Abstract
An 88-year-old man with congenital hemophilia A developed end-stage renal disease due to microscopic polyangiitis. He was at risk for catheter-related infection because he was taking immunosuppressive agents for the treatment of polyangiitis. He was also unable to manipulate the peritoneal dialysis device. Therefore, hemodialysis using an arteriovenous fistula was induced for renal replacement therapy. Recombinant coagulation factor VIII (1000 IU) was administered via the venous chamber of the hemodialysis circuit 10 min before the end of each hemodialysis session, and nafamostat mesylate (25 mg/h) was employed as an anticoagulant during hemodialysis. His clotting factor VIII activity level increased to > 50% and activated partial thromboplastin time decreased to 50 s at the end of each hemodialysis session. This method allowed him to achieve hemostasis at the puncture site of the arteriovenous fistula and undergo stable hemodialysis with no complications, including bleeding. This case suggests that hemodialysis using an arteriovenous fistula with coagulation factor replacement and nafamostat mesylate in each hemodialysis session is a therapeutic option for end-stage renal disease in patients of advanced age with hemophilia at high risk of bleeding.
Keywords: Hemophilia, Hemodialysis, Arteriovenous fistula, Microscopic polyangiitis, Nafamostat, Recombinant coagulation factor VIII
Introduction
Hemophilia A is an X-linked recessive inherited bleeding disorder characterized by a deficiency of coagulation factor VIII [1]. Replacement of coagulation factor VIII is necessary when patients with hemophilia sustain external injuries or undergo invasive procedures. The number of patients with hemophilia who develop age-related diseases, such as renal failure potentially requiring renal replacement therapy, has been increasing because of improvements in the treatments for patients with hemophilia [2].
Peritoneal dialysis or hemodialysis with a long-term tunneled central venous catheter has mainly been selected as the dialysis modality for patients with hemophilia and end-stage renal disease requiring renal replacement therapy because hemodialysis with an arteriovenous fistula may result in bleeding from the puncture site after each hemodialysis session [3, 4]. However, peritoneal dialysis cannot be performed if the patient and caregiver are unable to manipulate the peritoneal dialysis device for physical or cognitive reasons [5, 6]. Furthermore, hemodialysis with a long-term tunneled central venous catheter may increase the risk of serious catheter-related complications, such as fatal infection [7]. In these cases, hemodialysis with an arteriovenous fistula may be selected. Several previous reports have described the performance of hemodialysis using an arteriovenous fistula for treatment of end-stage renal disease in patients with hemophilia [8–11]. However, further studies that include the details of the hemodialysis condition are required for optimal management of hemodialysis using an arteriovenous fistula in patients with hemophilia at high risk of bleeding. The authors herein report a case involving a patient of advanced age with congenital hemophilia A and microscopic polyangiitis who safely underwent induction of hemodialysis using an arteriovenous fistula with coagulation factor VIII replacement in each hemodialysis session.
