Abstract
Real-time, high resolution, simultaneous measurement of multiple ionic species is challenging with existing chromatographic, spectrophotometric and potentiometric techniques. Potentiometric ion sensors exhibit limitations in both resolution and selectivity. Herein, we develop wafer scale graphene transistor technology for overcoming these limitations. Large area graphene is an ideal material for high resolution ion sensitive field effect transistors (ISFETs), while simultaneously enabling facile fabrication as compared to conventional semiconductors. We develop the ISFETs into an array and apply Nikolskii–Eisenman analysis to account for cross-sensitivity and thereby achieve high selectivity. We experimentally demonstrate real-time, simultaneous concentration measurement of K+, Na+, , , , and Cl− with a resolution of concentration units. The array achieves an accuracy of ±0.05 log concentration. Finally, we demonstrate real-time ion concentration measurement in an aquarium with lemnoideae lemna over three weeks, where mineral uptake by aquatic organisms can be observed during their growth.
Subject terms: Sensors; Electrical and electronic engineering; Electronic properties and devices; Design, synthesis and processing
The authors demonstrate wafer-scale, graphene-based ion sensitive field effect transistors arrays for simultaneous concentration measurement of K+, Na+, NH4+, NO3−, SO42−, HPO42− and Cl−, and use their technology for real-time ion concentration measurements in an aquarium with lemnoideae lemna over a period of three weeks.
Introduction
Measuring the ion content of analytes at low concentration, accurately, quickly, and reliably is important in a diverse range of applications, including genome sequencing1, medical diagnostics2, environmental monitoring3,4, and industrial process control5. Potentiometric ion sensors, especially solid-state sensors, have been attracting increasing interest as compared to state-of-the-art chromatographic6 and spectrophotometric7 techniques. Potentiometric sensors are compact, easy to integrate with electronics, and have the capacity for real-time, on-site measurements. The most common solid-state ion sensors are the ion sensitive field effect transistors (ISFETs), where the gate of the ISFET incorporates an ion selective membrane in the form of either a glass or ionophore mixture8. The principle of operation relies on Nernst’s law. Ions reversibly bind to the membrane, which acts as a buffer in a well-designed ISFET, thus creating an electric potential in proportion to the logarithm of ion concentration. The surface potential and transistor channel current of the ISFET is thus modulated by ion concentration. However, the selectivity of ion selective membranes is not absolute. Ions other than the target ion can also reversibly bind to the membrane, leading to an interfering response in potentiometric sensors that typically leads to unreliable measurement of ion concentration in an analyte with multiple ionic species. This can be overcome by assembling ISFETs for a variety of ionic species, including known interfering ions, and subsequently applying Nikolskii–Eisenman analysis to estimate ion concentration9–11. While the concept of ion sensitive arrays has been demonstrated in the past to overcome poor selectivity12–14, these arrays have faced challenges in achieving the resolution and detection limits required for many real-time sensing applications.
Current state-of-the-art ISFETs are silicon-based and fabricated using standard CMOS processes, where the ion selective membrane is added through back end of line processing. Silicon ISFETs are typically micro-scaled, and arrays of up to 107 ISFETs have been integrated for measuring pH1,15,16. However, as the active area of ISFETs is scaled down, the effects of low-frequency charge fluctuation become more pronounced, and it becomes more difficult to measure ion concentration with high resolution17. It has been previously shown that in order to improve resolution in ion concentration measurement, it is important to maximize channel area, charge carrier mobility, and capacitance between the ISFET channel and ion binding sites18. Consequently, it is impractical to fabricate large-area silicon ISFET arrays to account for ion interference with multiple ISFETs while simultaneously achieving reliable and high resolution sensor response. Efforts to improve resolution have been directed in recent years to the study of alternative materials and structures, including Si nanowires19–21, combined silicon FET and bipolar junction transistors22, AlGaN/GaN high electron mobility transistors (HEMTs)23,24, exfoliated MoS225,26, InN grown by molecular beam epitaxy27 and carbon nanotubes28 as summarized in Table 1. All sensor resolutions are reported in Table 1 with a 90% confidence level in accord with IUPAC guidelines29.
Table 1.
Comparison of recent ISFETs in literature.
| Channel material | Target ion | Sensitivity (mV per decade) | Resolution (log concentration (M)). |
|---|---|---|---|
| Si1,15,16 | H+ | 581, 4115, 4616 | 0.021, 0.0816 |
| Si nanowires19–21 | H+ | 4819, 4021 | 0.0521 |
| Si FET + BJT22 | H+ | 2022 | 0.00522 |
| AlGaN/GaN23,24 | 5523 | >0.224 | |
| MoS225 | H+ | 58.7 | 0.02 |
| InN27 | H+ | 58.3 | 0.03 |
| Carbon nanotube28 | K+ | – | >0.06 |
| Graphene18,34 | H+, K+ | 5518, 3734 | 0.000318, 0.00234 |
On the other hand, graphene ISFETs are optimal for overcoming these challenges because ~cm2 large-area devices with charge carrier mobilities reaching up to 7000 cm2 V−1 s−1 can be fabricated using facile chemical vapor deposition methods and thin film deposition techniques18. Due to challenges involved in functionalizing graphene with sensing layers30, initial work on graphene ISFETs reported modest performance in terms of sensitivity31, range of detection32, and resolution33. We have previously shown that both metal oxides and ionophore selective membranes can be integrated with graphene ISFETs for H+ and K+ concentration measurement, respectively, operating at both thermodynamic and quantum limits with record detection limits and resolutions for potentiometric sensing, and excellent stability over the course of 5 months18,34.
