Abstract
Bullous pemphigoid (BP) is the most common autoimmune subepidermal bullous diseases. Autoantibodies against hemidesmosomal adhesion proteins might be involved in the developing process. BP usually affects the elderly with high mortality whereas the drug-induced BP is often improved and rarely relapses after the withdrawal of the suspected drug. An accumulated evidence suggests that dipeptidyl peptidase-4 inhibitor (DPP-4I), which has been widely used as the antidiabetic drug improves glycemic control with little risk for hypoglycemia, could be an inducer of DPP-4I-associated BP (DPP-4I-BP). While the precise mechanism remains unclear, a unique immunological profile with human leukocyte antigen (HLA)-DQB1*03:01 could be a biomarker of genetic susceptibility to DPP-4I-BP. Here, we encountered an interesting case of DPP-4I-BP with HLA-DQB1*03:01, which was likely triggered by scabies. A 56-year-old Japanese male with type 2 diabetes on hemodialysis was referred to our hospital due to worsened blisters. Prior to his admission, he had been on linagliptin, a DPP-4I, for 5 months. He then suffered from scabies 2 weeks before his admission while the treatment with ivermectin failed to improve his symptom. Based on his clinical symptom, positive for anti-BP180 autoantibody in serum, and the pathological alterations of skin biopsy specimens, he was diagnosed with DPP-4I-BP. Importantly, he also carried an HLA-DQB1*03:01 allele. Oral prednisolone was subsequently administered after the discontinuation of linagliptin, and his symptom gradually disappeared. Given the fact that the DPP-4I-BP could be a life-threating disease, we should be cautious of prescribing DPP-4I in hemodialysis patients, whose immune system could be impaired.
Keywords: Bullous pemphigoid, Diabetes, Dipeptidyl peptidase-4 inhibitor, Human leukocyte antigen-DQB1*03:01, Scabies
Introduction
Bullous pemphigoid (BP) is the most common autoimmune subepidermal bullous disease typically manifesting generalized tense, pruritic blisters [1]. BP is basically classified into two clinical phenotypes: one is the inflammatory type, and the other is the non-inflammatory [2]. Noninflammatory BP tends to manifest milder skin symptoms with fewer erythema than inflammatory BP does [2, 3]. BP is characterized by the presence of autoantibodies that recognize hemidesmosomal adhesion components (known as BP180 and BP230) at the basement membrane zone (BMZ), which plays an important role for adhesion between the epidermis and dermis [4]. Noncollagenous 16A (NC16A), an extracellular domain of BP180, contains the major pathogenic epitope so that the serum level of anti-BP180-NC16A autoantibody often correlates with the severity of the disease [5]. In particular, anti-BP180-NC16A autoantibody could be involved in blister formation by activating complement-dependent inflammatory pathway and BP180 internalization at BMZ by complement-independent manner [4]. BP is a chronic skin disorder mainly affects the elderly with high mortality rate (1-year mortality rate, 23.5%) [6]. In contrast, drug-induced BP shows a good response to treatment after withdrawal of the suspected drug, and rarely relapses. More than 50 agents have been implicated as a cause of drug-induced BP [7].
Recently, dipeptidyl peptidase-4 inhibitors (DPP-4Is) have been known to cause the drug-induced BP [2, 7]. DPP-4 enzymatically degrades incretins such as glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) so that DPP-4 inhibition usually prolongs the GLP-1/GIP-dependent insulin secretion from pancreatic beta cells and exhibits anti-diabetic effect, less likely inducing hypoglycemia [8]. Importantly, oral treatment options are limited in cases of diabetic patients on hemodialysis but DPP-4Is are relatively safe to be utilized in such conditions. As a result, this agent is currently the mainstay drug to control blood sugar concentration in type 2 diabetic patients on hemodialysis [9]. Given the fact that diabetic nephropathy is the most common cause in the incident dialysis (43.2%) and the number of hemodialysis patients have been growing in Japan [10], perhaps the number of cases with DPP-4 inhibitor-associated BP (DPP-4I-BP) would increase in future. Importantly, DPP-4 is also known as cluster of differentiation 26 (CD26) and activates T-cell [11]. Thus, DPP-4I might alter immune regulation, causing autoimmune disorders. Recent studies showed predominance of noninflammatory phenotype in patients with DPP-4I-BP [2, 12]. Interestingly, the strong association between human leukocyte antigen (HLA)-DQB1*03:01 and non-inflammatory DPP-4I-BP was shown in Japanese patients [12].