Case report
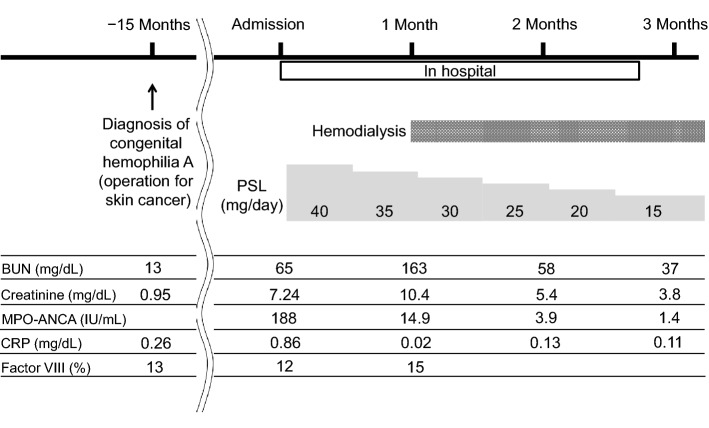
The patient was an 88-year-old man who had a family history of hemophilia in his brother and grandchild. He was diagnosed with congenital hemophilia A when he underwent an operation for skin cancer at the age of 87. The complete course of the patient is described in Fig. 1. The patient’s detailed preoperative laboratory data are shown in Table 1. His clotting factor level of 12% led to a diagnosis of mild hemophilia A because the blood clotting factor level of a patient with mild hemophilia ranges from 5 to 40%. At that time, 4000 IU of recombinant coagulation factor VIII was prophylactically administered immediately before the operation, and the operation was completed successfully with no bleeding complications. Fifteen months after surgery, he developed microscopic polyangiitis. He exhibited only signs of renal involvement, such as proteinuria, hematuria, and an elevated serum creatinine level. No hemorrhagic complications were observed, including purpura, nasal bleeding, pulmonary alveolar hemorrhage, or gastrointestinal bleeding. The detailed laboratory findings at the time of admission are shown in Table 1. The patient’s Birmingham vasculitis activity score on admission was 12 points. Regardless of immunosuppressive therapy including prednisolone, he progressed to end-stage renal disease necessitating renal replacement therapy. The authors presented the patient and his caregiver with the advantages and disadvantages of all available renal replacement therapy options, including peritoneal dialysis, hemodialysis with a long-term tunneled central venous catheter, and hemodialysis using an arteriovenous fistula. In the process of selecting the hemodialysis modality, he and his caregiver determined that they were unable to manipulate the peritoneal dialysis device because of age-related cognitive decline. They wished to avoid use of a long-term tunneled central venous catheter because of concerns regarding catheter-related problems, such as infection and bleeding. Additionally, the patient did not want to limit his daily physical activity by the presence of an indwelling tunneled hemodialysis catheter. Therefore, hemodialysis using an arteriovenous fistula was planned for renal replacement therapy. First, an arteriovenous fistula was produced in his right forearm with prophylactic administration of 4000 IU of recombinant coagulation factor VIII. Hemodialysis using the native arteriovenous fistula was conducted 44 days after admission. Nafamostat mesylate was administered continuously at a dose of 25 mg/h as an anticoagulant during hemodialysis. Coagulation-free dialysis was attempted, but it resulted in clotting of the hemodialysis circuit despite saline flushing. His target coagulation factor VIII activity level was > 40% at the end of the hemodialysis session to ensure hemostasis at the puncture site of the arteriovenous fistula [1]. Recombinant coagulation factor VIII (1000 IU) was administered via the venous chamber of the hemodialysis circuit 10 min before the end of each hemodialysis session. The administration dose of recombinant coagulation factor VIII was calculated by the following formula, which is based on a previous report [1]:
Fig. 1.
Clinical course of the patient. BUN blood urea nitrogen; MPO-ANCA myeloperoxidase–anti-neutrophil cytoplasmic antibodies; CRP C-reactive protein; PSL prednisolone
Table 1.
