Abstract
Introduction
Diabetes disproportionately affects American Indians/Alaskan Natives (AI/AN). Bisphenol A (BPA) and arsenic (As), environmental toxicants which may be associated with diabetes, have not been well studied in this population. Our objectives were to determine if urinary BPA and As are associated with diabetes among adults in the Cheyenne River Sioux Tribe (CRST), and to compare their urinary levels with the general US population.
Methods
We performed a case-control study among 276 volunteers. We matched our cases (persons with diabetes) and controls (persons without diabetes) using age. We collected questionnaire data and urine samples which were tested for BPA and speciated As analytes. We used paired t tests and McNemar’s chi-square test to compare continuous and categorical variables, respectively, between cases and controls and linear regression to assess the association between self-reported exposures and BPA and As levels. We used conditional logistic regression to investigate the association between case status and BPA and As levels. BPA and As levels among participants were compared with those from the 2011–2012 National Health and Nutrition Examination Survey (NHANES).
Results
The average age of participants was 46 years. The majority identified as AI/AN race (97%) and 58% were female. The geometric means from CRST participant urine specimens were 1.83 ug/L for BPA and 3.89 ug/L for total As. BPA geometric means of CRST participants were higher than NHANES participants while total As geometric means were lower. BPA and As were not associated with case status.
Conclusion
The results of this study are consistent with others that have reported no association between diabetes and exposure to BPA or As.
Keywords: Bisphenol A, Arsenic, Diabetes, Native Americans
Introduction
Type 2 diabetes is a major public health problem in the USA, affecting more than 30 million Americans and costing over $327 billion in 2017 [1]. American Indian/Alaskan Native (AI/AN) populations are more adversely impacted by this disease, likely due to a combination of factors including genetic predisposition, poor diet, and inadequate access to healthcare [2, 3]. While traditional risk factors such as diet and lifestyle practices have been studied in this population, studies investigating exposure to environmental toxicants associated with this disease are limited. Two toxicants that may be associated with type 2 diabetes and other adverse health effects are bisphenol A (BPA) and inorganic arsenic (As) [4, 5].
BPA is used extensively in the plastic industry; more than 5 million tons of this chemical were produced annually, and it can be detected in the body of more than 90% of the US population [6]. BPA was present in polycarbonate plastics of food and beverage containers, as well as some inner coatings and/or linings of preserved canned goods [6]. Media coverage of this chemical led to a large phase-out of consumer products such as bottles, containers, and canned good linings that contained BPA [7]. Despite these changes in exposure patterns, BPA use remained widespread, and the US Environmental Protection Agency (EPA) estimated that more than 1 million pounds were released into the environment each year [6].
BPA has been regarded as a chemical of concern, i.e., an “endocrine disruptor” that affects several molecular pathways in the body by interfering with the normal function of the endocrine or hormone system [6]. A widely cited analysis of the US National Health and Nutrition Examination Survey (NHANES) 2003–2004 data showed that persons with diabetes have higher urinary BPA levels [4]. An evaluation of the Nurses’ Health Study II (NHSII) cohort found a positive association between the incidence of type 2 diabetes and urinary BPA levels in premenopausal women [8]. Previous studies have shown variable urinary BPA levels across certain socioeconomic and ethnic groups, with Mexican Americans having the lowest urinary levels of BPA [9]. AI/AN populations have a higher prevalence of diabetes than the overall US population [3], and to our knowledge, BPA exposure and its possible association with diabetes have not been studied in this group.
Humans can be exposed to inorganic or organic forms of As, the latter of which is normally less toxic, through consumption of contaminated drinking water, certain food products such as seafood and grains, and through occupational exposure [5]. Inorganic As exposure is associated with significant health effects, including cardiovascular disease and malignancies of several organ systems, while organic arsenic from seafood is considered significantly less toxic [3]. In laboratory animals, exposure to high levels of inorganic As is associated with impaired glucose tolerance [10]. An analysis of NHANES 2003–2004 data found a positive association between urine As and prevalence of type 2 diabetes in the US population, generating much interest in this topic [5]. Subsequently, a National Toxicology Program review of epidemiologic studies performed in multiple countries including the USA concluded that sufficient evidence exists to support an association between chronic exposure to high levels of As in drinking water and diabetes [10].