Scabies, a parasitic infection caused by mite Sarcoptes scabiei, develops multiple clinical phenotypes and sometimes mimic BP which is known as bullous scabies [13]. Since skin lesions in patients with end-stage renal disease are diverse and sometimes difficult to diagnosis without histopathological evaluations [14], early diagnosis is critical to achieve a prompt remission from the disease. Scabies also becomes a serious problem in hemodialysis patients since it could be epidemic in dialysis unit [15]. In addition, diabetes is one of the prevalent comorbidities in patients with scabies [16]. Since steroid therapy could exacerbate scabies, ruling out scabies is essential before making diagnosis of BP in hemodialysis patients with diabetes. Importantly, some cases of BP could be triggered by scabies [16].
Case report
A 56-year-old Japanese male who had been on hemodialysis for 7 years was referred to our hospital because of worsening of cutaneous pruritus, which began 1 month prior to admission. Although antipruritic drugs were administered, his symptom was never resolved. Two weeks later, he was diagnosed with the common scabies as small number of mites was detected in the specimen taken from abdominal erythematous papules by microscopy. He was then treated with 200 µg/kg of oral ivermectin for treatment twice, 1 week apart. However, skin lesions were refractory to the treatment and tense blisters were rather spread into the whole-body. Since drug allergy was suspected as a cause, his daily oral medications were discontinued. However, skin lesions were not remarkably improved.
Originally, he had type 2 diabetes and initiated hemodialysis due to diabetic nephropathy. Although insulin therapy was initially required for treating diabetes, the patient could achieve successful withdrawal of insulin therapy 1 year prior to admission. However, 5 months prior to admission, he started linagliptin, a DPP-4I, for glycemic control. It was the first time for him to take a DPP-4I in his life. His past medical history also included hypertension, dyslipidemia, hyperuricemia, and peripheral artery disease. He had no recent histories of surgery and trauma. He had been also taking several daily drugs, including irbesartan 100 mg, carvedilol 5 mg, alfacalcidol 0.25 µg, clopidogrel sulfate 75 mg, and precipitated calcium carbonate 1500 mg for more than 2 years. The conditions of dialysis were as follows: mode, pre-dilution on-line hemodiafiltration; blood access, arteriovenous fistula; duration and frequency of dialysis, three 4 h sessions/week; dialysis membrane, MFX-30Seco (polyethersulphone membrane, Nipro, Japan); anticoagulant, heparin; and medications administered through the dialysis circuit, darbepoetin alfa 80 µg/week and saccharated ferric oxide 40 mg/week.
At initial presentation, his height was 181.0 cm, body weight was 63.5 kg. His vital signs included body temperature of 37.6 ℃, blood pressure of 185/95 mmHg, heart rate of 87 beats/min with regular rhythm, respiratory rate of 18 breathe/min, and oxygen saturation of 98% with room air. On physical examination, tense blisters were observed, and epidermal abrasion was remarkable since most of blisters were broke with time course (Fig. 1). Oral mucosa was intact. Other physical findings were not remarkable. Blood tests revealed normal white blood cell counts of 6600/µL with a slightly elevated eosinophil counts of 825/µL, an elevation of C-reactive protein of 12.57 mg/dL, and liver function test results within normal range. Immunoglobulin G (IgG) anti-BP180-NC16A autoantibody by chemiluminescent enzyme immunoassay (40.0 U/mL, normal range < 9.0 U/mL) was positive. Skin biopsy was performed to examine a blister of trunk of the body and pathological evaluations revealed a subepidermal bullae and infiltrations of inflammatory cells into dermis (Fig. 2), which did not indicate scabies. Although direct immunofluorescence was not performed due to the faculty’s limitation and we could not ascertain the deposition of IgG and complement component 3 on basement membrane of skin lesion, these findings were compatible with BP.
