Abstract
Objective:
Improved treatments for juvenile idiopathic arthritis (JIA) have increased remission rates. We investigated how patients and caregivers make decisions about stopping medications for well-controlled JIA.
Methods:
We performed a mixed-methods study of caregivers and patients affected by JIA, recruited through social media and flyers and selected by purposive sampling. Participants discussed their experiences with JIA, medications, and decision-making through recorded telephone interviews. Of 44 interviewees, 20 were patients (50% <18 years) and 24 were caregivers (50% of children ≤10 years). We evaluated characteristics associated with high levels of reported concerns about JIA or medicines using Fisher’s exact testing.
Results:
Decisions about stopping medicines were informed by competing risks between disease and treatment. Participants who expressed more concerns about JIA were more likely to report disease-related complications (P=0.002) and more motivated to continue treatment. However, participants expressing more concern about medicines were more likely to report treatment-related complications (P=0.04) and felt more compelled to stop treatment. Additionally, participants considered how JIA or treatments facilitated or interfered with their sense of normalcy and safety, expressed feelings of guilt and regret about previous or potential adverse events, and reflected on uncertainty and unpredictability of future harms. Decision-making was also informed by trust in rheumatologists and other information sources, e.g., family, online support groups.
Conclusion:
When deciding whether to stop medicines for well-controlled JIA, patients and caregivers weigh competing risks between disease and treatment. Based on our results, we suggest specific approaches for clinicians to perform shared decision-making around stopping medicines for JIA.
Juvenile idiopathic arthritis (JIA) contributes to numerous short- and long-term physical and psychosocial sequelae, including chronic pain, disability, depression, and impaired quality of life.(1, 2) About half of patients with JIA have persistently active disease into adulthood.(3, 4) However, with increasing availability of effective antirheumatic medicines, health outcomes in children with JIA have improved considerably in recent years. Many patients are able to achieve inactive disease, which may persist after stopping JIA drugs altogether.(5) These improvements in treatments and outcomes for patients with JIA have not been without costs. Conventional and biologic disease- modifying antirheumatic drugs (DMARDs) have a range of potential harms, ranging from mild side effects to more serious and uncertain complications, including serious infections and potentially malignancy.(6–11) JIA treatments, particularly biologics, can cause substantial financial burdens on families resulting from the high medicine costs and missed school and work for office visits, infusions, and hospitalizations.(12, 13)
Decisions about stopping effective medications are challenging and complex. A widely used and validated definition of inactive disease does not include patient-/parent-reported measures and may not account for factors most important to patients and families in decision-making.(14, 15) Additionally, clinical definitions of inactive disease and remission do not reflect the complex biology that predisposes some children to flare after withdrawing treatment.(16) Biomarkers for guiding decisions on treatment withdrawal have been tested (17) but not sufficiently validated for routine clinical use.(18) Choices about stopping treatment are further complicated by insufficient data on effective withdrawal strategies.(19, 20)
Given substantial uncertainty about how to manage inactive JIA, decisions about withdrawing treatment generally involve discussions between clinicians, patients, and families, each party with potentially different experiences, values, and priorities. Previous studies have examined clinicians’ motivations and priorities around stopping JIA medicines.(19, 21) Some studies have explored patients’ and parents’ perspectives on starting and adhering to JIA medicines (22, 23). Other research has examined decision-making around stopping treatment for rheumatoid arthritis (RA) (24, 25) and other pediatric conditions, e.g., ADHD and epilepsy.(26, 27) No study, however, has formally examined how patients with JIA and caregivers approach decision-making for well-controlled disease. We conducted a mixed-methods study of adolescents and young adults with JIA, and caregivers to identify important factors and priorities when deciding whether to stop medications for well-controlled JIA. We hypothesized that caregivers would prioritize long-term impacts (e.g., damage prevention, long-term drug toxicities), and patients would prioritize short-term impacts (e.g., symptoms, side effects).
PATIENTS AND METHODS
Study Design and Population
We performed a mixed-methods study based on semi-structured telephone interviews of patients with JIA and caregivers (Supplemental Methods). Eligible participants were required to live in the United States, be ≥13 years old, and report having either a JIA/JRA diagnosis or a child with JIA/JRA. We recruited participants via social media and flyers in pediatric rheumatology clinics. Interested caregivers and adults with JIA were asked to complete a preliminary online survey. Caregivers were asked for their permission to allow children with JIA ages 13–17 to participate. We purposively selected participants for interviews based on demographic and disease variables to ensure a broad range of participants (Table 1).
Table 1.
Self-reported characteristics of interview participants
| Characteristic | N (%) |
|---|---|
| Demographics and geography | |
| Group | |
| Patient, 13–17 years old | 10 (23%) |
| Patient, ≥18 years old (range 18–38) | 10 (23%) |
| Mother, child ≤10 years old and younger | 11 (25%) |
| Mother, child >10years old | 13 (30%) |
| Patient gender, female | 35 (80%) |
| Hispanic/Latino ethnicity | 10 (23%) |
| Non-white race | 6 (14%) |
| Public insurance | 10 (23%) |
| Maximum level of parental education (any parent or guardian) | |
| High school | 4 (9%) |
| College | 21 (47%) |
| Graduate school | 19 (43%) |
| Region of US | |
| Midwest | 9 (20%) |
| Northeast | 8 (18%) |
| South | 19 (43%) |
| West | 8 (18%) |
| Disease and drug experience | |
| JIA category | |
| Oligoarticular JIA | 11 (25%) |
| Polyarticular JIA | 18 (41%) |
| Psoriatic JIA | 4 (9%) |
| Enthesitis-related arthritis | 5 (11%) |
| Systemic JIA | 5 (11%) |
| Other | 1 (2%) |
| Years since JIA diagnosis | |
| <4 | 10 (23%) |
| 4–8 | 19 (43%) |
| >8 | 15 (34%) |
| Uveitis | 9 (20%) |
| Methotrexate use | |
| None | 7 (16%) |
| Prior | 17 (39%) |
| Current | 20 (45%) |
| Biologic use | |
| None | 7 (16%) |
| Prior | 5 (11%) |
| Current | 32 (73%) |
| History of inactive JIA, drug discontinuation | |
| Never inactive | 10 (23%) |
| Never stopped, inactive before | 2 (5%) |
| Never stopped, inactive now | 11 (25%) |
| Stopped, now active | 12 (27%) |
| Stopped, now inactive | 9 (20%) |
Interviews
After obtaining verbal consent/assent, research staff conducted telephone interviews approximately 30–45 minutes long using a semi-structured interview guide. This guide included open-ended questions followed by prompts to probe specific ideas and elicit additional thoughts and opinions (see Supplement, Interview Guides). Questions explored participants’ experiences with JIA, treatment, and factors that might influence treatment-related decisions, focusing on inactive disease and treatment withdrawal. Parallel interview scripts for caregivers and patients shared the same structure with age-appropriate questions. Interview scripts were developed through iterative revisions based on extensive feedback from study investigators (pediatric rheumatologists, two parents of children with JIA, a medical sociologist, and sociology trainees); prior literature on JIA,(28, 29) RA,(30) and patients’ perspectives on taking medicines (31); and input from social and behavioral scientists, patients, and parents not involved with the study. We also used software (Health Literacy Advisor, Bethesda, MD) to ensure that script was suitable for general understanding. Interviews were digitally recorded, professionally transcribed (ADA Transcription, Westampton, NJ), and de-identified for analysis.