Based on this information, we hypothesized that diabetes would be associated with higher urinary BPA and As levels among AI/AN populations. We conducted a case-control study among residents of the Cheyenne River Sioux Tribe (CRST) located in South Dakota to test the hypothesis that higher levels of urinary BPA and As would increase the odds of having diabetes. The study objectives were to determine if urinary BPA and As concentrations are associated with diabetes among adult CRST residents and to compare BPA and As levels in this community with the general US population.
Methods
We chose the CRST of South Dakota based on the presence of an established Department of Environmental Protection and Natural Resources program as well as a robust primary care medical clinic network that provides healthcare to a large number of patients with diabetes in the community. We determined that a sample size of 135 pairs, or 270 participants, would have a power of 80% to detect an odds ratio of 2.0. CRST Tribal Council and Centers for Disease Control and Prevention (CDC) institutional review boards approved the protocol. CRST study staff recruited a convenience sample of study participants from four primary care clinics on the CRST reservation on a volunteer basis while participants were waiting for a routine clinic visit from July of 2010 to June of 2011. Eligibility for the study was determined using a set of standard screening questions that included age over 18, not currently pregnant, and ability to provide a urine sample. We defined a “case” as any participant meeting eligibility criteria who reported a medical history of diabetes and a “control” as any participant meeting eligibility criteria who did not report a medical history of diabetes. Case status was confirmed using clinic records. Participants were matched on age (within 10 years) on a 1 to 1 ratio of cases to controls. CRST study personnel obtained informed consent, administered questionnaires, and collected random spot urine specimens from participants during routine clinical care to minimize burden on study participants. Demographic (including self-reported race/ethnicity), behavioral, and exposure data from all participants were collected via questionnaire. If a blood glucose measurement was performed as part of the clinic visit, the results were also recorded.
Spot urine samples collected from participants were frozen and shipped to the National Center for Environmental Health (NCEH), Division of Laboratory Sciences (CDC, Atlanta, GA) for analysis. Urinary BPA concentrations were determined using liquid chromatography and tandem mass spectrometry according to previously published methods [11]. The urinary total As (UTAS) and several arsenic species and metabolites (arsenic acid (UAS5), arsenobetaine (UASB), arsenocholine (UASC), arsenous acid (UAS3), monomethylarsonic acid (UMMA), dimethylarsonic acid (UDMA), and trimethylarsine oxide (UTMO)) were determined using inductively coupled plasma mass spectrometry according to previously published methods [12]. Arsenic speciation and metabolite analyses were conducted on samples with UTAS greater than 5 ug/L. Urine creatinine was also measured using a Roche-Hitachi 912 chemistry analyzer (Roche Diagnostics, Indianapolis, IN).
All laboratory and questionnaire data were de-identified and then entered into an Access 2010 (Microsoft, Redmond, WA) database. Statistical analyses were conducted using the SAS 9.3 (SAS Institute, Cary, NC). We used descriptive statistics to summarize questionnaire data. Laboratory values below the limit of detection (LOD; BPA = 0.4, UTAS = 1.25, UAS5 = 0.87, UASB = 1.19, UASC = 0.28, UAS3 = 0.48, UDMA = 1.8, UMMA = 0.89, UTMO = 0.25; all in ug/L) were substituted with the LOD divided by the square root of two [13]. Laboratory data were not normally distributed, so we used a logarithmic transformation to normalize the data for statistical analysis. We used paired t tests to compare the means of continuous variables (age, blood glucose, and BMI) and McNemar’s test to compare frequencies of categorical variables between cases and controls, and linear regression, adjusting for urinary creatinine, to assess the association between self-reported exposures (Table 1) and urinary BPA and total As levels in univariate and multivariate models. We determined whether the odds of being a case differed by levels of BPA and As using conditional logistic regression (univariate and multivariate models). We used Spearman’s correlation coefficients to assess the correlation between BPA and As levels. We used non-overlapping 95% confidence intervals (CI) to determine whether the geometric means for urine BPA, UTAS, and UDMA were statistically significantly different between CRST participants and the US population, ages 20 and older, from the 2011–2012 NHANES [14].