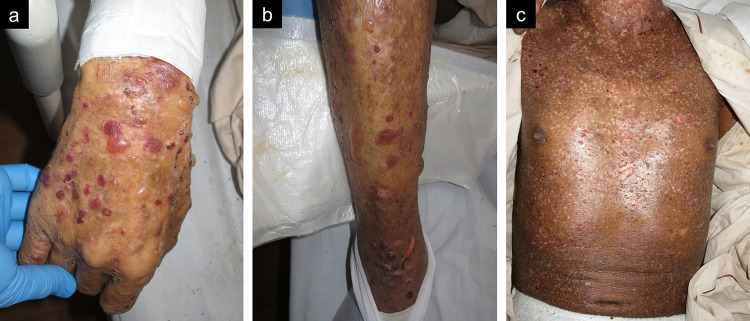
Fig. 1.
Cutaneous manifestations of the present case on the day of admission. Multiple bullae are observed on upper extremities (a), lower extremities (b). On body trunk, skin erosions are remarkable since most of bullae are broken during clinical course (c)
Fig. 2.
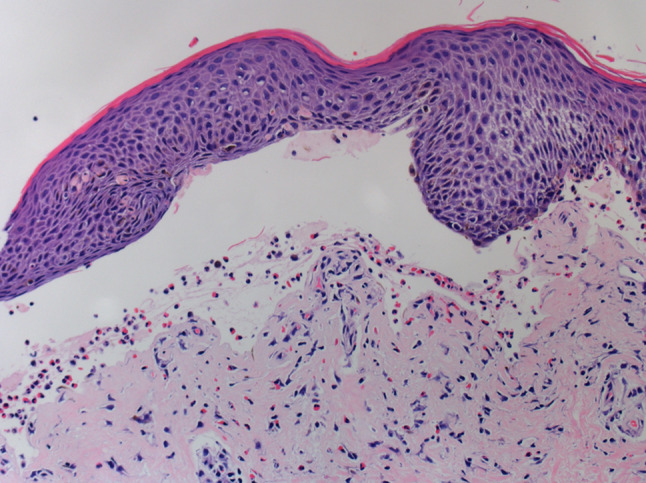
Light microscopic findings of skin biopsy. Subepidermal bullae are observed and superficial inflammation with infiltrations of eosinophils and lymphocyte are noted (hematoxylin and eosin stain, original magnification × 200)
Considering his clinical course, we suspected that linagliptin could trigger the development of BP. Oral prednisolone (PSL) therapy (20 mg/day) and 0.05% clobetasol propionate ointment were initiated from the day of admission based on severe cutaneous manifestation. Two types of antibiotics including vancomycin and ceftazidime were intravenously administered prophylactically to prevent his skin with compromised skin barrier function from infections. Intensive insulin therapy was required due to worsened glycemic control after the initiation of PSL therapy. His skin condition was gradually improved by PSL therapy and he was transferred to the former hospital to continue the treatment on day 12. During hospitalization, the recurrence of scabies was denied by repeated microscopic examinations. Later, we examined the patient’s HLA-DQB1 alleles by polymerase chain reaction-sequence-based typing method in the preserved blood sample and one of alleles was HLA-DQB1*03:01.
Discussion
To our knowledge, Skandalis et al. was the first group to document cases with DPP-4I-BP in 2011 [17]. Later, Haber et al. described the first two cases of linagliptin-associated BP in 2015 [18]. In 2016, the strong association between DPP-4I exposure and the development of BP was firstly demonstrated in a large-scale epidemiological study conducted by using French Pharmacovigilance Database [19]. Kawaguchi et al. subjected 9304 diabetic patients receiving DPP-4Is and found that the prevalence of DPP-4I-BP was 0.0859% [20]. To date, the number of patients with DPP-4I-BP has been likely increasing.