In this paper, we report the demonstration of real-time, high resolution measurement of multiple ion concentrations using large-area graphene ISFETs that overcomes the challenge of ion interference. With wafer-scale processing, we fabricated large area graphene ISFETs (~cm2) with ionophore membranes as sensing layers to measure the concentration of K+, Na+, , , , and Cl− down to concentrations lower than 10−5 M and a resolution of concentration units. These ions were selected due to their prominence in agricultural runoff, and the resultant need to measure these ions for water quality monitoring35,36. We used the separate solution method to calibrate the elements of the ISFET sensor array and extract the Nikolskii selectivity coefficients of each sensor element to each ionic species in our study. With all sensor elements simultaneously measuring the same analyte solution, we used Nikolskii–Eisenman analysis to account for ion interference and accurately estimate multiple ion concentrations. By this method, we achieved an accuracy of ±0.01 and ±0.05 log concentration for cations and anions respectively. As a demonstration of the graphene ISFET array in operation, we monitored the uptake of ions by duckweed, lemnoideae lemna, in an aquarium over a period of three weeks. The graphene ISFET array performance is suitable for applications such as real-time water quality monitoring of ions of environmental concern3.
Results
Device structure and characterization
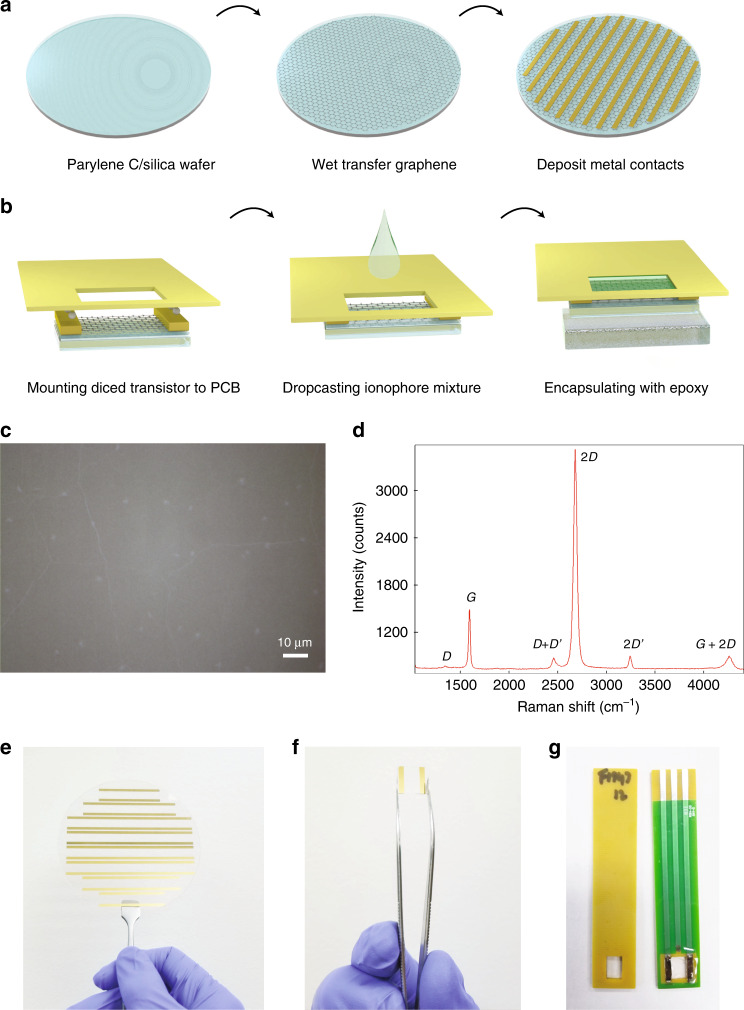
Graphene ISFETs were fabricated via wafer-scale processing methods, schematically illustrated in Fig. 1. A 100 mm diameter graphene monolayer grown via chemical vapor deposition (CVD) was wet-transferred onto a 100 mm target wafer. The wafer was 500 μm thick fused silica with a 115 nm layer of parylene C, a hydrophobic polymer that minimizes the unintentional doping of graphene and imparts stability37. A representative optical image of the transferred graphene with a ×100 magnification is shown in Fig. 1c. The quality of the transferred graphene was further confirmed via Raman spectroscopy, with a representative spectrum taken with a 532 nm pump wavelength shown in Fig. 1d along with peak assignments38,39. The D, G, and 2D peaks are located at 1341 ± 2 cm−1, 1593 ± 2 cm−1, and 2687 ± 2 cm−1, respectively. The D/G Raman intensity ratio is 0.045 ± 0.005 and the G/2D intensity ratio 0.027 ± 0.01, consistent with Raman spectra of monolayer graphene with a low density of defects and grain boundaries38. No particular effort was made at single-crystal graphene growth here, as grain boundaries in polycrystalline graphene have been shown to have a modest effect on graphene sheet resistance40.
Fig. 1. Graphene ISFET Fabrication.
a Schematic of the fabrication process of the graphene ISFETs. 115 nm of parylene C was grown on a 100 mm diameter fused silica wafer. Graphene was grown via CVD on a 100 mm diameter Cu foil and wet-transferred to the target wafer using a PMMA handle. Ti/Au contacts were evaporated with the aid of a shadow mask. b The wafer was then diced into individual 1.1 cm × 1.1 cm devices, which were then mounted onto a PCB using silver epoxy. The ionophore mixture was dropcasted into the opening. Epoxy was used to encapsulate the back gate of the transistor. c Optical image with ×100 magnification of graphene monolayer transferred onto parylene C/fused silca wafer. Scale bar is 10 μm. d Raman spectra of 100 mm graphene wet-transferred onto target substrate. e An optical image showing a 4" graphene wafer on fused silica and parylene with gold contacts, ready to be diced into individual devices. f A single graphene device after being diced, and ready to be mounted on a PCB. fabrication process. g Top and bottom view of graphene ISFETs mounted on PCBs.
After transferring the graphene onto the substrate, Ti/Au (20 nm/80 nm) contacts were evaporated onto the wafer to form source and drain contacts with the aid of a shadow mask. The wafer was then diced into individual 1.1 × 1.1 cm2 devices, and the devices were then mounted on a printed circuit board (PCB) with two part silver epoxy to contact the source and drain of each device (Fig. 1b). The PCBs have a 0.8 × 0.5 cm2 opening for the graphene FET to be exposed to the analyte solution. The 100 mm wafer was used to produce 52 graphene FETs, and the average two point resistance of the FET in air without an electrolytic gate was 277.0 Ω with a standard deviation of 11.9 Ω, indicative of the uniformity of wafer scale graphene FET processing.
