Abstract
Electronic cigarettes (e-cigarettes) are battery-operated devices to insufflate nicotine or other psychoactive e-liquid aerosols. Despite initial claims of e-cigarettes as a nicotine-cessation device, aggressive marketing of e-cigarettes has led to an explosion in adolescents’ and young adults’ use over the last few years. Coupled with a lack of adequate investigation and regulation of e-cigarettes, the USA is facing an outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) starting in mid-2019. While little long-term health hazard data are available, the components and constituents of e-cigarettes may adversely impact health. Propylene glycol and glycerin are humectants (water-retaining excipients) that generate pulmonary irritants and carcinogenic carbonyl compounds (e.g., formaldehyde, acetaldehyde, and acrolein) when heated in e-cigarettes. Metals contained in heating coils and cartridge casings may leach metals such as aluminum, chromium, iron, lead, manganese, nickel, and tin. Flavoring agents are considered safe for ingestion but lack safety data for inhalational exposures. Diacetyl, a common buttery flavoring agent, has known pulmonary toxicity with inhalational exposures leading to bronchiolitis obliterans. In 2019, clusters of lung injury associated with e-cigarette use were identified in Wisconsin and Illinois. Patients with EVALI present with a constellation of respiratory, gastrointestinal, and constitutional symptoms. Radiographically, patients have bilateral ground glass opacifications. As of February 18, 2020, the Centers for Disease Control has identified 2807 hospitalized patients diagnosed with either “confirmed” or “probable” EVALI in the US. Currently, vitamin E acetate (VEA) used as a diluent in tetrahydrocannabinol vape cartridges is implicated in EVALI. VEA cuts tetrahydrocannabinol oil without changing the appearance or viscosity. When inhaled, pulmonary tissue lacks the mechanism to metabolize and absorb VEA, which may lead to its accumulation. While most EVALI patients were hospitalized, treatment remains largely supportive, and use of corticosteroids has been associated with clinical improvement. The outbreak of EVALI highlights the need for regulation of e-cigarette devices and e-liquids. Clinicians need to be aware of the health hazards of e-cigarettes and be vigilant in asking about vaping.
Keywords: Electronic cigarettes, Vaping, Lung injury, Tetrahydrocannabinol, Nicotine
Introduction
Electronic cigarettes (e-cigarettes) are battery-operated devices that allow users to vape (the act of insufflating from an e-cigarette) aerosols containing nicotine and flavors. Since their appearance on the market, newer generations of e-cigarettes have been designed with refillable liquid chambers, introducing the potential for a wide array of additives including delta-9-tetrahydrocannabinol (THC). With growing popularity, these devices have assumed many different names including cig-a-likes, e-hookahs, electronic nicotine delivery systems (ENDS), mods, and vapes.
E-cigarettes were originally marketed as a safer alternative to traditional combustible cigarettes although they still expose users to known toxins and carcinogens. While little data is available on the long-term effects of e-cigarette use, thousands of users have fallen acutely ill in an epidemic of e-cigarette, or vaping, product use-associated lung injury (EVALI) in the USA since March of 2019 [1]. The outbreak highlights the heterogeneity of the constituents of e-cigarette liquid (also called e-liquid or e-juice) and casts doubt on the long-term safety of e-cigarette use.
Vitamin E acetate (VEA, also known as alpha-tocopherol acetate) has been linked to EVALI though no definitive causal relationship has been established. As the investigation is ongoing, the number of fatalities is rising, and the magnitude of impact on public health is expected to be vast. In this review, we describe e-cigarettes basics, summarize established risks of vaping, illustrate characteristics of patients with EVALI, discuss potential causative agent(s) in the outbreak, and present proposed treatments.
Methods
Relevant studies on EVALI were identified through PubMed using the query “((Vaping OR E-cigarette) AND “Lung injury“) OR EVALI” and searched through February 8, 2020. Additional references were identified through search of publication bibliography and primary references posted on the Centers for Disease Control and Prevention (CDC) “Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products” webpage [1].
Epidemiology
Among all e-cigarette users, demographic data show that e-cigarette use is more prevalent among men, individuals who identify themselves as Hispanic or non-Hispanic white, and individuals with incomes at least 4 times the federal poverty level [2–4].
Since e-cigarettes first appeared in the USA in 2006, use of these products has steadily increased and has disproportionately affected the country’s youth. Several national surveys of middle and high school students including the National Youth Tobacco Survey, Youth Risk Behavioral Surveillance, and Monitoring the Future (MTF) have consistently demonstrated an upward trend of e-cigarette use [5]. According to the results of these studies, the use of e-cigarettes has grown exponentially in middle and high school students since 2014 with the greatest growth between 2017 and 2019. In December 2018, annual survey results from MTF showed 13.5% of 8th graders have used nicotine vape products in their lifetime with 6% of those surveyed reporting use in the preceding 30 days. These numbers increased to 34% and 20%, respectively, in the 12th grade population [6]. Strikingly, early surveys from 2019 demonstrate even higher rates of frequent and daily use of e-cigarette products in middle and high school students, showing that prevalence more than doubled between 2017 and 2019 [7]. As of September 18, 2019, 40.5% of 12th graders reported ever vaping nicotine [7]. In contrast, rates of daily combustible cigarette smoking were low, at only 0.8% in 8th graders and 3.6% in 12th graders. Similarly, use of alcohol, opiates, and illicit use of prescription medications had steadily declined over the preceding five years [6].
E-cigarette use among adults is predominantly in the younger 18- to 24-year-old demographic with less frequent to rare use in older demographics [2]. In a large cohort of adults in 2018, only 3.2% of adults surveyed reported using e-cigarettes, demonstrating a small overall increase in use from 2.8% in 2014 [2, 8]. Older adults switch to vaping as an exit strategy for combustible cigarette smoking. Conversely, in adolescents and young adults, vaping is a means of social inclusion, pure enjoyment of flavors and serves as a gateway to future combustible cigarette use [5, 9]. The contrast between adult and adolescent use is striking and highlights a need to better understand the reasons for this divide.
Devices
The basic functional components of an e-cigarette include a battery, heating coil, wick, cartridge containing e-liquid, and a mouthpiece that the user inhales through [5]. With activation of the heating element, either through pressure changes initiated by inhalation or manually pressing an activation button on the device, e-liquid saturated on a wick is aerosolized using a heating coil and is inhaled [5]. Although the act of inhaling these compounds is colloquially called “vaping,” this term is misleading as it is in fact a superheated complex aerosol of semi-liquid particulate matter, not a gaseous vapor, that the user takes in [10, 11].
E-cigarette devices continue to evolve to meet the ever-changing demands and desires of users, generating a vast selection of devices of different shapes, sizes, and capabilities. For simplicity, these devices are now classified by generation from first through fourth.
First-generation devices, known commonly as “cig-a-likes” or “vape sticks,” are disposable, non-refillable electronic devices that have an appearance very similar to a traditional nicotine cigarette or pipe. Their long slender appearance made them ideal for individuals seeking an alternative to combustible cigarettes, closely simulating the cigarette smoking experience [5].
With the advent of second-generation e-cigarettes, the compact design was sacrificed for bulkier devices with more powerful batteries, which are often three to seven times heavier than that used in the first-generation [12]. These pen-sized devices have a cartomizer or clearomizer, which is made up of a cartridge containing a larger quantity of the e-liquid, a heating element, and an atomizer. Appropriately so, these devices are known as “personal vaporizers,” “tank systems,” or “vape pens” [5].
Third-generation devices are more diverse in appearance and capability, and many no longer resembled the esthetic of traditional combustible cigarettes in the least [12]. Third-generation “mods” come with the capability to customize features to individualize the vaping experience [5]. These devices are highly technical, allowing individuals to modify the battery voltage, coils/wicking configurations, and components of the e-liquid with commercial or homemade formulations. Common modifications allow the user to add more coils or use coils with less resistance so that more heat, and subsequently denser aerosols, may be generated. “Mods” have become particularly popular among youth who are drawn to the highly technical designs.
The most recent fourth-generation devices are less well defined. Some sources describe fourth-generation devices as an extension of third-generation “mods” to increase customizability, while other sources describe fourth-generation as commercial “pod” vape devices resembling small electronic devices such as flash drives [9, 12]. Of the multiple brands of fourth-generation “pod” e-cigarette devices, JUUL® is the most popular, especially among youth [9]. In 2018, sale of these devices and pods made up almost three-quarters of the legal e-cigarette sales in the USA [9]. The compact design enables discrete use of the product, even in prohibited areas such as school classrooms. Additionally, the manufacturers of JUUL® have heavily advertised their vast array of flavors using tactics that appeal to a young audience [9].
E-Liquid Constituents and Potential Health Hazards
Challenges in E-Cigarette Research
Numerous factors in smoking aerosol of e-liquids make conducting up-to-date peer-reviewed research difficult. First, e-cigarette industries innovate to bring newer and more potent delivery systems with little to no regulation and/or standardization of production. Markets can change dramatically between the inception and publication of a well-conducted peer-reviewed study. Second, since users can fill their own clearomizers, the permutation of possible e-liquid combinations is innumerable. Third, modifiable e-cigarette devices add complexity and heterogeneity to the aerosols inhaled by end-users. The chemicals in e-liquids degrade in the heating process, and degree of degradation varies based on the resistance of the heating coils in combination with the voltage and power applied to the atomizer [13]. At temperatures as high as 350°C, chemical reactions between e-liquid constituents can occur (e.g., formation of benzene) [13, 14]. Gas chromatography-mass spectrometry (GC-MS) analysis of the flavored e-cigarette aerosol by Eddingsaas et al. identified 19 unique compounds, which were generated in heating and emissions process and not detected in the unheated e-liquid [15]. Finally, the current published results of thermal decomposition products of e-cigarettes show wide ranging and conflicting concentrations of toxins [10].
