Abstract
Background:
High-fat diet, microbial alterations and lipopolysaccharide (LPS) are thought to cause enteric diabetic neuropathy and intestinal dysmotility. However, the role of the gut microbiota, lipoteichoic acid (LTA) from Gram-positive bacteria and short chain fatty acids (SCFAs) in the development of diabetic enteric neuropathy and intestinal dysmotility is not well understood. Our aim was to examine the role of the gut microbiota, LTA and SCFAs in the development of diabetic enteric neuropathy and intestinal dysmotility.
Methods:
We fed germ free (GF) and conventionally raised (CR) mice either a high-fat (HFD) or standard chow diet (SCD) for 8 weeks. We analyzed the microbial community composition in CR mice using 16S rRNA sequencing and damage to myenteric neurons using immunohistochemistry. We also studied the effects of LPS, LTA, and SCFAs on duodenal muscularis externa contractions and myenteric neurons using cultured preparations.
Key Results:
HFD ingestion reduced the total number and the number of nitrergic myenteric neurons per ganglion in the duodenum of CR but not in GF-HFD mice. GF mice had fewer neurons per ganglion compared to CR mice. CR mice fed a HFD had increased abundance of Gram-positive bacteria. LTA and LPS did not affect the frequency of duodenal muscularis contractions after 24 hours of cultured but reduced the density of nitrergic myenteric neurons, and increased oxidative stress and TNFα production in myenteric ganglia. SCFAs did not affect muscularis contractions or injure myenteric neurons.
Conclusions & Inferences:
Gut microbial alterations induced increase in Gram-positive bacterial LTA may contribute to enteric neuropathy.
Keywords: lipopolysaccharides, oxidative stress, gut bacteria, enteric neurons, neuroinflammation
Graphical Absract
1. INTRODUCTION
Over 70% of diabetic patients suffer from gastrointestinal (GI) motility disorders including dysphagia, gastroparesis, constipation, diarrhea, incontinence and pain.1,2 These diabetic GI motility-related disorders are associated with enteric nervous system (ENS) neuropathy, characterized by loss of nitrergic neurons expressing neuronal nitric oxide synthase (nNOS) and vasoactive intestinal peptide neurons (VIP) in the myenteric plexus.1 Enteric neuropathy in diabetes is often linked to alterations of the gut microbiota.3,4 Lipopolysaccharide (LPS), the cell wall component of Gram-negative bacteria is thought to be the microbial product that causes ENS neuropathy.4 However, it was recently shown that LPS and dietary palmitate act synergistically to damage ENS neurons mainly through oxidative stress, 3 and previous studies suggest that damage to enteric neurons occurs through AMPK activated protein kinase.5 These observations suggest that interaction between diet, gut microbes and the host have a crucial role in the pathophysiology of GI diabetic neuropathy and motility disorders.3,6 Nonetheless, the role of an intact gut microbiota itself in the development of enteric neuropathy is not well understood.
The composition of gut microbiota is mainly modulated by diet.7 High fat diet (HFD) consumption is associated with alterations of the gut microbiota in mice, 8–10 lower concentrations of SCFAs,11,12 type two diabetes (T2D) symptoms,10,13 enteric neuropathy,13 and intestinal dysmotility.3,4,6 All these conditions are commonly observed in diabetic patients.14 Microbiota alterations in T2D humans and animal models of T2D can be characterized by significant changes in the abundance ratios of Firmicutes, a phylum comprised mostly of Gram-positive bacteria, and in Bacteroidetes, comprising of Gram-negative bacteria.10,15,16 A previous study found the increased presence of Gram-positive gut bacteria in the blood of T2D patients,17 which combined with increased gut permeability18,19 and changes in the abundance of gram positive bacteria in the gut of diabetic subjects likely increases the intestinal and circulating levels of LPS20 and Gram-positive bacterial cell wall component, lipoteichoic acid (LTA). It has been shown that LTA binds to TLR-2 receptors,21 and induces neuronal damage in cultured rat cerebellum cells through glia activated oxidative- stress.22,23 LTA also enhances IL-6 expression in various cells,24 and could have a role in increasing IL-6 levels observed in mice fed HFD10 and T2D patients.18 Apart from LPS and LTA, gut microbes also influence host health by producing short chain fatty acids (SCFAs) derived from fermenting indigestible carbohydrates and proteins.25 Although increased concentrations of SCFAs are involved in reducing obesity and inflammation, and increasing insulin sensitivity,26,27 there is evidence suggesting that butyrate damages nitrergic myenteric neurons, ex vivo.28 Despite all of the observations above, most studies of enteric neuropathy and dysmotility have focused on LPS,3,4 leaving a knowledge gap regarding the role of LTA and SCFAs in the development of GI dysmotility and enteric neuropathy.
We have previously shown that gut microbiota alterations and the development of enteric neuropathy and GI dysmotility occur before the onset of diabetic conditions in female C57BL6 mice fed HFD for 8 weeks.10 We also showed that ileocecal contents from HFD mice damage nitrergic myenteric neurons and inhibit intestinal motility by blocking muscularis contractions.29 The aim of this study was to determine whether an intact gut microbiota is required for the development of enteric neuropathy, and what microbiota changes are present in the cecum of mice with enteric neuropathy. We also sought to determine whether LTA from Gram-positive bacteria, which are increased in mice fed HFD, and decreased levels of SCFAs contribute to GI dysmotility by damaging enteric neurons and disrupting muscle contractions in the duodenum. We chose to use the duodenum because previous studies have suggested that diabetic dysmotility and enteric neuropathy affects the proximal intestine first.30 In the later stage of diabetes, alterations of the ENS in the proximal part of the intestine leads to hypercontractility. 30 Furthermore, several studies have demonstrated that during diabetes there is a loss of inhibitory neurons and an increase in cholinergic innervations in the proximal part of the intestine.30 In fact, hypercontractility of the intestine 1) is associated with an increase of glucose absorption, and 2) generates an afferent signal that informs the brain to block glucose entry in the muscle. These “local” and “central” effects participate in the development of hyperglycemia and insulin resistance observed during T2D. Obese/diabetic mice and human exhibit an alteration of ENS activity, which is associated with intestinal hypercontractility. 31,32 For these reasons, we studied changes in motility and damage to inhibitory neurons in the duodenum.
2. MATERIALS AND METHODS
2.1. Mice
The Mayo Clinic Animal Care and Use Committee approved germ free (GF) mouse studies. Seven weeks old male Swiss GF mice were fed either a HFD,33,34 (n=5, containing 60% kcal fat diet; Research Diets, New Brunswick, NJ, D12492ii), or standard chow diet (SCD, n=5, containing 10% kcal fat diet; Research Diets, New Brunswick, NJ, D12450Bi) for 8 weeks in GF isolators. Duodenal tissues were collected post euthanasia at the end of the experiment. The University of Idaho Animal Care and Use Committee approved the study protocols for conventionally raised (CR) C57Bl/6J mice. Six to seven weeks old C57Bl/6J male mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the Laboratory Research Animal Facility. At 8 weeks, mice were separated into two groups (@ n=8) and fed either a SCD composed of 6.2% kcal fat (Teklad Global 2018, Teklad Diets, Madison WI) or a HFD whose composition was published previously10,20 containing 70% kcal fat diet with 2% additional corn oil (TestDiet, Richmond, IN) ad libitum for 8 weeks. Mice were euthanized by exsanguination under deep isoflurane anesthesia. The cecal contents were collected and immediately frozen at −80°C for microbial community profiling. For experiments with dihydroethidium (DHE), we used samples from eight to ten weeks old first generation (F1, n=8) GCaMP6f mice obtained by crossing female B6J.Cg-Gt(ROSA)26Sortm95.1(CAG-GCaMP6f)Hze/MwarJ (C57BL/6J; stock no. 028865), which express the Cre-dependent, fast fluorescent Ca2+ indicator protein GCaMP6 with B6.129-Nos1tm1(cre)Mgmj/J (C57BL/6J; stock no. 017526), which express Cre recombinase. Breeding mice were purchased from the Jackson Laboratories. We bred GCaMP6f mice for a Ca2+ imaging studies but use them here because they were readily available. These mice were fed SCD only.
2.2. 16S rRNA analysis of the gut microbiota and taxonomic assignment of reads
Genomic DNA was extracted from cecal contents using enzymatic and mechanical lysis of 0.01g of cecal contents from each mouse as previously described.35 The variable V1-V3 regions of bacterial 16S rRNA genes were amplified and sequenced as previously described.10 Hierarchical clustering and principal coordinate analysis (PCoA),36 were used to assess similarities and differences in bacterial community composition across conventional SCD and HFD mice with MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/).