To selectively measure the concentration of different ionic species in liquid, we used ionophore membranes for the following ions: K+, Na+, , , , and Cl−. These target ions were selected due to their prevalence in agricultural runoff35,36. The membranes were formed by drop-casting 50 μL of pre-prepared mixtures (see “Methods” section) onto the graphene through the PCB opening and left to dry overnight in ambient conditions. The membrane creates a seal between the graphene surface and PCB. Two component epoxy was applied and left to cure overnight to encapsulate the back of transistor and prevent electrical contact with the electrolytic solution. Figs. 1e–g show images image of the graphene ISFETs at different stages of the fabrication process.
To understand the evolution of the graphene monolayer through the ISFET fabrication process, electrical measurements were performed on graphene before and after the ionophore drop-casting. Back-gated field effect measurements were conducted on graphene ISFETs with similar substrates consisting of n-doped Si with 300 nm of dry thermal SiO2 and 115 nm of parylene C. Before the ionophore membranes were deposited, Hall mobilities up to 2050 cm2 V−1 s−1 were observed, and field effect mobilities up to ~2100 cm2 V−1 s−1 were observed (see Supplementary Table 1). The ionophore membrane electron dopes the graphene monolayer, with anion ionophore membranes giving higher electron doping than cation membranes as seen in representative measurements for K+ and Cl− ISFETs shown in Supplementary Fig. 1. The graphene FET carrier mobility does not change significantly during the ionophore deposition process, indicating that the ionophore membrane non-covalently functionalizes the graphene, preserving mobility while imparting ion sensitivity.
Additionally, measurements of Hall mobility and carrier density were made to study the uniformity of the graphene ISFETs from device to device. Based on a sampling of nine devices with active areas ranging from 25 μm2 to 26 mm2, the mean hole density was determined to be 8.27 × 1012 cm−2 with a standard deviation of 8.5 × 1011 cm−2. Likewise, the mean and standard deviation of the Hall mobility were 1720 cm2 V−1 s−1 and 240 cm2 V−1 s−1, respectively.
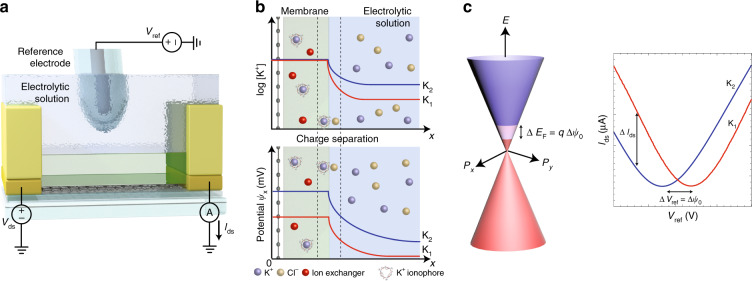
The response of each individual ISFET to its respective target ion was measured individually by immersion into electrolytic solutions of controlled concentration, as shown in Fig. 2a. The drain-source currents Ids of the graphene ISFETs were measured versus electrolytic gate potential Vref regulated through a Ag/AgCl reference electrode at a constant drain-source bias voltage Vds = 100 mV.
Fig. 2. Graphene ISFET sensing mechanism.
a Electrical setup for measuring the current through the graphene channel Ids for different electrolytic solutions. Ids was monitored at a constant bias Vds, while the electrolytic potential was varied through the reference electrode Vref. b Mechanism of how changes in analyte concentration affects the ISFET surface potential. The concentration of the target ion is buffered to a constant, and a charge separation layer appears at the membrane/electrolyte interface. A potential difference appears across the interface due to the difference in ion concentration between the membrane and the bulk solution. c A change in surface potential leads to a direct shift in the graphene Fermi level and is translated to a shift in the ISFET transfer curve.
In the electrolytic solution, the target ion binds to its respective ionophore in the permeable membrane. Fig. 2b illustrates the mechanism by which changes in ion concentration modulate graphene ISFET conduction. As the target ion concentration in the electrolytic solution changes from a1 to a2, the concentration in the membrane remains buffered to at a constant value41,42. A charge separation layer (diffusion layer) a few nm in thickness appears at the membrane/electrolyte interface. The concentration gradient between the bulk solution and the membrane generates a potential difference across the diffusion layer. Due to the buffered membrane and presence of counter ion exchange sites, the potential across the diffusion layer is ψ0:
| 1 |
where is a surface potential constant, α is a dimensionless sensitivity factor between 0 and 1, k is Boltzmann’s constant, T is temperature, q is electric charge, z is the valency and [a] is the activity of the primary target ion, equivalent to concentration for the range of concentrations explored in this study. As the concentration of the target ion changes from a1 to a2, the potential ψ0 also changes
| 2 |
As a result, Δψ0 is limited thermodynamically and at T = 300 K, for an ideal ion sensor with a sensitivity factor α = 1, for a target ion with valency z = 1, Δψ0 is 59.2 mV per decade change in ion concentration, known as the Nernstian limit.
In the presence of multiple (interfering) ions, we apply the Nikolskii–Eisenman equation, which is a generalization of the Nernst equation (Eq. (1)), to include the contribution to potential from multiple ionic species9,10. We use the index i to identify physical quantities associated with the primary target ion, and the index j to identify physical quantities associated with interfering ions. Thus, ai is the activity of the primary ion, aj is the activity of an interfering ion, and zi and zj are the valency of the primary and interfering ions, respectively. The potential ψ0i developed at a membrane for target ion i in the Nikolskii–Eisenman theory is,
| 3 |
where Kij is the selectivity coefficient of the i-selective sensor towards interfering ion j. For a set of N ions, a suite of ISFETs targeting each ion 1 ≤ i ≤ N can be characterized such that the selectivity coefficients Kij are known for all 1 ≤ i ≤ N and 1 ≤ j ≤ N, with Kii = 1 by definition. A measurement of N potentials ψ0i from N ISFETs will thus allow accurate determination of N ion concentrations ai, assuming Kij, and zi have been determined. Importantly, the determination of the ai from the measured ψ0i requires the simultaneous solution of N nonlinear equations. Additionally, it is important to select the N ions carefully such that no ionic species which may cause non-negligible interference is excluded from measurement. Therein lies an advantage of graphene ISFETs, where it is comparatively simple to fabricate multiple sensors as compared to other potentiometric devices.