Nicotine E-Liquids
Nicotine e-liquids usually contain a combination of nicotine, propylene glycol (PG, also called 1,2-propanediol), glycerin (also called vegetable glycerin [VG] or glycerol), water, and/or flavoring. Nicotine added to most e-liquids is isolated from the tobacco plant, although synthetic tobacco has become available [16]. Tobacco-based nicotine is highly purified and distilled, but trace impurities including nicotine-related alkaloids and tobacco-specific nitrosamines (TSNAs) are impossible to completely remove [17]. TSNAs are known carcinogens. While the concentration of TSNAs in e-cigarettes is significantly less than that of combustible cigarette products, the carcinogenic effect on users is unknown.
Nicotine concentrations within e-liquids are highly variable. In a study of e-cigarette sales data between March 2013 to November 2018, nicotine concentrations of over 1350 e-liquid containing products ranged from 0 to 87.2 mg/mL [18]. Interestingly, products with higher concentrations of nicotine correlated with better product sales, and thus nicotine concentrations have steadily increased over the years [5].
In 2016, Pax Labs Inc., the company that developed JUUL® e-cigarettes, announced that they received a US patent for an e-cigarette nicotine salt formulation by combining liquid nicotine with an acid (e.g., benzoic acid, acetic acid, or lactic acid). The nicotine salts delivered through a temperature-regulated device theoretically permits more efficient nicotine absorption with each inhalation than the previous free-base nicotine solutions [19]. Additionally, a JUUL® pod contains 61.6 mg/mL of nicotine, one of the highest nicotine concentrations on the market, and yields higher serum blood nicotine concentrations than other e-cigarettes [14, 20]. As of October 1, 2019, JUUL® still captured 66.7% of US e-cigarette sales despite their decreasing market share since the end of 2018 [21].
The high concentrations of nicotine in e-liquids pose a threat to exploratory toddlers. Nicotine e-cigarette–related calls to the US poison control centers are most frequently due to exposures in the five years and younger age group; accounting for 64.8% of all e-cigarette exposure calls between 2010 and 2018 [22]. Death in an 18-month-old was reported where the toddler drank from an open container of e-cigarette nicotine liquid and was found to have large concentrations of nicotine and metabolites in cardiac blood and gastric contents [23]. The lethal nicotine dose ranges between 1 and 7 mg/kg, and due to the high concentration of e-cigarette liquid, a miniscule amount ingested by a child will be enough to cause fatality [23]. After an initial surge of reported cases from 2012 to 2014, the Child Nicotine Poisoning Prevention Act of 2015 and individual states’ legislations mandated child-resistant packaging on all nicotine containing e-liquids and devices [24]. Nonetheless, e-cigarette and e-liquid exposures resurged in nearly all age groups from 2017 to 2018 in part due to the skyrocketing popularity of JUUL® products [22].
Humectants in Nicotine E-Liquids
Humectants are excipients added to food and cosmetics to help retain water. PG and VG are common organic humectants for e-liquids. The concept of delivering nicotine via heated PG or VG has been around since the 1960s, but the twenty-first century’s improved battery technology and heightened commercialization and industrialization practices ultimately popularized e-cigarettes in the USA and Europe [25, 26]. PG is a common solvent in intravenous (IV) medications (e.g., lorazepam, phenytoin) but is also considered a “toxic alcohol” at high doses [27]. The Food and Drug Administration (FDA) considers both PG and VG as “Generally Recognized as Safe” (GRAS) [28]. However, the GRAS designation applies only to dermal application or ingestion and does not address or imply the safety of inhalation exposure to these products or their thermal degradation products [5]. Chronic low dose exposure to PG may cause pulmonary irritation and allergic reactions [29]. Furthermore, PG/VG-containing e-liquids, when heated, generate pulmonary irritants as well as known and suspected carcinogenic carbonyl compounds (formaldehyde, acetaldehyde, and acrolein) [13, 14]. As mentioned previously, the generation of carbonyl compounds in heated e-liquids vary highly upon the specific brand of e-liquid and device used [5]. Although the full extent of damage of inhaling PG/VG is not fully known, some studies suggest PG/VG can cause significant damage. An animal study where mice inhaled e-cigarette smoke of nicotine and VG demonstrated a statistically significant increase in the development of lung adenocarcinoma and bladder urothelial hyperplasia [30]. Also, two randomized clinical trials of acute vaping of PG/VG with or without nicotine in young tobacco smokers induced airway epithelial injury and sustained decrease in transcutaneous oxygen tension [31].
Other potentially harmful humectants have been detected in nicotine-containing e-cigarettes, including diethylene glycol, ethylene glycol, and 1,3-propanediol [32–34]. However, a recent study out of Switzerland that tested common e-liquids sold out of the USA, UK, France, and Switzerland did not detect ethylene glycol, and the recent study by Etter et al. did not detect diethylene glycol [33, 34]. These studies highlight the variable and dynamic nature of e-liquids.
THC E-Liquids
Prevalence of vaping with cannabis products such as THC oil or loose-leaf marijuana has been steadily increasing. Nineteen percent of 18- to 24-year-old ever cannabis users reported vaping cannabis products [35]. E-cigarette users can make their own cartridge by filling it with various THC-containing products (oil, tincture, or concentrate) which may also contain propylene glycol or other excipients. Unfortunately, due to the wide variability of these products and accessibility, determination of the exact constituents in THC-containing products is difficult, if not impossible. Some individuals have described vaping THC in e-cigarettes as “dabbing,” and thus, the CDC included “dabbing” in the epidemiologic definition of EVALI, discussed below. However, “dabbing” is better known as a method of aerosolizing desired inhalants, most commonly cannabis concentrates. The wax form of a concentrate is applied or “dabbed” onto a heated nail attached to a modified water pipe. The aerosol is contained via a dome placed over the nail and passed through the water pipe and inhaled by the user [36].
Other Psychoactive E-Liquids
Multiple websites propose the addition of ethanol and caffeine to e-cigarettes. However, one study that looked at the effect of ethanol-containing e-cigarettes on young adult smokers showed no difference in subjective drug effects, and plasma alcohol levels were undetectable during testing, even when vaping a 23.5% ethanol concentration solution. The authors noted that there was not enough data to make definitive conclusions on the effects of vaping ethanol, but they strongly advised against its use [37]. Similarly, caffeine e-liquids have much lower concentrations of caffeine (3.3 to 703 μg/g) than traditional caffeinated drinks. The estimated daily caffeine-absorbed dose from most vaping is less than 1 mg with the highest at 27.9 mg of caffeine [38]. Other possible substances in e-liquids include, but are not limited to, powdered cocaine, ecstasy, hallucinogens, heroin, methamphetamine, prescription pain medications, and prescription stimulants [39, 40].
Flavoring Agents
Flavoring agents in e-liquids pose another threat to health. The addition of flavoring agents enhances appeal and reduces the risk perception of e-cigarettes in youth and nascent smokers, which is in part contributory to the discussed epidemiologic trend of youth e-cigarette use [41]. Similar to PG/VG, these agents are designated GRAS by the FDA but have limited inhalational exposure safety data [42]. For example, diacetyl (butanedione or butane-2,3-dione), a chemical with an intense buttery flavor, has known pulmonary toxicity causing bronchiolitis obliterans or “popcorn lung” in microwave popcorn workers via inhalation exposure [43]. Multiple studies have detected diacetyl and its diketone analogue, 2,3-pentanedione, in vape e-liquids [44, 45]. Although the exposure to diketones in flavored e-cigarette products is avoidable and unnecessary, the estimated quantitative exposure remains lower than that of combustible cigarettes. By one estimate, diacetyl generated as a combustion degradation product in traditional cigarettes may be 100-fold greater than that present in e-cigarettes [45]. Currently, no guidelines establish an acceptable risk for inhalation of diacetyl or its analogs in e-cigarettes. Extrapolating occupational risk levels of diacetyl to estimates of vaping exposure is fraught with potentially inaccurate assumptions, especially when applied to adolescents.
Metals in E-Liquids
E-cigarette device components that contact the e-liquid can leach metals into the e-liquid and subsequently, the aerosol. Additionally, cannabis plants, used to derive THC, absorb metals from the soil [46]. While studies have demonstrated detectable metal concentrations in e-liquids and aerosols such as aluminum, chromium, iron, lead, manganese, nickel, and tin, long-term studies are lacking to define the associated health risk of vaping posed by these metals [47]. E-cigarettes have a higher number of detectable metals (35 of out 36 tested elements) as compared with combustible cigarettes (15 out of 36 elements) [48]. Cadmium, a direct pulmonary toxin, was lower in e-cigarette aerosol (below level of detection at < 0.1 μg/kg) than in combustible cigarette smoke (between < 5 and 80 ng per cigarette), but other metals were comparable or higher [47].
Microbial Contaminants
Microbial agents in the form of bacterial endotoxin (lipopolysaccharides) and fungal (1,3)-beta-D-glucans have been detected in e-liquids [49]. However, live bacteria, mold, and yeast have not been found [34]. While endotoxin and glucans may cause respiratory irritation, long-term consequences at concentrations found in e-liquids have yet to be studied.
