills challenging (14, 38, 39). Since community-based or structural interventions to reduce societal stigma are unlikely in the near future, adherence-enhancing strategies in resource-limited settings need to be low cost and tailored to patient needs to support adaptive coping strategies that do not increase the risk of accidental HIV status disclosure and subsequent stigma and discrimination.
Multiple ART adherence intervention programs have been implemented and evaluated globally with mixed success. A 2017 review (40) showed that self-reported adherence frequently increased following interventions using SMS messages, both globally and in LMIC. In general, multi-component interventions appeared to have an additive effect, leading to better adherence than single sessions. Cognitive behavior therapy (CBT) and supportive interventions had an effect on viral load, but this effect was only seen among studies in the global network, not in LMIC and the effects typically waned over time. Similarly, a nurse-led self-management intervention (AIMS) in the Netherlands used MEMS caps as feedback mechanism and found that control participants had, on average, a 1.26 log higher viral load than AIMS participants post intervention (41). Economic incentives have also been found to improve adherence (measured by MEMS caps) in Uganda (42). Similarly, both cash and food assistance improved medication possession ratio in Tanzania and retention at 6-month follow up (43). However, neither study reported differences in viral load. There have also been some promising formative studies showing that targeting both depression and adherence in Zimbabwe among PLWH in Zimbabwe, who self-reported poor adherence and at least mild depression at baseline can improve both outcomes (44, 45).
To maximize the efficacy of limited antiretroviral regimens in LMIC, we thus need culturally-relevant interventions that empower patients to reduce their unique adherence barriers to long-term uninterrupted adherence. Such programs should meet the needs of adherence-challenged patients and include low cost strategies that can be quickly scaled up and sustained in collaboration with local groups in community settings and should ideally have an impact on both self-reported and clinical measures. The Chetana intervention was designed in collaboration with local PLWH to meet these requirements by targeting local patterns and barriers to adherence, using strategies that can easily be scaled up by NGOs. To accomplish this, this two-arm clinical trial (see figure 1) provided a general wellness intervention to participants in both study the Chetana intervention arm and the active control arm. In addition, Chetana intervention participants received adherence-enhancing strategies delivered by trained masters level staff and PLWH peer facilitators to reduce relevant adherence barriers.
Figure 1:
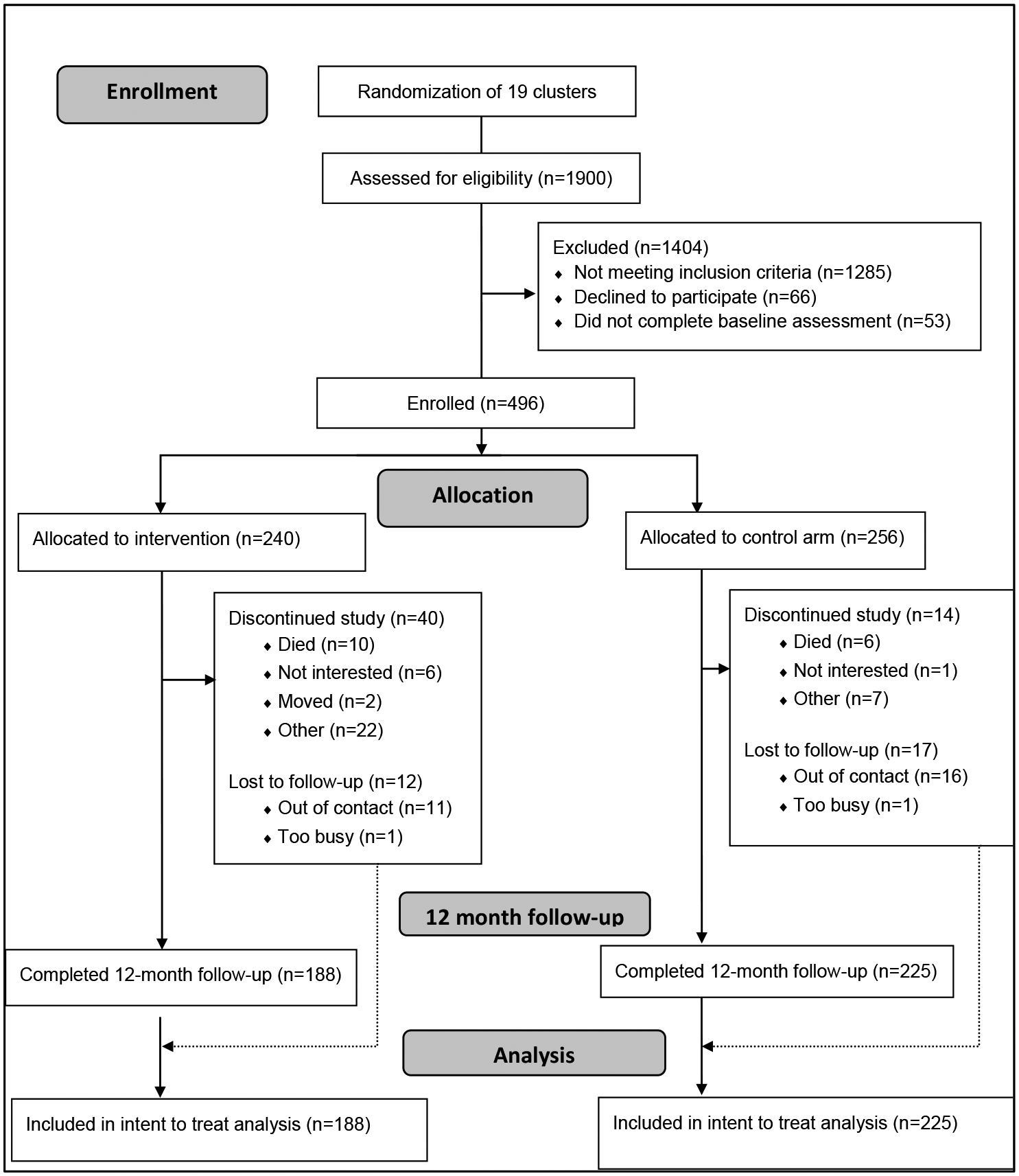
Chetana Study CONSORT Chart
METHODS:
Sample and setting:
This study was conducted among adherence-challenged PLWH in the state of Karnataka, India, which has been identified as a high HIV prevalence state by the Indian National AIDS Control Organization (NACO) (46). The specific government ART clinics were determined by the state arm of NACO, which provides virtually all HIV treatment in the state. Each clinic has a wide catchment area and includes patients from a range of SES, urban and rural settings. Inclusion criteria included being 18 years of age or older, currently on ART medication, fluent in Kannada (local language), residing within 40km of the intervention sites and self-reported to be