For a graphene ISFET, the graphene Fermi level is correlated with ψ0 directly
| 4 |
where Cq is the graphene quantum capacitance and Cgate is the gate capacitance. Since Cgate is much larger than Cq in a well-designed graphene ISFET18,34, the capacitive voltage division ratio approaches unity. Thus, at a constant reference potential Vref, the change in surface potential Δψ0 shifts the graphene Fermi level ΔEF = qΔψ0 as illustrated in Fig. 2c. This translates to a direct shift of the transfer characteristics curve by Δψ0. Consequently, there are two ways to measure the ion concentration with the graphene ISFETs, either by monitoring the reference potential Vnp that must be applied to reach charge neutrality where graphene conductivity is minimum, or by measuring the current Ids at a constant reference potential Vref and constant bias potential Vds. Importantly, the limited on/off current ratio in a graphene ISFET does not directly limit ISFET signal to noise ratio in either mode of operation. The graphene ISFET must provide sufficient small signal gain gm = ∂Ids/∂Vref ≈ CqμVds such that the signal to noise ratio is limited by potential fluctuations at the graphene ISFET input, and not the additive noise of subsequent amplifiers or signal acquisition stages. As has been previously shown, the combination of high capacitance Cq ≈ 1 μF cm−1 and high mobility μ ≈ 2000 cm2 V−1 s−1 with modest bias Vds = 100 mV gives sufficient gain gm ≈ 0.2 mA/V to reach input noise limited performance18.
ISFET response
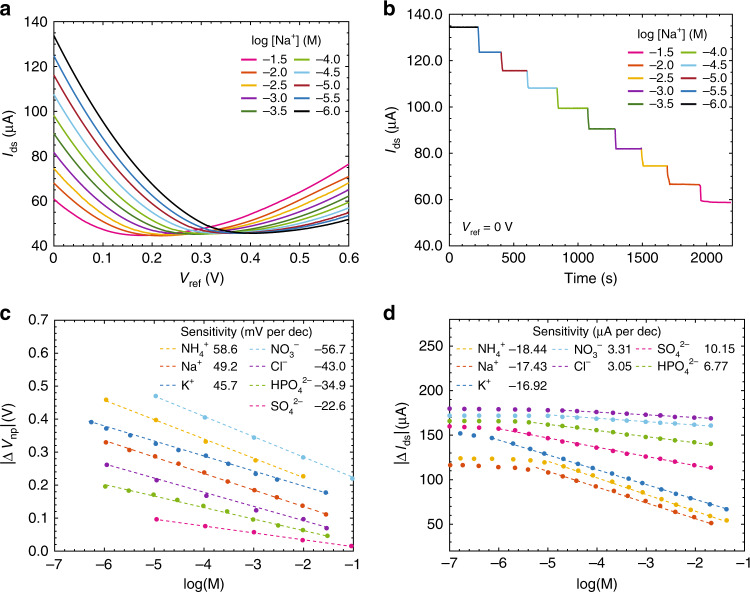
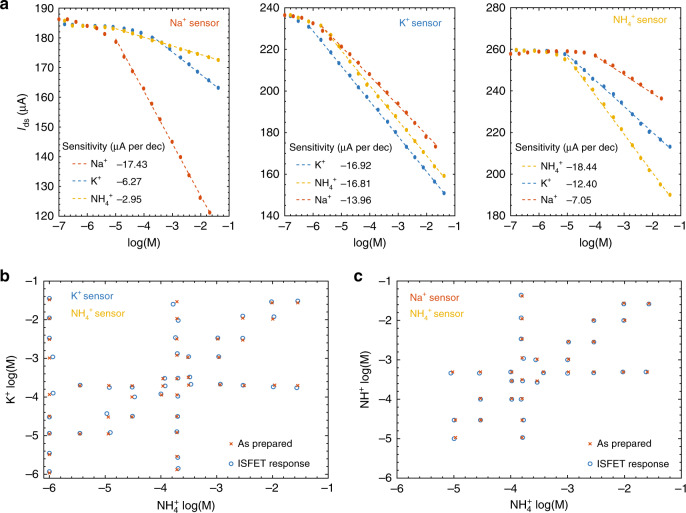
The ISFETs were first tested independently with respect to their target ion. A representative set of both Vnp and Ids measurements for a Na+ ISFET is shown in Fig. 3a, b when changing the concentration of NaCl from 10−6 to 10−1.5 M by half decade steps. The temperature and pH were closely monitored, since both can affect response as will be shown later and were 23 °C and 7.5 pH, respectively. At increasing Na+ concentration, the transfer curve shifts uniformly, and we observe a decrease in the potential Vnp required to reach charge neutrality. By measuring Ids in real-time at a constant Vref when changing the concentration, an instantaneous change in current is observed. The current values at different concentrations match those of Fig. 3a when Vref = 0 V. Similar measurements were performed for K+, , , , , and Cl− with their respective ISFETs.
Fig. 3. ISFET sensitivities with respect to their target ions.
a The channel current, Ids, of an Na+ ISFET versus Vref, for different Na+ molar concentrations. b Continuous real-time measurement of Ids for the same Na+ ISFET when increasing Na+ molar concentrations by half decade steps while keeping Vref = 0 V. c The changes in Vnp for the different ISFETs with respect to concentrations of their respective target ions, with a linear fit to extract their voltage sensitivities. d The changes in Ids for the different ISFETs with respect to concentrations of their respective target ions, with a linear fit to extract their current sensitivities.