Laboratory results before skin cancer operation and on admission
| Complete blood count and blood chemistry | |||
|---|---|---|---|
| Before skin cancer operation | On admission | ||
| White blood cells | 4270 | 4740 | /µL |
| Red blood cells | 347 | 208 | ×103/µL |
| Hemoglobin | 11.8 | 6.7 | g/dL |
| Hematocrit | 37.0 | 21.9 | % |
| Platelets | 17.8 | 12.2 | ×104/µL |
| Total protein | 7.4 | 6.6 | g/dL |
| Albumin | 4.1 | 3.4 | g/dL |
| Aspartate aminotransferase | 25 | 30 | IU/L |
| Alanine aminotransferase | 15 | 28 | IU/L |
| Lactate dehydrogenase | 226 | 257 | IU/L |
| Alkaline phosphatase | 222 | 175 | IU/L |
| C-reactive protein | 0.26 | 0.35 | mg/dL |
| Sodium | 142 | 141 | mmol/L |
| Potassium | 4.6 | 5.1 | mmol/L |
| Chloride | 110 | 114 | mmol/L |
| Blood urea nitrogen | 13 | 65 | mg/dL |
| Creatinine | 0.95 | 7.24 | mg/dL |
| Uric acid | 6.7 | 9.1 | mg/dL |
| Total cholesterol | N/A | 125 | mg/dL |
| Hemoglobin A1c | N/A | 5.6 | % |
| Glucose | N/A | 89 | mg/dL |
| Immunological test | |||
|---|---|---|---|
| Before skin cancer operation | On admission | ||
| IgG | N/A | 2057 | mg/dL |
| IgA | N/A | 154 | mg/dL |
| IgM | N/A | 51 | mg/dL |
| C3 | N/A | 88 | mg/dL |
| C4 | N/A | 25 | mg/dL |
| PR3-ANCA | N/A | (−) | IU/mL |
| MPO-ANCA | N/A | 188 | IU/mL |
| Anti-GBM Ab | N/A | (−) | IU/mL |
| HBs Ag | N/A | (−) | |
| HCV Ab | N/A | (−) | |
| HIV Ab | N/A | (−) | |
| Blood coagulation test | |||
|---|---|---|---|
| Before skin cancer operation | On admission | ||
| PT | 81.4 | 81.8 | % |
| APTT | 57.3 | 63.1 | second |
| Factor VIII | 13 | 12 | % |
| Factor VIII inhibitor | Negative | N/A | |
| von Willebrand factor activity | 262 | N/A | % |
| Urinalysis | |||
|---|---|---|---|
| Before skin cancer operation | On admission | ||
| Specific gravity | 1.013 | 1.008 | |
| pH | 6.0 | 6.5 | |
| Red blood cells | (−) | 100< | /HPF |
| Protein (qualitative) | (−) | (3+) | |
| Protein (quantitative) | N/A | 4.03 | g/gCr |
| NAG | N/A | 7.0 | IU/L |
| β2-MG | N/A | 39907 | µg/L |
Ab antibody; ANCA anti-neutrophil cytoplasmic antibody; Ag antigen; APTT activated partial thromboplastin time; β2-MG β2-microglobulin; GBM glomerular basement membrane; HBs hepatitis B surface; HCV hepatitis C virus; HIV human immunodeficiency virus; HPF high-power field; IgA immunoglobulin A; IgG immunoglobulin G; IgM immunoglobulin M; MPO myeloperoxidase; N/A not applicable; NAGN-acetyl-β-d-glucosaminidase; PR-3 proteinase-3; PT prothrombin time
Dose of recombinant coagulation factor VIII (IU) = desired coagulation factor VIII level (%) × patient’s body weight (kg) × 0.5.
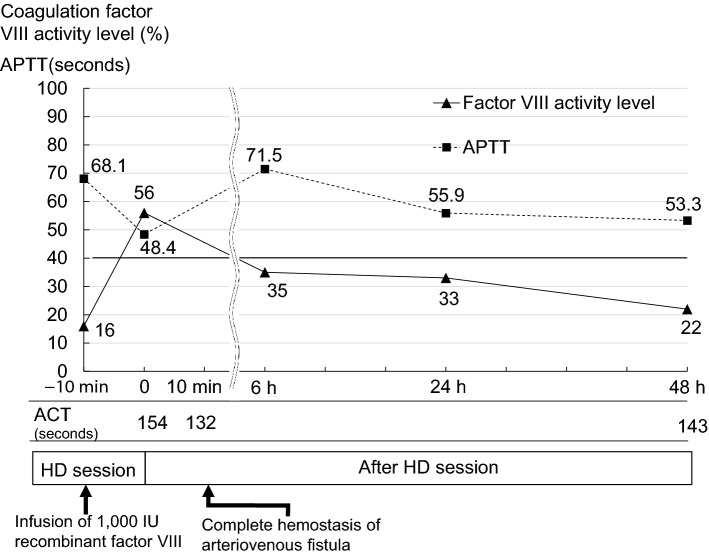
The details of the patient’s hemodialysis condition are shown in Table 2. After administration of 1000 IU of recombinant coagulation factor VIII, the patient’s coagulation factor VIII activity level increased to > 50% and his activated partial thromboplastin time (APTT) and activated clotting time (ACT) decreased to 48.4 and 132 s, respectively, at the end of each hemodialysis session (Fig. 2). Hemostasis at the puncture site of the arteriovenous fistula was achieved after 10 min of compression. Repeated monitoring of his APTT, ACT, and coagulation factor VIII activity level until his next hemodialysis session showed that his coagulation factor VIII activity level had decreased and that his APTT and ACT had increased to the basal levels at the next hemodialysis session (48 h after administration of recombinant factor VIII) (Fig. 2). On hospital day 80, he was discharged and continued to undergo regular hemodialysis in a community dialysis center with no problems, including bleeding and infection.
