Abstract
A 70-year-old man diagnosed with lung adenocarcinoma was referred to our department for an evaluation of acute onset of nephrotic syndrome with acute kidney injury (AKI) after the 7th course of pembrolizumab treatment. Renal biopsy could not be performed, because he needed anticoagulation therapy for venous thrombosis. Pembrolizumab was discontinued, and prednisolone was started. Hemodialysis was also started, because oliguria was not resolved, and dyspnea due to pulmonary congestion appeared even with the high dose of diuretics. Hemodialysis was successfully withdrawn within 5-week duration because of renal function recovery and increase of urine volume. Complete remission was achieved 4 months after initiating prednisolone. He has never experienced hemodialysis again and remains remission of nephrotic syndrome even the dose of prednisolone was tapered for 8 months. Renal pathology in the current case was uncertain. However, minimal change disease seemed to be a plausible cause of nephrotic syndrome with AKI because of a good response to steroid therapy and acute onset of nephrotic syndrome. In addition, renal pathology in all of the reported cases of pembrolizumab-associated nephrotic syndrome with AKI was minimal change disease. Our case shows for the first time that renal function could be reversible with prednisolone in pembrolizumab-associated nephrotic syndrome with severe AKI even after progression of renal failure which needs dialysis.
Keywords: Pembrolizumab, Nephrotic syndrome, Acute kidney injury, Lung cancer, Withdrawal from hemodialysis, Immune checkpoint inhibitor
Introduction
Pembrolizumab is an anti-programmed death 1 (PD-1) antibody approved for the treatment for several types of malignancies such as melanoma, Hodgkin’s lymphoma, non–small-cell lung cancer, and renal cell carcinoma [1]. Pembrolizumab is one of the immune checkpoint inhibitors that restore T-cell response toward cancer cells. Recent review demonstrated that the incidence of acute kidney injury (AKI) by these drugs was not rare, ranging from 9.9 to 29% [2]. The incidence of the apparent renal adverse events by pembrolizumab was 1.77% in a French single-center cohort [3]. The main renal pathophysiology in the report was acute tubulointerstitial lesions. Meanwhile, a few of cases in the glomerular lesions were also reported [3–6]. All of four cases with nephrotic syndrome exhibited minimal change disease, experienced AKI, and responded to steroid; however, a patient who had received hemodialysis could not withdraw from dialysis for a month, and died due to cancer progression [6]. Here, we report a case of pembrolizumab-associated nephrotic syndrome with severe AKI in a patient with lung cancer. The patient could successfully discontinue hemodialysis therapy within 5-week duration because of renal function recovery in response to steroid treatment.
Case report
A 70-year-old man was diagnosed with stage IV lung adenocarcinoma 6 months before referral. He was also diagnosed with venous thrombosis in brachiocephalic and brachial vein at the same time, and was started apixaban, direct oral anticoagulant. Pembrolizumab was initiated 5 months before referral (treatment dose, 200 mg intravenously every 3 weeks). The size of tumor assessed by computed tomography decreased after fifth course of pembrolizumab treatment. Erythroderma desquamation on the lower leg was noticed after sixth course of pembrolizumab treatment, but was suspected as an adverse effect of calcium channel blocker which was started over a year ago for the treatment of hypertension. Pembrolizumab was postponed, and topical corticosteroid and angiotensin receptor blocker, instead of calcium channel blocker, were prescribed. As the skin lesion was resolved a week later, pembrolizumab was re-administrated 1 month before referral. Seventeen days after the seventh course of pembrolizumab administration, the patient suddenly noticed anasarca with weight gain of 3.5 kg. Twenty-one days after the last treatment of pembrolizumab, he was referred to our department for an evaluation of massive proteinuria and hypoalbuminemia (1.3 g/dL). As urinary protein was negative and serum albumin level was normal (3.8 g/dL) at the last administration of pembrolizumab, and acute onset of nephrotic syndrome was diagnosed. A diuretic was started, but edema and body weight gain got worse. He was admitted for the treatment of uncontrollable edema and elevation of serum creatinine (SCr) level (from 0.70 to 1.63 mg/dL).
