Abstract
A 43-year-old male patient on maintenance hemodialysis had an enhanced computed tomography scan examination with iohexol for the first time 10 min before regular hemodialysis therapy. At the start of hemodialysis, no symptoms were observed, and the platelet count was 148,000/μl. Approximately 1 h after starting hemodialysis, dyspnea and chest discomfort appeared. Since oxygen saturation of the peripheral artery decreased to 87%, oxygen administration was immediately started while continuing hemodialysis therapy. Furthermore, gingival hemorrhage was observed, and the platelet count decreased to 5000/μl. We were carefully monitoring his conditions while continuing hemodialysis and oxygen administration, but no further deterioration was observed. Thereafter, these symptoms and severe thrombocytopenia gradually improved without additional treatment. At the end of hemodialysis, these symptoms completely disappeared. As well, the platelet count recovered to 35,000/μl at the end of hemodialysis and increased to 92,000/μl the next morning. From the clinical course, we diagnosed with contrast medium-induced thrombocytopenia. Acute thrombocytopenia is a rare complication induced by the contrast medium. Until now, 16 cases on contrast medium-induced thrombocytopenia have been reported. Our case spontaneously recovered from severe thrombocytopenia relatively earlier than previous reports. Our patient started hemodialysis therapy 10 min after an enhanced computed tomography examination. Early removal of contrast medium by hemodialysis might be associated with early improvement. We should acknowledge that contrast media have potential to induce severe thrombocytopenia, even in patients on maintenance hemodialysis.
Keywords: Contrast medium, Iohexol, Thrombocytopenia, Hemodialysis, Adverse effect
Introduction
Contrast media, also known as contrast agents, are commonly used to enhance the contrast of structures or fluids within tissues in medical imaging examinations. Contrast media also enables detection of abnormalities more clearly. However, use of contrast media is not completely devoid of risk, although currently available contrast media are generally considered to be relatively safe. Adverse complications induced by the administration of contrast media vary from minor physiological and mild allergic reactions to severe life-threatening events. Most of these are mild non-life-threatening events that usually require only observation, reassurance, and/or supportive measures. However, severe and potentially life-threatening adverse events can rarely and unpredictably be experienced. Contrast-induced nephropathy, characterized by development of acute renal injury after exposure to contrast media, is a serious condition, which is likely to develop in patients with renal dysfunction. Patients on maintenance hemodialysis can receive intravascular contrast medium without risk of further renal damage because their kidneys are no longer functioning. The American College of Radiology manual on contrast media states that there is no need for urgent dialysis after intravascular iodinated contrast medium administration, unless an unusually large volume of contrast medium is administered, or there is substantial underlying cardiac dysfunction [1]. Therefore, we tend to underestimate the risk of using contrast media in patients on maintenance dialysis. However, even in patients on maintenance dialysis, a variety of adverse reactions induced by contrast media occur, as well as in those without renal dysfunction. Therefore, the risk of using contrast media needs to be recognized and we need to know how to treat these patients properly.
Acute thrombocytopenia is a contrast medium-induced serious complication, but its incidence is rare. We report here a case of a 43-year-old male patient on maintenance hemodialysis who developed severe acute thrombocytopenia after his first exposure to intravenous non-ionic contrast medium.