2.3. Identifying the effects of LTA, LPS and SCFAs on intestinal muscle contractions using cultured duodenal muscularis preparations
Twelve-week-old C57Bl/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME), and housed in the Laboratory Research Animal Facility for a week. Mice were euthanized and the duodenum was removed and placed immediately in ice-cold Hepes buffer supplemented with 2% Penicillin-Streptomycin (Sigma, St. Louis, MO, USA). The samples were then pin-stretched in Sylgard lined petri dishes and opened along the mesenteric border. After multiple washes in Hepes Buffer with 2% Penicillin-Streptomycin, samples were dissected with fine forceps to obtain the muscularis externa (muscularis). Approximately 1.0 cm long pieces of muscularis were then cultured in Neurobasal A medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with the serum substitute B27 according to manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). Tissues were cultured in 24 well plates for 24 hours in a tissue culture incubator at a temperature of 37°C, relative humidity of 95% and CO2 concentration of 5%. 30-second long videos were recorded with a camera equipped Nikon light microscope using the 5x objective lens to determine the effect of test molecules on muscularis contractions. All test molecules were solubilized in culture media and filter sterilized before use at the following concentrations: 0.5 ng/ml LPS from Escherichia coli O111:B4 (L4391), 0.5 ng/ml LTA from Staphylococcus aureus (L2515), 30 mM sodium acetate (S2889), 30 mM sodium propionate (P5436), and 30 mM sodium butyrate (303410) (all from Sigma, St. Louis, MO, USA). Samples were videotaped after 1 hour of equilibration before test molecules were added (Time 0) and 24 hours later. Videos were analyzed by individuals not aware of the treatments to tissues by counting the number of contractions that occurred during 30 seconds. Data were normalized by dividing the frequency of contractions after 24 hours to the frequency of contractions recorded after 1 hour of equilibration and presented as percentages. The samples were then fixed and stained to access myenteric neuronal damage as described below.
2.4. Immunohistochemistry analysis of nerve cell damage
After filming tissues for measuring the rates of contractions, tissues were stretched and pinned on Sylgard, fixed overnight in 4% paraformaldehyde with 0.2% picric acid and dissected as previously described.37 Immunohistochemistry staining for neuronal nitric oxide synthase (nNOS, 1:300, Abcam, Cambridge, MA) and vasoactive intestinal peptide (VIP,1:1000, Abcam, Cambridge, MA) to localize myenteric inhibitory motor neurons was performed as previously described.13 Total neuron population analysis was completed using an antibody for anti-neuronal nuclear antibody 1 (ANNA1) positive human serum, 1:20,000, kind gift from Dr. Vanda A. Lennon, Mayo Clinic). Tissue inflammation was evaluated by staining with an antibody to TNFα (TNFα, 1:500, Abcam, Cambridge, MA). Increased antioxidant production in tissue was evaluated with an antibody to glutathione synthase (GS, 1:200, Abcam, Cambridge, MA). Nitrosative stress was measured by staining with an antibody to inducible nitric oxide synthase (iNOS, 1:500, Abcam, Cambridge, MA). We used Donkey anti-rabbit 594 or 488 and donkey anti-human 488 secondary antibodies (Jackson ImmunoResearch, West Grove, PA), both at 1:300, to label nNOS, iNOS, GS and ANNA1, respectively. Tissues were mounted on objective slides using VectaShield Mounting Medium (Vector Labs, Burlingame, CA).
2.5. Dihydroethidium Staining
Superoxide levels were measured by quantifying fluorescence of the superoxide marker dihydroethidium (DHE)38 as previously described.39 Briefly, LMMP whole mount preparations were incubated with 2 μM DHE (Life Technologies, Carlsbad, CA) dissolved in culture medium at 37°C for 1 hour. Fluorescence levels were quantified in live ganglia after mounting samples and capturing 15 to 20 random fields of view with a 40x objective lens and a wide field camera on a Nikon/Andor Spinning disk microscope (Nikon, Melville, NY) and analyzed with Nikon NIS Elements software using similar methods as previously described13.
2.6. Quantification of number of neurons and density indices
Tissues were photographed using a wide field camera on a Nikon/Andor Spinning disk microscope with a 40x objective lens (Nikon, Melville, NY) and analyzed with Nikon NIS Elements software using similar methods as previously described13.
2.7. Statistical analysis
Statistical analyses were conducted using GraphPad Prism 5 software (GraphPad Software Inc, La Jolla, CA) unless otherwise indicated. A repeated measure ANOVA with Bonferroni post- hoc test was used to correct for multiple comparisons. Statistical significance was determined at P < 0.05. The effects of the test molecules on muscle contractions were assessed by calculating the percentage of response compared to basal contractions recorded at time point 0 in the same samples. Data in bar graphs were analyzed by one-way ANOVA followed by Tukey’s post-tests and are expressed as mean ± SEM, or two-way ANOVA where appropriate (in Fig. 1& 2) to account for more than one independent variable.
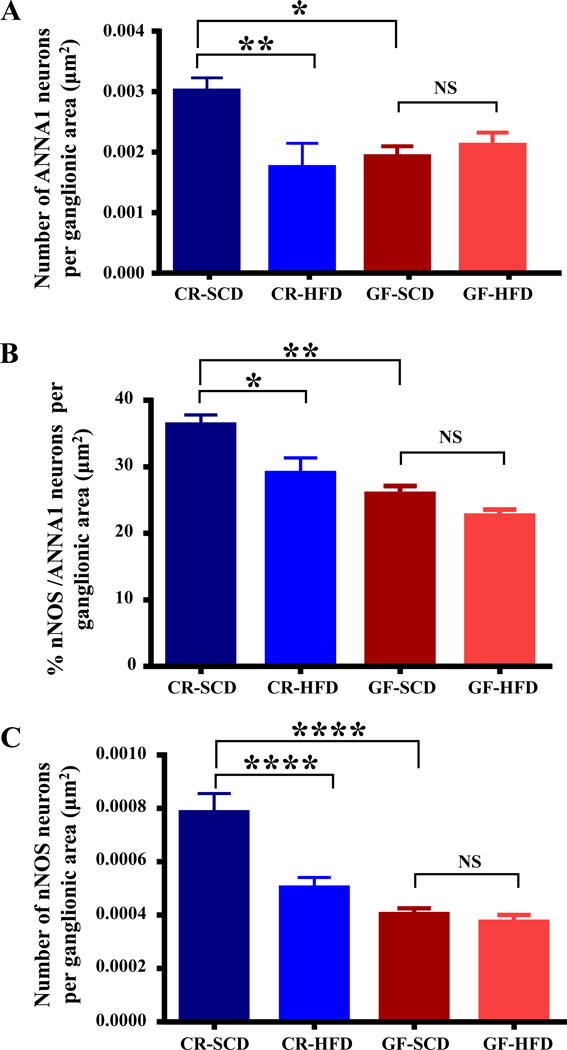
Figure 1.
The gut microbiota is required for HFD-induced injury to nitrergic myenteric neurons. A, HFD reduced the total number of duodenal myenteric neurons in conventionally raised mice (CR) but had no effect on myenteric neurons in GF mice after 8 weeks. B, HFD reduced the percentage of nNOS neurons out of the total number of myenteric neurons (labeled with ANNA1) per ganglionic area in CR-HFD mice but did not affect the percentage of nNOS neurons in GF mice. C-D, HFD reduced the number of nNOS neurons per ganglionic area (C), and the density of nNOS immunoreactive neurons and varicosities per ganglionic area (D) in CR mice. In contrast, HFD did not affect the number of duodenal myenteric nNOS in HFD-GF mice (C), but HFD GF mice had a higher density of nNOS immunoreactivity per ganglion (D). Noteworthy, GF mice had fewer total number of neurons and nNOS neurons per ganglion compared to CR mice (A-B). E, Sample images showing overlays of nNOS (red) and ANNA1 (green) staining in duodenal myenteric ganglia of CR-SCD, CR-HFD, GF-SCD and GF-HFD mice. Data were analyzed by one-way analysis of variance followed by Tukey’s post-tests and are expressed as mean ± SEM in all figures. Here and here after, number of animals (n) for experiments were 5–10 per group. Statistical significance is denoted non-significant by ns = P > 0.05 and significant by *P showing P≤ 0.05; **P showing P≤ 0.01; ***P showing P ≤ 0.001, and ****P, showing P value ≤ 0.0001.