E-Liquid Legislation
On December 19, 2019, the Tobacco 21 bill was signed into a law that set legal age to purchase tobacco and e-cigarettes products to 21 years of age throughout the USA [50]. On January 2, 2020, the FDA issued a policy to prioritize enforcement against illegally marketed ENDS, specifically any flavored, cartridge-based ENDS product (other than a tobacco- or menthol-flavored ENDS product); all other ENDS products for which the manufacturer has failed to take (or is failing to take) adequate measures to prevent minors’ access; and any ENDS product that is targeted to minors or likely to promote use of ENDS by minors. It is not specifically a ban on flavored or cartridge-based ENDS but rather a way for companies to demonstrate that a product meets the applicable standards set by Congress. The policy is also a way to increase education for the prevention of e-cigarette usage by youth [51].
Health Risks of Nicotine E-Cigarette Use
The National Academies of Science, Engineering, and Medicine reviewed the health literature of e-cigarettes in 2018 and provided evidence-based summary of health concerns [5]. The report on long-term health effects was limited by the absence of studies rather than the presence of negative studies.
Cardiovascular Effects
The report found no evidence on long-term cardiovascular outcomes such as coronary heart disease, stroke, and peripheral artery disease and insufficient evidence for long-term effects on heart rate, blood pressure, and cardiac function [5]. In the short term, nicotine increases heart rate and diastolic blood pressure [5]. Since the report, cross-sectional National Health Interview Surveys of daily e-cigarette smokers found a statistically significant increase odds ratio of myocardial infarction [52]. Other new studies in abstract form also suggest an association with myocardial infarction as well as an association with strokes [53].
Pulmonary Effects
The report also found no long-term evidence that e-cigarette use causes respiratory disease in humans [5]. The authors did find moderate evidence for increased asthma exacerbation, cough, and wheezing in adolescents who smoke e-cigarettes. The findings are further supported by two cross-sectional studies published in 2017 that suggest associations of e-cigarette use in adolescents with asthma exacerbation and chronic bronchitis [54, 55].
Prior to the current EVALI outbreak, scattered case reports and case series described cohorts of patients with a range of radiographic findings of pulmonary disease in the setting of e-cigarette use. The variation in radiographic patterns of pulmonary disease associated with vaping was summarized by Landman et al. and Henry et al. in a literature review spanning nearly two decades [56–58]. Lung injury with an organizing pattern (e.g., organizing pneumonia, acute fibrinous pneumonitis with organization) was the most common finding on their reviews though hypersensitivity pneumonitis, diffuse alveolar damage, and lipoid pneumonia were also reported in the literature [56–58].
Carcinogenicity
Similarly, no long-term studies on carcinogenicity were available at the time of publication [5]. Growing evidence is available on the carcinogenic potential of e-cigarette aerosol (e.g., carbonyl compounds and benzene) and on the deregulation of cancer-associated genes [53].
Secondhand Exposure
While secondhand exposure to combustible cigarettes has known health risks, the data surrounding secondhand exposure to the aerosols from vaping is limited. Secondhand smoke from combustible cigarettes differs from e-cigarette aerosol in genesis and constituents. Combustible cigarettes generate smoke that is mostly solid and semisolid material, whereas e-cigarette generates a semi-liquid aerosol. Additionally, 80% of secondhand exposure from combustible cigarettes occurs from the side-stream originating from the burning cigarette whereas nearly 100% of the e-cigarette aerosol is inhaled by the user in the mainstream with little to no side-stream. The resulting secondhand exposure originates from the exhaled vapors [5]. Finally, e-cigarette aerosol varies by the e-liquid constituent and the voltage applied to the vaping device as discussed above. Given these differences, health consequences from secondhand e-cigarette aerosol are not comparable with secondhand smoke.
Most studies have evaluated the chemical and environmental characteristics of secondhand e-cigarette aerosol rather than long-term clinically relevant endpoints. Studies of air quality after vaping demonstrate increased air concentrations of carbon dioxide, carbonyls, nicotine, particulate matter (ultrafine particles, 2.5 μm or less [PM2.5] and 10 μm or less [PM10]), and volatile organic compounds (e.g., benzene, diacetyl, and toluene) [5, 59]. One recent unblinded crossover study of secondhand aerosol exposure found increased frequency of respiratory irritation symptoms, breathlessness, and headaches [60]. The study was limited by the unblinded design, proximity (1.5 m away), and short duration of exposure (30 min). In a neonatal mouse model, exposure to 1.8% nicotine and PG e-cigarette aerosol impaired alveolar development at 10 days of life and negatively impacted weight gain [61]. While these studies suggest negative health consequences from secondhand e-cigarette aerosol, long-term studies and comparative studies with secondhand combustible cigarette smoke are needed.
Battery Risks
The batteries used in e-cigarettes have become more sophisticated and powerful as e-cigarettes have evolved; allowing users to alter power delivered to the coils and customize the vaping experience. Larger batteries allow for increased power delivery and extended use time but increased risk of serious injury. Thermal burns, alkali burns (from lithium hydroxide), house fires, and fatalities have been reported from e-cigarette use [62]. Battery malfunction occurs for several reasons including faulty casing, over-heating, over-charging, exposure to perspiration (e.g., from pant pockets), or short-circuit by metal contact (e.g., with coins or keys) [62].
E-Cigarette, or Vaping, Product Use-Associated Lung Injury
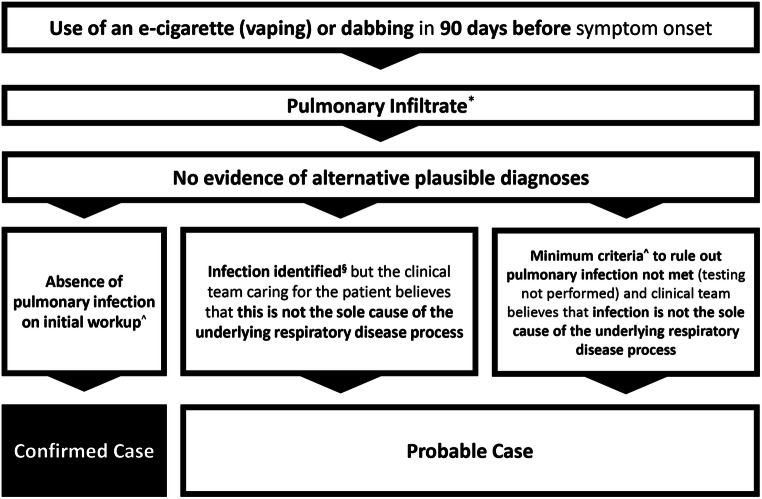
Since the introduction of e-cigarettes to China in 2004 and to the USA in 2006, cases of lung injury have been associated with their use as mentioned previously [56, 58]. The severity and consistent association of lung injury with e-cigarette use became more broadly recognized in July 2019 as the health departments of Wisconsin and Illinois published a case series of 53 patients presenting with pulmonary illnesses related to e-cigarette use [63]. Investigators at the Wisconsin and Illinois Departments of Health and the CDC used these patient data to generate case definitions to assist with disease surveillance in anticipation of a larger scale outbreak [63, 64]. What was first described as vaping-associated pulmonary injury is now termed EVALI [65]. “Confirmed” cases of EVALI include patients reporting use of an e-cigarette, “vaping,” or “dabbing” in the 90 days prior to symptom onset; pulmonary infiltrate on plain film chest radiograph or ground-glass opacities on chest computed tomography (CT); and absence of pulmonary infection or other plausible diagnoses. “Probable” cases are those meeting the above criteria but with findings of a pulmonary infection which the clinical team believes is not the sole cause of the underlying lung injury (Fig. 1) [64].
Fig. 1.
Centers for Disease Control and Prevention surveillance case definitions for e-cigarette, or vaping, product use-associated lung injury [64].*Opacities on plain-film radiograph of the chest or ground-glass opacities on chest computed tomography. ^Minimum criteria include negative respiratory viral panel and influenza polymerase chain reaction (PCR) or rapid test if local epidemiology supports testing. All other clinically indicated testing for respiratory infectious disease (e.g., urine antigen testing for Streptococcus pneumoniae and legionella, sputum culture if productive cough, bronchoalveolar lavage culture if done, blood culture, and presence of HIV-related opportunistic respiratory infections if appropriate) must be negative. §Identified by means of culture or PCR
Accurate and updated epidemiologic data remains dependent on voluntary reporting by state health departments. State and national task forces have coordinated their efforts for case reporting and monitoring, most notably state health departments, the Council of State and Territorial Epidemiologists, Vaping Associated Pulmonary Injury Epidemiology Task Force, and the CDC. Complicating these efforts are incomplete or inaccurate social histories because they are obtained through proxies (e.g., spouses, parents) as many patients are too critically ill, or reluctant to admit illicit substance use, to participate in information gathering.
Patient Characteristics
As of February 18, 2020, 2807 patients have been hospitalized with “confirmed” or “probable” EVALI reported to the CDC, including 64 (2%) associated deaths [1]. CDC last reported non-hospitalized cases in November 2019. At that time, only 110 (5%) cases out of 2016 were managed as outpatients [66]. Of the cases through December 2019, the median age was 24 years old, and most were white (75%) and male (67%) [67]. No cases have been reported in pregnant women. EVALI has disproportionately affected young adults and adolescents with 76% of patients aged < 35 years old and 15% < 18 years [68]. These demographics reflect the overall trends in e-cigarette use.