adherence-challenged. In this study, this was defined as reported less than 90% adherence to ART medication over the past 30 days or reporting more than two treatment interruptions of at least 48 hours each in the past year. Four hundred and ninety-six participant met eligibility criteria and were enrolled and assigned to one of two study arms, either the Chetana intervention arm, or the active control arm. Two hundred and forty participants were randomly allocated to one of nine Chetana intervention groups and 256 participants were assigned to one of 10 active control groups (see figure 1 CONSORT chart).
Procedures:
Screening and enrollment
Recruitment sites included collaborating non-governmental organizations (NGOs), hospital physicians, and government ART Centers in the Karnataka state cities of Bangalore, Chikkaballapur, Kolar, and Mysore. Letters describing the study were sent to potential recruitment and referral sites requesting permission and signed by the government officer in charge of the ART centers, or to the head of the NGO. Prior to referrals, study staff met with the medical officer, counsellor, NGO staff or person in charge of the recruitment site to explain the study objectives and referral procedures, and to provide study cards for them to give to interested participants. Study staff also distributed an information leaflet describing the study in more detail in the local languages to site officials, and staff. Study staff met with interested participants to provide additional information, collect their contact information and schedule them for screening either in person or over the phone. All interested and eligible participants were scheduled for consent and a baseline assessment visit within two weeks of screening. Field staff were assigned to either the intervention team or assessment team and traveled between sites. Assessment team members were blinded to intervention assignment. All study staff received extensive training in the ethics of human subjects research and the relevant portions of the study protocol and were certified based on performance in observed role plays. Ongoing supervision included both planned and unplanned observations by the study coordinators.
Assessment visits
Following consent, a trained staff interviewer conducted the baseline assessment and obtained tracking data. Consent and all assessments were held in a private room reserved for the study in our study office or at participating NGOs, hospitals, ART centers and clinic consultation centers in Bangalore, Chikkaballapur, Kolar, and Mysore. None were conducted in the medical clinics, given patient concerns about sharing sensitive information about their adherence challenges with the clinic staff.
Participants received reminder calls four days ahead of all study follow-up visits to improve appointment keeping. They were assessed every six months for 18 months using an interviewer-administered questionnaire. A study phlebotomist collected blood samples for viral load, and CD4 count tests. The six-month follow-up was completed after the last intervention group meeting. To encourage retention, the participants were given the option of receiving 200 INR (approximately $3) or three nutrition supplement packages. If participants were too sick, were unable to travel, or were out of contact, assessment staff completed home visits after receiving permission from the participant. Based on the preferences noted on their tracking forms, participants also had the option of being followed through the local ART clinic. All assessment staff members were blinded to group assignment.