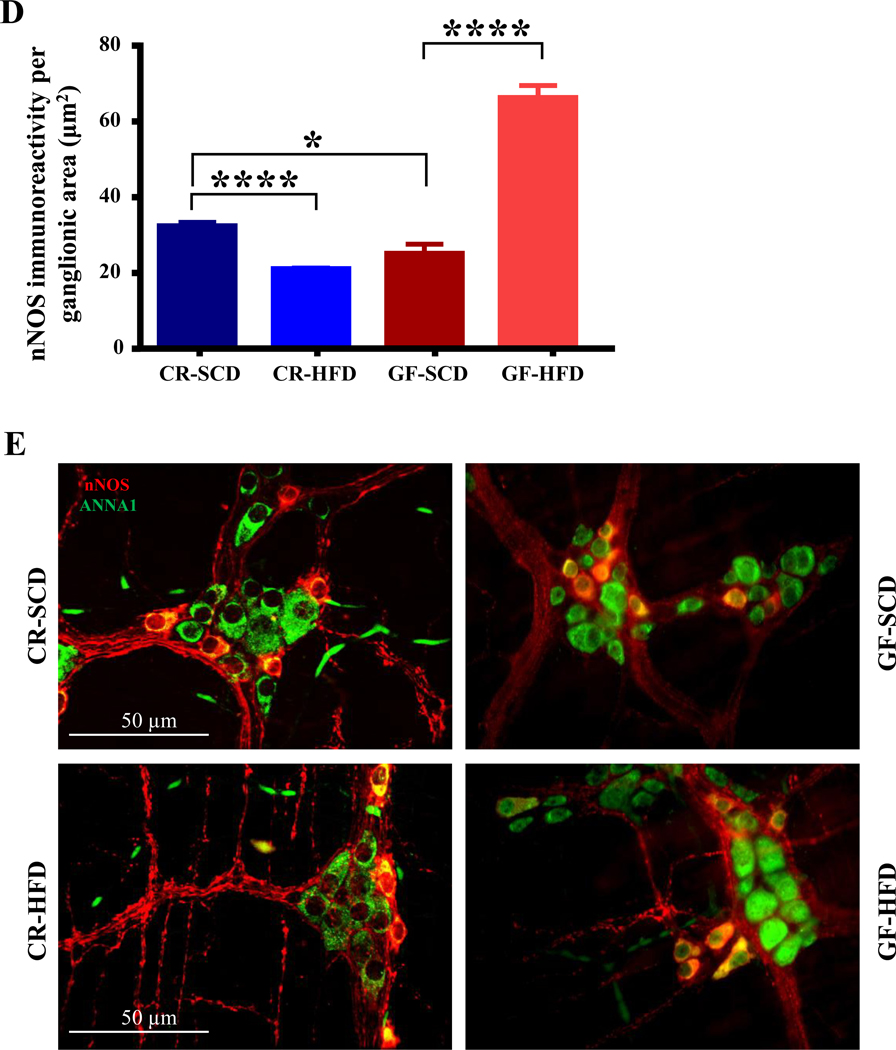
Figure 2.
The microbiota is necessary for HFD-induced injury to myenteric VIP neurons in mouse duodenum. A, In the duodenums of GF mice, HFD did not affect myenteric VIP immunoreactivity, but the VIP immunoreactivity was reduced in CR-HFD mice. GF-SCD mice had lower VIP immunoreactivity in duodenum myenteric plexus when compared to CR-SCD mice. B, Sample images showing overlays of VIP (red) and ANNA1 (green) staining in duodenal myenteric ganglia of CR-SCD, CR-HFD, GF-SCD and GF-HFD mice.
3. RESULTS
3.1. The gut microbiota is required for the development of enteric neuropathy and dysmotility
Gut microbiota alterations has been correlated with enteric neuropathy, however it is unclear if gut microbiota is directly involved in enteric neuropathy.10 To determine whether gut microbiota is involved in the loss of inhibitory motor neurons observed in the myenteric ganglia of CR HFD mice, we examined the duodenal myenteric plexus of GF and CR mice fed HFD or SCD for 8 weeks. HFD ingestion decreased the total number of neurons per ganglionic area in CR mice but not GF mice (Figure 1A). In addition, HFD decreased the number of nNOS neurons (Figure 1B), the density of nNOS immunoreactivity (Fig. 1C) and percentage of nNOS neurons per total number of neurons (Figure 1D) per ganglionic area in CR mice. In contrast, GF mice fed HFD did not display a decrease in either the number (Figure 1B) or percentage (Figure 1D) of nNOS neurons per ganglionic area. Of note, GF mice fed both SCD and HFD had fewer myenteric nitrergic neurons compared with CR mice (Figures 1A–D) but GF HFD mice had higher amounts of nNOS immunoreactive varicosities per ganglionic area (Figure 1C). HFD ingestion reduced the VIP immunoreactivity in CR mice, but not in GF mice (Figures 2A–B). These results suggest that the gut microbiota is required for the development of enteric neuropathy, and likely contributes molecules that play a role in the disruptive effects of HFD on motility and enteric neurons.
3.2. HFD ingestion increases the population of cecal Gram-positive bacteria
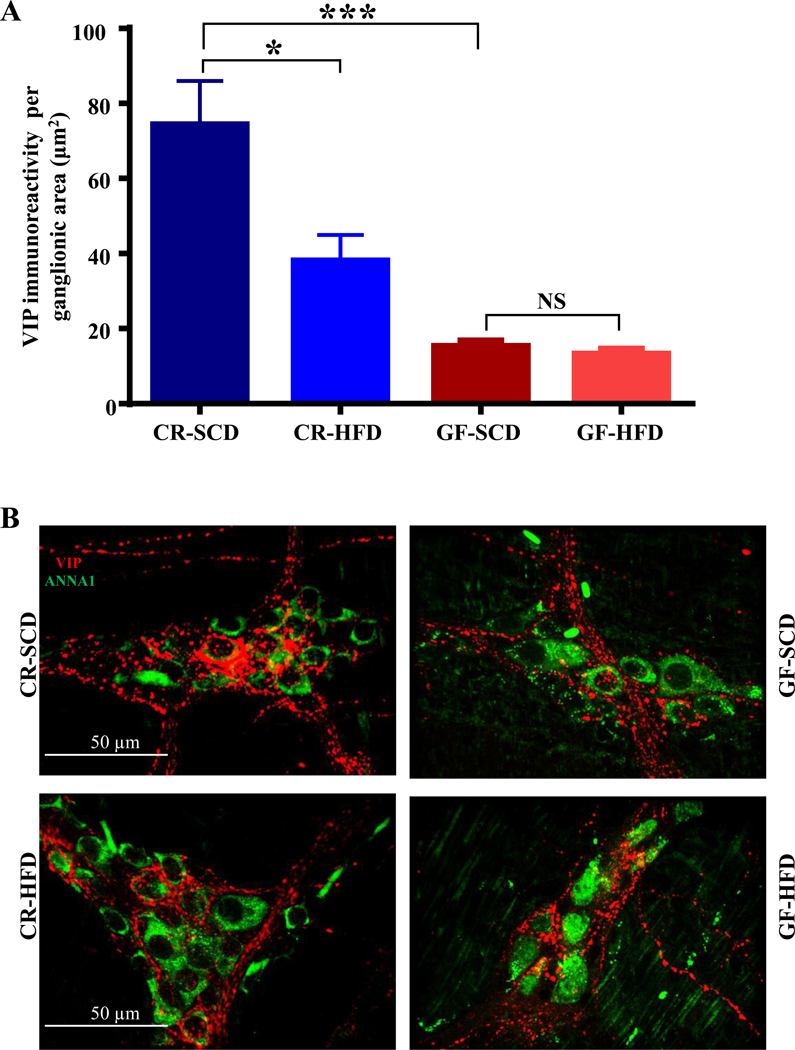
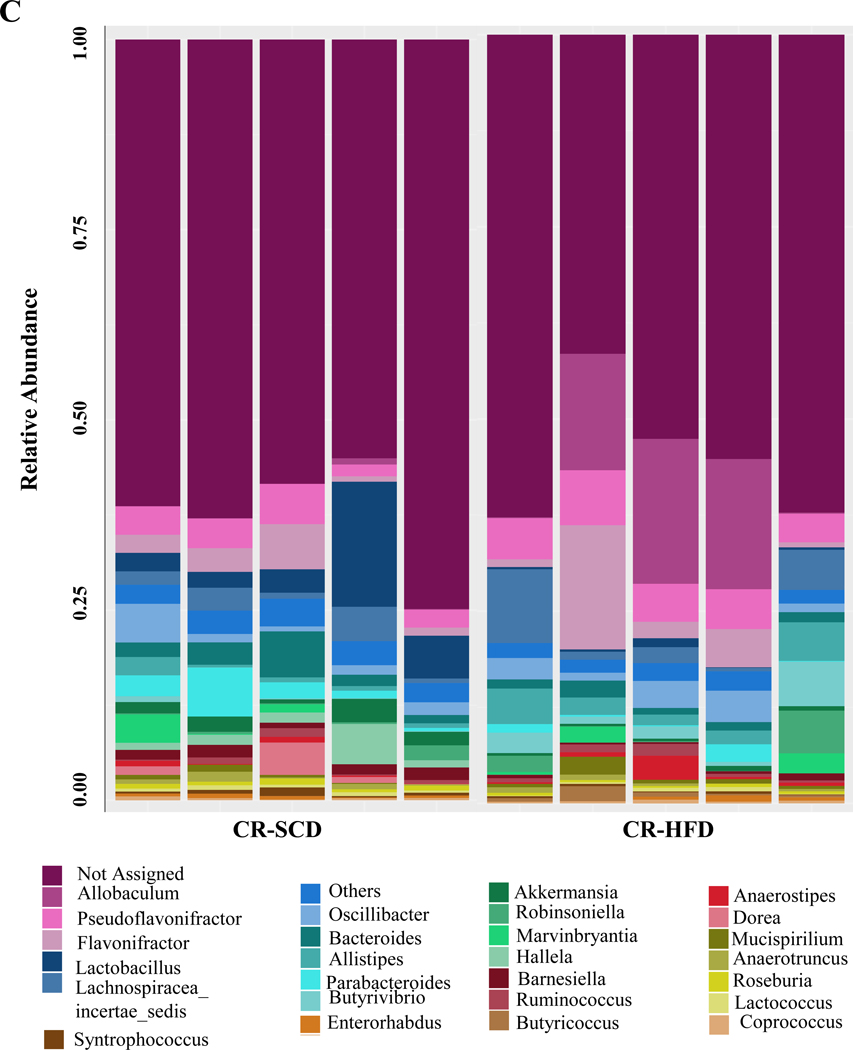
We have previously shown that neuropathy in CR mice is associated with shifts in the fecal microbiota including increased Firmicutes and decreased Bacteroidetes.10 We sought to determine whether HFD fed CR mice with neuropathy demonstrated alterations in the microbial community in the cecum, and in particular if Gram-positive bacteria increased. PCoA revealed that diet determined the composition of the microbial community (Figure 3A). HFD ingestion increased the abundance of Gram-positive Firmicutes in the cecum of CR-HFD while Gram-negative Bacteroidetes abundance was reduced (Figure 3B). Specific genera were also affected by HFD ingestion. Notably, Allobaculum was increased by HFD ingestion while Lactobacillus and Akkermansia were decreased (Figure 3C). These results match our previous observations using fecal samples.