Table 2.
Dialysis prescription
| At initiation of hemodialysis with AVF |
At discharge | |
|---|---|---|
| Frequency (times a week) | 3 | 3 |
| Time (hours) | 3 | 3 |
| Dialyzer | Polysulfone dialyzer (NV-13S; Toray Medical Co., Ltd.) | Polysulfone dialyzer (NV-18S; Toray Medical Co., Ltd.) |
| Blood flow (mL/min) | 120 | 150 |
| Dialysate flow (mL/min) | 500 | 500 |
| Anticoagulation | Nafamostat (25 mg/h) | Nafamostat (25 mg/h) |
| Factor VIII | 1000 IU via the venous chamber of the hemodialysis circuit 10 min before the end of the hemodialysis session | 1000 IU via the venous chamber of the hemodialysis circuit 10 min before the end of the hemodialysis session |
| Dry weight (kg) | 48.5 | 43.0 |
AVF arteriovenous fistula
Fig. 2.
Changes in coagulation factor VIII activity level and APTT after recombinant factor VIII infusion. The x-axis shows the time from the end of the HD session. The y-axis shows the factor VIII activity level and APTT concentration. The factor VIII activity peaked 10 min after administration of recombinant factor VIII. ACT activated clotting time; APTT activated partial thromboplastin time; HD hemodialysis
Discussion
The authors have herein described a patient with end-stage renal disease and hemophilia A who safely underwent initiation of hemodialysis using an arteriovenous fistula with coagulation factor VIII replacement in each hemodialysis session.
The life expectancy of patients with hemophilia has been increasing with the development of recombinant coagulation factor concentrates, which can prevent life-threatening bleeding and transfusion-related infection [12]. Given the growing population of advanced-age patients with hemophilia, the number of such patients who develop age-related diseases, such as renal disease, heart disease, and cancer, has increased [2]. Additionally, as shown in the present case, some patients with hemophilia may have rare concomitant diseases for which a common pathogenesis with hemophilia has not been elucidated. There is no accepted consensus for the selection of renal replacement therapy in patients with hemophilia. However, induction of hemodialysis with an arteriovenous fistula has been considered difficult for patients with hemophilia because of concerns regarding bleeding at the puncture site of the arteriovenous fistula. Therefore, peritoneal dialysis or hemodialysis with a long-term tunneled central venous catheter is indicated for most patients with hemophilia who need renal replacement therapy [13–15]. These renal replacement therapy modalities are considered appropriate for treatment of end-stage renal disease in patients with hemophilia. In the present case, however, the patient and caregiver determined that they were unable to manipulate the peritoneal dialysis procedure because of age-related cognitive decline. Additionally, the patient had hemophilia A and was taking immunosuppressive agents for the treatment of polyangiitis; therefore, they did not want to use a tunneled hemodialysis catheter because of concerns regarding catheter-related problems, such as infection and bleeding. Thus, hemodialysis with an arteriovenous fistula was selected as his dialysis modality. Patients with hemophilia A require coagulation factor VIII replacement when they undergo surgical procedures, such as operations or arterial puncture [1]. The dose of coagulation factor VIII that is needed to stop bleeding is adjusted according to the degree of invasiveness and is calculated based on the formula described in a previously established guideline [1]. In that guideline, the replacement dose of coagulation factor VIII for minor surgery ranges from 40 to 80% [1]. Additionally, the coagulation factor VIII concentration in blood reportedly peaks 10 min after administration [16]. Various types of coagulation factor VIII products are available, including human plasma-derived, recombinant, and PEGylated products. However, plasma-derived products contribute to concern of infectious diseases, and