Ethics approval:
This trial was approved by the Indian Health Ministry Screening Committee (HMSC) and the Ethics Committees at the University of California, San Francisco, and St. John’s Medical College and Hospital, Bangalore.
Intervention development
The development of the Chetana intervention was guided by social cognitive theory (SCT) (47) and motivational interviewing techniques (48) to target local adherence patterns and barriers experienced by PLWH (26) in a participant-centered manner. The content was also responsive to pilot data collected via focus groups with sub-optimally adherent participants in our previous cohort study during our formative research phase. Pilot participants stated that, while they wanted to learn how to better integrate their medical regimen into their lives, they did not only want to learn strategies to improve their HIV-related lab values and were unlikely to attend any information with such a narrow focus. There was a consensus that they would be much more likely to attend an intervention if it also provided skills, such as exercises and diets that would enable them to live positive, healthy lives and requested programs that addressed both needs. Finally, focus group participant noted that intervention participants may be reluctant to disclose some barriers, such as fear of intimate partner violence in a group format. They therefore requested that the intervention include individual session with a professional counselor, trained to provide participant-centered, non-judgmental adherence support. Motivational interviewing techniques meet these needs (48).
Content and format
Based on these considerations, the Chetana intervention included three components 1) Positive living groups 2) peer-led adherence groups to enable participants to obtain social support and learn techniques from each other and 3) individual counseling using motivational interviewing techniques to help them address individual barriers and family situations that they were unwilling to share with the group in a participant-centered, non-judgmental format.
Group session format:
Group sessions consisted of 10 two-hour sessions scheduled over six months. Sessions were held biweekly for the first three months and once a month for the last three months. The average group consisted of 13 same-sex participants with group size ranging from nine to 18 participants. To minimize stigma, the group sessions were held in a rented community space, that was not associated with HIV services. The first part of each session was devoted to a “positive living topic”. After a break for refreshments, the same group participated in an adherence support group session (also see figure 2). Participants were provided with meal and refreshments during the group sessions and received reimbursement for their transportation costs to each session.
Figure 2:

Outline of adherence and positive living group sessions
1). Positive living group sessions:
The “positive living” group sessions consisted of interactive sessions in yoga, nutrition, medical issues, legal aid and information on community resources for PLWH, in response to the requests made by focus group participants. Each session was led by an expert from the community who had experience working with PLWH. Participants were given a personal yoga mat, which they were allowed to keep following participation as well as a certificate of completion.
2). Adherence support group sessions:
Adherence support sessions focused on participant’s experiences and barriers to taking HIV medication and were facilitated by a Masters level staff member and a PLWH peer counselor. After the ground rules were established, each session began with a check-in and review of each participant’s health issues, successes and challenges experienced since the last group session, as well as their progress on their action plan. Although group discussions could include non-ART related issues as well, such as challenges with families or employers, the facilitators made sure that issues related to prescription refills, stigma, mental health, medication side effects, and other known adherence barriers were addressed in each session. The peer counselor facilitated a group brainstorm of potential strategies to reduce the identified barriers. At the end of the session each participant set-up or revised their action plan to overcome ongoing barriers, sometimes with the support of fellow group members.
Motivational interview individual sessions
In addition to the group sessions, participants assigned to the intervention arm also received six 30-minute individual sessions at least once a month for a period of six months to address unique adherence barriers in a participant-centered, non-judgmental format. Masters level counsellors were trained in Motivational Interviewing techniques to help participants develop internal motivation, readiness for changes, and learn how to eliminate adherence barriers using individualized tailored plans. Participants had the option to meet with the counselor either in person or by phone at any time to discuss urgent problems and solutions for adhering to their HIV medication treatment. The individual format also facilitated discussion of issues that participants did not want to share during the group sessions, which was requested during our formative research.
Control group
The control group received standard care from the ART center and four one-hour positive living group instructional sessions in yoga, nutrition, legal issues and information about community resources for PLWH. These sessions were scheduled for approximately one hour and occurred at least one month apart for the first six months of the study. The active control group did not receive any adherence related support groups or motivational interview individual sessions by the study.
Measures
Demographics:
We assessed several demographic characteristics, including gender, age, number of children, religion, and education.
HIV history:
Participants self-reported when they were first diagnosed with HIV and when they had started ART. These dates were validated using their ART clinic books, which patients keep. The dates were subsequently compared to the interview date to obtain the number of months since HIV diagnosis and months on ART.