Case report
A 43-year-old Japanese man with end-stage renal failure was hospitalized because of general fatigue, loss of appetite, and considerable body weight loss. He had regularly undergone maintenance hemodialysis at another hospital because of chronic renal failure from chronic glomerulonephritis for approximately 1 year. He had no history of blood disease or autoimmune disease. Since starting hemodialysis therapy, he continuously had general fatigue and loss of appetite, and then lost 14 kg in body weight in 1 year. Because of the reduction in his body weight, he attempted to appropriately adjust his dry weight, but this was insufficient. At admission to our hospital, electrocardiography and echocardiography examinations did not indicate the possibility of ischemic heart diseases. After admission, an enhanced computed tomography (CT) examination of the chest and abdomen was performed with a total of 150 ml of a non-ionic low osmolality contrast agent, iohexol. This examination was performed to rule out the complication of any malignant diseases or infectious diseases. The CT examination did not show any evidence of these diseases. The patient had never used a contrast medium before this time. During the enhanced CT scan examination, no specific symptoms were observed. Approximately 10 min later, he started regular hemodialysis therapy and his blood was drawn for a routine examination (Table 1). On examination at the beginning of hemodialysis, his blood pressure and pulse rate were 135/85 mmHg and 62 beats per minute, respectively. His temperature, respiratory rate, and oxygen saturation were normal. The platelet count, which was 244,000/μl in a routine examination at another hospital 2 months previously, was 148,000/μl (Fig. 1). He started hemodialysis with a polysulfone dialyzer, APS-21SA (Asahi Kasei Medical, Tokyo, Japan), and low molecular weight heparin of a 1000 U bolus, followed by 1000 U/h infusion, according to his previous hemodialysis condition. One hour after starting hemodialysis therapy, he complained of dyspnea and chest discomfort. At that time, his blood pressure was 105/64 mm Hg, pulse was 60 beats per minute, and oxygen saturation of the peripheral artery had decreased to 87%. No fever, urticaria, itches, or rash was observed. Electrocardiography did not show any specific abnormalities. Oxygen administration was immediately started. Two hours later, gingival hemorrhage appeared and severe thrombocytopenia with a platelet count of 5000/μl was simultaneously observed. We carefully monitored his condition while he continued hemodialysis therapy and oxygen administration, but no further deterioration of his condition was observed. Thereafter, his symptoms gradually improved without any specific treatment, except for hemodialysis and oxygen administration. At the end of hemodialysis, his symptoms had completely disappeared, and the platelet count spontaneously recovered to 35,000/μl (Fig. 1). From the next day onward, these contrast media-induced symptoms were not observed. The platelet count recovered to 92,000/μl on the following day and then increased to 139,000/μl 4 days later. As the changes in other blood cell counts except the platelet count, the white blood cell counts were temporarily increased at the end of hemodialysis and, then, were spontaneously recovered as well (Table 2). The level of platelet-associated immunoglobulin G (PAIgG) in this patient was 28.6 ng/107 platelets (normal range: < 30.2 ng/107 platelets). Anti-cardiolipin antibody and anti-nuclear antibody were negative, and the immature platelet proportion was not increased to 2.9%. This patient had no other diseases that could cause thrombocytopenia. In addition, the onset of severe thrombocytopenia in this patient appeared to be associated with the administration of contrast medium, considering from his clinical course. Thus, we finally diagnosed with contrast medium-induced severe thrombocytopenia. He was discharged on the 4th day after reducing his body weight to his appropriate dry weight. He has not recurred thrombocytopenia thereafter.
Table 1.
Laboratory data at the start of hemodialysis
| Peripheral blood | Blood chemistry | ||
|---|---|---|---|
| Hematocrit | 33.3% | Total protein | 6.6 g/dl |
| Hemoglobin | 11.3 g/dl | Albumin | 4.0 g/dl |
| White blood cells | 6500/μl | Aspartate aminotransferase | 7.0 IU/l |
| Neutrophils | 64.8% | Alanine aminotransferase | 6.0 IU/l |
| Lymphocytes | 21.5% | Lactate dehydrogenase | 146 IU/l |
| Eosinophil | 4% | Alkaline phosphatase | 123 IU/l |
| Platelets | 148,000/μl | Sodium | 136 mmol/l |
| Chloride | 101 mmol/l | ||
| Potassium | 4.8 mmol/l | ||
| Urea nitrogen | 38.7 mg/dl | ||
| Creatinine | 12.56 mg/dl | ||
| Uric acid | 4.3 mg/dl | ||
| Calcium | 8.6 mg/dl | ||
| Phosphorus | 4.6 mg/dl | ||
Fig. 1.
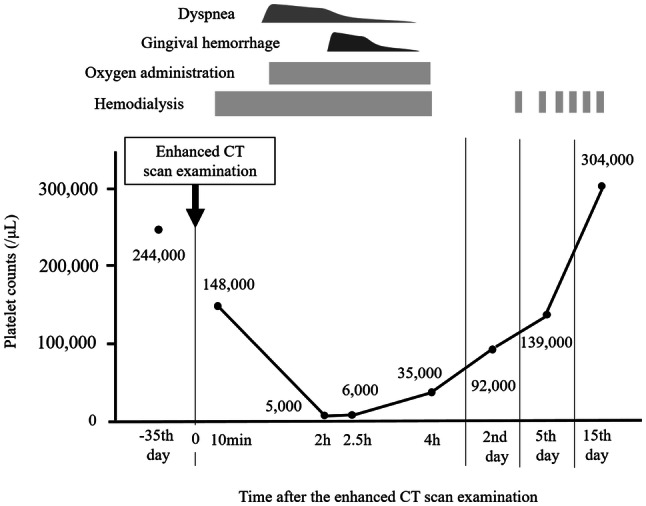
A clinical course after an enhanced CT scan examination. CT computed tomography
Table 2.