Analysis
We performed qualitative coding of transcripts using Dedoose, a cloud-based platform for storing and analyzing textual data with qualitative and mixed-methods approaches.(32) A hierarchical list of codes was developed based on the study questions using the Common Sense Model of Self-Regulation (CSM).(33) The CSM focuses on patients’ understanding of their illness to assess how perceptions of illness can shape actions. Understanding of illness includes the name and symptoms of a disease, its expected duration, perceived cause(s), the ways that disease can be controlled or cured, and its impact and consequences on health and lifestyle. The CSM is used to understand how experience-based beliefs affect treatment adherence and outcomes (34, 35).
Two research staff including the interviewer coded each transcript (Supplemental Methods). Investigators (DBH, MR) met with research staff monthly to review recently coded transcripts, resolve questions or discrepancies in coding and interpretation, discuss emerging themes, and determine when thematic saturation was reached. Upon completion of transcript coding and review, the team developed a conceptual model, matching codes to themes and identifying illustrative quotes for each theme. Differences and similarities were identified between responses from caregivers, adult patients, and adolescent patients.
Additionally, we assessed the level of concern or fear that participants expressed regarding future effects of JIA and medicines, respectively. We compared characteristics of subjects who did and did not express high levels of concern/fear by Fisher’s exact testing (Supplemental Methods).
RESULTS
We interviewed a varied group of 20 patients (50% under age 18) and 24 caregivers (all mothers, 50% of children ≤10 years old) (Table 1). Participants came from different demographic and educational backgrounds and different US regions. Participants or their children had different JIA categories, most commonly polyarticular. Most participants reported past or ongoing use of conventional (84%) or biologic (84%) DMARDs. Most reported having inactive disease (77%), either in the past (32%) or currently (45%). About half of participants (47%) had stopped all medicines for inactive disease, 20% of whom remained off medication (Table 1).
Balance of Competing Risks, Fears, and Adverse Experiences from JIA and from JIA Medicines
Decision-making about decreasing or stopping medicines generally hinged on a perceived balance between risks and fears from the disease and corresponding risks and fears from the medicines (Figure, Table 2). Participants reported numerous concerns about JIA, including flares and symptoms (e.g., pain, fatigue, vision loss, fevers); long-term damage of joints, eyes, or other organs; limited participation in activities, work, or school; loss of functional capacity through JIA-related joint or eye damage; and various psychosocial consequences, including mood disorders (e.g., depression, anxiety), behavioral problems, social isolation, bullying, and family disruption. Reported concerns about medicines closely paralleled concerns about JIA: toxicities and side effects (e.g., pain, fatigue, nausea, brain “fog”) analogous to JIA symptoms; long-term risks to health (e.g., cancer, immunosuppression) analogous to JIA-related damage; interference with school, work, and activities analogous to JIA-related functional and activity limitations; and various corresponding psychosocial consequences (e.g., anxiety around injections). Participants also reported concerns about the cost of JIA medicines and access to treatment.
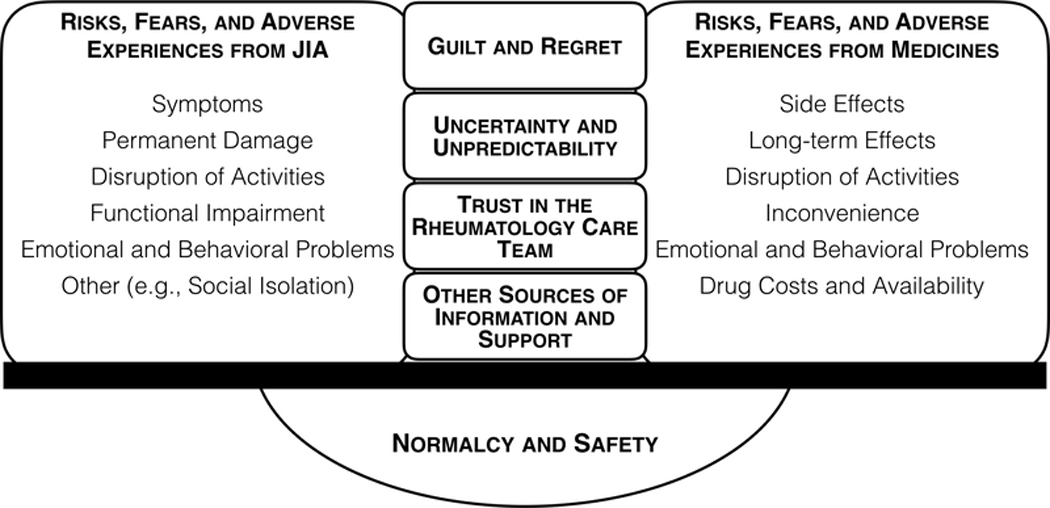
Figure.
Conceptual diagram of factors influencing decisions to stop or continue medicines for well-controlled JIA
JIA juvenile idiopathic arthritis.
This diagram illustrates the balancing that takes place between competing sets of risks, fears, and adverse experiences from JIA and from medicines, when patients and caregivers are deciding whether to stop medicines for well-controlled JIA. Each set contains analogous considerations. This balance is influenced by feelings of guilt and regret, by the uncertainty and unpredictability of future disease or treatment effects, and is informed by trust in the rheumatology care team, as well as other sources of information and support. Patients’ and caregivers’ decisions about stopping JIA treatment are often aimed at achieving or preserving a sense of normalcy and safety.
Table 2.