Patient deaths from EVALI has exhibited a similar racial distribution in comparison to e-cigarette users with a greater proportion of White or Caucasian patients (83%) compared with Hispanic (11%) and non-Hispanic, non-White patients (6%). The median age in EVALI-related deaths has been higher than the median age in EVALI-affected patients overall (45 years old vs 24 years old, respectively), though the range of ages has been similar [69]. Limited data have been available on the presence of co-morbid conditions for these patients. Some investigators have noted positive asthma history in subpopulations of EVALI patients, but no association has been linked between the two disease processes [70, 71]. The relative contribution of EVALI versus a co-morbidity to the death of these patients remains unclear.
Reported duration of exposure has varied widely. One study cited a median of 225 days of e-cigarette use before presentation with an upper range of five-years, but most patients (43%) have reported less than one year of use [70]. Frequency of e-cigarette use also has varied greatly among patients from as infrequently as one or two times per week to over 50 times per day but with no comment on associated severity of illness [70]. A high proportion of hospitalized EVALI patients have reported use of THC-containing products compared with those reporting traditional use of e-cigarettes for nicotine delivery. As of January 14, 2020, 82% have reported any use of THC-containing products with 33% having exclusive THC-containing product use, compared with 57% reporting any use of nicotine-containing products with only 14% having exclusive use of nicotine-containing products [1].
Clinical Presentation
Three reported case series detailing clinical presentation and patient management have provided some insight on the early stages of disease presentation and treatment strategies [63, 70, 72]. Aggregate data from these studies are provided in Table 1. Unsurprisingly, nearly all patients have presented with respiratory complaints (98%). Eighty percent of patients have presented with cough; 33% with productive cough. Interestingly, dyspnea has been the most common pulmonary symptom (86%) with 77% experiencing associated hypoxemia as defined by a peripheral capillary oxygen saturation (spO2) < 95% while breathing room air. A higher incidence of hypoxemia may have been expected as vaping of e-cigarette liquid alone has been linked to impaired pulmonary gas exchange [73].
Table 1.
| Symptoms reported at presentation no./total no. (%) | Values (%) |
|---|---|
| Respiratory symptoms | 122/125 (98) |
| Dyspnea | 107/125 (86) |
| Chest pain | 55/113 (49) |
| Pleurisy | 47/125 (38) |
| Cough | 100/125 (80) |
| Sputum | 4/12 (33) |
| Hemoptysis | 14/125 (11) |
| Gastrointestinal symptoms | 108/125 (86) |
| Nausea | 89/125 (71) |
| Vomiting | 88/125 (70) |
| Diarrhea | 26/65 (40) |
| Abdominal pain | 54/125 (43) |
| Constitutional symptoms | 118/125 (94) |
| Subjective fever | 99/125 (79) |
| Malaise | 9/12 (75) |
| Sweats | 5/12 (42) |
| Chills | 62/125 (50) |
| Weight loss | 21/113 (19) |
| Fatigue | 53/113 (47) |
| Headache | 35/125 (28) |
| Myalgias | 2/12 (17) |
| Vital signs at presentation no./total no. (%) | |
| Febrile, temperature ≥ 38 °C | 58/123 (47) |
| Heart rate > 100 beats per min | 92/125 (74) |
| Respiratory rate > 20 breaths per min | 68/123 (55) |
| SpO2 while breathing room air | |
| ≥ 95% | 27/124 (22) |
| 89–94% | 39/112 (35) |
| ≤ 88% | 47/112 (42) |
| Initial laboratory results no./total no. (%) | |
| White blood cell count > 11,000 per mm3 | 91/112 (81) |
| White-cell count with > 80% neutrophils | 34/36 (94) |
| Erythrocyte sedimentation rate > 30 mm/h | 35/36 (97) |
| Sodium < 135 mmol/l | 15/49 (31) |
| Potassium < 3.5 mmol/l | 16/46 (35) |
| Aspartate aminotransferase or alanine aminotransferase, or both | |
| > 35 U/L | 41/100 (41) |
| > 105 U/L | 10/100 (10) |
| Initial radiographic findings no./total no. (%) | |
| Abnormal chest radiograph | 117/124 (94) |
| Abnormal chest CT | 104/104 (100) |
| Treatment no./total no. (%) | |
| Bronchoscopy | 23/72 (32) |
| Antibiotics for lower respiratory tract infection | 110/122 (90) |
| Glucocorticoids | 111/122 (91) |
| Clinical course no./total no. (%) | |
| Hospitalization | 112/125 (90) |
| Admission to intensive care unit | 68/125 (54) |
| Respiratory support | |
| Supplemental oxygen | 103/125 (82) |
| High-flow nasal cannula | 34/72 (47) |
| Non-invasive positive pressure ventilation | 37/125 (30) |
| Invasive mechanical ventilation | 28/125 (22) |
| Outcome no./total no. (%) | |
| Discharged on supplemental oxygen | 17/72 (24) |
| Readmission to hospital or ICU | 6/58 (10) |
| Death | 3/125 (2) |
Gastrointestinal (GI) symptoms also have been a common chief complaint affecting 86% of patients. Nausea (71%) and vomiting (70%) have been the most common, while a smaller number of patients have experienced general abdominal pain (43%) and diarrhea (40%). In one case series of 60 patients, two patients were diagnosed with EVALI only after incidental findings of pulmonary ground glass opacities on abdominal CT obtained during workup for primary abdominal symptoms [70]. Constitutional symptoms have been common (94%) and included subjective fever (79%), chills, (50%), and fatigue (47%) [63, 70, 72].
Duration of symptoms prior to presentation has not been predictive of severity of illness, although a prospective study by Blagev et al. noted a trend toward milder presenting symptoms and earlier presentation to care as national media coverage heightened [70]. Of the patients included in the case series, 90% have been initially managed on inpatient services [63, 70, 72]. Fifty-four percent have presented with severe illness requiring intensive care unit (ICU) admission, slightly greater than CDC data that reported a 47% ICU admission rate. Most patients (82%) have required some degree of supplemental oxygen during hospital admission. Maximal level of respiratory support has included high-flow nasal cannula support (47%), non-invasive positive pressure ventilation (30%), and invasive mechanical ventilation (22%) [63, 70, 72]. Severe cases of EVALI have required veno-venous extracorporeal membrane oxygenation (VV-ECMO) with one case proceeding to lung transplantation [56, 71, 74].
Median hospital length of stay has been five-six days in patients < 50 years of age but increases to 12 days in patients > 50 years old. Twenty-four percent of patients have required supplemental oxygen upon discharge with a 10% readmission rate reported in one study [70, 72]. Half of the patients readmitted have reported continued e-cigarette use, highlighting the importance of integrating substance abuse and addiction therapy into treatment strategies [70].
Nationally, of the 1139 EVALI patients who have been discharged on or before October 31, 2019, 2.7% have required rehospitalization in addition to seven deaths after hospital discharge. Hospital readmission has occurred a median of four days after discharge, and death has occurred a median of three days after discharge. The median age of patients requiring rehospitalization (57 years) and those who die after discharge (27 years) have been higher compared with those who neither required rehospitalization nor died (23 years). Similarly, the presence of more than one chronic, co-morbid conditions has been significantly higher in those who required rehospitalization (70.6%) and those who died after discharge (83.3%) compared with those who neither require rehospitalization nor die (25.6%). Interestingly, the symptoms reported on presentation, duration of initial hospitalization, and exposure to corticosteroid therapy or antibiotic therapy during initial hospitalization do not differ among the three groups. All patients who have died after discharge had been admitted to an intensive care unit and had experienced respiratory failure requiring intubation and mechanical ventilation. Considering these data, the CDC recommends close follow-up for EVALI patients within 48 h after hospital discharge, particularly for older patients with chronic medical conditions and those requiring ICU admission with intubation and mechanical ventilation [75]. Further discussion on suggested management of EVALI is discussed below.
Radiographic Findings
Evaluation of disease burden has universally revealed opacities on chest radiograph with or without additional CT imaging. Consistent with epidemiologic definitions, all patients have had abnormal findings on either chest x-ray (CXR) or CT chest imaging. Ninety-four percent of patients in three case series have presented with abnormal initial chest x-rays [63, 70, 72]. On CT imaging, bilateral ground-glass pulmonary opacifications in a gravitational dependent gradient, with or without subpleural sparing, have been the most consistent finding among cases of EVALI. Kalininskiy et al. and Thakar et al. reported bilateral ground-glass opacification in all 23 combined cases with CT results; both patchy and/or confluent [72, 76]. These findings are suggestive of acute lung injury or a diffuse alveolar damage pattern [76]. In the same case series, subpleural sparing were found in 16 patients (70%), pleural effusions in three (13%), fibrotic features (reticulation, bronchiectasis, and/or honeycombing) in eight (35%), and mediastinal lymphadenopathy in 12 (52%) [72, 76]. Diffuse centrilobular nodularity suggestive of bronchiolitis was described in one case report and in 11 of the 12 cases by Thakar et al. [56, 76]. While Thakar et al. suggested hypersensitivity pneumonitis as a possible etiology for the bronchiolitis, the lower-lung distributions were not consistent with the diagnosis [76]. Air leak syndrome as defined by dissection of air out of the normal pulmonary airspace (e.g., pneumomediastinum and pneumothorax) has been a commonly reportedly complication; affecting 16% of patients in one case series [70, 77]. Spontaneous pneumothoraces are more common in young, male smokers, the same patient population as those who have been presenting with EVALI. Furthermore, underlying lung disease and the need for positive pressure ventilation have placed them at increased risk for developing secondary spontaneous pneumothoraces [78]. Interestingly, although lipoid pneumonia has been a common diagnosis based on positive oil-red-O stains from bronchoalveolar lavage (BAL) samples, neither case series by Maddock et al. and Henry et al. suggested the characteristic findings of fat attenuation on CT lung imaging expected with lipoid pneumonia [57, 63, 79].