Changes in blood cell counts before and after contrast medium infusion
| − 35 day | 10 min | 2 h | 2.5 h | 4 h | 2nd day | 5th day | 15th day | |
|---|---|---|---|---|---|---|---|---|
| PLTs(× 103/μl) | 244 | 148 | 5 | 6 | 35 | 92 | 135 | 304 |
| Ht (%) | 37.1 | 33.3 | 33.6 | 32.9 | 36.6 | 34.6 | 33.9 | 37.9 |
| Hb (g/dl) | 12.2 | 11.3 | 11.3 | 11.1 | 12.7 | 11.4 | 11.2 | 12.5 |
| RBC (× 104 /μl) | 389 | 357 | 357 | 352 | 398 | 366 | 360 | 401 |
| WBC(/μl) | 6,000 | 6,500 | 4,200 | 5,200 | 10,000 | 7,300 | 5,300 | 6,800 |
| Seg/neut (%) | 70.6 | 64.8 | 72.9 | 81.0 | 75.3 | 57.3 | 59.7 | 71.7 |
| Eosin (%) | 3.7 | 4.0 | 0.7 | 1.0 | 1.4 | 4.8 | 4.9 | 3.7 |
| Baso (%) | 0.5 | 0.5 | 0.2 | 0.2 | 0.2 | 0.5 | 0.8 | 0.7 |
| Lymph (%) | 18.8 | 21.5 | 24.5 | 15.9 | 13.5 | 27.2 | 27.0 | 17.3 |
| Mono (%) | 6.4 | 9.2 | 1.7 | 1.9 | 9.6 | 10.2 | 7.6 | 6.6 |
Discussion
Contrast-induced adverse reactions include a variety of manifestations, such as skin rashes, nausea, anaphylaxis, pulmonary edema, and nephropathy. Acute severe thrombocytopenia is an extremely rare and severe adverse complication induced by contrast media. To the best of our knowledge, 16 cases of this serious adverse reaction have been reported with different types of contrast media since the early 1980s (Table 3) [2–17]. In most patients, any symptoms, including bleeding, fever, chills, dyspnea, wheezing, abdominal pain, and blood pressure variability, were observed within 24 h after contrast administration and the platelet count was decreased to below 10,000/μl. The lowest reported value of the platelet count was 0/μl [16]. With regard to treatment, high-dose intravenous corticosteroids, which are widely used in management of anaphylaxis, were used in nine patients [2, 4, 6, 7, 10, 12, 13, 16, 17]. Eight patients received platelet transfusions [3, 5–8, 10, 12, 17]. However, five patients spontaneously recovered their platelet count in 1–9 days without any treatment [3, 9, 11, 14, 15]. For the underlying disease of patients, three patients were on maintenance hemodialysis [3, 11, 16] and five had renal dysfunction [3, 4, 6, 7, 10]. Taken together with our case and previous reports, patients with renal dysfunction appear to be at higher risk for contrast medium-induced thrombocytopenia.
Table 3.