Recently, a systemic review and adjusted meta-analysis conducted by Phan et al. also revealed a significant association between DPP-4I use and BP (odds ratio 2.13) [21]. In Japanese population, a disproportionality analysis based on the Japanese Adverse Drug Event Report Database (JADER) showed that odds ratio was high in patients receiving vildagliptin, teneligliptin, or linagliptin (reporting odds ratio, 12.09, 5.52, and 2.67, respectively). They also suggested that elderly was also a risk factor for DPP-4I-BP [22]. A study subjecting 769 cases of drug-induced BP (referred as pemphigoid in the original article) recorded in JADER between April 2004 and November 2017 examined causative agents of BP and found that the most causative agent of BP was vildagliptin (288 out of 769 cases) while 102 cases with sitagliptin, 86 cases with teneligliptin, and 64 cases with linagliptin were reported [23]. Although lower substrate selectivity for DPP-4 and different specificity against DPP-8 and DPP-9 could be associated with pathophysiology of the disease [24], further studies are required to explain different propensities to cause BP in each DPP-4I.
In terms of the latency period from DPP-4I administration to the onset of BP, Béné et al. reported that median time was 10 months (ranged from 8 days to 37 months) [19]. A large-scale cohort study in the United Kingdom population showed that a hazard ratio of DPP-4I-BP was peaked at 20 months after DPP-4I administration [25]. Although the prognosis and clinical courses of DPP-4I-BP are not well known, a suitable approach would be withdrawing the DPP-4I. Béné et al. reported that BP was improved relatively soon by DPP-4I withdrawal, and the median time was just 10 days [19]. Benzaquen et al. reported 95% of the cases of DPP-4I-BP achieved partial or complete remission by the DPP-4I withdrawal with a follow-up period of 3–30 months [26]. The importance of discontinuation of DPP-4I was illustrated by Shrestha et al. showing a hemodialysis patient who relapsed BP after withdrawal of steroid therapy because of continuous DPP-4I use [27].
Although the precise mechanism for DPP-4I-BP induction remains unclear, the production of autoantibodies in response to DPP-4I use could be a potential cause. Izumi et al. found that patients who were positive for anti-full-length BP180 autoantibodies (autoantibodies against non-NC16A domain of BP180) but negative for anti-BP180-NC16A autoantibodies would tend to develop noninflammatory BP with DPP-4I use [2]. Interestingly, even without any cutaneous symptoms, anti-full-length BP180 autoantibodies were more frequently found in the patients with DPP-4I use (10.9%), compared to those without DPP-4I use (5.6%) [28], suggesting that blocking DPP-4 might be involved in the induction of anti-full-length BP180 autoantibodies. Mai et al. reported patients of DPP-4I-BP without anti-BP180-NC16A autoantibodies had autoantibodies intensively react with LABD97, a 97-kDa processed extracellular domain of BP180 [29]. These unique autoantibody profiles against BP180 might be linked to clinical characteristics of DPP-4I-BP. Alternatively, the comorbidity of diabetes may contribute to autoantibody production in response to DPP-4I use as high frequency of diabetes was observed in patients with BP (20%) [30]. Although DPP-4I-BP also manifests either an inflammatory or noninflammatory phenotype [12], some studies reported the number of eosinophils infiltrating into the skin was significantly lower in patients with DPP-4I-BP compared to patients with non-drug induced BP [2, 12]. The lower eosinophil infiltration into skin lesions may contribute to the predominance of non-inflammatory phenotype in patients with DPP-4I-BP.