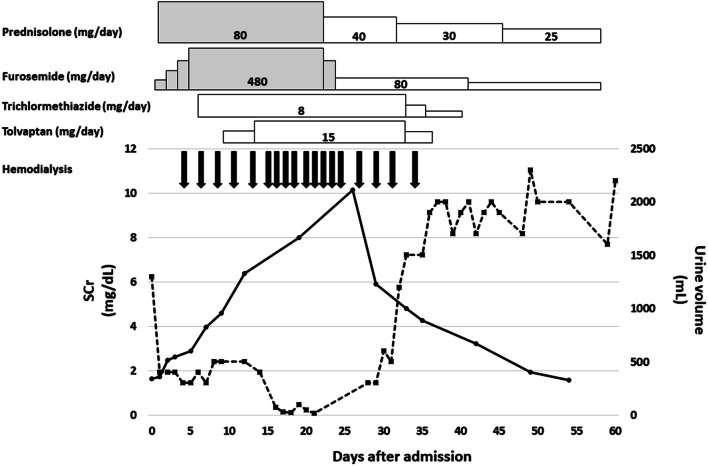
A physical examination on admission showed a blood pressure of 110/81 mmHg, a regular pulse rate of 92 beats/min. A laboratory test is shown in Table 1. The clinical course during hospitalization is shown in Fig. 1. The dose of diuretic was increased after admission, and 40 mg of intravenous injection of prednisolone sodium succinate (treatment dose; 0.8 mg/kg, twice in a day) was initiated on the second day after admission. However, oliguria was continued, even though the high dose of diuretics and dyspnea due to pulmonary congestion appeared on day 3 after admission. Therefore, hemodialysis was initiated, and excess fluid was removed. As edema was controlled by day 21, improvement of the drug absorption from intestine was considered. Hence, 40 mg of oral prednisolone was started instead of intravenous injection of prednisolone sodium succinate. Because urine output increased dramatically on day 34, hemodialysis was stopped. SCr level of the patient decreased gradually, and he was discharged on day 60. Afterward, the level of proteinuria gradually decreased, and reached to complete remission 50 days after discharge. At the last follow-up, 8 months after hospitalization, the levels of urinary protein-to-Cr ratio, serum albumin, and SCr were 60 mg/gCr, 4.0 g/dL, and 0.91 mg/dL, respectively. As the levels of tubular injury markers [urine β2 microglobulin and N-acetyl-beta-d-glucosaminidase (NAG)] on admission were elevated, tubular injury, as well as glomerular injury, was speculated. However, the levels were normalized at the last follow-up (urine β2 microglobulin 125 μg/L and NAG 8.0 IU/L), suggesting that the tubular injury was transient. There were no electrolyte abnormalities such as the low serum uric acid, potassium and phosphate, or excretion of urinary glucose after discharge, and thus, Fanconi syndrome was ruled out (Fig. 2).
Table 1.
Laboratory data on admission
| Complete blood count | |
|---|---|
| WBC | 6100/μL |
| Hb | 13.0 g/dL |
| Plt | 34.8 × 104/μL |
| Blood chemistry | |
| AST | 44 IU/L |
| ALT | 23 IU/L |
| ALP | 329 IU/L |
| TP | 4.6 g/dL |
| Alb | 1.0 g/dL |
| T-Chol | 319 mg/dL |
| BUN | 25 mg/dL |
| Cr | 1.63 mg/dL |
| eGFR | 34 mL/min/1.73 m2 |
| UA | 7.0 mg/dL |
| Na | 132 mEq/L |
| K | 4.9 mEq/L |
| Cl | 105 mEq/L |
| Ca | 7.0 mg/dL |
| iP | 4.5 mg/dL |
| CRP | 0.1 mg/dl |
| HbA1C | 5.8% |
| BS | 103 mg/dL |
| Serology | |
| C3 | 145 mg/dL |
| C4 | 32 mg/dL |
| CH50 | 38.1 IU/mL |
| IgG | 1,103 mg/dL |
| IgA | 459 mg/dL |
| IgM | 366 mg/dL |
| ANA | 1/160 |
| Anti-DNA Ab | < 2.0 IU/mL |
| Urine analysis | |
| pH | 5.0 |
| Protein | 15.1 g/gCr |
| RBC | 1–5/HPF |
| NAG | 54.4 IU/L |
| β-2MG | 1,018 μg/L |
| FeNa | 0.11% |
| FeUN | 18.4% |
| Selectivity index | 0.16 |
WBC white blood cell count, Hb hemoglobin, Plt platelet, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, TP total protein, Alb albumin, T-Chol total cholesterol, BUN blood urea nitrogen, Cr creatinine, UA uric acid, iP inorganic phosphate, CRP C-reactive protein, HbA1c glycosylated hemoglobin, BS blood sugar, C3 complement component 3, C4 complement component 4, CH50 complement activities, Ig immunoglobulin, ANA antinuclear antibodies, anti-DNA Ab anti-DNA antibody, RBC red blood cell, NAG N-acetyl-β-D-glucosaminidase, β-2MG β-2 microglobulin, Fe fraction excretion
Fig. 1.