A parabolic fit was used to find the potential Vnp of minimum conductance from the measured ISFET transfer curves (Fig. 3a) at every ion concentration, with the resulting dependence on ion concentration plotted in Fig. 3c. A line of best fit was used to calculate ISFET sensitivity. The sensitives between cations and anions differ in sign due to the charge of the target ion. The sensitivity of ISFETs targeting and were 58.6 and −56.7 ± 0.2 mV per decade, respectively, approaching the Nernstian limit of 58.8 mV per decade at 23 °C. The sensitivity of ISFETs targeting Na+, K+, and Cl− were slightly lower at 49.2, 45.7, and −43.0 ± 0.2 mV per decade. The sub-Nernstian response is possibly due to the ionophore to lipophilic salt ratio42, which may require further optimization to achieve higher sensitivity. Both and are divalent, with a corresponding Nernstian limit of ~29.4 mV per decade, and measured sensitivities of −22.6 and −34.9 ± 0.2 mV per decade, respectively. The apparent super-Nernstian response for the ISFET is due to speciation of phosphate at different pH levels. At a pH of 7.5, 40% of phosphate has the monovalent form 43. As a result, a sensitivity falling between the monovalent and divalent Nernstian limits is observed. All ISFETs exhibit linear response down concentrations of 10−5 M or lower, which is sufficient for many of the aforementioned applications. Importantly, in all the measurements, a change in counter-ion in the electrolytic solution had no observable effect on the measured sensitivities.
Figure 3d is a plot of the change in steady state graphene ISFET current Ids of the different ISFETs versus the molar concentration of their respective target ions, extracted from Fig. 3b and other similar measurements. The current sensitivities of the ISFETs are extracted from linear fits of Ids versus ion concentration. The current sensitivity is a function of both the voltage sensitivity and the transistor transconductance. The relatively low current sensitivity for both the and Cl− ISFETs is due to lower graphene ISFET transconductance, arising from variation in graphene ISFET fabrication. A similar linear response down to concentrations of 10−5 M or lower is observed in the current measurements. A typical root mean square noise 30 nA in a 60 Hz electrical bandwidth is observed for the ISFETs, resulting in a minimum resolvable concentration of concentration for the cation ISFETs, concentration for the ISFET, concentration for the , and concentration for both the and Cl− ISFETs, with a 90% confidence level following IUPAC guidelines29. Multiple ISFETs for the same target ion were also fabricated and tested to compare the variability in the fabrication process and performance. It has been found that ionophore membrane induced doping varies amongst graphene ISFETs, but detection limits, sensitivities, and resolution limits are similar. Improved control over the ionophore membrane preparation and drop-casting method is expected to reduce doping variation. Further details are provided in Supplementary Table 2.
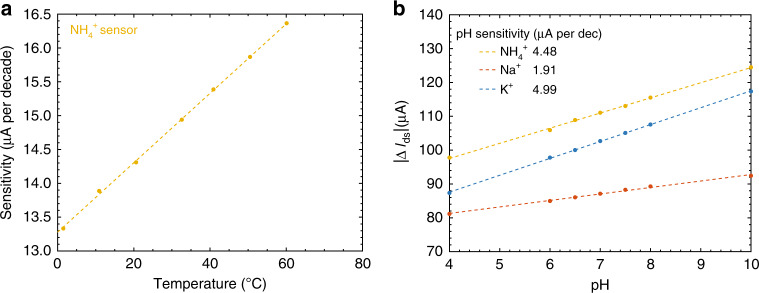
The effects of temperature on graphene ISFET sensitivity was studied by varying the temperature from 1 °C up to 60 °C, and measuring the ISFET current across different concentrations of the target ion under similar bias conditions. A representative measurement is shown for an ISFET in Fig. 4a. The Nernst Eq. (2) and generalized Nikolskii–Eisenman Eq. (3) both indicate that ISFET sensitivity is expected to vary linearly with absolute temperature T, tending to zero sensitivity at 0 K. As shown in Fig. 4a, the measured sensitivity of the ISFET varies linearly with T, with an intercept of T = 12 K for zero ion sensitvity inferred from the linear fit, in approximate agreement with theory. The pH of the analyte solution also affects ISFET response, as protons can interfere with the ionophore. The response of ISFETs targeting the cations Na+, K+, and to pH was also measured at a constant temperature under similar bias conditions, as shown in Fig. 4b. The ISFETs exhibit a pH sensitivity much smaller than the sensitivity to the target ions as shown in Fig. 3d, and interference from other ionic species are of greater concern as discussed below.
Fig. 4. The effects of temperature and pH on ISFET performance.
a The changes in current sensitivity for an ISFET with respect to temperature. Following the Nernst equation, the sensitivity varies linearly with temperature. b The changes in Ids for the different cation ISFETs with respect to H+ concentrations.
Discussion
The ISFETs’ response to interfering ions was studied by the separate solution method and confirmed with mixed solution method (the fixed interference method)44,45. For the separate solution method, the ISFETs were placed in the same solution and the ISFET currents Ids were measured concurrently in real-time while varying the ionic concentration. Only one salt was added to de-ionized water to prepare the solution for each experiment. The currents Ids were measured with one common reference electrode, where Vref = −0.2 V, and a bias Vds = 0.1 V was applied to each ISFET. The reference potential Vref was chosen to ensure that all ISFETs were operating at a point of high transconductance, far from their respective conductance minima where transconductance vanishes. Figure 5a illustrates the Na+, K+, and ISFETs’ response to the different cations used in this study, with linear fits applied to the measured current versus log concentration. The response to the other cations is much higher than with respect to pH from the earlier measurements. Similar measurements were performed for ISFETs sensitive to anions, as shown in Supplementary Fig. 2.
Fig. 5. Overcoming ISFET cross sensitivities.
a The changes in Ids for the different cation ISFETs with respect to concentrations of the cations, using the separate solution method. Linear fits were used to extract the Nikolskii selectivity coefficients. b Comparison of the measured K+ and concentrations by applying the Nikolskii-Eisenman formalism and the as prepared concentrations. The as prepared concentrations are determined from the solution volume and mass of the added salts. c Comparison of the measured Na+ and concentrations by applying the Nikolskii-Eisenman formalism and the as prepared concentrations.