Table 1.
Characteristics and self-reported exposures among cases (participants with diabetes), controls (participants without diabetes), and all participants (n = 276), Cheyenne River Sioux Tribe (SD, 2010–2011).
| Characteristic | Cases (n = 138) | Controls (n = 138) | All participants (n = 276) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 47.0 (10.7) | 45.6 (9.9) | 46.3 (10.3) |
| Finger-stick blood glucose levels (mg/dL) | 206.2 (94.5)* | 109.7 (26.6)* | 158.1 (84.5) |
| Body mass index (BMI) (kg/m2) | 31.7 (6.1) | 30.9 (6.3) | 31.3 (6.2) |
| n (%) | n (%) | n (%) | |
| Sex | |||
| Female | 78 (57) | 82 (59) | 160 (58) |
| Male | 59 (43) | 56 (41) | 115 (42) |
| Missing | 1 (< 1) | 0 (0) | 1 (< 1) |
| Race | |||
| White | 1 (1) | 2 (1) | 3 (1) |
| Native Hawaiian | 1 (1) | 0 (0) | 1 (< 1) |
| American Indian | 134 (97) | 135 (98) | 269 (97) |
| Missing | 2 (1) | 1 (< 1) | 3 (1) |
| Education level | |||
| High school or less | 79 (57) | 81 (59) | 160 (58) |
| Bachelor’s degree or some college | 58 (42) | 56 (41) | 114 (41) |
| Missing | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| Total household income | |||
| < $10,000 | 95 (69)** | 79 (57)** | 174 (63) |
| ≥ $10,000 | 41 (30) | 57 (41) | 98 (36) |
| Missing | 2 (1) | 2 (1) | 4 (1) |
| Cigarette smoking | |||
| Never | 64 (46) | 57 (41) | 121 (44) |
| Former | 6 (4) | 3 (2) | 9 (3) |
| Current | 68 (49) | 78 (57) | 146 (53) |
| Chewing tobacco, snuff, or snus | |||
| Every day/some days | 9 (7) | 15 (11) | 24 (9) |
| Not at all | 129 (93) | 122 (88) | 251 (91) |
| Missing | 0 (0) | 1 (< 1) | 1 (< 1) |
| Self-reported exposures | |||
| Main source of drinking water, bottled water | 38 (28) | 35 (26) | 73 (27) |
| Drank cold beverages from plastic bottles, cups, or containers in the past 24 hours | 116 (85) | 107 (79) | 223 (81) |
| Drank hot beverages from plastic bottle, cups, or containers in the past 24 hours | 48 (36) | 55 (40) | 103 (38) |
| Eaten canned food in the past 24 hours | 63 (46) | 50 (38) | 113 (42) |
| Eaten cold or unheated foods from plastic dishes or containers in the past 24 hours | 28 (20) | 35 (26) | 63 (23) |
| Eaten hot food from plastic dishes or containers in the past 24 hours | 59 (43) | 47 (35) | 106 (39) |
| Consumed fish in last 7 days | 22 (16) | 25 (18) | 47 (17) |
| Previous occupation with BPA exposure | 2 (1) | 1 (1) | 3 (1) |
| Previous occupation with arsenic exposure | 1 (1) | 2 (1) | 3 (1) |
*p < 0.0001 (paired t test); **p = 0.03 (McNemar’s test)
Results
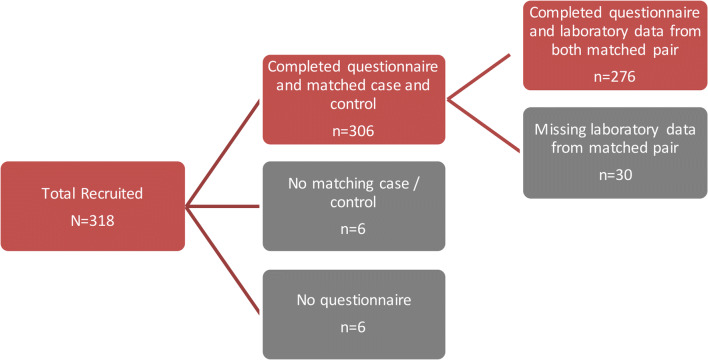
A total of 318 patients were enrolled into the study over a 12-month period from July 2010 to June 2011. A total of 42 participants who did not have an age-matched pair or did not have both questionnaire and laboratory data were excluded from the analysis, resulting in 138 matched pairs for analysis (Fig. 1).