Representative quotes related to decisions around stopping JIA medicines: risks, fears, adverse experiences, and trade-offs
| Concept | Participant | Quote |
|---|---|---|
| Risks, fears, and adverse experiences from JIA | patient with JIA, age 38, F | “I’m in pain from the moment I open my eyes till I fall asleep at night, until whenever the flare goes away. And sometimes it’s not horrific pain. And sometimes it’s just being uncomfortable, but uncomfortable over a lot of hours wears on you.” |
| patient with JIA, age 13, F | “Well, I can’t do PE most of the time. I can’t do a lot of things with other girls in PE. But I also sometimes – my sister has to help me out – just get up and get my dinner kinda thing because I can’t get off the couch because my legs hurt or open my water bottles and cans – things like that.” | |
| mother of child with JIA (age 4, F) | “… especially when she’s in more pain than usual, she just doesn’t deal well with even … little disappointments. She’ll just go into a full-fledged tantrum – crying, screaming, throwing herself on the floor. Where the normal child would be upset or disappointed, but she just takes it to a whole new level.” | |
| Risks, fears, and adverse experiences from medicines | patient with JIA, age 22, F | “I was on [medicine] a month ago, and [it] was giving me stomach ulcers. And I was so desperate because I was in so much pain that I was taking [it] anyway… but it was giving me so many side effects that I was almost like, ‘maybe I should just deal with the pain and stop taking the NSAID because I’m so miserable. I have the worst heartburn of my life. I can’t deal with this every day.’” |
| mother of child with JIA (age 3, F) | “… it was not good for her because it could stunt her growth and damage her organs. And that’s what I was scared about. And now once we had her at the doctor’s appointment, we found out the [medicine] was stunting her growth, so we had to stop that.” | |
| patient with JIA, age 19, F | “I worry about with all the medications I’m taking affecting my fertility… now going on 20 … I worry about if my medications will affect my chances of that. How will it affect me being a mom?” | |
| patient with JIA, age 13, F | “I think about that a lot. Especially when I’m like watching TV and some commercials come on for the medicine that I take and the side effects. I get really worried and scared because there’s some severe side effects … my mom has been putting it off for giving it to me for years because it’s hard core. It’s very – I don’t know the word. Risky I guess.” | |
| patient with JIA, age 13, M | “Usually the day I take the medicine, I can’t – I mean I go to school but I don’t really function that much because I don’t feel that great and usually if I take my medicine and I’m feeling nauseated that day, I’ll get checked out after my fourth period because my core classes are first, second, third, and fourth so.” | |
| Trade-off between competing risks | patient with JIA, age 13, F | “I think that if it’s so painful or there’s just a lot of side effects, is it really worth it? If it’s getting my arthritis better, but it’s putting me through so much pain, then it’s not really taking away the pain. So then I don’t see any point on going on that specific medicine.” |
| mother of child with JIA (age 14, F) | “The thing is always in the back of your mind. How long can she do it before the side effects in the future – it’s – you’re playing with fire and with fate. You just don’t know how it’s going to affect [your child] in the future and what’s the magic number? When do you stop so that you don’t have a reaction in the future? So that’s why we stopped, because there was so much uncertainty. And do the risks outweigh the benefits?” | |
| mother of child with JIA (age 10, F) | “I mean, there’s always worries about all of the horrible side effects with all of the drugs that are used to treat it. So I mean, there’s always those worries, like those things. But I always try to put them in the back of my head because it’s way worse to leave her arthritis untreated than it is to worry about side effects that may or not happen when we know what is currently happening.” | |
| mother of child with JIA (age 4, F) | “So it’s kind of – again, benefit versus risk. Right now, the benefit of the medications currently is going to be protecting her future as far as keeping her from being permanently disabled versus well, okay, there might be a risk of something that we don’t know in the future. I’m not gonna let a possibility of some unknown thing happening prevent me from protecting her now.” |
Participants with past or ongoing adverse experiences from JIA or JIA medicines were more likely to express salient concerns and fears related to those experiences (Table 3). Adolescents, adults with JIA, and caregivers reflected on such adverse experiences to a similar degree. Nonetheless, many concerns focused on possible future complications that might result from decisions to either continue or stop medicines. These fears of future complications sometimes, but not always, reflected past experiences and were more commonly reported by adults with JIA or caregivers than by adolescents.
Table 3.
Relationship between participant characteristics and high reported levels of fear of JIA or medicines
| High level of concern or fear of JIA1 |
High level of concern or fear of medicines2 |
|||
|---|---|---|---|---|
| Characteristic | N (%) | P-value3 | N (%) | P-value3 |
| All participants | 20 (45%) | - | 9 (20%) | - |
| Demographics and geography | ||||
| Group | 0.65 | 0.46 | ||
| Patient, ages 13–17 years old | 4 (40%) | 3 (30%) | ||
| Patient, ages ≥18 (range 18–38) | 3 (30%) | 1 (10%) | ||
| Mother, child 10 and younger | 6 (55%) | 1 (9%) | ||
| Mother, child older than 10 | 7 (54%) | 4 (31%) | ||
| Patient gender | 0.48 | 0.17 | ||
| Female | 17 (49%) | 9 (26%) | ||
| Male | 3 (33%) | 0 | ||
| Race/ethnicity | 0.62 | 0.99 | ||
| White, non-Hispanic/Latino | 12 (43%) | 6 (21%) | ||
| Hispanic/Latino | 4 (40%) | 2 (20%) | ||
| Other | 4 (67%) | 1 (17%) | ||
| Public insurance | 0.002 | 0.02 | ||
| Private | 11 (32%) | 4 (12%) | ||
| Public | 9 (90%) | 5 (50%) | ||
| Maximum level of parental education (any parent or guardian) | 0.76 | 0.70 | ||
| No Bachelor’s degree | 8 (50%) | 4 (25%) | ||
| Bachelor’s degree | 12 (43%) | 5 (18%) | ||
| Region of US | 0.24 | 0.05 | ||
| Midwest | 5 (56%) | 4 (44%) | ||
| Northeast | 1 (13%) | 0 | ||
| South | 10 (53%) | 5 (26%) | ||
| West | 4 (50%) | 0 | ||
| Disease and drug experience | ||||
| JIA category | 0.58 | 0.84 | ||
| Oligoarticular JIA | 3 (27%) | 3 (27%) | ||
| Polyarticular JIA | 9 (50%) | 4 (22%) | ||
| Spondyloarthritis | 4 (44%) | 2 (22%) | ||
| Systemic JIA | 3 (60%) | 0 | ||
| Other | 1 (100%) | 0 | ||
| Years since JIA diagnosis | 0.79 | 0.31 | ||
| <4 | 4 (40%) | 2 (20%) | ||
| 4–8 | 8 (42%) | 2 (11%) | ||
| >8 | 8 (53%) | 5 (33%) | ||
| Uveitis | 0.99 | 0.36 | ||
| No | 16 (46%) | 6 (17%) | ||
| Yes | 4 (44%) | 3 (33%) | ||
| Methotrexate use | 0.66 | 0.47 | ||
| None | 2 (29%) | 0 | ||
| Prior | 8 (47%) | 4 (24%) | ||
| Current | 10 (50%) | 4 (25%) | ||
| Biologic use | 0.70 | 0.50 | ||
| None | 2 (29%) | 1 (14%) | ||
| Prior | 2 (40%) | 2 (40%) | ||
| Current | 16 (50%) | 6 (19%) | ||
| History of inactive JIA, drug discontinuation | 0.37 | 0.33 | ||
| Never inactive | 5 (50%) | 1 (10%) | ||
| Never stopped, inactive before | 2 (100%) | 0 | ||
| Never stopped, inactive now | 5 (45%) | 1 (9%) | ||
| Stopped, now active | 6 (50%) | 5 (42%) | ||
| Stopped, now inactive | 2 (22%) | 2 (22%) | ||
| Complications from JIA4 | 0.002 | 0.72 | ||
| No | 3 (16%) | 3 (17%) | ||
| Yes | 17 (65%) | 6 (23%) | ||
| Complications from medicines5 | 0.53 | 0.04 | ||
| No | 12 (41%) | 3 (10%) | ||
| Yes | 8 (53%) | 6 (40%) | ||
Quantitated in response to the question, “Are there any particular fears or concerns about the disease that are especially important in this decision [whether to stop medicines]?”