Laboratory Findings and Evaluation
EVALI remains a diagnosis of exclusion necessitating an extensive workup. Initial laboratory findings from selected patients are available in two case series by Layden et al. and Blagev et al. and are summarized in Table 1. EVALI seems to cause an acute inflammatory response as demonstrated by a white blood cell count > 11,000 per cubic millimeter in 81% of patients, with > 80% neutrophils on differential in 94% of patients, and an erythrocyte sedimentation rate > 30 mm/h in 97% of patients [63, 70]. Localized, acute inflammatory response in the lower airways as measured by serum levels of specific pneumoproteins is a known association with vaping of e-liquid [73]. Mild elevations of transaminases have occurred in a small proportion of cases. Forty-one percent of EVALI patients have had > 35 units/L of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT), and 10% have had > 105 units/L AST and/or ALT. Mild hyponatremia (< 135 mmol/L) and mild hypokalemia (< 3.5 mmol/L) have been noted in 31% and 35% of patients, respectively [63, 70].
Secondary epithelial cell damage from e-cigarette use has been associated with an increased risk of pulmonary infections; however, EVALI patients have had negative infectious workup as per the CDC case definition [80]. Current recommendations for evaluation include respiratory viral panel testing including influenza testing during flu season, testing for community-acquired pneumonia including Streptococcus pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, endemic mycoses, and opportunistic infections [65]. Although the CDC case definition includes the possibility of co-infection in patients with “probable” EVALI, some case series have only reported on confirmed cases of EVALI [63, 70, 72]. Limited data has been published specifically highlighting patient characteristics of “probable” EVALI cases.
The urine drug screen for THC metabolite may be a useful adjunct. As most cases (80%) have reported some THC use, the screen may identify recent cannabis product use, especially in cases where the history may be less reliable (e.g., adolescents who are reticent historians about substance use). Very few false positives have been reported with the immunoassays but includes efavirenz, ibuprofen, and naproxen [81]. While urine quantitative analysis for THC may indicate the frequency of recent use, a risk factor for EVALI, the test has not been studied to correlate with severity of symptoms or outcomes [82]. Additionally, urine testing of THC metabolite does not differentiate between the source of THC—marijuana versus cannabis concentrates versus vape oils.
Pathology Findings
In patients with BAL, most cases have shown either neutrophilic or macrophage predominance with few findings of eosinophilic dominance [70, 79]. Four case series describing BAL findings have reported 22/33 (67%) with lipid-laden macrophages (LLM) with oil-red-O staining [63, 70, 72, 79]. In one case series, the quantification of LLM exceeded 50% in four of six cases with the highest greater than 75% [79]. Some of the heterogeneity reported in BAL findings may have resulted from the administration of antibiotics and/or corticosteroids prior to bronchoscopy. The findings of LLM are also non-specific and do not discriminate between exogenous and endogenous lipoid pneumonia. Whereas exogenous lipoid pneumonia occurs from aspirated oily substances, endogenous lipoid pneumonia does not represent a true disease but rather a histologic finding of phagocytosed phospholipid membranes by macrophages [83]. Blount et al. reported the detection of VEA in 48/51 (94%) patients’ BAL samples submitted to CDC from 16 states [84]. VEA will be discussed in the next section.
Biopsy results from EVALI patients have been similarly heterogeneous and confounded by corticosteroid use. Reported findings have included nonspecific inflammation, foamy macrophages, acute diffuse alveolar damage, interstitial/peribronchiolar granulomatous pneumonitis, hyaline membranes, and pneumocyte vacuolation [63, 85]. Biopsy results have not demonstrated evidence of exogenous lipoid pneumonia but rather have suggested a chemical pneumonitis [83, 85].
Etiology
On September 5, 2019, New York State’s Department of Health announced that most of the cannabis-containing samples obtained from affected patients and analyzed by the Wadsworth Center laboratory contained VEA [86, 87]. The laboratory also found VEA as the primary component of two of three tested THC diluents, and one of three THC oil “thickeners” [87]. In Minnesota, 52% of the 46 THC cartridges submitted by patients contained VEA, and all 20 THC cartridges confiscated by Minnesota law enforcement in September 2019 contained VEA [88]. A case series in Utah found 89% of THC cartridges provided by patients contained VEA [89]. Testing by the FDA as of February 12, 2020, found VEA in 50% of the 511 THC-containing product samples tested. The concentration of VEA ranged from 23 to 88% by weight. The FDA has further tested samples linked to 70 patients reported to the CDC. Of the patients using THC containing products, 81% of cases had a product containing VEA as a diluent [90]. The CDC detected VEA in BAL samples from EVALI patients and did not detect in BAL samples from negative control patients [84]. Most recently, a mouse model of VEA aerosol provided evidence of pulmonary injury on BAL and pathology, including elevated albumin in BAL fluid, leukocytosis in lung tissue, and lipid-laden macrophages [91].
In THC vape cartridges, VEA dilutes the amount of THC product needed in each cartridge without affecting the apparent viscosity of the oil. Prior to the use of VEA, propylene glycol and other excipients have been used as cutting agents or diluents. Those diluted products became less viscous, allowing trapped air bubbles to move rapidly up the cartridge when inverted. Consumers easily identified diluted cartridges and avoided purchasing them. VEA as a diluent slows the bubble movement in cartridges, inaccurately reassuring an unsuspecting consumer that the “bubble test” is that of an unadulterated product. The first company to market VEA as a cutting agent in THC vape cartridges was Honey Cut in 2018. As market demand gradually increased for the product, multiple other companies started marketing VEA to THC cartridge distributors and wholesale markets [92]. Despite the high price markup of VEA, distributors sell VEA at $16–60 per milliliter when mixed into THC cartridges [93]. The exposure to VEA as a diluent in THC cartridges is supported by the apparent risk factors for EVALI: (1) use of THC containing products, (2) use more than five times per day, (3) acquisition of products from “informal sources,” and (4) use of Dank vapes (a brand that relies on lay persons to fill cartridges and distribute) [82].
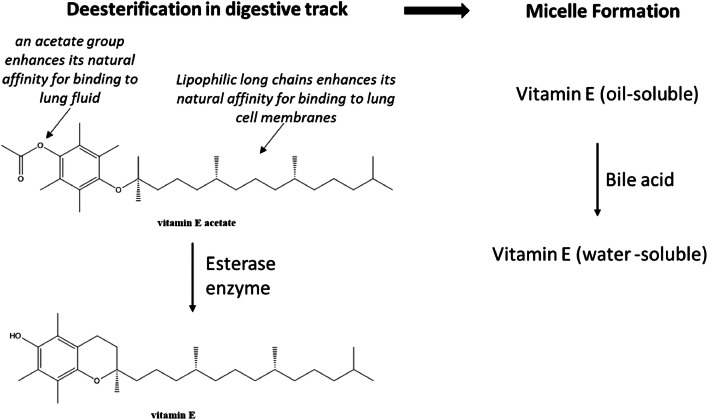
Many dietary supplements and skincare products contain VEA for its antioxidant properties. For those products, VEA is considered GRAS by the FDA. VEA has the advantage over vitamin E because of shelf stability in heat and light. The chemical structure of VEA is comprised of hydrophilic acetate group and a long lipophilic chain contributing a lipo-hydrophilic property. Generally, supplemental VEA is safe in the digestive tract through a two-step metabolic process (Fig. 2). First, VEA is de-acetylated by an esterase enzymatic reaction to vitamin E. Then, vitamin E is converted to a water-soluble form by conjugations to bile salts and circulated nonspecifically by lipoproteins in the plasma [94–96]. The normal intestinal bioconversion and absorption processes of the acetate form of vitamin E do not exist in the lungs.
Fig. 2.
Digestion steps for vitamin E acetate in gastrointestinal tract
While VEA is safe for ingestion, inhaled VEA may injure the lungs and lead to an inflammatory response. Three current hypotheses postulate mechanisms of lung injury. First, the lipo-hydrophilic chemical properties of VEA allow it to permeate pulmonary surfactant layers where VEA may transition phospholipids from a gel to a liquid crystalline state [84]. Surfactant in the liquid crystalline state loses ability to decrease alveolar surface tension. Second, heated VEA releases a ketene (an organic compound with the form R′R″C=C=O) which induces a chemical lung injury [97]. Third, the BAL findings of LLM may have resulted from exogenous VEA [79, 98]. This hypothesis is supported by the accumulation of VEA in lung tissue after IV exposure in a neonatal pig model [99], but as discussed above, LLM may simply be endogenous lipoid pneumonia and macrophage clearance of cellular debris. To further complicate effects of VEA on pulmonary tissue, one study demonstrated the presence of THC and VEA bonded heterodimer in both e-liquid and aerosol [100]. The clinical significance of this complex is unknown but worthy of future investigations. The CDC is currently performing emissions testing on case samples to evaluate the constituents of e-liquid aerosol including ketenes.