Fig. 1.
Flowchart illustrating how the final number of cases (Cheyenne River Sioux Tribe (CRST) members with diabetes) and their age-matched controls (CRST members without diabetes) were determined (n = 276) (SD, 2010–2011).
The average age of participants was 46 years; 97% (n = 269) identified as AI/AN race, 58% (n = 160) were female, and 53% (n = 146) were current smokers. The majority of participants (63%, n = 174) had a total household income of less than $10,000 per year. About a quarter of participants (26%, n = 73) reported bottled water as their main source of drinking water, and 38% (n = 103) drank hot beverages from plastic bottles, cups, or containers in the past 24 hours. Additional demographic and exposure factor characteristics of the study population are detailed in Table 1. Cases and controls did not differ significantly in any of the categorical variables measured except for income; a higher proportion of cases had an annual household income < $10,000 (p = 0.04). As expected, cases had significantly higher finger-stick blood glucose levels than did controls (p < 0.0001).
Among participants with diabetes, 96% reported having type 2 diabetes, over half had obesity with a BMI greater than 30 kg/m2, and 23% reported using insulin (Table 2). Medians and geometric means for BPA, UTAS, UDMA, and creatinine by diabetes status are reported in Table 3. Although arsenic speciation was done on all samples with UTAS greater than 5 ug/L, we found that several species/metabolites were less than the LOD among 60% or more of study subjects (percent detected, UAS5 = 15.38%, UASB = 17.09%, UASC = 0.85%, UAS3 = 51.28%, UMMA = 40.17%, UTMO = 2.56%). Thus, geometric means, confidence intervals, and p values were not calculated for these analytes. For all participants, the median and geometric mean for urinary BPA were 1.90 and 1.83 ug/L and for UTAS were 4.15 and 3.89 ug/L. Paired t tests showed no statistical differences in the levels of BPA, total As, UDMA, or urinary creatinine between cases and controls.
Table 2.
Self-reported medical characteristics among participants with diabetes, Cheyenne River Sioux Tribe (n = 138, SD, 2010–2011).
| Characteristic | n (%) |
|---|---|
| Diabetes type | |
| Type 2 | 133 (96) |
| Missing | 5 (4) |
| Self-reported use of Insulin | |
| Yes | 32 (23) |
| No | 100 (72) |
| Missing | 6 (4) |
| Oral diabetic medication | |
| Yes | 98 (71) |
| No | 35 (25) |
| Missing | 5 (4) |
| Finger-stick Blood Glucose Category at time of urine collection | |
| Normal range (< or = 125 mg/dL) | 28 (20) |
| Outside normal range (> 125 mg/dL) | 110 (80) |
| Self-reported average blood sugar level at home | |
| ≤ 150 mg/dL | 52 (38) |
| 151–199 mg/dL | 38 (28) |
| 200–299 mg/dL | 29 (21) |
| 300–399 mg/dL | 12 (9) |
| Do not know/missing | 7 (5) |
| Categories of body mass index (BMI) (kg/m2) | |
| < 18.5 | 0 |
| 18.5–< 25 | 14 (10) |
| 25–< 30 | 48 (35) |
| > 30 | 76 (55) |
| # of times seen a provider for diabetes | |
| ≤ 6 times in the past 12 months | 113 (82) |
| > 6 times in the past 12 months | 13 (9) |
| Missing | 12 (9) |
| Emergency department visit due to high blood sugar, in past 12 months | |
| None | 117 (85) |
| > 1 | 15 (11) |
| Missing | 6 (4) |
Table 3.