From these measurements, the selectivity coefficients Kij were extracted and used to generate a series of equations to estimate ion concentration in multiple analyte solutions. Real-time ISFET response is more easily measured in the form of ISFET current Ids rather than neutrality point voltage Vnp, since the latter requires a sweep of reference voltage Vref. The Nikolskii–Eisenman Eq. (3) is thus rewritten for current analysis,
| 5 |
where Ii is the current Ids for sensor i, is the current constant, and sii is the current sensitivity for the ith sensor for the target ion i. Since the ISFETs have different sensitivities to different ions despite having the same valency, the activity aj of the interfering ions is raised to the power of the sensitivity ratio instead of the valency ratio. For an ISFET placed in a solution only containing its respective target ion i, Eq. (5) becomes
| 6 |
which is a linear equation between ISFET current Ii and log ion concentration , allowing sii to be identified. When the current of ISFET i is measured versus the concentration of an interfering ion j with all other ion concentrations negligible, Eq. (5) becomes
| 7 |
which is also a linear equation between current Ii and log concentration . The sensitivity sij can be identified from a linear fit of Ii versus in Fig. 5a. The constant current allows the Nikolskii selectivity coefficient Kij to be determined,
| 8 |
Tables 2 and 3 show the Nikolskii coefficients for the cation and anion ISFETs, respectively. For the cations, the K+ ISFET is the least selective, giving the greatest response to other cations. For the anions, due to the difference in valency, both SO and ISFETs are more sensitive to than their respective primary ions. As for the Cl− ISFET, it is more sensitive to than Cl−, an evident limitation of the ionophore membrane. The Nikolskii selectivity coefficients Kij extracted by the separate solution method were confirmed via the mixed solution fixed interference method. In the fixed interference method, each ISFET’s response was measured against a constant background (BG) activity of an interfering ion aj(BG), while varying the activity of the target ion ai. The Nikolskii selectivity coefficients Kij are then calculated as , where ai(DL) is the ISFET detection limit for the target ion in the presence of aj(BG)45,46. A comparison of Kij extracted by the two methods reveals that the coefficients agree to within 1% in log activity units (see Supplementary Table 3), signifying that either method can be used for coefficient extraction. Furthermore, the selectivity coefficients are within the same order of magnitude of values reported in the literature for the same Na+ 47, K+ 48,49 and 50,51 ionophores, with variation amongst literature results attributed to variation in the ionophore/salt ratio of the ionophore membrane46.
Table 2.
Nikolskii selectivity coefficients Kij for the three cation ISFETs with respect to three cations.
| Na+ | K+ | ||
|---|---|---|---|
| Na+ ISFET | 1 | 9.13 × 10−4 | 1.99 × 10−4 |
| K+ ISFET | 6.45 × 10−2 | 1 | 3.16 × 10−1 |
| ISFET | 2.13 × 10−3 | 2.14 × 10−2 | 1 |
Table 3.
Nikolskii selectivity coefficients Kij for the four anion ISFETs with respect to four anions.
| NO | SO | HPO | Cl− | |
|---|---|---|---|---|
| ISFET | 1 | 1.47 × 10−2 | 6.11 × 10−3 | 1.98 × 10−2 |
| ISFET | 8.85 × 103 | 1 | 8.27 × 10−3 | 3.75 × 10−1 |
| ISFET | 1.66 × 100 | 1.09 × 10−4 | 1 | 2.10 × 10−3 |
| Cl− ISFET | 1.51 × 10−3 | 1.38 × 10−7 | 1.28 × 102 | 1 |
With offset current , sensitivity sij and selectivity Kij determined for all ions i, j, ion concentrations ai can be estimated from simultaneously measured ISFET currents Ii using Eq. (5) in multi-analyte solutions containing ions i, j. As will be shown, ion concentration estimation of a multi-ion analyte is accurate in the absence of strongly interfering ions in the analyte that are absent in the ISFET array. Note that numerical methods are required to solve for the ion concentrations ai from the currents Ii because of the non-linear nature of Eq. (5) in the presence of more than one non-zero concentration ai.
For simplicity, the Nikolskii–Eisenman formalism was first tested with two ISFETs working simultaneously in electrolytic solutions containing two ions of the same charge across a wide range of concentrations. In one case, K+ and concentrations were measured with ISFETs targeting K+ and , and in a second case Na+ and concentrations were measured with ISFETs targeting Na+ and . These pairs were chosen because of the strong cross-sensitivity of the ISFETs to these ion pairs. The respective ISFETs were placed in the electrolytic solutions, where their currents were measured with reference potential Vref = −0.2 V and an independently applied bias Vds = 0.1 V. The ion concentrations were calculated from the ISFET currents using the Nikolskii–Eisenman equations Eq. (5), and compared with the as prepared concentrations determined from the volume and mass of the added salts, with the resulting comparison shown in Figs. 5b and c. The concentrations calculated from the ISFET currents are accurate to within concentration units, even when the ratio of ion concentrations exceeds four orders of magnitude.
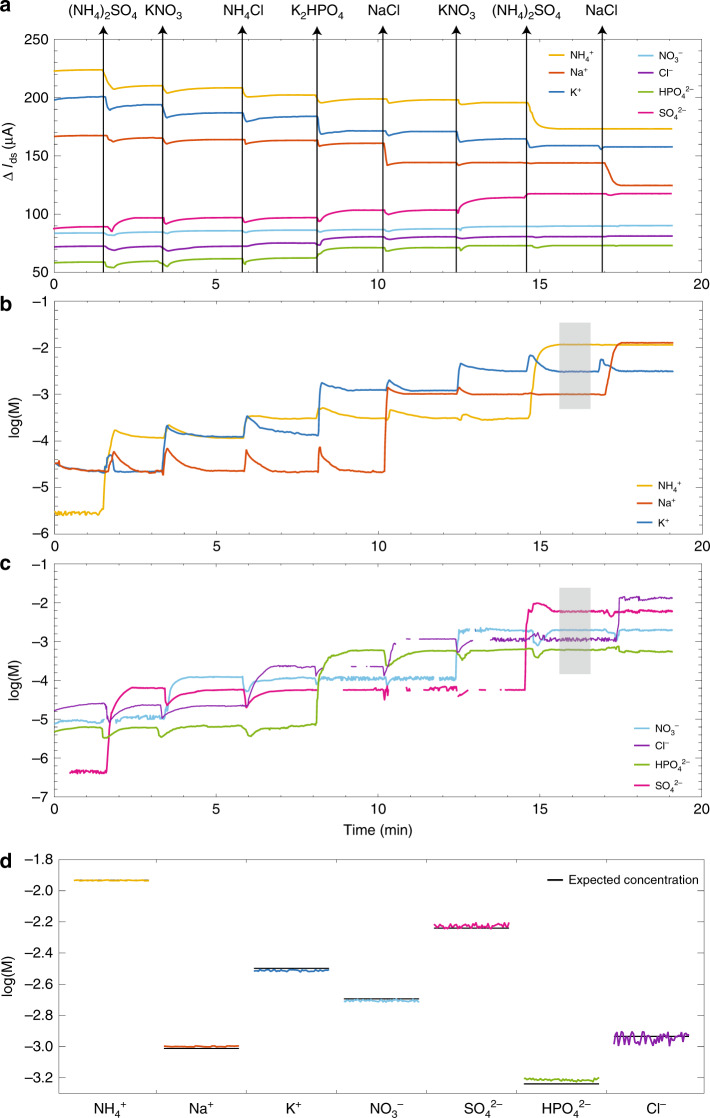
The complete ISFET array performance was then tested with multi-ion electrolytic solutions containing all seven ions. The ISFETs were placed in a solution and their respective currents Ids = Ii were measured in real time with reference potential Vref = −0.2 V and independently applied bias Vds = 0.1 V. The electrolytic solution was spiked with different salt mixtures to change ion concentrations, and the instantaneous changes in the ISFET currents versus time t were observed as shown in Fig. 6a.