Figure 3.
HFD ingestion caused microbial alterations in the cecum, which was marked by increased Gram-positive bacteria. A, Principal coordinate analysis revealed that cecal microbial communities from CR mice clustered together by diet, indicating HFD shifted the community composition. B, Cecal microbiota of CR-HFD mice had higher Firmicutes and lower Bacteroidetes compared to CR-SCD mice, indicating an increase in Gram-positive bacteria in the cecum of CR-HFD mice. C, HFD induced shifts in specific genera in the cecum of CR mice. Notable shifts include HFD increased Allobaculum and decreased Akkermansia and Lactobacillus abundances.
3.3. Microbiota LTA, LPS, and SCFAs do not affect contractions of cultured duodenal muscularis externa
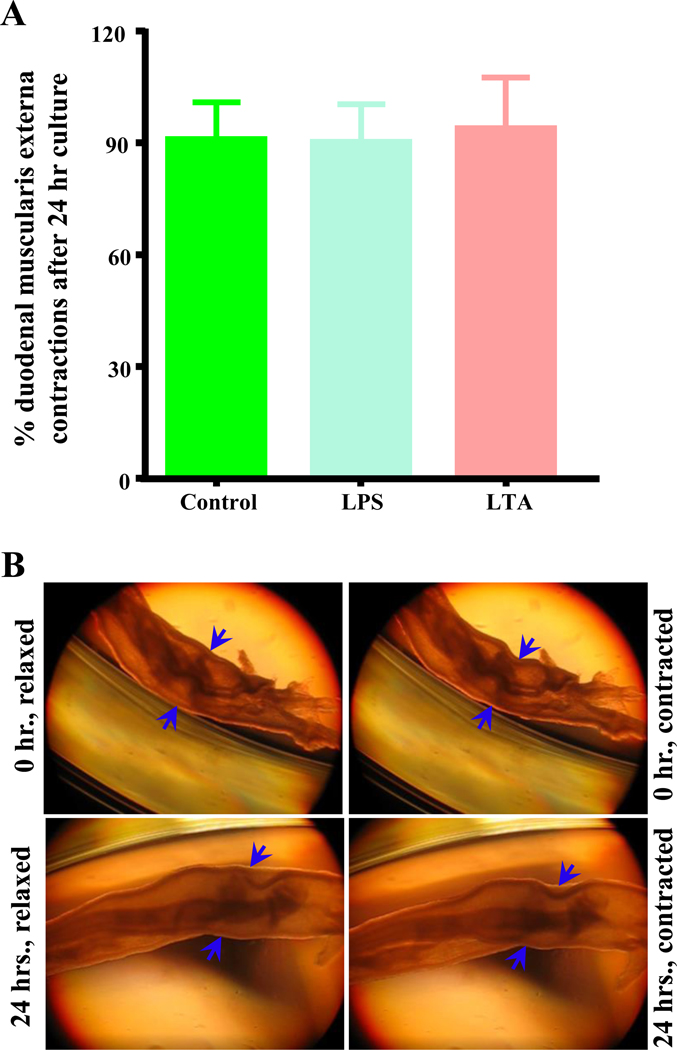
We have previously shown that ileocecal supernatants from CR mice fed a HFD inhibits contractions in duodenum and in cultured duodenal muscularis externa.29 We next determined whether LTA, LPS and SCFAs inhibit intestinal smooth muscle contractions using cultured muscularis externa preparations from CR mice fed SCD. After 24 hours of culture, the frequency of contractions in untreated muscularis preparations was unchanged compared to initial contractions recorded after 1-hour equilibration (0 hour time point). Treatment with LPS and LTA did not affect the frequency of muscle contractions after 24 hours (Figures 4A–B). Similarly, 30 mM concentrations of acetate, propionate and butyrate had no effect on contractions (Figure 5A). These results suggest that these bacterially derived molecules do not affect muscle contractions at used concentrations.
Figure 4.
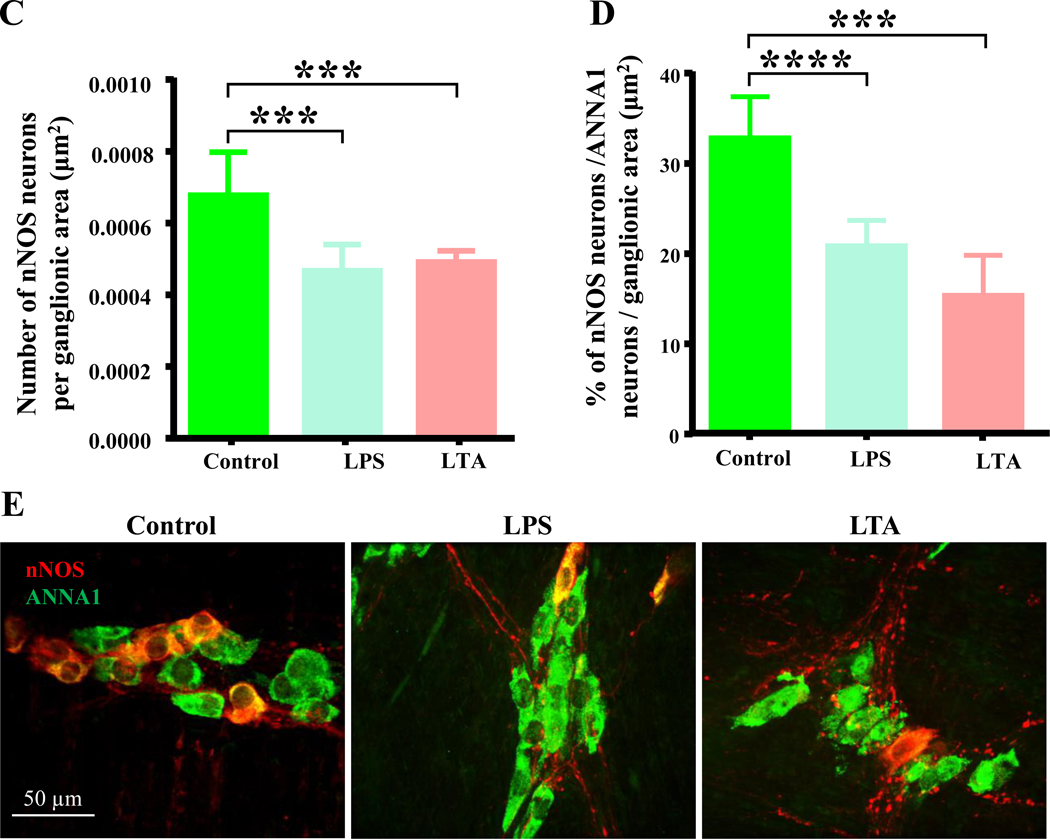
Bacterially derived cell wall components LTA and LPS damage nitrergic myenteric neurons without affecting contractions of intact duodenal muscularis externa in culture. A, The number of muscle contractions per second was not affected by 24 hours of treatment with LPS and LTA (0.5 μg/ml) when compared with untreated control samples. B, Representative pictures of cultured duodenal muscularis external demonstrating contraction and relaxation at 0 hour (hr.) and 24 hours (hrs.). Compare the width changes at sites shown by blue arrows in the relaxed and contracted pictures. C-D, Similar to LPS treatment, LTA triggered damage to nitrergic myenteric neurons in cultured duodenal muscularis samples. Both LTA and LPS reduced the percentage of nNOS neurons per total number of neurons in myenteric ganglia and the number of myenteric of nNOS neurons per ganglionic area after 24 hours. E, Sample images showing overlays of nNOS (red) and ANNA1 (green) staining in duodenal myenteric ganglia of untreated control, LPS and LTA treated duodenal muscularis externa.
Figure 5.