Median, geometric mean, and 95% confidence interval of BPA and As metabolites among all participants, cases with diabetes, and controls without diabetes, Cheyenne River Sioux Tribe (n = 276, SD, 2010–2011).
| Variable | n | % detected | Median (range) | Geometric mean (95% CI) | p valuea |
| Bisphenol A (ug/L) (BPA) | 0.90 | ||||
| Total | 276 | 86 | 1.90 (< LOD–64.30) | 1.83 (1.62–2.07) | |
| Cases | 138 | 89 | 1.90 (< LOD–64.30) | 1.90 (1.61–2.24) | |
| Controls | 138 | 83 | 1.85 (< LOD–35.40) | 1.77 (1.47–2.13) | |
| Urinary total arsenic (ug/L) (UTAS) | 0.51 | ||||
| Total | 276 | 88 | 4.15 (< LOD–44.51) | 3.89 (3.51–4.31) | |
| Cases | 138 | 90 | 4.41 (< LOD–44.51) | 4.12 (3.58–4.75) | |
| Controls | 138 | 86 | 3.95 (< LOD–33.85) | 3.68 (3.17–4.27) | |
| Urinary dimethylarsenic acid (ug/L) (UDMA) | 0.21 | ||||
| Total | 117b | 97 | 4.97 (< LOD–21.60) | 5.27 (4.82–5.76) | |
| Cases | 63 | 97 | 4.83 (< LOD–13.50) | 5.21 (4.60–5.90) | |
| Controls | 54 | 98 | 5.10 (< LOD–21.60) | 5.34 (4.68–6.08) | |
| Urinary creatinine (ug/L) | 0.63 | ||||
| Total | 276 | 100 | 103.7 (3.58–436.68) | 86.47 (77.76–96.15) | |
| Cases | 138 | 100 | 98.70 (3.58–75.53) | 86.72 (75.00–100.30) | |
| Controls | 138 | 100 | 107.61 (7.65–436.68) | 86.22 (73.71–100.80) | |
ap values are from paired t tests comparing geometric means between cases and controls
bArsenic speciation and metabolite analyses conducted on samples with UTAS greater than 5 ug/L
We used linear regression to assess the association between the self-reported exposures in Table 1 and BPA and UTAS levels (data not shown). Only fish consumption in the last 7 days was associated with a significantly higher level of UTAS (p = 0.0002). Activities that might be expected to increase exposure to BPA such as consuming bottled water; drinking hot beverages from plastic bottles, cups, or containers; and eating hot food from plastic dishes or containers in the past 24 hours were not significantly associated with BPA levels. BPA and UTAS were statistically correlated (p = <0.001); however the Spearman’s correlation coefficient was 0.53.
Results for BPA and UTAS were not significant in univariate or multivariate conditional logistic regression models. BPA and UTAS levels were evaluated as continuous variables and by quartiles. Odds ratios for both BPA and UTAS were close to 1 and therefore not clinically relevant. Age was consistently a significant variable in the multivariate models, with higher ages being associated with increased odds of being in the diabetes group (cases). The multivariate models were adjusted for BMI, continuous age, and sex, and were performed with and without creatinine adjustment (data not shown). None of the other variables in the multivariate models achieved statistical significance.
The 95% confidence intervals (CIs) around the geometric means for BPA, UDMA, and UTAS were compared between the CRST participants and the US population, aged 20 and older from the 2011–2012 NHANES. The CRST participants had significantly lower mean levels of total As and higher mean levels of UDMA and BPA when compared with the 2011–2012 NHANES (Table 4).
Table 4.