Adherence:
We assessed the proportion of pills taken in the past month via a Visual Analogue Scale (VAS) in which participants point to a spot on a line with endpoints 0 and 100 to indicate what percentage of their prescribed pills they took in the preceding month. Scores were dichotomized as <95% vs. ≥95% adherence. Data from our previous observational cohort study showed that this measure was significantly associated with viral load in a similar sample (5, 25).
Treatment interruptions:
Participants also reported the number of times in the past six months that they had missed all their ART pills for at least two consecutive days (>48 hours). This measure was dichotomized into any vs. no treatment interruptions. Our previous cohort study found that adding this measure of treatment interruptions improved our ability to identify people with detectable viral load (5) and that a combined “optimal adherence” measure of VAS and treatment interruptions was significantly associated with both viral load and drug resistant mutations (5, 25).
Adherence barriers:
Participants indicated how often (0 ‘Never’ to 3 ‘Most of the time’) they missed their ART medication for various reasons. The items were originally generated based on focus groups and interviews with local providers and interviews with representatives from PLWH networks. They were subsequently updated based on the results of our prior adherence cohort study (26) and found to be associated with self-reported adherence. The barriers were divided into four categories: individual barriers (seven items, e.g. forgetting to take pills, being asleep at time for medication), family level barriers (three items, e.g. financial difficulties to pay for travel to clinic or lab tests), regimen or clinic barriers (nine items, e.g. medication stock outs, problems taking pills according to instructions) and social/structural barriers (seven items, e.g. no privacy or no time to take pills at work, religious leader advised against medication). Four additional items assessing barriers to attend the ART clinic (e.g. difficult getting time off work or household duties, clinic wait times) were combined into a fifth category. Respondents answering ‘Never’ to all items in a category were classified as not having any barriers of that kind; those who endorsed at least one item were scored as having adherence barriers in that category (26).
Viral load (VL):
HIV plasma VL levels were determined using a real-time PCR assay with a fluorescein-labeled TaqMan probe for quantification of HIV particles (49). Detectable VL was defined as ≥100 copies/ml of blood. This method was used in our previous India studies (5, 25, 26)
Data Analyses
Frequencies and percentages for categorical variables and means with standard deviation or medians with interquartile range for continuous variables were used to describe the sample. Comparisons on baseline data between the two intervention groups and between those with and without 12 mo. follow-up data were done via X2-test for categorical variables, t-test for age and Mann-Whitney U-test for other continuous variables, respectively.
Post intervention differences of the outcome variables between the intervention and control group were analyzed via logistic linear regression, controlling for baseline differences on those outcome measures that showed variability in outcome at baseline (VL and treatment interruptions). We also controlled for gender and location of the intervention site (Bengaluru, Chikkaballapur, Mysuru, Kolar). An interaction effect between gender and intervention was initially added as a predictor, but since the intervention effect was not significantly different for male and female participants, the interaction term was not retained in the final regression models. We also explored if it was necessary to control for any demographic imbalances between the two arms at baseline, but the only variable showing a difference was having children (see details in results section), which was not associated with any of the adherence outcomes bivariately, hence it was not included in the multivariate logistic regression analyses.
For the treatment interruptions and family-level barriers outcomes, we faced an issue of complete separation in the logistic regression analysis, due to the small number of respondents without treatment interruptions at baseline and an empty cell for barriers for one of the towns, respectively. We used Firth’s penalized maximum likelihood estimation to address the issue (50). Dropping the problem variable from the logistic regressions resulted in similar results.
Descriptive analyses were conducted in SPSS v25 and the regression in Stata v15. All p-values reported are two-sided.
RESULTS:
As shown in the Figure 1 Consort chart, 1,900 participants were screened for eligibility, of whom 1285 were found ineligible. Among the remaining 615 participants, 66 refused participation, 53 did not attend their scheduled baseline assessment, leaving 496 who were enrolled into the study.
Attrition bias:
Eighty-three participants did not have a 12mo follow-up data, 16 of whom had been confirmed deceased by the 12-month follow-up (10 in intervention and 6 in the control group), resulting in 67 participants considered lost to follow-up (LTFU). Attrition bias analyses showed that the 83 participants had on average been diagnosed more recently (median of 44 vs 56 months prior to baseline, MW-U=19,614.5, p=.038) and had been on ART for a shorter time (31 vs. 38 months, MW-U=20,025.5, p=.015) than panel members. They were also more likely to be male (62.7% vs. 50.6%, X2=4.022, p=0.045). There was no difference at baseline between deceased/LTFU participants and panel members in terms of age, education, or any of the adherence-related outcome variables. Participants who withdrew from the study (see figure 1) most frequently reported that this was due to time constraints, including inability to take time off from work or household duties. Some female participants also reported not being allowed to travel alone, which made attendance at intervention groups challenging.