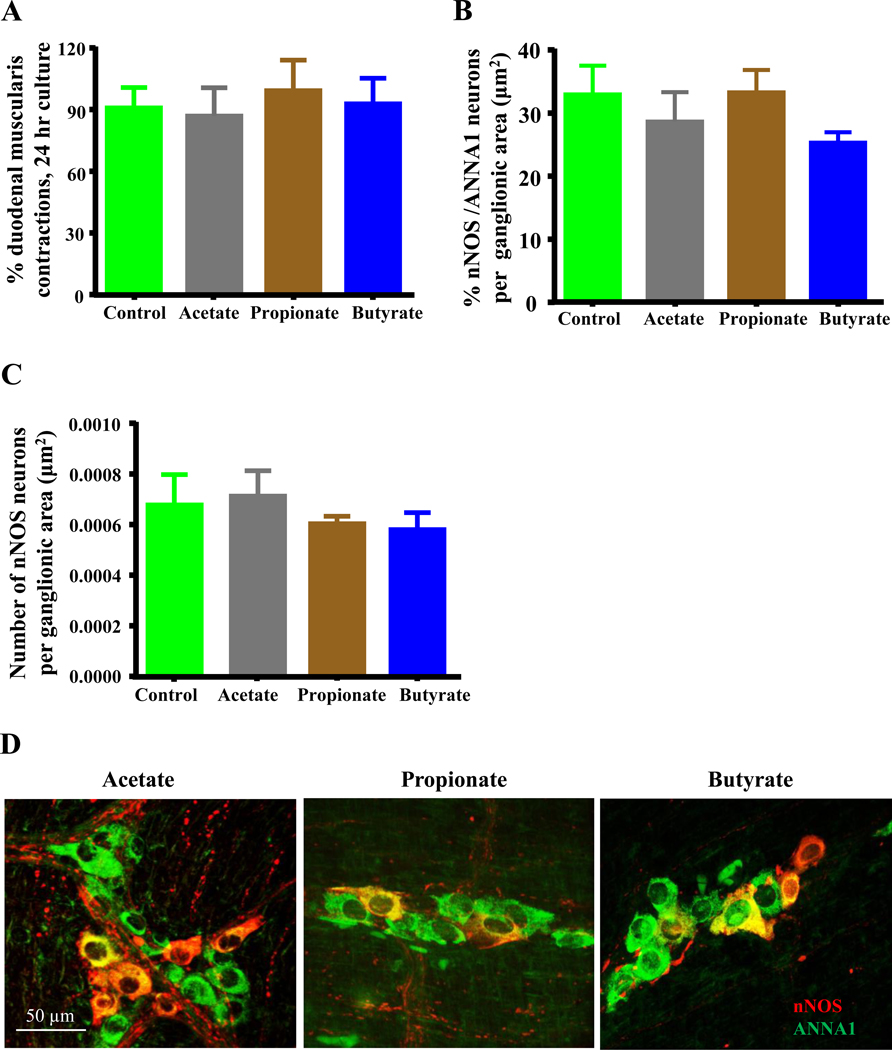
Bacterially derived SCFAs do not affect contractions or injure nNOS neurons in cultured intact duodenal muscularis externa. A, The number of muscle contractions per second was not affected by 24 hours of treatment with 30mM concentrations of SCFAs when compared with untreated control samples. B-C, SCFAs did not affect the percentage of nNOS neurons per total number of neurons in myenteric ganglia and the number of myenteric of nNOS neurons per ganglionic area after 24 hours. D, Sample images showing overlays of nNOS (red) and ANNA1 (green) staining in duodenal myenteric ganglia of untreated control, acetate, propionate and butyrate treated duodenal muscularis externa.
3.4. LTA induces injury to myenteric neurons which is similar to that elicited by LPS
LPS is known to damage ENS neurons and reduce the number of nitrergic myenteric neurons, which is similar to the neuropathy elicited by HFD and diabetes in animal models and diabetic humans.4 We next determined whether LTA could also reduce the number of myenteric nitrergic neurons using cultured duodenal muscularis preparations. We observed that in muscularis cultured for 24 hours, LPS and LTA decreased the number of nNOS neurons per ganglionic area (Figure 4C) and the percentage of nNOS neurons per total number of neurons per ganglionic area (Figures 4D–E). These results suggest that similar to HFD ingestion and LPS, LTA reduces the number of myenteric nitrergic neurons.
3.5. SCFAs do not affect duodenal myenteric neurons
It was previously shown that 1 mM butyrate decreased the proportion of nNOS neurons in primary rat myenteric neurons cultured from colon,28 and 30 mM SCFAs impaired colon propulsion in guinea pigs.40 We studied whether 30mM acetate, propionate and butyrate could also reduce the number of myenteric nitrergic neurons in cultured duodenal muscularis. We found that compared to untreated controls, SCFAs did not affect the number (Figure 5B) and percentage (Figures 5C–D) of nNOS neurons in cultured muscularis preparations. 30 mM concentrations of SCFAs did not cause a reduction in the number of myenteric nitrergic neurons.
3.6. LTA induces oxidative stress
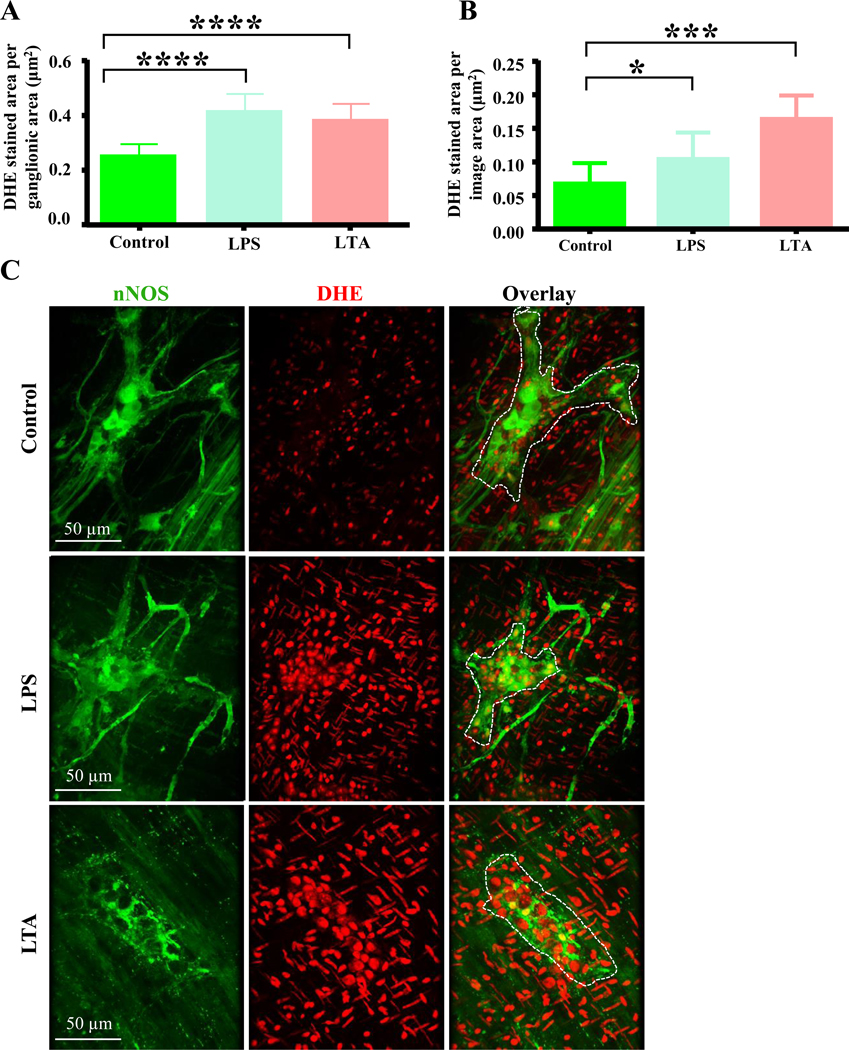
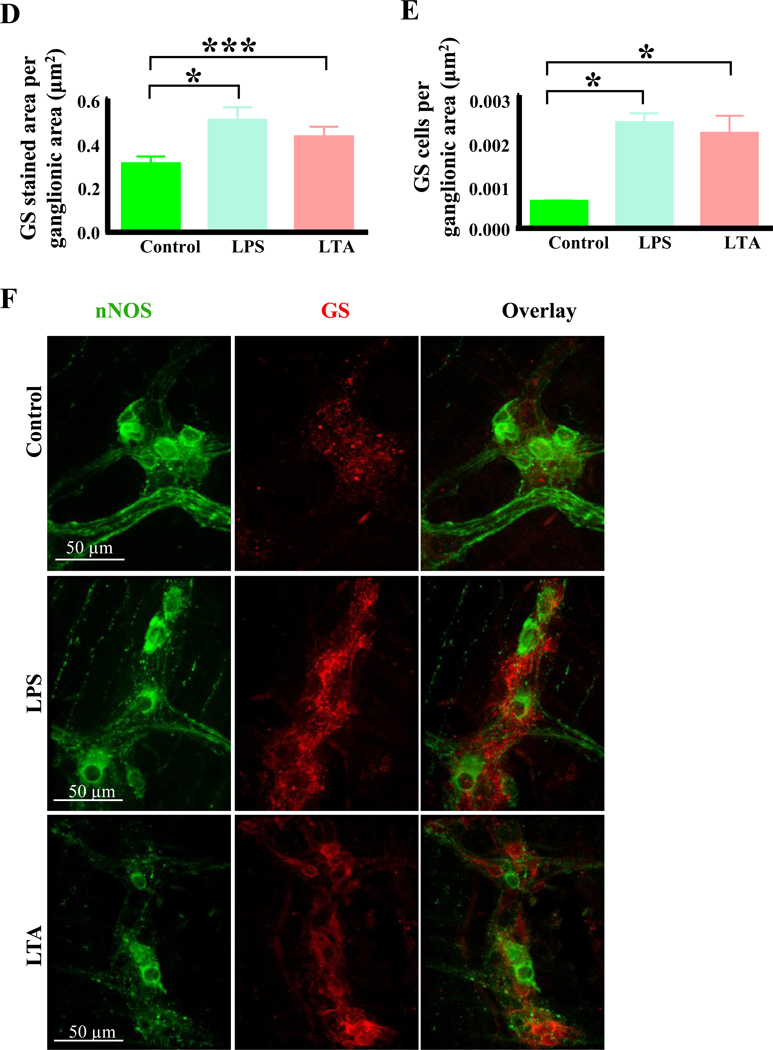
Previous studies have demonstrated that oxidative stress is the main pathway through which LPS-induced enteric nerve cell injury occurs.4,41 We therefore hypothesized that, similar to LPS, LTA induced damage to myenteric nitrergic neurons was associated with oxidative stress. To test this idea, duodenal muscularis samples were cultured in LTA or LPS and stained with the superoxide-specific fluorescent indicator dihydroethidium (DHE).38,42 We also stained fixed treated muscularis samples with an anti-glutathione antibody to measure the expression of glutathione, an essential antioxidant produced in response to oxidative stress.42 Compared to untreated control samples, the total DHE staining per ganglionic area (Figure 6A) and in the entire field of view (Figure 6B) was increased by LTA and LPS treatment after 12 hours (Figures 6A–C). Additionally, the anti-glutathione staining per ganglionic area (Figure 6D) and the number of anti-glutathione positive cells (Figures 6E–F) was dramatically increased in LTA and LPS treated samples. This suggests that like LPS, LTA causes increased oxidative stress in myenteric ganglia.