Geometric mean BPA, As, and Dimethylarsinic levels from Cheyenne River Sioux Tribe study participants (n=276, South Dakota, 2010-2011) and US population, 20 years and older, from 2011-2012 NHANES (n~1700).
| Geometric Mean (95% CI) | ||
|---|---|---|
| Analyte (ug/L) | CRST Participants | NHANES Participants |
| Bisphenol A | 1.83 (1.62–2.07) | 1.48 (1.35–1.61) |
| Urinary Total Arsenic | 3.89 (3.51–4.31) | 7.09 (6.03–8.33) |
| Urinary Dimethylarsinic acid | 5.27 (4.82–5.76) | 3.51 (3.22–3.82) |
Discussion
After a single, low-dose exposure, approximately 70% of As is excreted in urine after undergoing methylation within a few days of ingestion [15]. BPA is rapidly absorbed after low-dose oral ingestion and rapidly excreted in the urine as a glucuronide metabolite with a terminal half-life of less than 6 hours [16]. We did not find any statistically significant differences in UTAS and BPA levels between cases and controls in our study. Overall results in both cases and controls showed low urinary levels of As, As metabolites and BPA in our study population. This may have been due to a lack of any recent significant exposure to these toxicants. This study is novel because it reports urinary BPA levels in an AI/AN population and is also one of few studies examining urinary As levels in this population.
Our case-control study findings for BPA are similar to those previously reported in a cross–sectional, population-based Korean study that did not find a link between BPA and type 2 diabetes after adjusting for confounders [17]. While the first study by Lang showed a positive association between BPA and type 2 diabetes, LaKind et. al. (2012) performed a re-analysis of the NHANES data and found that after applying additional exclusion criteria and outcome definitions, there were no significant associations between urinary BPA and heart disease or diabetes [18]. According to NHANES data, urinary BPA concentrations vary by ethnicity and race in U.S. populations [14]. The urinary BPA geometric mean among the CRST participants during this study (1.83 ug/L) was higher than all other race/ethnicity groups in the 2011–2012 NHANES with the exception of non-Hispanic blacks (2.12 ug/L) [14]. The reason for this finding is unclear but might be related to socioeconomic status, diet and/or a particular cultural behavior. Another possibility could be a yet to be identified genetic differences in the metabolism or elimination of BPA.
Our case-control study findings for As differ from Navas’ original analysis of NHANES 2003–2004 data [5], however they are similar to a subsequent analysis of the same NHANES dataset published by Steinmaus, which found no evidence of increased risk of diabetes with arsenic exposure after controlling for UASB [19]. Similar to Navas’ NHANES analysis, later studies of Strong Heart Study participants concluded that total urinary As is associated with diabetes prevalence, but the studies did not control for organic As, a less toxic form of As commonly found in seafood [2]. Our study found lower UTAS concentrations in all CRST participants (geometric mean 3.89 ug/L) compared to the Strong Heart Study participants (geometric mean; 11.9 ug/L) [2]. The Strong Heart Study included other AI/AN populations which likely have different exposures compared to our participants because their study encompassed several communities that were distributed across several U.S. states.
Interestingly, we also found that UDMA was higher than either inorganic arsenic forms (UAS3 and UAS5) or MMA, a finding that was also reported in the Strong Heart Study [2]. The median UDMA level among CRST participants was higher than all racial/ethnic groups in the NHANES 2011–2012 data with the exception of Asians [14]. This finding was also noted by investigators of the Strong Heart Study, which looked at the relative proportion of each urine arsenic species and found higher percentages for UDMA and UMMA as a proportion of the UTAS [2]. The significance of this finding is unknown and the investigators hypothesized that this may possibly represent a unique genetic-related difference in arsenic metabolism found in AI/AN populations [3]. Unfortunately in our study, we had a high proportion of UMMA results that were < LOD and thus we could not test this hypothesis using accurate statistical analysis.
We found that UTAS levels were associated with self-reported consumption of seafood; this result was expected and agrees with previous studies [20]. BPA levels were not associated with self-reported recent use of plastic containers/dishes for water and food consumption, a factor which was thought to be a major source of exposure to BPA [21]. We found a statistically significant correlation between urinary BPA and UTAS levels, however, the correlation coefficient of 0.53 does not indicate a strong association. Possible scenarios that could explain this finding include exposure to a common source that has both BPA and As (such as a particular food product or drinking water) and/or less likely, a common toxicokinetic mechanism that has yet to be elucidated. This finding merits further investigation but was outside the scope of our study.