Quantitated in response to the question, “Are there any particular fears or concerns about the treatment that are especially important in this decision [whether to stop medicines]?”
P-value calculated from Fisher’s exact tests
Reported history of systemic JIA with macrophage activation syndrome; permanent damage to the joints, eyes, or other organs; severe flare; physical disability; or severe comorbidities
Reported history of severe side effects or other harms from medicines or aversion to medicines
Several individuals articulated explicit trade-offs between these competing sets of concerns, reflecting on the tension between the risks of continuing unnecessary treatment that relate to future complications (e.g., cancer) and the risks of premature treatment discontinuation that lead to future disease complications (e.g., pain, disability, damage, adverse impacts on school or work). Decisions about withdrawing treatment often related to whether individuals perceived that the disease or medicines represent the greater risk and threat to health. Those who worried more about what JIA had done or could do in the future expressed more motivation to continue treatment. On the other hand, participants who viewed medicines as noxious, toxic, disruptive, or otherwise dangerous to present or future health expressed more motivation to stop medicines sooner. Individuals with stronger beliefs about one set of risks being greater than the other expressed stronger preferences about either treating for a long time or stopping treatment quickly. For others whose views about the trade-off were more balanced or uncertain, decisions about withdrawing medicines seemed to be more fluid and dependent on context and external input, such as from rheumatologists, family, friends, and others affected by JIA (see sections below on “Trust in the Rheumatology Care Team” and “Other Sources of Information and Support”).
Among other demographic, disease-related, and treatment-related factors examined, publicly insured participants were more likely than privately insured individuals to report high levels of fear of JIA and of medicines (Table 3). Other numeric differences in reported fears were observed across other domains (e.g., gender, region, JIA type) that, nonetheless, did not achieve traditional statistical significance (Table 3).
Normalcy and Safety
In reflecting positively on the competing concerns about JIA and medicines, participants discussed striving to achieve and maintain good health and wellness, free from ill effects (Table 4). Participants reflected on the importance of feeling normal and safe—for example, to grow and develop like other children, to participate fully and without limitations in school, work, or sports, and to be considered equal to and not different from siblings or peers. For some participants, medicines were the means for maintaining freedom from adversity and living more normal lives, by controlling flares and preventing future disabilities and/or complications from the disease. These participants perceived stopping medicine as a threat to that sense of normalcy and safety. Other participants who felt that they or their children were particularly threatened or endangered by medicines, including the possibility of developing severe treatment-related harms or disruption of daily activities because of side effects, viewed stopping medicines as necessary means to achieving more normal and safe lives.
Table 4.
Representative quotes related to decisions around stopping JIA medicines: safety and normalcy, guilt and regret, uncertainty and unpredictability
| Concept | Participant | Quote |
|---|---|---|
| Safety and normalcy | mother of child with JIA (age 9, F) | “… she’s a child and you want them to be happy and you want them to play and you want them to run and you want them to be as normal as – live as normal of a life as they can while they’re still living an abnormal life because it’s not normal to get poked two or three times a day. But it’s just that’s what’s most important to me is to keep her healthy and keep her active.” |
| patient with JIA, age 16, F | “I feel that stopping medication obviously is going to make you feel more quote, unquote normal than you were before with all the medication.” | |
| mother of child with JIA (age 3, F) | “… That’s for me to stop medicine for my child is just have the naturals in her body without the chemicals – medicines that’s infecting her body where it’s gonna – who knows if it’s gonna affect her in the long run. It may help her now, but I don’t know.” | |
| patient with JIA, age 14, F | “I don’t want anything else to be put at risk of getting worse or happening. I feel a lot more safe on the medicine. I know I’m okay right now, and that’s good to know.” | |
| mother of child with JIA (age 15, F) | “if you read one of those god-awful 8,000-page packets that come with your prescription about the potential side effects, that’s enough to scare anybody out of that… I don’t mind as long as it’s gonna work, but none of this stuff said yes, we can cure her. It was just a band-aid. And I wasn’t willing to gamble those side effects for a Band-Aid… | |
| Guilt and regret | mother of child with JIA (age 12, F) | “So the worst part for her was the pain, and for us it was the pain and the feeling we couldn’t – for the first time in our lives as parents, we couldn’t fix what we were supposed to fix for her.” |
| mother of child with JIA (age 7, F) | “She didn’t know how she would feel without the medications. She didn’t know how life would be different without the medications. So by stopping them and restarting them, that’s when we saw the biggest changes in her behaviors and attitudes … And she did have some damage to her eyesight because of the recurrence and the uveitis. So perhaps that may not have occurred. It’s a lot of what-ifs because you can’t turn back the clock.” | |
| patient with JIA, age 38, F | “I mentioned something before about believing that there was a genetic factor. And I wish I would have known that sooner … And then as an adult, it was at my daughter’s rheumatology appointment that the suggestion made that I have genetic testing done for myself. And I wish that would have happened differently.” | |
| mother of child with JIA (age 10, F) | “I hate it. Every time – for the first year, every time I’d give her her shot I would feel like I was gonna be physically ill after. But I always have to rationalize in my brain that this is way better than her having damage from untreated arthritis …” | |
| Uncertainty and unpredictability | patient with JIA, age 14, F | “I think I feel more comfortable knowing what [the disease is] doing. I feel like if I were in remission, it would make me a little nervous because I wouldn’t know when it would come up again. I’d like to know what’s going on with it. I don’t like things being up to chance.” |
| mother of child with JIA (age 12, F) | “And so it was – it was terrifying because you’d have no control. At first we didn’t know what it was. Then we knew it was arthritis, but it was uncontrolled with the [medicine]. And so you don’t know is your kid going to be disabled for life? Are you ever going to be able to get them out of pain?” | |
| patient with JIA, age 28, F | “I know that there’s a lot of research out there about how varied it is between physicians and their opinions. As a patient, I am leery of stopping medications because I know how quickly antibodies can build up and make the medication stop working. So you wouldn’t necessarily be able to go on the same medication again when you restart if you needed to.” | |
| mother of child with JIA (age 9, F) | “it can be scary too because you don’t know what the long-term effects could be from these medications. So they’re helping them right now but in the years to come is this gonna cause other issues … Because these are some pretty high-powered medications these kids take.” | |
| patient with JIA, age 14, F | “I’m a little worried because this is a newer medicine, and not many people have weaned off of it. So I’m a little worried because they don’t really know anything. I kind of feel like a guinea pig, so that’s a little strange.” | |
| patient with JIA, age 24, F | “Then I think also there’s the medical piece to it which is not knowing or being a little concerned about what it may mean to take immune-suppressants in the long term. And so I think the sooner I can get off of it, the less I have to be concerned about that.” |
Guilt and Regret
Some participants expressed feelings of guilt and regret about their perceived involvement in or responsibility for past events (Table 4). These prior events included the diagnosis of JIA (a common source of guilt among parents), severe flares occurring after prior treatment discontinuation, and severe reactions to medicines. This sense of guilt and regret was projected forward in anticipation of future complications from the disease and/or medicines related to perceived actions (e.g., giving or stopping medicines) or inactions (e.g., not stopping or not giving medicines). A heightened sense of guilt or regret over what the disease had done or could do if poorly controlled in the future (e.g., damage to joints or eyes) motivated participants to continue treatments. In contrast, guilt or regret over experiencing harms from treatment led to preferences to stop treating sooner. For example, caregivers were concerned with allergic reactions or side effects that severely limited patients’ ability to achieve “normalcy.” Caregivers were generally more likely to express guilt and regret for past or future actions/inactions than patients, although the attribution of these feelings to JIA or medicines did not differ between caregivers and patients.