Table 1 shows baseline sample characteristics. About half the sample (209/413) was male, 58.8% (n=243) were married, and most were Hindu (92.7%, n=383). Participants were between 20 and 75 years old, with a mean (SD) age of 38.9 (8.4) and had been on ART for a median duration of just over 3 years at the time of study enrollment (IQR 16 – 67 months). The only statistically significant demographic difference between the two arms was that the proportion of participants with children was larger in the intervention (88.8%) than in the control arm (77.3%, p=0.002).
Table 1.
Demographic characteristics of the sample at baseline
| Overall | Intervention (n=188) | Control (n=225) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| % | (n) | % | (n) | % | (n) | ||
| 50.6 | (209) | 47.3 | (89) | 53.3 | (120) | ||
| Married | 58.8 | (243) | 61.7 | (116) | 56.4 | (127) | 0.280 |
| Has children | 82.6 | (341) | 88.8 | (167) | 77.3 | (174) | 0.002 |
| ≥ 10 years schooling | 32.9 | (136) | 29.8 | (56) | 35.6 | (80) | 0.214 |
| Hindu religion | 92.7 | (383) | 93.1 | (175) | 92.4 | (208) | 0.803 |
| Age: mean (SD) | 38.9 | (8. 4) | 38.9 | (8. 5) | 38.9 | (8. 3) | 0.963 |
| Months since HIV diagnosis: Median (IQR) | 56 | (28 – 91) | 57 | (25 – 89) | 56 | (28 – 93) | 0.797 |
| Months on ART: Median (IQR) | 38 | (16 – 67) | 32 | (13 – 69) | 41 | (18 – 67) | 0.118 |
Based on χ2 test for categorical variables, t-test for age and Mann-Whitney U-test for months diagnosed/on ART
There were no baseline differences between the intervention and the control arm (Table 2, column “Pre”) with respect to the adherence outcomes. All participants reported below 95% adherence in the past month as measured by the VAS and nearly all (95.9%) had treatment interruptions of at least 48h in the 6-month period before baseline. All participants, in both groups, endorsed experiencing adherence barriers (result not shown), especially individual, social/structural and regimen/clinic barriers (see Table 2). In both arms, 46.8% had an undetectable VL at BL.
Table 2.
Pre- and 12 mo follow-up outcomes for control (n=225) and intervention (n=188) groups
| Pre % (n) | Post (12mo) % (n) | AORa | (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Adherence past mo (VAS) ≥ 95% | |||||
| Intervention | 0 (0) | 83.5 (157)* | 1.86 | (1.09 – 3.15) | .022 |
| Control | 0 (0) | 73.8 (166) | (ref) | ||
| No Tx interruptions past 6 mob | |||||
| Intervention | 5.3 (10) | 79.8 (150) | 1.43b | (0.87 – 2.36) | .163 |
| Control | 3.1 (7) | 72.4 (163) | (ref) | ||
| No family-level adherence barriersb | |||||
| Intervention | 75.5 (142) | 92.0 (173) | 0.93 | (0.44 – 1.97) | 0.841 |
| Control | 72.0 (162) | 92.0 (207) | (ref) | ||
| No individual adherence barriers | |||||
| Intervention | 4.8 (9) | 62.2 (117)*** | 2.33 | (1.51 – 3.62) | <.001 |
| Control | 3.6 (8) | 45.3 (102) | (ref) | ||
| No social/structural adherence barriers | |||||
| Intervention | 12.8 (24) | 64.9 (122) | 1.49 | (0.96 – 2.32) | 0.078 |
| Control | 12.4 (28) | 59.1 (133) | (ref) | ||
| No regimen/clinic adherence barriers | |||||
| Intervention | 18.1 (34) | 78.2 (147) | 1.07 | (0.65 – 1.77) | 0.795 |
| Control | 23.1 (52) | 76.9 (173) | (ref) | ||
| No clinic attendance barriers | |||||
| Intervention | 57.5 (108) | 83.0 (156)** | 2.01 | (1.20 – 3.38) | 0.008 |
| Control | 62.7 (141) | 70.7 (159) | (ref) | ||
| Undetectable VLc | |||||
| Intervention | 46.8 (88) | 52.7 (99)* | 1.98 | (1.22 – 3.23) | .006 |
| Control | 46.8 (102) | 40.8 (89) | (ref) |
p< 0.05;
p<0.01;
p≤ 0.001
Adjusted for gender and location for all outcomes, as well as for pre-intervention value on outcomes with pre-intervention variability.