Given that BP is an autoimmune disorder, immune dysregulation often observed in hemodialysis patients could be involved in the pathogenesis of BP. For example, an increase in serum soluble CD 23 (sCD23) levels was found to be associated with the disease activity of BP along with the aberrant activation of B cell and IgE production [31-33]. In turn, CD23 expression, which is also expressed in the surface of B cells, was correlated with serum IgE levels in patients with BP [34]. Furthermore, IgE autoantibodies against anti-BP180-NC16A were correlated with the disease activity in patients with BP [35]. These immune abnormalities in hemodialysis patients could be involved in the development of severe skin manifestation and remarkable eosinophil infiltrations into the superficial skin of the present case.
In terms of genetic susceptibility, Sun et al. emphasized the importance of HLA-DQB1*03:01 on BP development as this allele was detected in about half of the patients with BP; however, HLA-DQB1*03:03 and HLA-DQB1*06:01 were found to be significant protective alleles [36]. In Japanese patients, Ujiie et al. reported that HLA-DQB1*03:01 was presented in 86% (18/21) cases with noninflammatory DPP-4I-BP whereas it was detected in only 44% (4/9) cases with inflammatory DPP-4I-BP, 31% (19/61) cases with diabetes treated by DPP-4Is, 26% (19/72) cases with non-DPP-4I-BP, and 18% (156/873) cases in Japanese general population [12]. Although HLA-DQB1 genotyping is not commonly performed in daily clinical settings, it might provide useful information to evaluate the susceptibility to develop DPP-4I-BP. To lower the risk of BP, it might be safe not to use DPP4-I in patients with HLA-DQB1*03:01.
Importantly, BP itself could be triggered by various factors, such as radiotherapy, trauma, surgical procedure, burns, and infections [37]. In hemodialysis patients, the placement of the fistula and/or hemodialysis catheter could be also a trigger [38]. In this regard, epidermal injuries could stimulate inflammatory reactions with increased antigen-autoantibody interactions and scabies could be a potential trigger. A Taiwanese population study demonstrated that hazard ratio for BP for subjects with scabies was 21.61 within 1-year follow-up period after receiving the first-time diagnosis of scabies, compared to subjects without scabies [16]. In the present case, we considered exposure of BMZ antigens following physical injury by mites or lytic enzymatic digestion [13] might contribute to antigen-autoantibody interactions to develop DPP-4I-BP.
We encountered an interesting case of linagliptin-associated BP, likely triggered by scabies. We assumed that HLA-DQB1*03:01 was also partially involved in the development of linagliptin-associated BP in the present case. DPP-4I-BP could be considered as a distinct entity in drug-induced BP. We should be aware of the risk of DPP-4I-BP since BP is life-threatening disease especially in hemodialysis patients, considering the high risk of infections. Scabies could be one of predisposing factors of DPP-4I-BP and must be ruled out before initiating steroid treatment. The risk of DPP-4I-BP could be lowered if we avoid using DPP-4I especially in patients carry an HLA-DQB1*03:01 allele.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests associated with this manuscript.
Human and animal rights
The article does not contain involving human or animal participants.