Uncertainty and Unpredictability
Participants discussed coping with the uncertainty and unpredictability of having JIA and treating it with potentially harmful medicines, using words such as “terrifying,” “scary,” and “worried” (Table 4). Uncertainty was expressed as not knowing how likely events were to occur, such as eye damage or cancer. Unpredictability was expressed as not knowing whether or when events might occur, such as flares. Some individuals felt more concern about the potential for harm from JIA, including harm that was not readily detected (e.g., clinically silent damage). Several participants were concerned about whether the same medicine that controlled their or their child’s disease would still work if restarted after flares. Some participants expressed distress from uncertainty and unpredictability about the disease when discussing preferences to continue treatment. Others expressed more concerns about the possibility of future adverse effects from medicines, however likely or unlikely those outcomes were; these individuals preferred stopping treatment sooner. The sense of uncertainty or unpredictability sometimes was linked by participants to doctors’ inability to give detailed or accurate assessments of risks or to predict how a patient might respond to a particular treatment.
Trust in the Rheumatology Care Team
Participants commonly reflected on the importance of trusting the rheumatologists and staff when making decisions about whether to stop medicines (Table 5). This trust encompassed a sense of interacting well with informed, knowledgeable clinicians who listened, cared, and included patients and caregivers in the decision-making process. Some participants expressed high levels of trust in rheumatologists, which often corresponded to support for following the rheumatologists’ recommendations about continuing or stopping medicine. In contrast, other individuals recounted experiences that led them to lose trust in the rheumatologist, such as feeling unheard, disregarded, or judged for their health beliefs. These feelings motivated some to find new clinicians and others to avoid returning to rheumatology clinic for treatment, managing symptoms on their own or in some cases turning to alternate care providers. Lower levels of trust in rheumatologists were associated with expressing greater fears about medicines and less willingness to continue them. Some participants expressed frustration about variations in medical care and about differences in doctors’ professional opinions and treatment recommendations. Levels of trust in rheumatologists did not appreciably differ between patients and caregivers.
Table 5.
Representative quotes related to decisions around stopping JIA medicines: trust in rheumatology team, other sources of information and support
| Trust in the rheumatology care team | patient with JIA, age 19, F | “I tell my rheumatologist everything. We’re very close. I’m very blessed to have that kind of relationship with my doctor, like I said. She’s known me since before I could even remember – at 14 months old when she first met me. And while I was seeing a different rheumatologist at the beginning, she’s always been there … And actually, most of the time, if I have any sort of medical issue, I go to them instead of my primary care doctor because I trust them more than my PCP because they know me better.” |
| patient with JIA, age 13, F | “I think I get an input exactly. Like they would like to ask me, how does this make you feel, and would you be okay with this or would this make you uncomfortable. I don’t get to decide completely. I don’t get to say like ‘okay. We’re going to start this,’ because I am only 13 and I only know so much. Yeah, my doctor is very – he’s very – he cares a lot about what I say and how I feel about it.” | |
| Mother of child with JIA (age 10, F) | “… I trust my rheumatologist to make the best decision. So I would say it’s probably an 80/20 type scenario. I leave 80 percent of it up to him and then I just ask questions to make sure that we’re making the best decision … I trust him and I would leave the decision up to him, even though sometimes I have doubts and I ask lots of questions, but he’s managed her this far. | |
| patient with JIA, age 15, F | “My last rheumatologist, he wanted to do all those infusions and all those shots and every single day get an infusion. That was a lot. But I don’t think that was a great idea … when we started the different diet and that helped me a lot and he wasn’t about that. He wasn’t about diet because he wanted to do all the [medications] and stuff. But we didn’t wanna do that …” | |
| mother of child with JIA (age 8, M) | “… well, listening to my doctor who I trust which is strange because I don’t trust most doctors. We’ve had a lot of doctors in our family with the different things, and half of them contradict each other. Or they say one thing and another doctor says another. So I don’t trust them as a whole. But I really like this doctor and he knows his research, and so I would trust him.” | |
| mother of child with JIA (age 4, F) | “But we’re lucky to have a really wonderful rheumatologist who will take the time to sit and explain and give me options and allow me to say, well, okay, this is what I want for her or let’s try something different. So we have a really good team...I feel like I’m a member of that team decision, not just a parent being told, okay, well, this is what we’re doing for your kid and I don’t have a say.” | |
| Other sources of information and support | patient with JIA, age 16, F | “I have friends that I met at the arthritis convention. And I usually talk to them about it because I just don’t think other people understand … And we listen to each other and how each of us are doing. And we talk about medicines and stuff. And we talk about other things too, but like mostly – that’s what we talk about with our arthritis.” |
| patient with JIA, age 28, F | “I am connected with a lot of other people who have the same or similar diseases. And so I try whenever I am on the fence about a medical decision or just trying to kind of toss around some ideas to myself, I do try to reach out to some of them and see what their thoughts are. Because a lot of them have been on medications I haven’t been on yet or have been through kind of this whole process of stopping meds and then waiting and then having to come back on and those kinds of things. So I really value the opinions of my friends who have been in the same or similar positions as well.” | |
| mother of child with JIA (age 14, M) | “There’s a good Facebook group, and so it’s always interesting to see what other people with similar situations, what advice they’re getting from their physicians. And so it’s information that I read and I digest and it might help prompt questions I need to be asking.” | |
| patient with JIA, age 22, F | “So for me, a very important part of making decisions about medication is being informed about it. And so I personally read a lot of studies of things that have been published. I don’t think it’s good to just Google something and read anything on the Internet. I want to read something that’s like gone through a peer review process. So something that’s on Google Scholar, or PubMed or something like that. I think it’s important to know things like that to see what response rates are, what different types of JIA have been seen to respond better to different types of medications, what side effects there are.” |
Other Sources of Information and Support
When making decisions about stopping, patients and caregivers turned to various sources of information and support besides the treating rheumatologists, including family members, friends, websites, social media, others with arthritis, and the medical literature (Table 5). Participants reported feeling empowered and in more control by having access to more knowledge and understanding about JIA and medicines. Many reflected on the importance of camaraderie, community, and having support from others who have gone through similar experiences and could offer both advice and validation. Online groups were important sources of community for young patients with JIA. Several individuals also commented on the diversity of information and opinions online and the need to remain skeptical. Many reported a preference for reading peer-reviewed articles or reports by foundations or non-profits. Some acknowledged the diversity of individual experiences, and that what worked for some people (e.g., stopping treatment) might not work for others in similar situations.