Clinical course during hospitalization. Serum creatinine level and 24-h urine volume are shown in a solid line and dotted line, respectively. The gray/white colored box indicates intravenous/oral administration of the drugs. Hemodialysis was withdrawn, because urine output was dramatically increased
Fig. 2.
Change of proteinuria after discharge. The amount of proteinuria and serum creatinine (SCr) level are shown in a solid line and dotted line, respectively
Discussion
Pembrolizumab is a humanized, monoclonal IgG4-kappa isotype antibody against PD-1 which gets rid of the checkpoints from the immune system and activates T cells, and thus, it is referred to as immune checkpoint inhibitor. Currently, two types of immune checkpoint inhibitors are available. One is PD-1-blocking antibodies including pembrolizumab/nivolumab, and another is Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-blocking antibodies such as ipilimumab/tremelimumab. Immune-related toxicities such as colitis, dermatitis, pneumonitis, hepatitis, and thyroiditis are common with these drugs [7, 8]. On the other hand, the incidence of the renal adverse events by immune checkpoint inhibitors was considered to be low [9]. However, recent reports revealed that the incidence was not rare [2]. Main pathology by these drugs was acute tubulointerstitial lesions [9], whereas other pathologies were also reported, including minimal change disease, IgA nephropathy, lupus nephropathy, thrombotic microangiopathy, and pauci-immune glomerulonephritis [3–6, 10–12]. In our case, we could not perform renal biopsy, because he was treated with direct oral anticoagulant against venous thrombosis. We speculated that the renal pathology of our patient was minimal change disease as all of the reported cases in pembrolizumab-associated nephrotic syndrome had exhibited minimal change disease [3–6]. In addition, the clinical course of the patient (acute onset of nephrotic syndrome with AKI and response to steroid therapy) was consistent with minimal change disease. In the present case, urinary NAG and β2-microglobulin at the time of onset showed high values, which might derive from tubular injury as others have reported [9]. However, it was difficult to distinguish whether the cause of the elevation of these parameters was due to AKI or tubular injury by pembrolizumab.
The onset of nephrotic syndrome with AKI in our case occurred after the seventh course of pembrolizumab, which means 5 months after pembrolizumab initiation. Median time to the onset of renal adverse event by immune checkpoint inhibitors was highly variable, ranging from 6 to 30 weeks [13]. Regarding PD-1 inhibitors, median time in nivolumab was from 6 to 10 weeks [14, 15]; on the other hand, that in pembrolizumab has not been reported yet. The timing of pembrolizumab-associated nephrotic syndrome with AKI was reported to occur after the second-dose administration [3–6]. An atypical case of patient with mild AKI and nephrotic range of proteinuria without decline of serum albumin was reported [6]. The onset of renal adverse event in the case occurred 18 months after pembrolizumab initiation. The reason for the relatively late onset of nephrotic syndrome with AKI in our case is not clear. Cancer-induced minimal change disease might be considered as an etiology of nephrotic syndrome. Minimal change disease in association with cancers including lung cancer has been reported [16]. The extent of proteinuria was correlated with tumor progression/regression in the cases, suggesting that factors released from cancer cells induce minimal change disease [16]. However, the size of tumor in the current case was regressed before fifth course of pembrolizumab administration, and had not been progressed again until discharge, denying the possibility of minimal change disease induced by cancer.