PEGylated recombinant coagulation factor VIII might be associated with a risk of inhibitor development [17]. Coagulation factor VIII is not considered to be dialyzed because the molecular weight of factor VIII (light chain weight of 80,000 Da and heavy chain weight of 200,000 Da) is greater than that of albumin (69,000 Da) [18]. The authors selected a polysulfone membrane as a dialysis membrane because of its high biocompatibility, low protein adsorption capacity, and low platelet adhesion property [19]. However, the possibility of adhesion and trapping of recombinant coagulation factor VIII by such a dialyzed membrane cannot be denied. The authors therefore administered 1000 IU of recombinant coagulation factor VIII via the venous chamber of the hemodialysis circuit 10 min before the end of each hemodialysis session. As a result, the patient’s coagulation factor VIII activity level increased to > 50% and his APTT and ACT decreased to 48.4 and 132 s, respectively, at the end of each hemodialysis session, resulting in hemostasis at the puncture site of the arteriovenous fistula with 10 min of compression. Because his coagulation factor VIII activity level decreased and his APTT and ACT increased to basal levels 48 h after administration of recombinant coagulation factor VIII, the administration of recombinant coagulation factor VIII at each hemodialysis session was considered to be required. Coagulation factor VIII products should be administered at the lowest frequency to prevent the development of inhibitors of coagulation VIII products. ACT is widely used as an indicator of anticoagulation during hemodialysis. Heparin is administered to prevent clotting in the extracorporeal circuit with a target ACT of > 150 s during the hemodialysis session and is discontinued before the end of the hemodialysis session to achieve hemostasis with a target ACT of < 140 s at the end of hemodialysis [20]. Therefore, factor VIII supplementation might be able to be omitted when the ACT is < 140 s at the end of the hemodialysis session. In the present case, the authors carefully monitored the patient’s APTT, ACT, and coagulation factor VIII activity level until his next hemodialysis session after administration of recombinant coagulation factor VIII (Fig. 2). Several previous reports have described hemodialysis with administration of recombinant coagulation factors in patients with hemophilia using an arteriovenous fistula [10, 11]. Kato et al. [10] reported the safe conductance of hemodialysis in a patient with hemophilia A using an arteriovenous fistula with administration of recombinant coagulation factor VIII (1000 IU) immediately before removal of the dialysis needle at the end of every hemodialysis session. Fujii et al. [11] also reported the safe conductance of hemodialysis in a patient with hemophilia B using an arteriovenous fistula with administration of recombinant coagulation factor IX (20 IU/kg body weight) immediately after every hemodialysis session. Amberker et al. [21] suggested administration of a coagulation factor VIII (25–40 IU/kg body weight) three times per week after hemodialysis using an arteriovenous fistula in patients with hemophilia. In the present case, recombinant coagulation factor VIII (1000 IU) was administered 10 min before the end of each hemodialysis session by monitoring the patient’s APTT, ACT, and coagulation factor VIII activity level. Close monitoring of these parameters may be important to confirm the optimal dose of recombinant coagulation factor for conductance of safe hemodialysis in patients with hemophilia. However, a consensus has not been reached regarding the timing and dose of administration of coagulation factors for hemodialysis using an arteriovenous fistula in patients with hemophilia. Thus, accumulation of more cases involving hemodialysis with administration of coagulation factors in patients with hemophilia is needed to clarify the optimal dose and timing of coagulation factors for these patients.