Used Firth’s penalized maximum likelihood estimation to address complete separation problem
control group: missing data for 1 case at BL and 6 more cases at 12mo; hence results based on n=225–7=218; intervention group: no missing data, n=188.
The most prevalent adherence barriers were related to disruptions of people’s daily lives, such as being away from home (endorsed by 83.3%), being busy with other things (79.9%), or not being on one’s daily routine (22.0%). Nearly 70% of participants said that they ‘simply forgot’. Other common barriers included running out of medication before being able to obtain a refill (47.7%), lack of privacy (31.7%), feeling sick (32.3%) or feeling depressed (30.3%).
At the 12-month follow-up visit (Table 2, “Post column”), Viral load tests showed that intervention participants were significantly more likely to have undetectable VL (52.7% vs. 40.8%, p=0.017) at 12-month follow-up. Intervention arm participants were also significantly more likely than control arm participants to report VAS ≥95% (83.5% vs. 73.8, p=0.017) and having eliminated all individual adherence barriers (62.2% vs. 45.3%, p=0.001) and clinic attendance barriers (83.0% vs. 70.7%, p=0.003).
Multiple logistic regression analyses showed that these results remained significant after controlling for location, gender, and pre-intervention values (on outcomes with pre-intervention variability). Intervention arm participants were almost twice as likely to be virally suppressed at their 12-month follow-up visit (AOR = 1.98; 95% CI [1.2, 3.23]). They were also about twice as likely as control group participants to report ≥95% VAS adherence (AOR = 1.86, 95% CI [1.09, 3.15]), and as having eliminated individual adherence barriers (AOR = 2.33, 95% CI [1.51, 3.62]) and clinic attendance barriers (AOR = 2.01, 95% CI [1.20, 3.38]) (Table 2).
An examination of gender differences showed that female participants were nearly twice as likely to have undetectable VL at 12mo follow-up as male participants (AOR:=1.94, 95% CI [1.24, 3.05], p=0.004 – not shown in Table 2). There were no significant gender differences for any of the other study outcomes.
DISCUSSION:
The data described here demonstrate that our comprehensive behavioral wellness intervention that incorporates motivational interviewing techniques and peer-led group adherence support and was based on formative research resulted in a significantly greater proportion of virally suppressed and optimally adherent participants at the 12-month follow-up assessment, compared to participants who received only wellness sessions unrelated to adherence. The success of the program points to the importance of designing adherence programs that meet the perceived needs of adherence-challenged PLWH, in addition to providing evidence-based adherence-specific support. It also indicates that while public health professionals may consider adherence to be a priority for optimizing health, sub-optimally adherent PLWH may have different priorities, such as fitness and access to legal and other community resources. Unless we incorporate their priorities in our adherence interventions, it can be challenging to get their buy-in and attendance.
Although there were no between-group differences in terms of reasons for withdrawal, it appears that the greater time commitment of the intervention group presented an unanticipated challenge for some participants, which likely led to the greater number of drop outs from this study arm. Future intervention programs may consider flexible scheduling, providing sessions in the evening and on Sundays, or offering groups on a drop-in basis. Although we did not formally collect these data, some participants suggested these modifications during their exit interviews.
Analyses of post-intervention change in the baseline adherence barriers suggest, not surprisingly, that individual-level barriers were most amenable to change and that many participants learned how to deal with structural barriers as well. In contrast, family and regimen/clinic barriers, which often represented factors over which participants had little or no control, were the most resistant to change.
Limitations of this study include the fact that we were unable to recruit a large number of participants from key populations (KP), such as female sex workers (FSW) and men who have sex with men (MSM), which prevents us from generalizing to those communities. When referred to us, several members of these key populations told us anecdotally that their irregular daily schedules prevented them from attending regularly scheduled group sessions and that they would prefer either drop-in groups or individual peer navigation programs. This has implications for future programs, which might benefit from exploring if such an approach is more likely to enroll and retain key populations. Secondly, while the comprehensive, multi-component nature of the Chetana intervention resulted in improved adherence, it does not allow us to tease out the relative impact of the different components. Future studies may want to randomly assign participants to different combinations of these components in order to examine their relative impact. Finally, as with all studies, the Chetana study has geographical limitations and, while many of the cultural factors are similar across India, we cannot generalize the findings beyond the Southern states, from where our participants came.
In spite of these limitations, we are encouraged by the findings that this comprehensive intervention program led to a significantly increased proportion of virally suppressed and adherent participants, as well as a decrease in perceived adherence barriers. In addition, since Chetana uses low cost strategies that can be implemented by local NGOs, it is both scalable and sustainable in this and similar settings.