DISCUSSION
This paper represents the first mixed-methods study focused on the perspectives of patients and caregivers on stopping JIA medicines. Patients and caregivers described their decisions around stopping treatments based on a key trade-off: risks and fears of the disease itself compared to risks and fears of medicines for the disease. For many participants, this requires weighing parallel risks: symptoms versus side effects; disease-related damage versus long-term treatment-related toxicities; limitations in participation and function from disease sequelae versus disruptions in life and activities from treatments; and the emotional toll of having JIA or experiencing treatment-related harms. These judgments are based on both prior personal experiences and concerns about future consequences of treatment-related decisions. Other factors that appeared important in balancing these competing risks included: optimizing a sense of normalcy and safety (having well-controlled JIA versus not taking medicines); distress from uncertainty and unpredictability of JIA-related or treatment-related complications; feelings of guilt and regret about taking or not taking action; levels of trust in physicians; and reliance on other sources of information and support, including family, friends, and various online venues.
Stopping antirheumatic medicines appropriately is important, given their toxicities, inconvenience, and costs to society.(36) However, stopping treatment also poses risks of subsequent disease flares, which may or may not respond to the same regimens that once kept the disease well-controlled. Decisions about when to stop effective medicines are particularly challenging given the lack of high-quality evidence on individual risks of flare after treatment withdrawal, reliable strategies for withdrawing treatment, and the likelihood of regaining disease control after treatment re-initiation (recapture).(20) Clinicians may have different priorities (e.g., maintaining remission) from patients and families (e.g., achieving a sense of normalcy, eliminating ongoing side effects or costs, or avoiding long-term treatment-related harms). We hypothesized that patients and caregivers would worry differently about long-term vs. short-term risks. Instead, we found a divide between age lines, with adolescents tending to consider short-term risks to well-being, as previously reported,(45, 46) while adults with JIA, like caregivers, were often more future-oriented. We did not observe any significant age-related differences in perceived levels of fear towards JIA or medicines more broadly. Notably, publicly insured participants were more likely to express high levels of fear about both JIA and JIA medicines compared to privately insured participants, another unexpected finding that bears confirmation in future research. We did not find parallel differences based on level of parental education.
An existing body of research has described the attitudes and behaviors of patients who take or choose not to take medication for conditions other than JIA.(31) Many studies, including an older study on use of nonsteroidal medications for RA, have focused on adherence and reasons why people stop medications despite ongoing disease activity (e.g., for lack of efficacy).(31, 37) In pediatric rheumatology, more qualitative research has focused on decisions about starting disease-modifying antirheumatic drugs including studies on the perspectives of patients and families.(21, 23) Prior research about stopping JIA medications has focused on clinicians’ perspectives through interviews and surveys.(19, 21) Research examining the perspectives of adults with RA on stopping antirheumatic medicines has also highlighted similar tensions between risks of arthritis and risks of treatments, the role of uncertainty and unpredictability, the importance of achieving normalcy, and impacts of prior disease experiences.(24, 25) However, these studies have not touched on other themes identified in our study, including the perspectives of family members, impacts of disease and treatment on family dynamics, the financial costs of treatment, the influence of guilt and regret on decision-making, and other issues specific to pediatrics, including growth, development, and bullying. Research examining decision-making around treatment withdrawal for other pediatric diseases has mainly focused on neuropsychiatric conditions, including ADHD and epilepsy, citing adverse side effects and decreased efficacy as main factors for withdrawal.(26, 27, 38, 39) None of these studies have elicited or compared the perspectives of both patients and caregivers. Furthermore, clinical differences distinguish JIA medicines from those for neuropsychiatric diseases, including concerns and uncertainties about long-term drug toxicities (e.g., malignancy) as well as unpredictability of treatment response to future disease flares. Additionally, no prior study in RA or other pediatric diseases has quantified the impact of participants’ characteristics or experiences on reported fears of disease and treatment.
Previous studies have explored topics of general importance in the experiences of children with JIA and their families, including the importance of understanding medications and their potential toxicities and their impact on everyday life.(28, 29) Other research has also discussed issues mentioned by participants in this study, including impacts of JIA on physical and psychosocial function, disruption of activities, “striving for normality,” social isolation, and uncertainty.(40, 41) Other studies have also demonstrated the importance of different sources for information and support, including rheumatologists, support groups and trusted Web-sources, all of which have a role in decision-making more broadly for JIA.(42, 43) This study helps illustrate the complex interplay of these and other themes in the decision-making process that patients and families commonly confront when managing JIA. In particular, the roles of guilt and regret have not been well described in research on decision-making in JIA. Among participants in our study, these feelings appeared more prominent among caregivers than patients. Guilt and regret have been reported in other pediatric research on health care decisions, including decision-making around end-of-life care for children and adolescents.(47, 48)
Our conceptual model of competing risks and fears raised by our participants, along with their goals of achieving normalcy and safety, echoes the traditional balance of benefits and risks that applies to medical decision-making. Many participants we interviewed were risk-averse and stated preferences to avoid negative consequences through their decisions, whether those consequences related to JIA, medicines, or both. This risk aversion was reflected in associations between reported complications from JIA or treatment and higher levels of fear regarding future effects of the disease or medicines, respectively. Not surprisingly, severe consequences such as irreversible joint damage from relapse after treatment withdrawal, or development of cancer, appeared particularly influential in participants’ stated preferences, even if such consequences were rare or of uncertain connection to treatment.(11) These concepts of loss aversion and overweighting of low-probability events have been well-described.(44)
In the face of medical uncertainty and an unpredictable future, trust in the treatment team, having access to trustworthy information, and having agency in the decision-making process were key moderating influences that helped participants make difficult decisions. Patients and parents alike valued relationships with trusted, caring clinicians to help them weigh these competing risks, relying on their clinical experience and knowledge to guide the decision-making process.(49, 50)
Our study had certain limitations. While we purposefully selected and analyzed responses from a diverse group of participants affected by JIA, participants may not have fully represented the greater population of individuals making decisions about stopping JIA medicines. For example, compared to non-participants, participants in our study may have been affected by more severe forms of JIA and may have been more motivated and proactive (e.g., in advocacy, reading primary medical literature). Furthermore, all disease and treatment history was self-reported. Finally, quantitative comparisons were limited by small sample sizes and their exploratory nature, thus warranting replication in larger, more representative samples.