Finally, several questions about VEA and EVALI remain unresolved. (1) We do not know if VEA mediates the gastrointestinal symptoms seen in EVALI patients [85]. The gastrointestinal effects may be the result of ingested hydrocarbons or a manifestation of cannabinoid hyperemesis syndrome [101, 102]. (2) While VEA is a proposed etiology associated with EVALI, VEA may not be the sole etiology responsible for the outbreak. Other diluents (e.g., medium-chain triglycerides oil, squalene) and pesticides (e.g., myclobutanil, piperonyl butoxide) have been detected in THC cartridges from EVALI patients, but the associations with EVALI appear far weaker though we cannot exclude them as contributing factors [84, 87]. The CDC and FDA continue to investigate these other potential toxins. (3) We do not fully understand why 14% of all EVALI cases reported only nicotine-product use [1]. While nicotine solutions do not need VEA to thicken, 17% of EVALI cases obtained nicotine cartridges from informal sources [68]. Thus, we cannot exclude the possibility of VEA contamination, but neither the CDC nor FDA have reported to date that VEA has been detected in nicotine-containing cartridges. More likely, however, we suspect patients, especially adolescents and young adults in THC prohibited states, are underreporting THC use.
Analytical Testing of E-Liquid Constituents
Mass spectrometry analysis has become the main interest in cannabis testing and regulations for the determination of “potency” and the percentage of cannabinoids in the material [46]. Currently, high-performance liquid chromatography coupled with photodiode array (HPLC/PDA) detectors and gas chromatography with flame ionization (GC/FID) detectors is popular and is accepted because of low-cost and secure handling. While PDA and FID detectors are relatively specific in identifying cannabinoids, they fail to provide information about potential interferences co-eluted with endogenous plant compounds. On the other hand, a mass spectrometer (MS) detector is capable of performing higher sensitivity and specificity, which facilitates qualitative and quantitative identification of trace amounts of targeted material, but at increased cost and technical expertise [46]. Electrospray ionization (ESI), a gentler ionization technique, can be coupled with MS for analysis of VEA and cannabinoids. Therefore, GC and HPLC separations combined with mass spectrometry have been employed to analyze cannabinoids, terpenes, VEA, and over 100 pesticides in low parts-per-billion range [46]. Meng et al. have validated the HPLC/MS technique as reliable, sensitive, and specific with a lower limit of quantification of cannabinoids at 0.195–50.0 ng/mL and a correlation of > 0.99 accuracies [103]. Similar methods can also be applied to analysis of e-liquid emissions [15]. Duffy et al. at the Laboratory of Organic Analytical Chemistry, Wadsworth Center, New York State Department of Health, used a combination of untargeted GC-MS and HPLC in tandem with a high resolution (HR) quadropole time-of-flight mass spectrometer (LC-HRMS/MS) to detect VEA in the initial samples [87].
Metal testing uses atomic absorption spectrometry (AAS) and inductively coupled plasma mass spectrometry (ICP/MS) [46]. In general, flame techniques, such as AAS, are not as sensitive as ICP/MS and are only capable of measuring one element at a time. ICP/MS is typically employed for multi-metal analysis with ten times the sensitivity of other techniques [46].
Suggested Treatment Algorithm
Management of EVALI is anecdotal and based on limited experience from treating similar disease processes. Treatment algorithms for EVALI have been suggested by multiple groups [65, 70, 72, 104]. After careful evaluation of exposure history and severity of symptoms, a decision for outpatient versus inpatient management should be based on O2 saturation > 95% on room air, absence of respiratory distress, absence of comorbidities that may contribute to respiratory decline or resumption of e-cigarettes, having reliable access to care, and reliability of follow-up within 24–48 h [104]. Clinical data collected from 2016 EVALI patients showed that only 5% were not hospitalized. Among those not hospitalized, 81% were sent home with corticosteroids [66]. Adequate return instructions, coordination of follow-up and access to care, including with social/mental health/substance use disorder services, are key [104].
While some patients qualify for outpatient management, most EVALI patients present with an ill appearance and look floridly “septic” owing to tachycardia, tachypnea, and fever. Respiratory failure in EVALI may be unpredictable but may progress quickly. They may require admission for dehydration, respiratory failure, and further evaluation. Inpatient management should take a multidisciplinary approach and involve the inpatient medicine or critical care team, pulmonology, and medical toxicology. Infectious disease, addiction medicine, and psychiatry may also be of benefit when determining the diagnosis and aiding the patient in withdrawal from nicotine and/or THC. Initiation of antimicrobial coverage until confirmation of EVALI is prudent because it may take several days to rule out non-EVALI causes.
Corticosteroids are the main suggested treatment for EVALI. While outpatient corticosteroid treatment regimens have been cautioned, a corticosteroid taper is more likely of benefit with anecdotal evidence in other forms of pneumonitis. Authors of the recent CDC interim clinical guidance warned that the use of corticosteroids for outpatient management may worsen undiagnosed infectious etiologies [104]. Improvement with systemic corticosteroids is reported, but no controlled clinical trials of corticosteroids for EVALI exists [63]. Although treatment algorithms recommend consideration of corticosteroids, inpatient steroid regimens lack standard dosing and often depend on pulmonologist preference and/or perceived severity of disease. Kalininskiy et al. suggest starting methylprednisolone 40 mg every 8 h until the patient shows improvement, then moving to an oral prednisone taper for two weeks [72]. Blagev et al. reported 41 out of 60 patients with EVALI received an average dose of IV methylprednisolone 125 mg daily (range 120–240 mg) for an average of two days prior to taper of oral prednisone that started at 40–60 mg daily for an average of 11 days [70]. They attributed clinical improvement to corticosteroids in 84% of their overall cohort, but the authors did not report how many improved without corticosteroids. Pulse dose steroids (IV methylprednisolone up to 1 g per day) have also been administered in severe cases.
In cases of severe respiratory failure refractory to invasive ventilatory methods, VV-ECMO can be life sustaining until lung injury improves. However, VV-ECMO may need to be used for prolonged periods of time. Many cases of VV-ECMO in EVALI have been reported, but the long-term outcome is still unclear [63]. Those patients with worsening clinical deterioration despite prolonged VV-ECMO may be evaluated for lung transplantation. As the majority of EVALI patients have been otherwise healthy adolescents and young adults, a discussion of transfer to a tertiary transplant center or with the transplant team can be lifesaving if decompensation occurs. To date, only one EVALI patient, a 17-year-old male, successfully received a double lung transplant [74].
Readiness for hospital discharge is challenging in EVALI patients. Guidelines support discharge in patients that are clinically stable for at least 24–48 h. Due to high rates of hospital readmission and a large number of deaths having occurred within two days of discharge, patients should have primary care follow-up established within 48 h of discharge [104]. Initial follow-up with a pulmonologist within two-four weeks is suggested. Subsequent pulmonology follow-up for pulmonary function testing and/or repeat imaging should occur within one-two months [104].
Conclusions
Electronic cigarettes have been promoted as “safer” alternatives to combustible cigarettes and as a smoking cessation tool. However, the recent outbreak of EVALI highlights the potential threat unregulated e-liquids pose to the increasing number of young users of e-cigarettes. VEA has been associated with constellation of pulmonary, gastrointestinal, and constitutional symptoms in EVALI. While the underlying pathophysiology remains unclear, clinicians need to be vigilant when managing patients with severe respiratory and gastrointestinal symptoms. Asking about e-cigarette use is paramount to the diagnosis. The treatment of EVALI requires multidisciplinary coordination, and the use of corticosteroids may lead to improvement of symptoms.
Sources of Funding
None
Compliance with ethical standards
Conflicts of Interest
None
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Center for Disease Control and Prevention. 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed 20 Feb 2020.
- 2.Bao W, Liu B, Du Y, Snetselaar LG, Wallace RB. Electronic cigarette use among young, middle-aged, and older adults in the United States in 2017 and 2018. JAMA Intern Med. 2019. 10.1001/jamainternmed.2019.4957. [DOI] [PMC free article] [PubMed]
- 3.Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, et al. Tobacco use among middle and high school students - United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2017;66(23):597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulenbeg JE, Johnston LD, O’Malley PM, Bachman JG, Miech R, Patrick ME. Monitoring the future national survey results on drug use, 1975–2017. Ann Arbor: Institute for Social Research, The University of Michigan; 2018. [Google Scholar]
- 5.Eaton DL, Kwan LY, Stratton K, editors. Public health consequences of E-cigarettes. Washington DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 6.Monitoring the future survey: high school and youth trends. national institute on drug abuse. National Institute on Drug Abuse. 2018. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Accessed 20 Oct 2019.