Summary of our case and previous cases reported
| References | Age/sex | Contrast media | Nadir platelet counts | Time after contrast medium infusion | Coexisting kidney disordera | Treatment | Recovery time |
|---|---|---|---|---|---|---|---|
| Our case | 43 M | Iohexol | 5000 | 2 h | HD | HD | 4 h |
| 2 | 29 M | Diatrizoate | 4000 | Next day | NA | Steroid | NA |
| 3 | 57F | Diatrizoate | 21,000 | Next day | HD | (–) | 9 days |
| 3 | 60 M | Diatrizoate | 6000 | 12 h | AKI | PT | 4 days |
| 4 | 79 M | Diatrizoate | 2000 | Next day | AKI | PD, steroid | 8 days |
| 5 | 66F | Diatrizoate | 9000 | 3 h | NA | PT | Next day |
| 6 | 52 M | Diatrizoate | 8000 | 4 h | AKI | PT, steroid | NA |
| 7 | 66F | Iopamidol | 1000 | 2 h | AKI | HD, PT, steroid | NA |
| 8 | 50 M | Iopamidol | 19,000 | 5 h | NA | PT | NA |
| 9 | NA/F | Iopamidol | 8000 | 48 h | NA | (–) | NA |
| 10 | 70 M | Iopamidol | 5000 | 3 h | CKD | PT, steroid | Next day |
| 11 | 74 M | Iopamidol | 30,000 | 1 h | HD | (–) | Next day |
| 12 | 72 M | Ioversol | 2000 | Next day | (–) | PT, steroid | Next day |
| 13 | 22F | Iopamidol | 4000 | Several hours | (–) | Steroid | Next day |
| 14 | 75 M | Iodixanol | 9000 | 4.5 h | (–) | (–) | 3 days |
| 15 | 71F | NA | 1000 | 6 h | (–) | (–) | 4 days |
| 16 | 47 M | Ioversol | 0 | 6 h | HD | steroid | NA |
| 17 | 63 M | Ioversol | 2000 | 6 h | (–) | PT, steroid | Next day |
PD peritoneal dialysis, PT platelet transfusion, NA data not available
aPatients with chronic kidney disease (CKD), acute kidney injury (AKI), or on maintenance hemodialysis (HD) when contrast medium is administered
The mechanism of contrast medium-induced thrombocytopenia remains unclear. An immunological mechanism is proposed as a plausible mechanism [3]. According to this hypothesis, high-dose intravenous corticosteroids have been used as a treatment in some reports. Some reports have shown that radiocontrast media induce excessive coagulation and activate the complement cascade [18–22], which result in abnormal platelet aggregation with resultant thrombocytopenia. In our case, iohexol (non-ionic low osmolar contrast media) as a contrast medium for enhanced CT examinations was used. Adverse symptoms appeared in our patient approximately 2 h after administration of contrast medium. The nadir platelet count was 5000/μl. However, his symptoms and platelet count spontaneously recovered without any specific treatment, except for hemodialysis and oxygen administration. The time required for recovery was 4 h, which is relatively shorter than that in other previous cases. In our case, he coincidentally started regular hemodialysis therapy approximately 10 min after an enhanced CT examination. Most contrast media must be readily eliminated from the body by hemodialysis therapy. Therefore, removal of contrast media by hemodialysis therapy might be associated with early recovery from severe thrombocytopenia. Further accumulation of evidence is required to prove this possibility.
There is a large number of contrast-mediated diagnostic and therapeutic procedures worldwide. Therefore, complications induced by contrast media need to be recognized and any changes in the general condition need to be carefully observed over several hours after the use of contrast agent, even in maintenance hemodialysis.
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Human animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
We have received a permission and written informed consent on the publication of this case report from this patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American College of Radiology. ACR manual on contrast media. v10.3. American College of Radiology, 2018; pp. 33–40. ISBN: 978-1-55903-012-0
- 2.Wein P, Handler M, Chadda KD. Severe thrombocytopenia as a result of contrast left ventricular angiography. Cathet Cardiovasc Diagn. 1982;8(5):495–499. doi: 10.1002/ccd.1810080511. [DOI] [PubMed] [Google Scholar]
- 3.Shojania AM. Immune-mediated thrombocytopenia due to an iodinated contrast medium (diatrizoate) Can Med Assoc J. 1985;133(2):123. [PMC free article] [PubMed] [Google Scholar]
- 4.Lacy J, Bober-Sorcinelli KE, Farber LR, Glickman MG. Acute thrombocytopenia induced by parenteral radiographic contrast medium. AJR Am J Roentgenol. 1986;146(6):1298–1299. doi: 10.2214/ajr.146.6.1298. [DOI] [PubMed] [Google Scholar]