ACKNOWLEDGEMENTS:
We gratefully acknowledge the staff at the Karnataka ART Centres for referring the study participants as well as the Chetana study staff for their hard work and dedication to the study participants. Finally, we are grateful to the many study participants who so generously shared their time and thoughts with us.
This manuscript was supported by (R01MH095659)
Footnotes
Disclosure of potential conflicts of interest
Funding: this study was funded by the National Institutes of Health (R01MH095659)
Conflict of Interest: The authors declare they have no conflict of interest
Research involving Human Participants
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standard of the Ethics Committees of the University of California, San Francisco and St. John’s Research Institute and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–29. [DOI] [PubMed] [Google Scholar]
- 2.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science (New York, NY). 2013;339(6122):961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. UNAIDS Data 2018. Geneva, Switzerland; 2018. [Google Scholar]
- 4.Nance RM, Delaney JAC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV Viral Suppression Trends Over Time Among HIV-Infected Patients Receiving Care in the United States, 1997 to 2015: A Cohort Study. Ann Intern Med. 2018;169(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstrand ML, Chandy S, Heylen E, Steward W, Singh G. Developing useful highly active antiretroviral therapy adherence measures for India: the Prerana study. Journal of acquired immune deficiency syndromes (1999). 2010;53(3):415–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10(6):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachega JB, Stein DM, Lehman DA, Hlatshwayo D, Mothopeng R, Chaisson RE, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Hum Retroviruses. 2004;20(10):1053–6. [DOI] [PubMed] [Google Scholar]
- 10.Pasquet A, Messou E, Gabillard D, Minga A, Depoulosky A, Deuffic-Burban S, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Cote d’Ivoire. PloS one. 2010;5(10):e13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reda AA, Biadgilign S. Determinants of Adherence to Antiretroviral Therapy among HIV-Infected Patients in Africa. AIDS Res Treat. 2012;2012:574656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV Stigma Mechanisms and Well-Being Among PLWH: A Test of the HIV Stigma Framework. AIDS Behav. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdottir TB, Richter C, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steward WT, Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Stigma is associated with delays in seeking care among HIV-infected people in India. Journal of the International Association of Providers of AIDS Care. 2013;12(2):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, Musoke P, et al. Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav. 2009;13 Suppl 1:82–91. [DOI] [PubMed] [Google Scholar]
- 16.Kong MC, Nahata MC, Lacombe VA, Seiber EE, Balkrishnan R. Association between race, depression, and antiretroviral therapy adherence in a low-income population with HIV infection. J Gen Intern Med. 2012;27(9):1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PS, Campsmith ML, Nakamura GV, Begley EB, Schulden J, Nakashima AK. Patient and regimen characteristics associated with self-reported nonadherence to antiretroviral therapy. PloS one. 2007;2(6):e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orrell C, Cohen K, Mauff K, Maartens G, Wood R, DR B. Randomised Controlled Trial of Text-Message Dosing Reminders in Patients Starting ART. 22nd Conference on Retroviruses and Opportunistic Infections (CROI),, Abstract 559. Seattle, WA February 23–26, 2015. [Google Scholar]
- 19.Weiser SD, Tuller DM, Frongillo EA, Senkungu J, Mukiibi N, Bangsberg DR. Food insecurity as a barrier to sustained antiretroviral therapy adherence in Uganda. PloS one. 2010;5(4):e10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. [DOI] [PubMed] [Google Scholar]
- 21.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safren SA, Biello KB, Smeaton L, Mimiaga MJ, Walawander A, Lama JR, et al. Psychosocial predictors of non-adherence and treatment failure in a large scale multi-national trial of antiretroviral therapy for HIV: data from the ACTG A5175/PEARLS trial. PloS one. 2014;9(8):e104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: A systematic review and meta-analysis. AIDS Care. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekstrand ML, Shet A, Chandy S, Singh G, Shamsundar R, Madhavan V, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. International health. 2011;3(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallabhaneni S, Chandy S, Heylen E, Ekstrand M. Reasons for and correlates of antiretroviral treatment interruptions in a cohort of patients from public and private clinics in southern India. AIDS Care. 2012;24(6):687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batavia AS, Balaji K, Houle E, Parisaboina S, Ganesh AK, Mayer KH, et al. Adherence to antiretroviral therapy in patients participating in a graduated cost recovery program at an HIV care center in South India. AIDS Behav. 