- 7.Miech R, Johnston L, O'Malley PM, Bachman JG, Patrick ME. Trends in adolescent vaping, 2017-2019. N Engl J Med. 2019;381(15):1490–1491. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. doi: 10.15585/mmwr.mm6845a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6). 10.1542/peds.2018-2741. [DOI] [PubMed]
- 10.Gilman G. Analytical testing of e-cigarette aerosol. In: Farsalinos KE, Gillman IG, Thornburg JW, Hecht SS, Polosa R, editors. Analytical assessment of E-cigarettes: from contents to chemical and particle exposure profiles. Amsterdam: Elsevier; 2017. pp. 9–35. [Google Scholar]
- 11.Petrucci RH, Harwood WS, Herring FG. General chemistry : principles and modern applications. 8. Prentice Hall: Upper Saddle River; 2002. [Google Scholar]
- 12.Protano C, Avino P, Manigrasso M, Vivaldi V, Perna F, Valeriani F, et al. Environmental electronic vape exposure from four different generations of electronic cigarettes: airborne particulate matter levels. Int J Environ Res Public Health. 2018;15(10). 10.3390/ijerph15102172. [DOI] [PMC free article] [PubMed]
- 13.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, Peyton DH. Benzene formation in electronic cigarettes. PLoS One. 2017;12(3):e0173055. doi: 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddingsaas N, Pagano T, Cummings C, Rahman I, Robinson R, Hensel E. Qualitative analysis of E-liquid emissions as a function of flavor additives using two aerosol capture methods. Int J Environ Res Public Health. 2018;15(2). 10.3390/ijerph15020323. [DOI] [PMC free article] [PubMed]
- 16.NextGenerationLabs. TFN. 2019. https://www.nextgenerationlabs.com. Accessed 29 Oct 2019.
- 17.Nasrin S, Chen G, Watson CJ, Lazarus P. Evaluation of tobacco-specific nitrosamine (TSNA) content in smokeless tobacco products in Bangladesh. FASEB J. 2019;33(1_supplement):672.7–.7. doi: 10.1096/fasebj.2019.33.1_supplement.672.7. [DOI] [Google Scholar]
- 18.Romberg AR, Miller Lo EJ, Cuccia AF, Willett JG, Xiao H, Hair EC, Vallone DM, Marynak K, King BA. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013-2018. Drug Alcohol Depend. 2019;203:1–7. doi: 10.1016/j.drugalcdep.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowen A, Xing C, inventors; Juul Labs Inc, assignee. Nicotine salt formulations for aerosol devices and methods thereof. United States patent 2014–0345631-A1. 2015 October 28.
- 20.Yingst JM, Hrabovsky S, Hobkirk A, Trushin N, Richie JP, Jr, Foulds J. Nicotine absorption profile among regular users of a pod-based electronic nicotine delivery system. JAMA Netw Open. 2019;2(11):e1915494. doi: 10.1001/jamanetworkopen.2019.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J. E-cigarette sales slowing, led by Juul, amid negative headlines. CNBC. 2019. https://www.cnbc.com/2019/10/01/e-cigarette-sales-slowing-led-by-juul-amid-negative-headlines.html. Accessed 15 Dec 2019.
- 22.Wang B, Liu S, Persoskie A. Poisoning exposure cases involving e-cigarettes and e-liquid in the United States. Clin Toxicol (Phila) 2010;2019:1–7. doi: 10.1080/15563650.2019.1661426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggleston W, Nacca N, Stork CM, Marraffa JM. Pediatric death after unintentional exposure to liquid nicotine for an electronic cigarette. Clin Toxicol (Phila) 2016;54(9):890–891. doi: 10.1080/15563650.2016.1207081. [DOI] [PubMed] [Google Scholar]
- 24.Child Nicotine Poisoning Prevention Act of 2015, S.142. Sect. 114th Congress (2015).
- 25.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 26.Vardavas CI, Filippidis FT, Agaku IT. Determinants and prevalence of e-cigarette use throughout the European Union: a secondary analysis of 26 566 youth and adults from 27 countries. Tob Control. 2015;24(5):442–448. doi: 10.1136/tobaccocontrol-2013-051394. [DOI] [PubMed] [Google Scholar]
- 27.Zosel A, Egelhoff E, Heard K. Severe lactic acidosis after an iatrogenic propylene glycol overdose. Pharmacotherapy. 2010;30(2):219. doi: 10.1592/phco.30.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ATSDR . Toxicological profile for propylene glycol. Atlanta: U.S. Department of Health and Human Services, Public Health Service; 1997. [Google Scholar]
- 29.Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med. 2001;58(10):649–655. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang MS, Wu XR, Lee HW, Xia Y, Deng FM, Moreira AL, Chen LC, Huang WC, Lepor H. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci U S A. 2019;116(43):21727–21731. doi: 10.1073/pnas.1911321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L705–LL19. doi: 10.1152/ajplung.00492.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23. doi: 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etter JF, Bugey A. E-cigarette liquids: Constancy of content across batches and accuracy of labeling. Addict Behav. 2017;73:137–143. doi: 10.1016/j.addbeh.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796–4815. doi: 10.3390/ijerph120504796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S. High school students’ use of electronic cigarettes to vaporize Cannabis. Pediatrics. 2015;136(4):611–616. doi: 10.1542/peds.2015-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struble CA, Ellis JD, Lundahl LH. Beyond the bud: emerging methods of Cannabis consumption for youth. Pediatr Clin N Am. 2019;66(6):1087–1097. doi: 10.1016/j.pcl.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Valentine GW, Jatlow PI, Coffman M, Nadim H, Gueorguieva R, Sofuoglu M. The effects of alcohol-containing e-cigarettes on young adult smokers. Drug Alcohol Depend. 2016;159:272–276. doi: 10.1016/j.drugalcdep.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisko JG, Lee GE, Kimbrell JB, Rybak ME, Valentin-Blasini L, Watson CH. Caffeine concentrations in coffee, tea, chocolate, and energy drink flavored E-liquids. Nicotine Tob Res. 2017;19(4):484–492. doi: 10.1093/ntr/ntw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulder HA, Patterson JL, Halquist MS, Kosmider L, Turner JBM, Poklis JL, Poklis A, Peace MR. The effect of electronic cigarette user modifications and E-liquid adulteration on the particle size profile of an aerosolized product. Sci Rep. 2019;9(1):10221. doi: 10.1038/s41598-019-46387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenne DR, Fischbein RL, Tan AS, Banks M. The use of substances other than nicotine in electronic cigarettes among college students. Subst Abus. 2017;11:1178221817733736. doi: 10.1177/1178221817733736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang LL, Baker HM, Meernik C, Ranney LM, Richardson A, Goldstein AO. Impact of non-menthol flavours in tobacco products on perceptions and use among youth, young adults and adults: a systematic review. Tob Control. 2017;26(6):709–719. doi: 10.1136/tobaccocontrol-2016-053196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safety assessment and regulatory authority to use flavors: focus on e-cigarettes. Flavors and Extracts Manufacturers’ Association. 2014. https://www.femaflavor.org/node/24344. Accessed 25 Nov 2019.
- 43.Criteria for a recommended standard: occupational exposure to diacetyl and 2,3-pentanedione. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH); 2016.
- 44.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes. Environ Health Perspect. 2016;124(6):733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farsalinos KE, Gillman IG, Thornburg JW, Hecht SS, Polosa R. Analytical assessment of e-cigarettes : from contents to chemical and particle exposure profiles. Emerging issues in analytical chemistry. Amsterdam: Elsevier; 2017. [Google Scholar]
- 46.Nie B, Henion J, Ryona I. The role of mass spectrometry in the Cannabis industry. J Am Soc Mass Spectrom. 2019;30(5):719–730. doi: 10.1007/s13361-019-02164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, Chen R, Hilpert M, Cohen JE, Navas-Acien A, Rule AM. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect. 2018;126(2):027010. doi: 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 2017;12(4):e0175430. doi: 10.1371/journal.pone.0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee MS, Allen JG, Christiani DC. Endotoxin and (1,3)-β-D-glucan contamination in electronic cigarette products sold in the United States. Environ Health Perspect. 2019;127(4):47008. doi: 10.1289/EHP3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobacco 21 Laws: Raising the minimum sales age for all tobacco products to 21. American Lung Association. 2020. https://www.lung.org/our-initiatives/tobacco/cessation-and-prevention/tobacco-21-laws.html. Accessed 21 Feb 2020.
- 51.FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. U.S. Food & Drug Administration. 2020. https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children. Accessed 21 Feb 2020.