Figure 6.
LTA induces oxidative stress in myenteric neurons. A-B, Like LPS, LTA increased the amount of DHE staining per ganglionic area (A) and in the entire field of view (B) after 12 hours. C, Sample images showing nNOS (green) and DHE (red) staining in duodenal myenteric ganglia (outlined in overlay) of control, LPS and LTA treated samples. D-E, Like LPS, LTA increased the anti-glutathione synthase (GS) immunoreactivity (D) and increased the GS positive cells per ganglionic area (E). F, Sample images showing nNOS neurons (green) and anti-glutathione synthase (red) immunoreactivities in duodenal myenteric ganglia of control, LPS and LTA treated (12 hours) muscularis.
3.7. LTA triggers nitrosative stress and inflammation
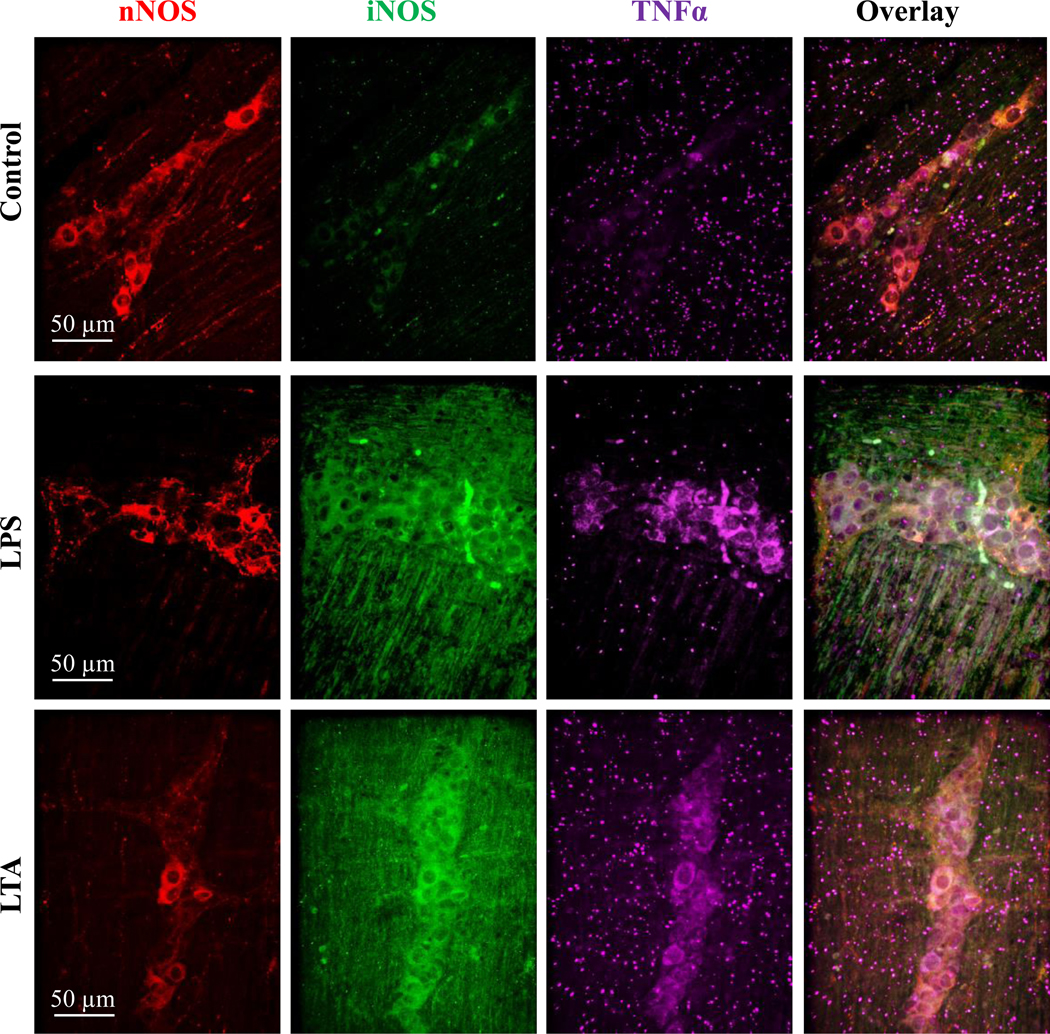
TNFα mediated inflammation leading to oxidative-nitrosative stress is the major pathway through which LPS induced enteric neuropathy occurs.43 We therefore determined if LTA-induced TNFα-mediated inflammation and nitrosative stress by staining muscularis preparations with antibodies to TNFα and iNOS. Samples treated with LPS and LTA demonstrated more TNFα and iNOS staining (Fig. 7) when compared to untreated controls. TNFα and iNOS labeling was observed in nNOS and other types of myenteric neurons in ganglia from samples treated with LPS and LTA. This suggests that similar to LPS, LTA induces inflammation and nitrosative stress, which are known to damage to enteric neurons.
Figure 7.
LTA treatment caused nitrosative stress and activated the production of TNFα. Images to demonstrate qualitative analysis of inducible nitric oxide synthase (iNOS, green) and TNFα immunoreactivity (purple) in nNOS neurons (red) in control, LPS and LTA treated samples, after 12 hours. Similar to LPS treatment, LTA treatment increased the number of neurons expressing TNFα, and increased the number of neurons expressing iNOS compared to control samples.
4. DISCUSSION
The goals of this study were to: a) determine whether the gut microbiota plays an essential role in the development of HFD-induced enteric neuropathy and b) determine the role of gut microbiota derived LTA and SCFAs on enteric neuropathy and dysmotility. Our results demonstrate that GF mice fed a HFD do not develop the enteric neuropathy observed in CR mice fed HFD suggesting that gut microbiota is required for the development of enteric neuropathy. The data presented herein also demonstrates that HFD caused alterations of the gut microbiota in CR mice, resulting in higher abundance of Gram-positive Firmicutes bacteria in cecum. Furthermore, LTA from Gram-positive bacteria, like LPS from Gram-negative bacteria, induced enteric neuropathy, oxidative stress- and TNFα-mediated inflammation, but did not affect duodenal muscle contractions. The major SCFAs produced as a result of gut bacterial fermentation (acetate, propionate and butyrate) did not damage myenteric neurons or inhibit duodenal muscle contractions. This is the first study, to our knowledge, to establish an association between the increase of Gram-positive gut bacteria caused by HFD ingestion and the development of enteric neuropathy through LTA.
One of the main findings from this study is that the gut microbiota is required for the development of HFD-induced enteric neuropathy including injury to nitrergic myenteric neurons. We report for the first time that HFD ingestion does not affect the total number of neurons and the number of nitrergic neurons in the myenteric plexus of GF mouse duodenum, which is in contrast to findings in CR mice also fed HFD for 8 weeks.3,10,13 The HFD-induced reduction in the total number of neurons and the number of nitrergic myenteric neurons in duodenal myenteric plexus of CR mice observed in this study correspond with previous observations from our lab and other labs utilizing HFD of different compositions ranging from 45% to 70% calorific intake from fat.3,10,13 Three potential explanations support the observed differences between GF and CR HFD mice and the involvement of gut microbiota in caused enteric neuropathy. First, our results strongly suggest that HFD induced changes to the gut microbiota are involved in the development of enteric neuropathy likely through end toxemia. This view is supported by previous studies showing that LPS is increased in the bloodstream of mice fed the same diet41 as well as other animal models of T2D and T2D patients because of disrupted gut epithelial barrier.18,44 Additional support for this view include findings suggesting that LPS acts synergistically with dietary saturated fat (palmitate) to cause enteric neuropathy in diabetic mice,3,4 and findings of the current study that like LPS, LTA damages myenteric neurons via inflammation and oxidative stress. Second, it has been shown that hyperglycemia and hyperlipidemia are involved in causing enteric neuropathy in CR mice.45,46 Interestingly, GF HFD mice do not develop hyperlipidemia, hyperglycemia and obesity after 8 weeks,47,48 however they develop diabetic conditions upon receiving gut microbiota transplants from diabetic patients,47,48 which highlights further the important role of the microbiota in the pathophysiology of diabetic conditions including enteric neuropathy. Finally, recent studies proposing that HFD-induced damage to ENS neurons can occur in CR mice in the absence of hyperglycemia,10,49 is an additional fact suggesting microbial factors have a key role in the intricate interaction of factors that cause enteric neuropathy associated with HFD ingestion and T2D.