2010;14(4):794–8. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh KK, Srikrishnan AK, Mayer KH, Kumarasamy N, Raminani S, Thamburaj E, et al. Predictors of Nonadherence to Highly Active Antiretroviral Therapy Among HIV-Infected South Indians in Clinical Care: Implications for Developing Adherence Interventions in Resource-Limited Settings. AIDS patient care and STDs. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh KK, Srikrishnan AK, Mayer KH, Kumarasamy N, Raminani S, Thamburaj E, et al. Predictors of nonadherence to highly active antiretroviral therapy among HIV-infected South Indians in clinical care: implications for developing adherence interventions in resource-limited settings. AIDS patient care and STDs. 2010;24(12):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinman NJ, Manhart LE, Mohanraj R, Kumar S, Jeyaseelan L, Rao D, et al. Antiretroviral therapy adherence measurement in non-clinical settings in South India. AIDS Care. 2015;27(2):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi B, Chauhan S, Pasi A, Kulkarni R, Sunil N, Bachani D, et al. Level of suboptimal adherence to first line antiretroviral treatment & its determinants among HIV positive people in India. Indian J Med Res. 2014;140(1):84–95. [PMC free article] [PubMed] [Google Scholar]
- 32.Hamide A, Shamanna SB, Balaguru S, Subbian M. Long-term outcome of HIV-infected patients treated at a tertiary care hospital in southern India. Natl Med J India. 2014;27(3):134–7. [PubMed] [Google Scholar]
- 33.Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41(3):315–22. [DOI] [PubMed] [Google Scholar]
- 34.Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45(3):334–41. [DOI] [PubMed] [Google Scholar]
- 35.Cauldbeck MB, O’Connor C, O’Connor MB, Saunders JA, Rao B, Mallesh VG, et al. Adherence to anti-retroviral therapy among HIV patients in Bangalore, India. AIDS research and therapy. 2009;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meena LP, Pandey SK, Rai M, Bharti A, Chakravarty J, Sundar S. Study the drug adherence and possible factor influencing drug adherence in HIV/AIDS patients in north eastern part of India. J Educ Health Promot. 2014;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekstrand ML, Bharat S, Srinivasan K. HIV stigma is a barrier to achieving 90-90-90 in India. The lancet HIV. 2018;5(10):e543–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, et al. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med. 2008;67(8):1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steward WT, Chandy S, Singh G, Panicker ST, Osmand TA, Heylen E, et al. Depression is not an inevitable outcome of disclosure avoidance: HIV stigma and mental health in a cohort of HIV-infected individuals from Southern India. Psychology, health & medicine. 2011;16(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. The lancet HIV. 2017;4(1):e31–e40. [DOI] [PubMed] [Google Scholar]
- 41.de Bruin M, Oberjé EJ, Viechtbauer W, Nobel H-E, Hiligsmann M, van Nieuwkoop C, et al. Effectiveness and cost-effectiveness of a nurse-delivered intervention to improve adherence to treatment for HIV: a pragmatic, multicentre, open-label, randomised clinical trial. The Lancet Infectious Diseases. 2017;17(6):595–604. [DOI] [PubMed] [Google Scholar]
- 42.Linnemayr S, Stecher C, Mukasa B. Behavioral economic incentives to improve adherence to antiretroviral medication. AIDS (London, England). 2017;31(5):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCoy SI, Njau PF, Fahey C, Kapologwe N, Kadiyala S, Jewell NP, et al. Cash versus food assistance to improve adherence to antiretroviral therapy among HIV-infected adults in Tanzania: a randomized trial. AIDS (London, England) 2017;31(6):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abas M, Nyamayaro P, Bere T, Saruchera E, Mothobi N, Simms V, et al. Feasibility and Acceptability of a Task-Shifted Intervention to Enhance Adherence to HIV Medication and Improve Depression in People Living with HIV in Zimbabwe, a Low Income Country in Sub-Saharan Africa. AIDS Behav. 2018;22(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bere T, Nyamayaro P, Magidson JF, Chibanda D, Chingono A, Munjoma R, et al. Cultural adaptation of a cognitive-behavioural intervention to improve adherence to antiretroviral therapy among people living with HIV/AIDS in Zimbabwe: Nzira Itsva. J Health Psychol. 2017;22(10):1265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Aids Control Organization (NACO) MoHaFW, Governemnt of India;. National Strategic Plan for HIV/AIDS and STI 2017–2014. New Delhi, India: NACO; 2017. [Google Scholar]
- 47.Bandura A Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–64. [DOI] [PubMed] [Google Scholar]
- 48.Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational Interviewing Improves Medication Adherence: a Systematic Review and Meta-analysis. J Gen Intern Med. 2016;31(8):929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. Journal of clinical microbiology. 2003;41(10):4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinze G A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat Med. 2006;25(24):4216–26. [DOI] [PubMed] [Google Scholar]