Informed consent
Informed consent was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano AV. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. 2019 doi: 10.3389/fimmu.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izumi K, Nishie W, Mai Y, Wada M, Natsuga K, Ujiie H, et al. Autoantibody profile differentiates between inflammatory and noninflammatory bullous pemphigoid. J Investig Dermatol. 2016;136:2201–2210. doi: 10.1016/j.jid.2016.06.622. [DOI] [PubMed] [Google Scholar]
- 3.Nakama K, Koga H, Ishii N, Ohata C, Hashimoto T, Nakama T. Clinical and immunological profiles of 14 patients with bullous pemphigoid without IgG autoantibodies to the BP180 NC16A domain. JAMA Dermatol. 2018;154:347–350. doi: 10.1001/jamadermatol.2017.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Li L, Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol. 2017 doi: 10.3389/fimmu.2017.01752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai SC, Lim YL, Li W, Allen JC, Chua SH, Tan SH, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in asian patients with bullous pemphigoid. Ann Acad Med Singapore. 2015;44:119–126. [PubMed] [Google Scholar]
- 6.Kridin K, Shihade W, Bergman R. Mortality in patients with bullous pemphigoid: a retrospective cohort study, systematic review and meta-analysis. Acta Derm Venereol. 2019;99:72–77. doi: 10.2340/00015555-2930. [DOI] [PubMed] [Google Scholar]
- 7.Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]
- 8.Langley AK, Suffoletta TJ, Jennings HR. Dipeptidyl peptidase IV inhibitors and the incretin system in type 2 diabetes mellitus. Pharmacotherapy. 2007;27:1163–1180. doi: 10.1592/phco.27.8.1163. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Hasegawa H, Tsuji M, Udaka Y, Mihara M, Shimizu T, et al. Diabetes therapies in hemodialysis patients: dipeptidase-4 inhibitors. World J Diabetes. 2015;6:840–849. doi: 10.4239/wjd.v6.i6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, et al. Annual dialysis data report 2016, JSDT Renal Data Registry. Ren Replace Ther. 2018 doi: 10.1186/s41100-018-0183-6. [DOI] [Google Scholar]
- 11.Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin Exp Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ujiie H, Muramatsu K, Mushiroda T, Ozeki T, Miyoshi H, Iwata H, et al. HLA-DQB1*03:01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP-4 inhibitors. J Investig Dermatol. 2018;138:1201–1204. doi: 10.1016/j.jid.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Luo DQ, Huang MX, Liu JH, Tang W, Zhao YK, Sarkar R. Bullous scabies. Am J Trop Med Hyg. 2016;95:689–693. doi: 10.4269/ajtmh.16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol. 2014;9:201–218. doi: 10.2215/CJN.05900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lempert KD, Baltz PS, Welton WA, Whittier FC. Pseudouremic pruritus: a scabies epidemic in a dialysis unit. Am J Kidney Dis. 1985;5:117–119. doi: 10.1016/S0272-6386(85)80006-8. [DOI] [PubMed] [Google Scholar]
- 16.Chung SD, Lin HC, Wang KH. Increased risk of pemphigoid following scabies: a population-based matched-cohort study. J Eur Acad Dermatol Venereol. 2014;28:558–564. doi: 10.1111/jdv.12132. [DOI] [PubMed] [Google Scholar]
- 17.Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249–253. doi: 10.1111/j.1468-3083.2011.04062.x. [DOI] [PubMed] [Google Scholar]
- 18.Haber R, Fayad AM, Stephan F, Obeid G, Tomb R. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224–226. doi: 10.1001/jamadermatol.2015.2939. [DOI] [PubMed] [Google Scholar]
- 19.Béné J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case–noncase study in the French Pharmacovigilance Database. Br J Dermatol. 2016;175:296–301. doi: 10.1111/bjd.14601. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Shimauchi R, Nishibori N, Kawashima K, Oshitani S, Fujiya A, et al. Dipeptidyl peptidase-4 inhibitors-associated bullous pemphigoid: a retrospective study of 168 pemphigoid and 9,304 diabetes mellitus patients. J Diabetes Investig. 2019;10:392–398. doi: 10.1111/jdi.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan K, Charlton O, Smith SD. Dipeptidyl peptidase-4 inhibitors and bullous pemphigoid: a systematic review and adjusted meta-analysis. Australas J Dermatol. 2019 doi: 10.1111/ajd.13100. [DOI] [PubMed] [Google Scholar]