Another important finding of our study is the observation suggesting that LTA plays a role in HFD-induced and T2D enteric neuropathy. The majority of previous studies linking gut microbiota alterations to enteric neuropathy have focused on LPS which is derived from Gram-negative bacteria.3,4 However, like several other studies in HFD mouse models and in diabetic humans,10,50 the current study found HFD-induced alterations of microbiota in cecum was characterized by the increased abundance of Firmicutes (Gram -positive) and decreased abundance of Bacteroidetes (Gram-negative) bacteria. These increases in Gram-positive bacteria correlates with the increase in Gram-positive gut bacteria in the bloodstream of T2D patients.17 Gram-positive bacteria are distinguished from Gram-negative bacteria by the absence of an outer cell membrane,51 and an inner cellular membrane composed of LTA and peptidoglycan instead of LPS.51 Therefore, an increase in Gram-positive bacteria in the cecum of CR mice fed HFD led us to hypothesize that Gram-positive LTA induces enteric neuropathy through oxidative- stress and TNFα mediated neuroinflammation. In testing this idea, we have established that like LPS, LTA induces damage to ENS neurons, particularly nitrergic myenteric neurons, likely through oxidative stress and inflammation. It has been shown that LTA damages cerebellar granule neurons by activating glial cells to release pro-inflammatory cytokines (TNFα, IL-1β and IL-6), which damage neurons via oxidative-nitrosative stress and caspase activation.22 LPS acts via TLR4 to damage nitrergic myenteric neurons via oxidative-nitrosative stress and inflammation.3,4 LTA is a TLR2 agonist that induces oxidative and nitrosative stress through a similar pathway as LPS,21,52 mainly by activating myd88, NF-kB and Nrf2.21 Therefore, it is likely that these mechanisms were involved in the observed LPS and LTA-induced activation of inflammation, increased oxidative stress and damage to nitrergic myenteric neurons in myenteric ganglia. Nitrergic myenteric innervation modulates the relaxation component of intestinal motility, and is therefore essential for the maintenance of healthy motility. Decreased nitrergic innervation underlie dysmotility in diabetic patients.1,53,54 Mice and isolated neurons that express GCaMP6f in myenteric nNOS neurons could be useful in determining HFD-, T2D- and endotoxins-induced alterations in transmission from nNOS neurons, and the cellular mechanisms associated with degenerative changes in these neurons. Collectively, our results support the need for determining the levels of components of Gram-positive bacteria cell wall, including peptidoglycan and LTA in the blood stream, elucidating the involvement of glia in LTA-induced enteric neuropathy and analyzing the cellular mechanisms by which LTA damages ENS neurons in future studies.
The short chain fatty acids acetate, propionate and butyrate appear to have no role in HFD induced damage to nitrergic myenteric neurons and impaired duodenal contractions. We have previously shown that HFD causes loss of nitrergic myenteric neurons and impairs duodenal contractions in conventionally raised male and female mice.10,13 Other studies have demonstrated that HFD ingestion in a rat model of T2D and alterations of the gut microbiota in diabetic humans is associated with lower fermentative bacteria and impaired production of the major short chain fatty acids acetate, propionate and butyrate.12,55–57 The concentrations of all three fatty acids is also reduced by HFD ingestion in humans.11 At the 30 mM concentration used in this study, acetate, propionate and butyrate did not affect duodenal muscle contractions and the number of nitrergic myenteric neurons. These concentrations were selected because a previous study had determined that 30 mM concentrations affected colonic motility ex vivo, 58 and because we considered 30 mM to be representative of the lower SCFA concentrations found in the gut of diabetic subjects.58 These findings suggest that low concentrations of these SCFAs do not cause neuropathy or impair intestinal motility by disrupting muscle contractions. Effects of SCFAs on myenteric nitrergic neurons have not been evaluated thoroughly, warranting future studies with different concentrations and combinations of SCFAs.
This study found that the intact gut microbiota is required for the development of the optimal number of all neurons and nitrergic neurons in the myenteric plexus of mice. GF mice fed SCD had lower myenteric neuronal densities and a lower proportion of nitrergic neurons in the duodenum compared to CR mice fed SCD. Our results support previous findings showing that GF mice have fewer myenteric neurons and the conclusion that the gut microbiota is needed for a healthy development of the ENS.59,60 However, in contrast with our study in duodenum, other studies reported an increased proportion of nNOS neurons in the jejunum and ileum of GF mice fed SCD. These differences are likely due to regional differences in the gut.
Some studies have showed that LPS intraperitoneal injections and in vitro exposure to intestinal muscle preparations inhibit muscle contractions. However, responses to LPS differ between mouse strains.59,60 For example, the intraperitoneal exposure of intestine to LPS (6 or 12 mg/kg) for 6 hours decreased in vivo intestinal propulsive motility and ex vivo ileal muscularis contractions in BALB/c but increased these parameters in C57BL/6 mice.59,60 In contrast, incubating ileal muscularis externa from C57BL/6J mice in 100 μg/ml LPS for 4 hours inhibited carbachol-induced smooth muscle contraction.59,60 Therefore, observed lack of effect of 1 μg/ml LPS on duodenal contractions is likely due to strain differences and the concentration of LPS tested. It is thought that endotoxins from Gam-positive bacterial cause intestinal Intussusception by inhibiting motility,51 but the effect of LTA on intestinal muscle contractions has not been studied.
5. CONCLUSIONS
In conclusion, our data strongly supports the hypothesis that gut microbiota alterations are directly involved in causing enteric neuropathy through LPS and LTA. Additional studies need to identify the specific mechanisms used by LTA to trigger neuropathy and investigate the role of glial cells.
Key Points.
Alterations of the gut microbiota have a potential role in causing diabetic enteric neuropathy, which is characterized by loss of nitrergic myenteric neurons. However, whether gut microbiota is actually required for high fat diet-induced myenteric nerve injury to occur has not been established. By using germ free mice fed high fat diet, we show that the gut microbiota is required for the development high fat diet- (HFD)-induced loss of nitrergic myenteric neurons.
Diabetes and HFD consumption are associated with increased Gram-positive bacteria in the gut. These bacteria are also increased in the blood of diabetes patients. We determined whether lipoteichoic acid, a Gram-positive bacterial cell wall component, can damage nitrergic myenteric neurons in vitro. Like lipopolysaccharide, lipoteichoic acid damaged and caused loss of nitrergic myenteric neurons, and induced neuroinflammation and oxidative stress.
At the concentration found in mice with HFD-induced diabetes conditions and enteric neuropathy, short chain fatty acids (acetate, butyrate and propionate) did not damage nitrergic myenteric neurons suggesting they do not play a role in HFD-induced and diabetic enteric neuropathy.
ACKNOWLEDGEMENTS
We would like to thank animal research facility personnel at the University of Idaho and Mayo Clinic for assistance with animal care. We thank Charles Salmonson, Roan Wilson, Forrest Potter and Ann Norton (IBEST Optical Imaging Core) for assistance with sampling, imaging and/or data analysis. We are grateful to Drs Tanya Miura and Lee Fortunato for assistance with tissue culture, and Dr. Vanda A. Lennon for assistance obtaining and using ANNA1 antibody. We thank Mr. Weston Durland for help with preparing the graphical abstract.
GRANT SUPPORT
Research reported in this publication was supported by the University of Idaho—Dyess Faculty Fellowship and Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20 RR016454 and the National Center for Research Resources grant number P20 GM103408 through IDAHO INBRE. Additional support was through National Institutes of Health grant numbers DK114007 to Purna C. Kashyap and R01DK106011 to David. R Linden.