The administration of nafamostat mesylate was also necessary in the present case because its temporary cessation during hemodialysis resulted in blood coagulation within the hemodialysis circuit. This patient was of advanced age, had microscopic polyangiitis, and was undergoing treatment with a corticosteroid, all of which reportedly increase the risk of bleeding such as gastrointestinal bleeding, intracranial bleeding, and subcutaneous bleeding [22–24]. Nafamostat mesylate has been used as an effective and safe anticoagulant during hemodialysis in patients at high risk of bleeding because of its short half-life of 8 min [25]. The superiority of nafamostat mesylate over heparin is especially evident in patients at high risk of bleeding [26, 27]. Therefore, nafamostat mesylate was used as a hemodialysis anticoagulant instead of heparin in this case. The above-described conditions, including the use of nafamostat mesylate, could facilitate the induction and conduction of stable maintenance hemodialysis with no complications, including bleeding and infection. However, low-molecular-weight heparin or heparin with the neutralization agent protamine may also be used for anticoagulation in patients undergoing hemodialysis. Further studies are needed to investigate the safety and effects of various anticoagulant agents for hemodialysis in patients with hemophilia who have concomitant diseases.
In conclusion, the authors have herein presented a case of safe treatment for end-stage renal disease in a patient with hemophilia A and microscopic polyangiitis by initiating hemodialysis using an arteriovenous fistula with coagulation factor VIII replacement and nafamostat mesylate in each hemodialysis session. This method may be a therapeutic option for patients with end-stage renal disease, hemophilia A, and a high risk of bleeding who require renal replacement therapy.
Acknowledgments
We thank Angela Morben, DVM, and ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient described in this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hiroki Ishii and Chiaki Miyoshi contributed equally.
References
- 1.Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 2.Canaro M, Goranova-Marinova V, Berntorp E. The ageing patient with haemophilia. Eur J Haematol. 2015;94(Suppl 77):17–22. doi: 10.1111/ejh.12497. [DOI] [PubMed] [Google Scholar]
- 3.Lambing A, Kuriakose P, Lanzon J, Kachalsky E. Dialysis in the haemophilia patient: a practical approach to care. Haemophilia. 2009;15(1):33–42. doi: 10.1111/j.1365-2516.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 4.Esposito P, Rampino T, Gregorini M, Fasoli G, Gamba G, Dal Canton A. Renal diseases in haemophilic patients: pathogenesis and clinical management. Eur J Haematol. 2013;91(4):287–294. doi: 10.1111/ejh.12134. [DOI] [PubMed] [Google Scholar]
- 5.Bieber SD, Mehrotra R. Patient and technique survival of older adults with ESRD treated with peritoneal dialysis. Perit Dial Int. 2015;35(6):612–617. doi: 10.3747/pdi.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong B, Venturato L, Oliver MJ, Quinn RR, Ravani P, Holroyd-Leduc J. Selection of peritoneal dialysis among older eligible patients with end-stage renal disease. Nephrol Dial Transplant. 2017;32(2):384–392. doi: 10.1093/ndt/gfw367. [DOI] [PubMed] [Google Scholar]
- 7.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusztal M, Kuzniar J, Weyde W, Klinger M. Haemodialysis in a patient with haemophilia B. Nephrol Dial Transplant. 2008;23(1):424–425. doi: 10.1093/ndt/gfm556. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa Silva PA, Soares SM, Fráguas G, de Oliveira Vaz FM, da José Silva M, da Gabriel Silva J. Management of a hemophilia patient in renal replacement therapy. Dial Transplant. 2011;40(6):262–263. doi: 10.1002/dat.20580. [DOI] [Google Scholar]