- 5.Chang JC, Lee D, Gross HM. Acute thrombocytopenia after i.v. administration of a radiographic contrast medium. AJR Am J Roentgenol. 1989;152(5):947–949. doi: 10.2214/ajr.152.5.947. [DOI] [PubMed] [Google Scholar]
- 6.Kudoh Y, Kijima T, Iimura O. Acute thrombocytopenia after intravenous infusion of radiographic contrast medium. Nihon Jinzo Gakkai Shi. 1991;33(9):885–888. [PubMed] [Google Scholar]
- 7.Wiemer M, Kreuzpaintner G, Lauer B, et al. Recurrent immune thrombocytopenia: a rare complication after contrast medium injection. Dtsch Med Wochenschr. 1995;120:1236–1240. doi: 10.1055/s-2008-1055470. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara T, Takenaka T, Horimoto K, Bannba C, Sakuma K. Thrombocytopenia induced by nonionic radiographic contrast medium. Rinsho Hoshasen. 1997;42:393–396. [Google Scholar]
- 9.Ural AU, Beyan C, Yalçin A. Thrombocytopenia following intravenous iopamidol. Eur J Clin Pharmacol. 1998;54:575–576. doi: 10.1007/s002280050516. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh T, Saiki M, Sawada U, Kawamura N, Tohno H, Horie T. Severe thrombocytopenia induced by radiographic non-ionic contrast medium. Rinosho Ketsueki. 2001;42(6):507–511. [PubMed] [Google Scholar]
- 11.Bonnard P, Sarraj A, Cagny B, Lassoued K, Slama M. Recurrent severe thrombocytopenia and non cardiogenic pulmonary edema following intravenous radiographic contrast medium. Eur J Radiol Extra. 2004;50:27–29. doi: 10.1016/j.ejrex.2003.11.001. [DOI] [Google Scholar]
- 12.Bata P, Tarnoki AD, Tarnoki DL, Horvath E, Berczi V, Szalay F. Acute severe thrombocytopenia following non-ionic low-osmolarity intravenous contrast medium injection. Korean J Radio. 2012;13(4):505–509. doi: 10.3348/kjr.2012.13.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCaulley JA, Deering SH, Pates JA. Severe thrombocytopenia after contrast infusion in pregnancy. Obstet Gynecol. 2013;121(2 Pt 2 Suppl 1):473–475. doi: 10.1097/aog.0b013e31826d67cb. [DOI] [PubMed] [Google Scholar]
- 14.Keach JW, Huang J, Garcia JA, Krantz MJ, Hass EE. Acute severe thrombocytopenia secondary to intraarterial low-osmolar iodinated contrast administered during coronary angiography. J Vasc Interv Radiol. 2014;25(12):2003–2005. doi: 10.1016/j.jvir.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira RM, Mansur Filho J, Villela PB, et al. Contrast-induced thrombocytopenia following percutaneous coronary intervention. J Saudi Heart Assoc. 2017;29(3):227–229. doi: 10.1016/j.jsha.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubero-Gómez JM, Guerrero Márquez FJ, Diaz de la-Llera L, Fernández-Quero M, Guisado-Rasco A, Villa-Gil-Ortega M. Severe thrombocytopenia induced by iodinated contrast after coronary angiography: the use of gadolinium contrast and intravascular ultrasound as an alternative to guide percutaneous coronary intervention. Rev Port Cardiol. 2017;36(1):61.e1–61.e4. doi: 10.1016/j.repc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Park M, Kim M, Park J, Cho J. Life-threatening thrombocytopenia following intravenous contrast media infusion. Yonsei Med. 2018;59(1):158–161. doi: 10.3349/ymj.2018.59.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szebeni J. Hypersensitivity reactions to radiocontrast media: the role of complement activation. Curr Allergy Asthma Rep. 2004;4:25–30. doi: 10.1007/s11882-004-0038-9. [DOI] [PubMed] [Google Scholar]
- 19.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Simon RA, Schatz M, Stevenson DD, et al. Radiographic contrast media infusions. Measurement of histamine, complement, and fibrin split products and correlation with clinical parameters. J Allergy Clin Immunol. 1979;63:281–288. doi: 10.1016/0091-6749(79)90114-3. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Gabriel DA. Differences between contrast media in the inhibition of platelet activation by specific platelet agonists. Acad Radiol. 1997;4:108–114. doi: 10.1016/S1076-6332(97)80157-2. [DOI] [PubMed] [Google Scholar]
- 22.Aspelin P, Stacul F, Thomsen HS, et al. Effects of iodinated contrast media on blood and endothelium. Eur Radiol. 2006;16:1041–1049. doi: 10.1007/s00330-005-0081-5. [DOI] [PubMed] [Google Scholar]