- 22.Arai M, Shirakawa J, Konishi H, Sagawa N, Terauchi Y. Bullous pemphigoid and dipeptidyl peptidase 4 inhibitors: a disproportionality analysis based on the Japanese Adverse Drug Event Report Database. Diabetes Care. 2018;41:e130–e132. doi: 10.2337/dc18-0210. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Ishii T. Analysis of patients with drug-induced pemphigoid using the Japanese Adverse Drug Event Report database. J Dermatol. 2019;46:240–244. doi: 10.1111/1346-8138.14741. [DOI] [PubMed] [Google Scholar]
- 24.Murakami T, Yabe D, Inagaki N. Bullous pemphigoid with dipeptidyl peptidase-4 inhibitors: clinical features and pathophysiology. J Diabetes Investig. 2019 doi: 10.1111/jdi.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douros A, Rouette J, Yin H, Yu OHY, Filion KB, Azoulay L. Dipeptidyl peptidase 4 inhibitors and the risk of bullous pemphigoid among patients with type 2 diabetes. Diabetes Care. 2019;42:1496–1503. doi: 10.2337/dc19-0409. [DOI] [PubMed] [Google Scholar]
- 26.Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard MA, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2018;78:1090–1096. doi: 10.1016/j.jaad.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha GR, Matsumoto A, Tarutani M. Bullous pemphigoid induced by vildagliptin: a case involving a maintenance hemodialysis patient. J Jpn Soc Dial Ther. 2017;50:647–652. doi: 10.4009/jsdt.50.647. [DOI] [Google Scholar]
- 28.Izumi K, Nishie W, Beniko M, Shimizu H. A cross-sectional study comparing the prevalence of bullous pemphigoid autoantibodies in 275 cases of type II diabetes mellitus treated with or without dipeptidyl peptidase-IV inhibitors. Front Immunol. 2019 doi: 10.3389/fimmu.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mai Y, Nishie W, Izumi K, Shimizu H. Preferential reactivity of dipeptidyl peptidase-IV inhibitor-associated bullous pemphigoid autoantibodies to the processed extracellular domains of BP180. Front Immunol. 2019 doi: 10.3389/fimmu.2019.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuang TY, Korkij W, Soltani K, Clayman J, Cook J. Increased frequency of diabetes mellitus in patients with bullous pemphigoid: a case–control study. J Am Acad Dermatol. 1984;11:1099–1102. doi: 10.1016/S0190-9622(84)70266-0. [DOI] [PubMed] [Google Scholar]
- 31.Nakao K, Nagake Y, Okamoto A, Ichikawa H, Yamamura M, Makino H. Serum levels of soluble CD26 and CD30 in patients on hemodialysis. Nephron. 2002;91:215–221. doi: 10.1159/000058395. [DOI] [PubMed] [Google Scholar]
- 32.Descamps-Latscha B, Herbelin A, Nguyen AT, de Groote D, Chauveau P, Verger C, et al. Soluble CD23 as an effector of immune dysregulation in chronic uremia and dialysis. Kidney Int. 1993;43:878–884. doi: 10.1038/ki.1993.123. [DOI] [PubMed] [Google Scholar]
- 33.Maekawa N, Hosokawa H, Soh H, Kasahara M, Izumi H, Yodoi J, et al. Serum levels of soluble CD23 in patients with bullous pemphigoid. J Dermatol. 1995;22:310–315. doi: 10.1111/j.1346-8138.1995.tb03394.x. [DOI] [PubMed] [Google Scholar]
- 34.Inaoki M, Sato S, Takehara K. Elevated expression of CD23 on peripheral blood B lymphocytes from patients with bullous pemphigoid: correlation with increased serum IgE. J Dermatol Sci. 2004;35:53–59. doi: 10.1016/j.jdermsci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. 2017;177:141–151. doi: 10.1111/bjd.15114. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Liu H, Wang Z, Fu X, Wang C, Mi Z, et al. The HLA-DQB1*03:01 is associated with bullous pemphigoid in the Han Chinese population. J Investig Dermatol. 2018;138:1874–1877. doi: 10.1016/j.jid.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Dănescu S, Chiorean R, Macovei V, Sitaru C, Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134–140. doi: 10.1111/1346-8138.13031. [DOI] [PubMed] [Google Scholar]
- 38.Osipowicz K, Kalinska-Bienias A, Kowalewski C, Wozniak K. Development of bullous pemphigoid during the haemodialysis of a young man: case report and literature survey. Int Wound J. 2017;14:288–292. doi: 10.1111/iwj.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]