In summary, decisions around the withdrawal of medicines for well-controlled JIA emerge from trade-offs between sets of competing risks and fears from the disease and the treatment, with the goal to attain and preserve a sense of normalcy and safety. Individuals weigh these risks differently based on past experiences, how they cope with uncertainty and unpredictability, and feelings of guilt and regret. Trusting relationships with the rheumatology care team and other sources of information and support inform, enable, and empower people to make these decisions. Future research should better quantify these considerations and priorities for decision-making in larger populations. When discussing the benefits and risks of JIA treatment withdrawal, medical professionals may ask patients and families about what they fear most about the disease and treatment, and whether they would feel more normal and safe by continuing medicines or by stopping them. Clinicians might also ask about what patients and families would regret more in scenarios of future outcomes (e.g., flares, treatment-associated toxicities) and what other information would help with decision-making. Greater awareness of the trade-offs that patients and caregivers face, along with their concerns and priorities, will help improve shared decision-making around JIA treatment withdrawal.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
This is the first study to jointly examine patients’ and caregivers’ perspectives on stopping medicines for well-controlled juvenile idiopathic arthritis (JIA) or any other chronic pediatric disease.
Decisions about stopping JIA medicines involve a trade-off between competing risks and fears of the disease and risks and fears of the medicines; the magnitude of these reported fears is associated with prior complications from the disease and treatments, respectively.
Unlike prior research on decisions to stop medicines for rheumatoid arthritis, the current study highlights the influence of guilt and regret on decision-making, the financial costs of treatment, the perspectives of family members, impacts of disease and treatment on family dynamics, and other pediatric-specific issues.
These considerations can inform shared decision-making with clinicians around stopping medicines for JIA.
Acknowledgments:
The study team thanks all the participants in this study for sharing their time, thoughts, feelings, and experiences. The study team would also like to thank Carolina Mejia, Marcela Riano, and Carlos Rose for help with Spanish translation; Beth Savage for assistance with interviews and coding; Jen Horonjeff for help with recruitment; and Patients, Advocates, and Rheumatology Teams Network for Research and Service (PARTNERS) for help with recruitment and use of readability software. The authors wish to acknowledge CARRA, and the ongoing Arthritis Foundation financial support of CARRA.
Grants: This project was supported by funding from the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (K23-AR070286).
Footnotes
Disclosures: Dr. Horton has received research support from CARRA and Bristol-Myers Squibb. Dr. Beukelman has received honoraria and/or consulting fees from Bristol‐Myers Squibb, Novartis, Sobi, and UCB. Dr. Boneparth has received research support from CARRA. Dr. Mannion has received research support from CARRA. Dr. Ringold has received research support from CARRA.
REFERENCES
- 1.Adam V, St-Pierre Y, Fautrel B, Clarke AE, Duffy CM, Penrod JR. What is the impact of adolescent arthritis and rheumatism? Evidence from a national sample of Canadians. J Rheumatol. 2005;32(2):354–61. [PubMed] [Google Scholar]
- 2.Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Psychosocial outcomes and health status of adults who have had juvenile rheumatoid arthritis. A controlled, population-based study. Arthritis Rheum. 1997;40(12):2235–40. [DOI] [PubMed] [Google Scholar]
- 3.Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(9):2809–18. [DOI] [PubMed] [Google Scholar]
- 4.Bertilsson L, Andersson-Gare B, Fasth A, Petersson IF, Forsblad-D’elia H. Disease course, outcome, and predictors of outcome in a population-based juvenile chronic arthritis cohort followed for 17 years. J Rheumatol. 2013;40(5):715–24. [DOI] [PubMed] [Google Scholar]
- 5.Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;74(10):1854–60. [DOI] [PubMed] [Google Scholar]
- 6.Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62(8):2517–24. [DOI] [PubMed] [Google Scholar]
- 7.Bulatović M, Heijstek M, Verkaaik M, van Dijkhuizen EH, Armbrust W, Hoppenreijs EP, et al. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 2011;63(7):2007–13. [DOI] [PubMed] [Google Scholar]
- 8.Beukelman T, Xie F, Chen L, Baddley JW, Delzell E, Grijalva CG, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(8):2773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beukelman T, Xie F, Baddley JW, Chen L, Delzell E, Grijalva CG, et al. Brief report: incidence of selected opportunistic infections among children with juvenile idiopathic arthritis. Arthritis Rheum. 2013;65(5):1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beukelman T, Xie F, Baddley JW, Chen L, Mannion ML, Saag KG, et al. The risk of hospitalized infection following initiation of biologic agents versus methotrexate in the treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2016;18(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beukelman T, Xie F, Chen L, Horton DB, Lewis JD, Mamtani R, et al. Risk of malignancy associated with paediatric use of tumour necrosis factor inhibitors. Ann Rheum Dis. 2018;77(7):1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minden K, Niewerth M, Listing J, Mobius D, Thon A, Ganser G, et al. The economic burden of juvenile idiopathic arthritis-results from the German paediatric rheumatologic database. Clin Exp Rheumatol. 2009;27(5):863–9. [PubMed] [Google Scholar]
- 13.Ungar WJ, Costa V, Hancock-Howard R, Feldman BM, Laxer RM. Cost-effectiveness of biologics in polyarticular-course juvenile idiopathic arthritis patients unresponsive to disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken). 2011;63(1):111–9. [DOI] [PubMed] [Google Scholar]
- 14.Wallace C, Giannini E, Huang B, Itert L, Ruperto N, Alliance CARR, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 2011;63(7):929–36. [DOI] [PubMed] [Google Scholar]
- 15.Morgan EM, Munro JE, Horonjeff J, Horgan B, Shea B, Feldman BM, et al. Establishing an Updated Core Domain Set for Studies in Juvenile Idiopathic Arthritis: A Report from the OMERACT 2018 JIA Workshop. J Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 16.Knowlton N, Jiang K, Frank M, Aggarwal A, Wallace C, McKee R, et al. The meaning of clinical remission in polyarticular juvenile idiopathic arthritis: gene expression profiling in peripheral blood mononuclear cells identifies distinct disease states. Arthritis Rheum. 2009;60(3):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothmund F, Gerss J, Ruperto N, Dabritz J, Wittkowski H, Frosch M, et al. Validation of relapse risk biomarkers for routine use in patients with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2014;66(6):949–55. [DOI] [PubMed] [Google Scholar]