Fig. 6. Real time and simultaneous measurements of ion concentrations in a multi-ion electrolytic solution.
a The change in current ΔIds = ΔIi versus time t for all seven ISFETs measured concurrently in a multi-ion electrolytic solution, spiked at different time intervals with salt mixtures as illustrated by the arrows. b, c The calculated concentrations ai of the cations and anions, respectively, from the measured currents as determined from the Nikolskii–Eisenman equations. d The accuracy of concentration estimation by the ISFET sensor array is analyzed by comparing the concentrations calculated from the ISFET array currents in the shaded time interval with the concentration determined from the volume and mass of the added salts.
The ion concentrations of the solution were calculated using the Nikolskii–Eisenman equations Eq. (5) applied to the measured currents Ii at each time t. The cations and anions were solved separately since the counter-ions were not found to have any observable effect on sensor response. Figs. 6b and c show the calculated concentrations ai for cations and anions, respectively, versus time t corresponding to the ISFET currents measured in Fig. 6a. Despite the ISFETs responding to interfering ions, the changes in the calculated concentrations only occurred when their respective ions were added. The solution of the nonlinear system of equations failed at some points in time for the anions, where no real solutions can be determined, and these points are omitted from the plot. The failure is most likely due to the comparatively poor current sensitivity of both the Cl− and ISFETs.
The seven ion concentrations calculated from the seven ISFET currents Ii are compared with the theoretical concentrations determined from the volume and mass of the added salts in Fig. 6d. A one minute time interval represented by the gray shaded area in both Fig. 6b and c was chosen for the comparison. The cation concentrations calculated from ISFET currents are accurate to within concentration units. The anion concentrations are accurate to within concentration units due to lower anion selectivity and lower anion sensitivities. These accuracies are on par with those achieved by spectrophotometric and chromatographic techniques. Importantly, changes in ISFET performance will require recalibration of selectivity coefficients to ensure accurate results.
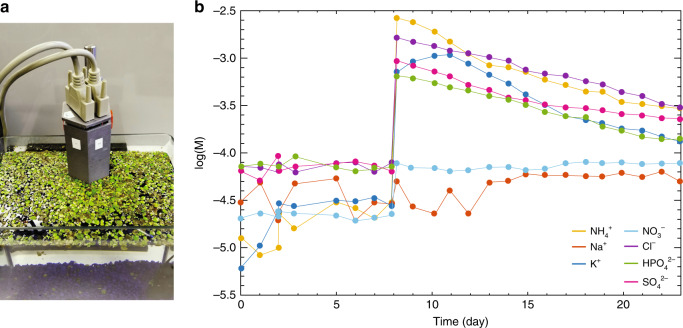
Lastly, the ISFET array was tested in an aquarium environment. The ion concentrations were monitored in an aquarium with lemnoideae lemna, commonly known as duckweed, as shown in Fig. 7. Lemnoideae lemna is a flowering aquatic plant that is ubiquitous throughout the world. To sustain growth, lemnoideae lemna absorbs nutrients including the ions K+, , Cl−, and 52,53. After placing the plants in an aquarium only containing tap water, the ion concentrations were monitored with the ISFET array once a day over a period of three weeks. The plants were provided light 12 hours per day. After one week, (NH4)2SO4, K2HPO4 and NH4Cl were added to the aquarium to set the ion concentrations at ~3 × 10−3 M for and Cl−, and ~5 × 10−4 for K+, and . The ion concentrations ai versus time t as measured by the ISFET array are shown in Fig. 7b, where it can be seen that the K+, , Cl−, , and concentrations decrease with time upon introduction to the aquarium. By the end of the third week of observation, the ion concentrations are reduced by 70–80%, as reported in previous studies of lemnoideae lemna52,53. To confirm that the decrease in ion concentrations was indeed due to intake of nutrients by the aquatic plants, a control experiment was performed simultaneously, under the same conditions, but in the absence of aquatic plants. The ion concentrations as measured by the ISFET array remained constant after adding the salts to the control sample (see Supplementary Fig. 3). It is thus demonstrated that the graphene ISFET array can be applied to ion concentration measurement in more complex environments, such as that of an aquarium.
Fig. 7. Measuring ion concentrations in an aquarium.
a An optical image of the ISFET array in an aquarium containing duckweed. b The measured ion concentrations in the aquarium over time. The concentrations were calculated from the measured currents using the series of Nikolskii–Eisenman equations. After the eighth day, salts were added to the aquarium as nutrients for the plants.