Limitations
There were several limitations to our study. One limitation of our study was the recruitment of participants on a volunteer basis which may have caused selection bias. Another limitation was that some participants with undiagnosed diabetes may have been included in the control group; however, recruitment from a local primary care clinic of patients with established medical history is likely to have reduced misclassification. We used corroborating clinic information when available, in addition to self-reported past medical history of diabetes, to prevent misclassification in the assignment to the control group vs. the case group. We also used validated approaches for self-reported diabetes diagnosis used in other scientific studies, such as screening questions (i.e., “Have you ever been told that you had diabetes by a doctor?” and “Do you have diabetes type 1 or type 2?”), a list of reported medications, and finger-stick blood glucose measurement [22]. All participants in the case group were able to provide very specific information about diabetes therapy and monitoring (Table 2).
Another limitation was that all our case patients who answered the question about diabetes reported that they had type 2 diabetes. Consequently, we could not evaluate any association between type 1 diabetes and environmental exposure to BPA and As. Previous studies reporting an association between BPA and arsenic in urine and a diagnosis of diabetes were also performed using participants that had type 2 diabetes (given that type 2 diabetes is more common compared with type 1 diabetes [3, 5, 8]). While five of our case patients with diabetes did not indicate whether they had type 1 or type 2 diabetes, this small number (4%) did not affect the results or conclusions of this study when excluded and, thus, were retained in the analyses. A second limitation is potential laboratory measurement artifacts and/or inaccuracies due to urine freezing, shipping, and thawing procedures. While this possibility always exists with any laboratory testing, we used the collection, shipping, and testing procedures for NHANES urine samples, which are analyzed at the same CDC laboratory (NCEH, Division of Laboratory Sciences, Atlanta, GA).
Conclusion
We did not find any statistically significant differences in BPA and arsenic levels between CRST study participants with and without diabetes. In our study, BPA and As were not significant predictors of case status in conditional logistic regression models.
Sources of Funding
This work was produced by US Government employees (except D.N.) as part of their official duties. Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Compliance with Ethical Standards
Conflict of Interest
None
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad F, Rossen L, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. 2018. [Google Scholar]
- 2.Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, Goessler W, Lee E, Guallar E, Navas-Acien A. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care. 2015;38(4):620–627. doi: 10.2337/dc14-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grau-Perez M, Kuo C-C, Gribble MO, Balakrishnan P, Spratlen MJ, Vaidya D, et al. Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environ Health Perspect. 2017;125(12). [DOI] [PMC free article] [PubMed]
- 4.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 5.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300(7):814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 6.Seachrist DD, Bonk KW, Ho S-M, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. 2016;59:167–182. doi: 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146–155. doi: 10.1056/NEJMra1202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122(6):616–623. doi: 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environ Health. 2012;11(1):10. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(12):1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett JMJR, Caldwell KL, Verdon CP. Total urine arsenic measurements using inductively coupled plasma mass spectrometry with a dynamic reaction cell. At Spectrosc. 2007;28(4):113–122. [Google Scholar]
- 13.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 14.Fourth report on human exposure to environmental chemicals, Updated Tables, (March, 2018). http://www.cdc.gov/exposurereport/ Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. ; 2018.
- 15.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, Silbergeld EK, Guallar E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117(9):1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hyg Environ Health. 2013;216(4):467–471. doi: 10.1016/j.ijheh.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.LaKind JS, Goodman M, Naiman DQ. Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS One. 2012;7(12):e51086. doi: 10.1371/journal.pone.0051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmaus C, Yuan Y, Liaw J, Smith AH. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology. 2009;20(6):807–815. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- 20.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res 2011;111(1):110-118. [DOI] [PMC free article] [PubMed]
- 21.Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastorino S, Richards M, Hardy R, Abington J, Wills A, Kuh D, Pierce M, National Survey of Health and Development Scientific and Data Collection Teams Validation of self-reported diagnosis of diabetes in the 1946 British birth cohort. Prim Care Diabetes. 2015;9(5):397–400. doi: 10.1016/j.pcd.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]