- 18.Hinze CH, Foell D, Johnson AL, Spalding SJ, Gottlieb BS, Morris PW, et al. Serum S100A8/A9 and S100A12 Levels in Children With Polyarticular Forms of Juvenile Idiopathic Arthritis: Relationship to Maintenance of Clinically Inactive Disease During Anti-Tumor Necrosis Factor Therapy and Occurrence of Disease Flare After Discontinuation of Therapy. Arthritis Rheumatol. 2019;71(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton DB, Onel KB, Beukelman T, Ringold S. Attitudes and Approaches for Withdrawing Drugs for Children with Clinically Inactive Nonsystemic JIA: A Survey of the Childhood Arthritis and Rheumatology Research Alliance. J Rheumatol. 2017;44(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halyabar O, Mehta J, Ringold S, Rumsey DG, Horton DB. Treatment withdrawal following remission in juvenile idiopathic arthritis: a systematic review of the literature. Paediatr Drugs. 2019;21(6):469–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipstein EA, Brinkman WB, Sage J, Lannon CM, Morgan Dewitt E. Understanding treatment decision making in juvenile idiopathic arthritis: a qualitative assessment. Pediatr Rheumatol Online J. 2013;11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Ramirez O, Gibbon M, Berard R, Jurencak R, Green J, Tucker L, et al. A recurring rollercoaster ride: a qualitative study of the emotional experiences of parents of children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipstein EA, Muething KA, Dodds CM, Britto MT. “I’m the one taking it”: adolescent participation in chronic disease treatment decisions. J Adolesc Health. 2013;53(2):253–9. [DOI] [PubMed] [Google Scholar]
- 24.Markusse IM, Akdemir G, Huizinga TW, Allaart CF. Drug-free holiday in patients with rheumatoid arthritis: a qualitative study to explore patients’ opinion. Clin Rheumatol. 2014;33(8):1155–9. [DOI] [PubMed] [Google Scholar]
- 25.Baker KF, Isaacs JD, Thompson B. “Living a normal life”: a qualitative study of patients’ views of medication withdrawal in rheumatoid arthritis. BMC Rheumatol. 2019;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong IC, Asherson P, Bilbow A, Clifford S, Coghill D, DeSoysa R, et al. Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY)--a pharmacoepidemiological and qualitative study. Health Technol Assess. 2009;13(50):iii-iv, ix-xi, 1–120. [DOI] [PubMed] [Google Scholar]
- 27.Chiu YP, Lee TY, Lin KL, Laadt VL. Adjusting to a seizure-free “new normal” life following discontinuation of antiepileptic drugs during adolescence. Epilepsy Behav. 2014;33:54–8. [DOI] [PubMed] [Google Scholar]
- 28.Tong A, Jones J, Craig JC, Singh-Grewal D. Children’s experiences of living with juvenile idiopathic arthritis: a thematic synthesis of qualitative studies. Arthritis care & research. 2012;64(9):1392–404. [DOI] [PubMed] [Google Scholar]
- 29.Guzman J, Gomez-Ramirez O, Jurencak R, Shiff NJ, Berard RA, Duffy CM, et al. What matters most for patients, parents, and clinicians in the course of juvenile idiopathic arthritis? A qualitative study. J Rheumatol. 2014;41(11):2260–9. [DOI] [PubMed] [Google Scholar]
- 30.van Tuyl LH, Hewlett S, Sadlonova M, Davis B, Flurey C, Hoogland W, et al. The patient perspective on remission in rheumatoid arthritis: ‘You’ve got limits, but you’re back to being you again’. Ann Rheum Dis. 2015;74(6):1004–10. [DOI] [PubMed] [Google Scholar]
- 31.Pound P, Britten N, Morgan M, Yardley L, Pope C, Daker-White G, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med. 2005;61(1):133–55. [DOI] [PubMed] [Google Scholar]
- 32.Dedoose Version 8.0.35, web application for managing, analyzing, and presenting qualitative and mixed method research data [Internet]. SocioCultural Research Consultants, LLC. 2018. Available from: dedoose.com. [Google Scholar]
- 33.Cameron LD, Leventhal H. The self-regulation of health and illness behaviour: Psychology Press; 2003. [Google Scholar]
- 34.Toupin April K, Higgins J, Ehrmann Feldman D. Application of Rasch analysis to the parent adherence report questionnaire in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.April K, Feldman D, Zunzunegui M, Duffy C. Association between perceived treatment adherence and health-related quality of life in children with juvenile idiopathic arthritis: perspectives of both parents and children. Patient preference and adherence. 2008;2:121–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Gidman W, Meacock R, Symmons D. The humanistic and economic burden of juvenile idiopathic arthritis in the era of biologic medication. Curr Rheumatol Rep. 2015;17(5):31. [DOI] [PubMed] [Google Scholar]
- 37.Donovan JL, Blake DR. Patient non-compliance: deviance or reasoned decision-making? Soc Sci Med. 1992;34(5):507–13. [DOI] [PubMed] [Google Scholar]
- 38.Brinkman WB, Sherman SN, Zmitrovich AR, Visscher MO, Crosby LE, Phelan KJ, et al. Parental angst making and revisiting decisions about treatment of attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(2):580–9. [DOI] [PubMed] [Google Scholar]
- 39.Cuenca J, Glazebrook C, Kendall T, Hedderly T, Heyman I, Jackson G, et al. Perceptions of treatment for tics among young people with Tourette syndrome and their parents: a mixed methods study. BMC Psychiatry. 2015;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cartwright T, Fraser E, Edmunds S, Wilkinson N, Jacobs K. Journeys of adjustment: the experiences of adolescents living with juvenile idiopathic arthritis. Child Care Health Dev. 2015;41(5):734–43. [DOI] [PubMed] [Google Scholar]
- 41.Tong A, Jones J, Craig JC, Singh-Grewal D. Children’s experiences of living with juvenile idiopathic arthritis: a thematic synthesis of qualitative studies. Arthritis Care Res (Hoboken). 2012;64(9):1392–404. [DOI] [PubMed] [Google Scholar]
- 42.Condon C, O’Regan D, MacDermott E, Killeen O. Self-management needs of children with JIA in Ireland: a qualitative survey of families. . European Journal of Physiotherapy. 2017;19:237–42. [Google Scholar]
- 43.Cai RA, Beste D, Chaplin H, Varakliotis S, Suffield L, Josephs F, et al. Developing and Evaluating JIApp: Acceptability and Usability of a Smartphone App System to Improve Self-Management in Young People With Juvenile Idiopathic Arthritis. JMIR Mhealth Uhealth. 2017;5(8):e121. [Google Scholar]
- 44.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47(2):263–91. [Google Scholar]
- 45.Grootens-Wiegers P, Hein IM, van den Broek JM, de Vries MC. Medical decision-making in children and adolescents: developmental and neuroscientific aspects. BMC Pediatr. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipstein E, Dodds C, Lovell D, Denson L, Britto M. Making decisions about chronic disease treatment: a comparison of parents and their adolescent children. Health Expectations. 2016;19:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu SM, Lin HR, Lu FL, Lee TY. Taiwanese parents’ experience of making a “do not resuscitate” decision for their child in pediatric intensive care unit. Asian Nurs Res (Korean Soc Nurs Sci). 2014;8(1):29–35. [DOI] [PubMed] [Google Scholar]
- 48.Himelstein B Palliative Care for Infants, Children, Adolescents, and Their Families. Journal of palliative medicine. 2006;9:163–81. [DOI] [PubMed] [Google Scholar]
- 49.Konstantynowicz J, Marcinowicz L, Abramowicz P, Abramowicz M. What Do Children with Chronic Diseases and Their Parents Think About Pediatricians? A Qualitative Interview Study. Maternal and child health journal. 2016;20:1745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anyfantakis D, Symvoulakis E. Medical decision and patient’s preference: ‘much ethics’ and more trust always needed. International journal of medical sciences. 2011;8:351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.