Glossary
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- SCFAs
short chain fatty acids
- GF
germ free mice
- CR
conventionally raised mice
- HFD
high-fat diet
- SCD
standard chow diet
- GI
gastrointestinal
- ENS
enteric nervous system
- nNOS
neuronal nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- VIP
vasoactive intestinal peptide neurons
- T2D
type two diabetes
- DHE
dihydroethidium
- PCoA
principal coordinate analysis
- GS
glutathione synthase
- ANNA1
anti-neuronal nuclear antibody 1
Footnotes
Competing Interests: the authors have no competing interests.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26(5):611–624. doi: 10.1111/nmo.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98(3):378–384. [DOI] [PubMed] [Google Scholar]
- 3.Reichardt F, Chassaing B, Nezami BG, et al. Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J Physiol. 2017;595(5):1831–1846. doi: 10.1113/JP273269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anitha M, Reichardt F, Tabatabavakili S, et al. Intestinal Dysbiosis Contributes to the Delayed Gastrointestinal Transit in High-Fat Diet Fed Mice. Cell Mol Gastroenterol Hepatol. 2016;2(3):328–339. doi: 10.1016/j.jcmgh.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss U, Ekblad E. Lipopolysaccharide-induced loss of cultured rat myenteric neurons - Role of AMP-activated protein kinase. PLoS One. 2014;9(12):1–17. doi: 10.1371/journal.pone.0114044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyavor YEA, Balemba OB. Diet-induced dysmotility and neuropathy in the gut precedes endotoxaemia and metabolic syndrome: the chicken and the egg revisited. J Physiol. 2017;595(5):1441–1442. doi: 10.1113/JP273888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144(5):967–977. doi: 10.1053/j.gastro.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hul M, Geurts L, Plovier H, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Metab. 2018;314(4):E334–E352. doi: 10.1152/ajpendo.00107.2017 [DOI] [PubMed] [Google Scholar]
- 9.Everard A, Lazarevic V, Gaïa N, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–2130. doi: 10.1038/ismej.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyavor Y, Estill R, Edwards H, et al. Intestinal nerve cell injury occurs prior to insulin resistance in female mice ingesting a high-fat diet. Cell Tissue Res. 2019;376(3):325–340. doi: 10.1007/s00441-019-03002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219 [DOI] [PubMed] [Google Scholar]
- 12.Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M. High-Fat Diet Reduces the Formation of Butyrate, but Increases Succinate, Inflammation, Liver Fat and Cholesterol in Rats, while Dietary Fibre Counteracts These Effects. Bassaganya-Riera J, ed. PLoS One. 2013;8(11):e80476. doi: 10.1371/journal.pone.0080476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenkamp-Strahm CM, Kappmeyer AJ, Schmalz JT, Gericke M, Balemba O. High-fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell Tissue Res. 2013;354(2):381–394. doi: 10.1007/s00441-013-1681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winzell MS, Ahren B. The High-Fat Diet-Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes. 2004;53(Supplement 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.S215 [DOI] [PubMed] [Google Scholar]
- 15.Gérard C, Vidal H. Impact of Gut Microbiota on Host Glycemic Control. Front Endocrinol 2019;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Assal K Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-en-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients. 2020;12(2):278. doi: 10.3390/nu12020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato J, Kanazawa A, Ikeda F, et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients With Type 2 Diabetes. Diabetes Care. 2014;37(8):2343–2350. doi: 10.2337/dc13-2817 [DOI] [PubMed] [Google Scholar]
- 18.Jayashree B, Bibin YS, Prabhu D, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388(1–2):203–210. doi: 10.1007/s11010-013-1911-4 [DOI] [PubMed] [Google Scholar]
- 19.de Kort S, Keszthelyi D, Masclee AAM. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12(6):449–458. doi: 10.1111/j.1467-789X.2010.00845.x [DOI] [PubMed] [Google Scholar]
- 20.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 21.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and Lipoteichoic Acid-induced Cell Activation Is Mediated by Toll-like Receptor 2. J Biol Chem. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406 [DOI] [PubMed] [Google Scholar]
- 22.Kinsner A, Pilotto V, Deininger S, et al. Inflammatory neurodegeneration induced by lipoteichoic acid from Staphylococcus aureus is mediated by glia activation, nitrosative and oxidative stress, and caspase activation. J Neurochem. 2005;95(4):1132–1143. doi: 10.1111/j.1471-4159.2005.03422.x [DOI] [PubMed] [Google Scholar]
- 23.Neher JJ, Brown GC. Neurodegeneration in models of Gram-positive bacterial infections of the central nervous system. Biochem Soc Trans. 2007;35(5):1166–1167. doi: 10.1042/BST0351166 [DOI] [PubMed] [Google Scholar]
- 24.Tang C-H, Hsu C-J, Yang W-H, Fong Y-C. Lipoteichoic acid enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, PKCδ and c-Src dependent pathways. Biochem Pharmacol. 2010;79(11):1648–1657. doi: 10.1016/j.bcp.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 25.Walker AW, Duncan SH, McWilliam Leitch EC, Child MW, Flint HJ. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl Environ Microbiol. 2005;71(7):3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HV, Frassetto A, Kowalik EJ, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. Brennan L, ed. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141(5):874–880. doi: 10.1038/sj.bjp.0705682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soret R, Chevalier J, De Coppet P, et al. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology. 2010;138(5):1772–1782.e4. doi: 10.1053/j.gastro.2010.01.053 [DOI] [PubMed] [Google Scholar]
- 29.Nyavor Y, Brands C, Cox KK, Starks K, Balemba OB. 552 High Fat Diet-Microbiota-Host Interactions Result in the Production of Substances That Cause Intestinal Dysmotility and Enteric Neuropathy in Type Two Diabetic Mice. Gastroenterology. 2016;150(4):S116–S117. doi: 10.1016/S0016-5085(16)30498-X [DOI] [Google Scholar]
- 30.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19(12):951–960. doi: 10.1111/j.1365-2982.2007.01023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fournel A, Drougard A, Duparc T, et al. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 2017;66(2):258–269. doi: 10.1136/gutjnl-2015-310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abot A, Lucas A, Bautzova T, et al. Galanin enhances systemic glucose metabolism through enteric Nitric Oxide Synthase-expressed neurons. Mol Metab. 2018;10:100–108. doi: 10.1016/j.molmet.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22(4):658–668. doi: 10.1016/j.cmet.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soares A, Beraldi EJ, Ferreira PEB, Bazotte RB, Buttow NC. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015;15(1):3. doi: 10.1186/s12876-015-0228-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS One. 2012;7(3):e33865. doi: 10.1371/journal.pone.0033865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl:4680–4687. doi: 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenkamp-Strahm CM, Nyavor YEA, Kappmeyer AJ, Horton S, Gericke M, Balemba OB. Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res. 2015;361(2):411–426. doi: 10.1007/s00441-015-2132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25(7):826–831. doi: 10.1016/S0891-5849(98)00163-4 [DOI] [PubMed] [Google Scholar]
- 39.Brown IAM, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric Glia Mediate Neuron Death in Colitis Through Purinergic Pathways That Require Connexin-43 and Nitric Oxide. Cell Mol Gastroenterol Hepatol. 2016;2(1):77–91. doi: 10.1016/j.jcmgh.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26(11):1586–1596. doi: 10.1111/nmo.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 42.Brown IAM, Gulbransen BD. The antioxidant glutathione protects against enteric neuron death in situ, but its depletion is protective during colitis. Am J Physiol Liver Physiol. 2018;314(1):G39–G52. doi: 10.1152/ajpgi.00165.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei B, Mace B, Dawson HN, Warner DS, Laskowitz DT, James ML. Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia. Peterson KE, ed. PLoS One. 2014;9(7):e103969. doi: 10.1371/journal.pone.0103969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am J Pathol. 2013;182(2):375–387. doi: 10.1016/j.ajpath.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nezami BG, Mwangi SM, Lee JE, et al. MicroRNA 375 Mediates Palmitate-Induced Enteric Neuronal Damage and High-Fat Diet-Induced Delayed Intestinal Transit in Mice. Gastroenterology. 2014;146(2):473–483.e3. doi: 10.1053/j.gastro.2013.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344–356. doi: 10.1172/JCI26295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24(12):4948–4959. doi: 10.1096/fj.10-164921 [DOI] [PubMed] [Google Scholar]
- 49.Rivera LR, Leung C, Pustovit R V, et al. Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high-fat diet. Neurogastroenterol Motil. 2014;26(8):1188–1199. doi: 10.1111/nmo.12385 [DOI] [PubMed] [Google Scholar]
- 50.Larsen N, Vogensen FK, van den Berg FWJ, et al. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. Bereswill S, ed. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silhavy TJ, Kahne D, Walker S. The Bacterial Cell Envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414-a000414. doi: 10.1101/cshperspect.a000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long EM, Millen B, Kubes P, Robbins SM. Lipoteichoic Acid Induces Unique Inflammatory Responses when Compared to Other Toll-Like Receptor 2 Ligands. Hartl D, ed. PLoS One. 2009;4(5):e5601. doi: 10.1371/journal.pone.0005601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen MB. The Enteric Nervous System II: Gastrointestinal Functions. Pharmacol Toxicol. 2003;92(6):249–257. doi: 10.1034/j.1600-0773.2003.920601.x [DOI] [PubMed] [Google Scholar]
- 54.Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20 Suppl 1:32–38. doi: 10.1111/j.1365-2982.2008.01094.x [DOI] [PubMed] [Google Scholar]
- 55.Vrieze A, Van Nood E, Holleman F, et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology. 2012;143(4):913–916.e7. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 56.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 57.Udayappan SD, Hartstra AV., Dallinga-Thie GM, Nieuwdorp M Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin Exp Immunol. 2014;177(1):24–29. doi: 10.1111/cei.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the Gut Microbiota Short-Chain Fatty Acids as Key Pathophysiological Molecules Improving Diabetes. Mediators Inflamm. 2014;2014:1–9. doi: 10.1155/2014/162021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107. doi: 10.1111/nmo.12236 [DOI] [PubMed] [Google Scholar]
- 60.De Vadder F, Grasset E, Mannerås Holm L, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci. 2018;115(25):6458–6463. doi: 10.1073/pnas.1720017115 [DOI] [PMC free article] [PubMed] [Google Scholar]