- 10.Kato N, Chin-Kanasaki M, Tanaka Y, Yasuda M, Yokomaku Y, Sakaguchi M, et al. Successful renal replacement therapy for a patient with severe hemophilia after surgical treatment of intracranial hemorrhage and hydrocephalus. Case Rep Nephrol. 2011;2011:824709. doi: 10.1155/2011/824709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii T, Takata N, Saito S, Naito T, Kimura A. Management of haemostasis during haemodialysis in a patient with haemophilia B. Haemophilia. 2008;14(5):1135–1137. doi: 10.1111/j.1365-2516.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 12.Mannucci PM, Iacobelli M. Progress in the contemporary management of hemophilia: the new issue of patient aging. Eur J Intern Med. 2017;43:16–21. doi: 10.1016/j.ejim.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Bajo MA, del Peso G, Jimenez V, Aguilera A, Villar A, Jimenez C, et al. Peritoneal dialysis is the therapy of choice for end-stage renal disease patients with hereditary clotting disorders. Adv Perit Dial. 2000;16:170–173. [PubMed] [Google Scholar]
- 14.Solak Y, Turkmen K, Atalay H, Turk S. Successful peritoneal dialysis in a hemophilia A patient with factor VIII inhibitor. Perit Dial Int. 2010;30(1):114–116. doi: 10.3747/pdi.2009.00011. [DOI] [PubMed] [Google Scholar]
- 15.Gopalakrishnan N, Usha T, Thopalan B, Dhanapriya J, Dineshkumar T, Thirumalvalavan K, et al. Hemodialysis in a patient with severe hemophilia A and factor VIII inhibitor. Hemodial Int. 2016;20(4):E11–E13. doi: 10.1111/hdi.12429. [DOI] [PubMed] [Google Scholar]
- 16.Shirahata A, Fukutake K, Takamatsu J, Shima M, Yoshioka A. Pharmacokinetics, prophylactic effects, and safety of a new recombinant FVIII formulated with sucrose (BAY 14–2222) in Japanese patients with hemophilia A. Int J Hematol. 2000;72(1):101–107. [PubMed] [Google Scholar]
- 17.Powell JS. Recombinant factor VIII in the management of hemophilia A: current use and future promise. Ther Clin Risk Manag. 2009;5(2):391–402. doi: 10.2147/tcrm.s4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei B, Pan C, Jiang H, Tjandra H, Strauss J, Chen Y, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270–279. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 19.Oshihara W, Ueno Y, Fujieda H. A new polysulfone membrane dialyzer, NV, with low-fouling and antithrombotic properties. Contrib Nephrol. 2017;189:222–229. doi: 10.1159/000450805. [DOI] [PubMed] [Google Scholar]
- 20.Jannett TC, Wise MG, Shanklin NH, Sanders PW. Adaptive control of anticoagulation during hemodialysis. Kidney Int. 1994;45(3):912–915. doi: 10.1038/ki.1994.121. [DOI] [PubMed] [Google Scholar]
- 21.Amberker D, Li TT, Rampure R. Successful use of arterio-venous graft for hemodialysis in patient with hemophilia. Hemodial Int. 2018;22(S2):S88–s91. doi: 10.1111/hdi.12706. [DOI] [PubMed] [Google Scholar]
- 22.Di Minno G, Tufano A. Challenges in the prevention of venous thromboembolism in the elderly. J Thromb Haemost. 2004;2(8):1292–1298. doi: 10.1111/j.1538-7836.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 23.Savige J, Gillis D, Benson E, Davies D, Esnault V, Falk RJ, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA) Am J Clin Pathol. 1999;111(4):507–513. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 24.Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open. 2014;4(5):e004587. doi: 10.1136/bmjopen-2013-004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo T, Kario K, Nakao K, Yamada T, Matsuo M. Anticoagulation with nafamostat mesilate, a synthetic protease inhibitor, in hemodialysis patients with a bleeding risk. Haemostasis. 1993;23(3):135–141. doi: 10.1159/000216866. [DOI] [PubMed] [Google Scholar]
- 26.Yang JW, Han BG, Kim BR, Lee YH, Kim YS, Yu JM, et al. Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Ren Fail. 2009;31(8):668–675. doi: 10.3109/08860220903180616. [DOI] [PubMed] [Google Scholar]
- 27.Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S. Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs. 2016;39(1):16–21. doi: 10.5301/ijao.5000465. [DOI] [PubMed] [Google Scholar]