- 52.Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med. 2018;55(4):455–461. doi: 10.1016/j.amepre.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glantz SA. The evidence of electronic cigarette risks is catching up with public perception. JAMA Netw Open. 2019;2(3):e191032. doi: 10.1001/jamanetworkopen.2019.1032. [DOI] [PubMed] [Google Scholar]
- 54.Kim SY, Sim S, Choi HG. Active, passive, and electronic cigarette smoking is associated with asthma in adolescents. Sci Rep. 2017;7(1):17789. doi: 10.1038/s41598-017-17958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195(8):1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landman ST, Dhaliwal I, Mackenzie CA, Martinu T, Steele A, Bosma KJ. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ. 2019;191(48):E1321–E1E31. doi: 10.1503/cmaj.191402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 58.Henry TS, Kligerman SJ, Raptis CA, Mann H, Sechrist JW, Kanne JP. Imaging findings of vaping-associated lung injury. AJR Am J Roentgenol. 2019:1–8. 10.2214/AJR.19.22251. [DOI] [PubMed]
- 59.Li L, Lin Y, Xia T, Zhu Y. Effects of electronic cigarettes on indoor air quality and health. Annu Rev Public Health. 2020. 10.1146/annurev-publhealth-040119-094043. [DOI] [PMC free article] [PubMed]
- 60.Tzortzi A, Teloniatis S, Matiampa G, Bakelas G, Tzavara C, Vyzikidou VK, et al. Passive exposure of non-smokers to E-cigarette aerosols: sensory irritation, timing and association with volatile organic compounds. Environ Res. 2019;182:108963. doi: 10.1016/j.envres.2019.108963. [DOI] [PubMed] [Google Scholar]
- 61.McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, Chen G. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One. 2015;10(2):e0118344. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones CD, Ho W, Gunn E, Widdowson D, Bahia H. E-cigarette burn injuries: comprehensive review and management guidelines proposal. Burns. 2019;45(4):763–771. doi: 10.1016/j.burns.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - preliminary report. N Engl J Med. 2019. 10.1056/NEJMoa1911614. [DOI] [PubMed]
- 64.Schier JG, Meiman JG, Layden J, Mikosz CA, VanFrank B, King BA, et al. Severe pulmonary disease associated with electronic-cigarette-product use - Interim Guidance. MMWR Morb Mortal Wkly Rep. 2019;68(36):787–790. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, Cates JE, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury - United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(41):919–927. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chatham-Stephens K, Roguski K, Jang Y, Cho P, Jatlaoui TC, Kabbani S, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury - United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1076–1080. doi: 10.15585/mmwr.mm6846e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lozier MJ, Wallace B, Anderson K, Ellington S, Jones CM, Rose D, et al. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injuries - United States, December 2019. MMWR Morb Mortal Wkly Rep. 2019;68(49):1142–1148. doi: 10.15585/mmwr.mm6849e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellington S, Salvatore PP, Ko J, Danielson M, Kim L, Cyrus A, et al. Update: product, substance-use, and demographic characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury - United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(2):44–49. doi: 10.15585/mmwr.mm6902e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moritz ED, Zapata LB, Lekiachvili A, Glidden E, Annor FB, Werner AK, et al. Update: characteristics of patients in a national outbreak of E-cigarette, or vaping, product use-associated lung injuries - United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(43):985–989. doi: 10.15585/mmwr.mm6843e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019;394(10214):2073–2083. doi: 10.1016/S0140-6736(19)32679-0. [DOI] [PubMed] [Google Scholar]
- 71.Bradford LE, Rebuli ME, Ring BJ, Jaspers I, Clement KC, Loughlin CE. Danger in the vapor? ECMO for adolescents with status asthmaticus after vaping. J Asthma. 2019:1–5. 10.1080/02770903.2019.1643361. [DOI] [PubMed]
- 72.Kalininskiy A, Bach CT, Nacca NE, Ginsberg G, Marraffa J, Navarette KA, McGraw M, Croft DP. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026. doi: 10.1016/S2213-2600(19)30415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaumont M, Tagliatti V, Channan EM, Colet JM, Bernard A, Morra S, et al. Short halt in vaping modifies cardio-respiratory parameters and urine metabolome: a randomized trial. Am J Physiol Lung Cell Mol Physiol. 2019. 10.1152/ajplung.00268.2019. [DOI] [PMC free article] [PubMed]
- 74.Williams C. Michigan teen receives double lung transplant after ‘enormous’ damage from vaping. Time. 2019. https://time.com/5726491/michigan-teen-vaping-damage-lung-transplant. Accessed 9 Dec 2019.
- 75.Mikosz CA, Danielson M, Anderson KN, Pollack LA, Currie DW, Njai R, et al. Characteristics of patients experiencing rehospitalization or death after hospital discharge in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1183–1188. doi: 10.15585/mmwr.mm685152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakrar PD, Boyd KP, Swanson CP, Wideburg E, Kumbhar SS. E-cigarette, or vaping, product use-associated lung injury in adolescents: a review of imaging features. Pediatr Radiol. 2020;50(3):338–344. doi: 10.1007/s00247-019-04572-5. [DOI] [PubMed] [Google Scholar]
- 77.Bonilla A, Blair AJ, Alamro SM, Ward RA, Feldman MB, Dutko RA, Karagounis TK, Johnson AL, Folch EE, Vyas JM. Recurrent spontaneous pneumothoraces and vaping in an 18-year-old man: a case report and review of the literature. J Med Case Rep. 2019;13(1):283. doi: 10.1186/s13256-019-2215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sajadi-Ernazarova KR, Martin J, Gupta N. Acute pneumothorax evaluation and treatment. Treasure Island: StatPearls; 2019. [PubMed] [Google Scholar]
- 79.Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, Aberegg SK. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 80.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9):e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387–396. doi: 10.1093/jat/bku075. [DOI] [PubMed] [Google Scholar]
- 82.Navon L, Jones CM, Ghinai I, King BA, Briss PA, Hacker KA, et al. Risk factors for E-cigarette, or vaping, product use-associated lung injury (EVALI) among adults who use E-cigarette, or vaping, products - Illinois, July–October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(45):1034–1039. doi: 10.15585/mmwr.mm6845e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, Brenner DS, Maldonado F, Choi H, Ghobrial M. Lung biopsy findings in severe pulmonary illness associated with E-cigarette use (Vaping) Am J Clin Pathol. 2020;153(1):30–39. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 84.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2019. 10.1056/NEJMoa1916433.
- 85.Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, Khoor A, Mira-Avendano I, Patel A, Larsen BT. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 86.New York State Department of Health announces update on investigation into vaping-associated pulmonary illnesses. New York State Department of Health. 2019. https://www.health.ny.gov/press/releases/2019/2019-09-05_vaping.htm. Accessed 29 Oct 2019.
- 87.Duffy B, Li L, Lu S, Durocher L, Dittmar M, Delaney-Baldwin E, et al. Analysis of cannabinoid-containing fluids in illicit vaping cartridges recovered from pulmonary injury patients: identification of vitamin E acetate as a major diluent. Toxics. 2020;8(1). 10.3390/toxics8010008. [DOI] [PMC free article] [PubMed]
- 88.Taylor J, Wiens T, Peterson J, Saravia S, Lunda M, Hanson K, et al. Characteristics of E-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68(47):1096–1100. doi: 10.15585/mmwr.mm6847e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis N, McCaffrey K, Sage K, Cheng CJ, Green J, Goldstein L, et al. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury - Utah, April–October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(42):953–956. doi: 10.15585/mmwr.mm6842e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lung illnesses associated with use of vaping products. U.S. Food & Drug Administration. 2019. https://www.fda.gov/news-events/public-health-focus/lung-illnesses-associated-use-vaping-products. Accessed 14 Dec 2019.
- 91.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N Engl J Med. 2020. 10.1056/NEJMc2000231. [DOI] [PMC free article] [PubMed]
- 92.Wenzke M, Downs D. From ‘Veronica Mars’ to toxic vapes: the rise and fall of honey cut. Leafly. Leafly. 2019. https://www.leafly.com/news/health/toxic-vaping-vapi-evali-lung-injury-rise-and-fall-of-vitamin-e-oil-honey-cut. Accessed 6 Dec 2019.
- 93.Downs D, Howard D, Barcott B. Journey of a tainted vape cartridge: from China’s labs to your lungs. Leafly. 2019. https://www.leafly.com/news/politics/vape-pen-injury-supply-chain-investigation-leafly. Accessed 6 Dec 2019.
- 94.Drevon CA. Absorption, transport and metabolism of vitamin E. Free Radic Res Commun. 1991;14(4):229–246. doi: 10.3109/10715769109088952. [DOI] [PubMed] [Google Scholar]
- 95.Hung SS, Moon TW, Hilton JW, Slinger SJ. Uptake, transport and distribution of DL-alpha-tocopheryl acetate compared to D-alpha-tocopherol in rainbow trout (Salmo gairdneri) J Nutr. 1982;112(8):1590–1599. doi: 10.1093/jn/112.8.1590. [DOI] [PubMed] [Google Scholar]
- 96.Mayer S, Weiss J, McClements DJ. Behavior of vitamin E acetate delivery systems under simulated gastrointestinal conditions: lipid digestion and bioaccessibility of low-energy nanoemulsions. J Colloid Interface Sci. 2013;404:215–222. doi: 10.1016/j.jcis.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 97.Dan W, Donal OS. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. 2019. [DOI] [PMC free article] [PubMed]
- 98.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211(3):139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hale TW, Rais-Bahrami K, Montgomery DL, Harkey C, Habersang RW. Vitamin E toxicity in neonatal piglets. J Toxicol Clin Toxicol. 1995;33(2):123–130. doi: 10.3109/15563659509000461. [DOI] [PubMed] [Google Scholar]
- 100.Lanzarotta A, Falconer TM, Flurer R, Wilson RA. Hydrogen bonding between tetrahydrocannabinol and vitamin E acetate in unvaped, aerosolized, and condensed aerosol e-liquids. Anal Chem. 2020;92(3):2374–2378. doi: 10.1021/acs.analchem.9b05536. [DOI] [PubMed] [Google Scholar]
- 101.Richards JR. Cannabinoid hyperemesis syndrome: pathophysiology and treatment in the emergency department. J Emerg Med. 2018;54(3):354–363. doi: 10.1016/j.jemermed.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 102.Victoria MS, Nangia BS. Hydrocarbon poisoning: a review. Pediatr Emerg Care. 1987;3(3):184–186. doi: 10.1097/00006565-198709000-00014. [DOI] [PubMed] [Google Scholar]
- 103.Meng Q, Buchanan B, Zuccolo J, Poulin MM, Gabriele J, Baranowski DC. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS One. 2018;13(5):e0196396. doi: 10.1371/journal.pone.0196396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Evans ME, Twentyman E, Click ES, Goodman AB, Weissman DN, Kiernan E, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected E-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge - United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189–1194. doi: 10.15585/mmwr.mm685152e2. [DOI] [PMC free article] [PubMed] [Google Scholar]