Erythematous rash on the lower extremities was preceded nephrotic syndrome in this case. Erythematous rash on the trunk or extremities is one of the typical skin adverse effects of immune checkpoint inhibitors [17]. It has been reported that the extrarenal adverse events preceded AKI in half of the cases treated with pembrolizumab [6]. Therefore, the onset of extrarenal symptoms may predict a subsequent nephrotic syndrome in patients treated with pembrolizumab. Further case accumulation is needed to clarify the precursory symptoms of nephrotic syndrome associated with pembrolizumab.
Recently, the underfilling hypothesis in the nephrotic syndrome is controversial [18]. It has been reported that plasma volume was preserved during edema removal in the patients with nephrotic syndrome [19]. Therefore, in the present case, high dose of diuretics was used for the treatment of pulmonary congestion. However, the guideline from Kidney Disease: Improving Global Outcomes or Japanese Societies did not recommend the use of loop diuretics for the prevention or treatment of AKI [20, 21]. We should consider about stopping such high-dose diuretics when the patient became anuric and hemodialysis started, because we can control plasma volume with hemodialysis. The cytotoxicity of high-dose furosemide has been reported [22].
The case of a patient with lung cancer who had experienced pembrolizumab-associated nephrotic syndrome with severe AKI was herein described. The patient could successfully discontinue hemodialysis 5 weeks after initiation of steroid treatment. One should consider initiating steroid treatment and dialysis to similar cases even if the patients are in the advanced stage of cancer as the renal function could be reversible. Since we could not exclude the possibility of a sporadic case of nephrotic syndrome, further case accumulation is necessary to conclude that steroid therapy is effective in pembrolizumab-associated nephrotic syndrome.
Authors’ contributions
KI prepared abstract, case presentation, and figures. TI prepared the remaining part of manuscript, and corrected abstract/case presentation. MK, HF, and RF reviewed and corrected the manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare no competing interest.
Informed consent
Informed consent was obtained from the patient in the case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kento Ishibuchi and Takamasa Iwakura have contributed equally to this study as co-first authors.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockadeincancertherapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–169. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 3.Bickel A, Koneth I, Enzler-Tschudy A, Neuweiler J, Flatz L, Früh M. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer. 2016;16:656. doi: 10.1186/s12885-016-2718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Brom RR, Abdulahad WH, Rutgers A, et al. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology (Oxford) 2016;55:1143–1145. doi: 10.1093/rheumatology/kew063. [DOI] [PubMed] [Google Scholar]
- 5.Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, Reich HN. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70:581–585. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Izzedine H, Mathian A, Champiat S, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 8.Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 2015;6:3479–3492. doi: 10.18632/oncotarget.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, Mathian A. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32:936–942. doi: 10.1093/ndt/gfw467. [DOI] [PubMed] [Google Scholar]
- 10.Kishi S, Minato M, Saijo A, et al. IgA nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med. 2018;57(9):1259–1263. doi: 10.2169/internalmedicine.9814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 12.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remon J, Mezquita L, Corral J, Vilariño N, Reguart N. Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. J Thorac Dis. 2018;10:S1516–S1533. doi: 10.21037/jtd.2017.12.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyrier A, Delahousse M, Callard P, Rainfray M. Minimal change nephrotic syndrome revealing solid tumors. Nephron. 1992;61:220–223. doi: 10.1159/000186877. [DOI] [PubMed] [Google Scholar]
- 17.Lacouture ME, Wolchok JD, Yosipovitch G, Kähler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161–169. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Siddall EC, Radhakrishnan J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012;82:635–642. doi: 10.1038/ki.2012.180. [DOI] [PubMed] [Google Scholar]
- 19.Anton B, Hendrik A, Jan C, et al. Preservation of blood volume during edema removal in nephrotic subjects. Kidney Int. 1985;28:652–657. doi: 10.1038/ki.1985.179. [DOI] [PubMed] [Google Scholar]
- 20.Kellum JA, Lameire N. KDIGO AKI guideline work group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi K, Nishida O, Shigematsu T, et al. The Japanese clinical practice guideline for acute kidney injury 2016. J Intensive Care. 2018;6:48. doi: 10.1186/s40560-018-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuengsrigul A, Chin TW, Nussbaum E. Immunosuppressive and cytotoxic effects of furosemide on human peripheral blood mononuclear cells. Ann Allergy Asthma Immunol. 1999;83:559–566. doi: 10.1016/S1081-1206(10)62870-0. [DOI] [PubMed] [Google Scholar]