In conclusion, we have demonstrated that graphene ISFET arrays can overcome the challenge of poor selectivity in potentiometric sensing. Large-area graphene ISFETs can be fabricated from wafer scale graphene by facile methods. The large active area is critical to achieving a high signal-to-noise-ratio and high resolution sensing. We demonstrate that a modified Nikolskii–Eisenman theory to be applied to estimate the concentration ai of multiple ions from the ISFET currents Ii. ISFET array calibration can be performed simply by the separate solution method, by which selectivity and sensitivity coefficients can be determined. Despite the presence of heavily interfering ions, we have demonstrated ion detection limits down to at least 10−5 M concentration. Concentrations in multiple ion electrolytes were accurate to within ±0.01 and concentration units for cations and anions, respectively, and were resolvable to at least M. We demonstrated the operation of the ISFET array in an aquatic environment, monitoring the uptake of ions in an aquarium by aquatic plants over the course of three weeks. These sensor array characteristics exceed the requirements for many real-time ion monitoring applications. The approach outlined here could be expanded upon, by increasing the number of ISFETs to target a wider range of ions tailored to different problems in environmental sensing.
Methods
Device preparation
Graphene ISFETs were fabricated via wafer-scale processing methods, schematically illustrated in Fig. 1. A 100 mm diameter graphene monolayer was grown on poly-crystalline Cu foil catalyst (18 μm) via chemical vapor deposition (CVD) in a cold wall CVD Reactor (Aixtron BM) at Graphenea. The Cu foil was chemically treated and thermally annealed prior to graphene growth at 1000 °C at low pressure. The graphene was wet-transferred via a semi-automated process from the Cu foil to a target wafer using a poly(methyl methacrylate) (PMMA) handle and sacrificial etch of the Cu growth substrate.
The target wafers were 100 mm diameter, 500 μm thick fused silica with a 115 nm layer of parylene C, a hydrophobic polymer that minimizes the unintentional doping of graphene and imparts stability37. After transferring the graphene onto the substrate, Ti/Au (20 nm/80 nm) contacts were evaporated onto the wafer to form source and drain contacts with the aid of a shadow mask. The wafer was then diced into individual 1.1 × 1.1 cm2 devices, and the devices were then mounted on a printed circuit board (PCB) with two part silver epoxy (EpoTek-H20E) to contact the source and drain of each device (Fig. 1b). The PCBs have a 0.8 × 0.5 cm2 opening for the graphene FET to be exposed to the analyte solution.
The ionophore membranes were formed on the graphene FETs by drop-casting 50 μL of a pre-prepared mixture onto the graphene through the PCB opening and left to dry overnight in ambient conditions. The membrane creates a seal between the graphene surface and PCB. Two component epoxy (EpoTek-302) was applied and left to cure overnight to encapsulate the back of transistor and prevent electrical contact with the electrolytic solution.
Ionophore membrane preparation
We prepared ionophore membrane mixtures for K+, Na+, , and , using potassium ionophore III (2-dodecyl-2-methyl-1,3-propanediyl bis[N-[5’-nitro(benzo-15-crown-5)-4’-yl] carbamate]), ammonium ionophore I, sodium ionophore X (4-tert-Butylcalix[4]arene-tetraacetic acid tetraethyl ester), and sulfate ionophore I (1,3-[Bis(3-phenylthioureidomethyl)]benzene) as the ionophore, respectively. To prepare the ionophore mixture, 20–25 mg of the ionophore was mixed with 10 mg of lipophilic salt, 330 mg of poly(vinyl chloride) (PVC) and 660 mg of dioctyl sebacate (DOS) plasticizer. The lipophylic salt was potassium tetrakis (4-cholorophenyl) borate (K-TCPB) for cation sensors, and tridodecylmethylammonium chloride (TDMAC) for the anion sensors. The salt provides exchange sites, thus lowering the electrical resistance of the membrane, reducing anionic interference and consequently improving sensitivity42,46. The 1:2 ratio of PVC to plasticizer is a standard matrix for ionophore membranes46,54. The mixture was dissolved in 4 mL of tetrahydrofuran (THF) and sonicated overnight. All chemicals were sourced from Sigma Aldrich. Commercially available ionophore membrane cocktails from CleanGrow were used to produce ionophore membranes for Cl−, , and .
Solution preparation
KCl, KNO3, K2HPO4, NaCl, Na2HPO4, NH4Cl, and (NH4)2SO4 anhydrous salts with ≥99% purity were used to prepare solutions for studying ISFET sensitivity and selectivity. The concentrations were carefully prepared and diluted with de-ionized water (>10 MΩ), using a combination of microbalance and micropipette. All devices, glassware and components were thoroughly rinsed with de-ionized water prior to, and in between, all measurements to minimize the effects of cross-contamination.
Raman measurements
To investigate the quality of the transferred graphene, the Raman spectrum was measured using a 532 nm laser, with a spot size less than 1 μm and an incident power <30 μW with a ×100 magnification lens.
Hall measurements
To investigate the graphene carrier density and Hall mobility, samples were prepared with a van der Pauw contact geometry. A lock-in amplifier (SRS, SR-830) was used to provide a 1 μA rms 17 Hz AC bias current IB and simultaneously monitor the transverse AC Hall voltage VH while a normally incident magnetic field B was swept between ±800 mT. From the relative orientation of IB, B, and VH, as well as the sign of ∂VH/∂B, the charge carriers were determined to be holes. The hole density p was then extracted using the equation p = IB/(e∂VH/∂B) and the Hall mobility μp was determined using μp = 1/(qnRs), where Rs is the sheet resistance of the sample inferred from van der Pauw measurements.
Individual ISFET characterization
The response of each individual ISFET to its respective target ion was measured individually by immersion into electrolytic solutions of controlled concentration, as previously reported for example in 34. The drain-source currents Ids of the graphene ISFETs were measured via a semiconductor analyzer (Keithley 1500B) versus electrolytic gate potential Vref regulated through a Ag/AgCl reference electrode (RE, MF-2078, BASi with 3 M NaCl filling electrolyte) at a constant drain-source bias voltage Vds = 100 mV. For voltage sensitivity measurements, the range of Vref was controlled to ±0.8 V to prevent electrolysis of the analyte and to limit the current through the electrolytic gate to no more than 0.5% of the measured channel current Ids. While for current sensitivity measurements, Vref was kept constant and at potentials far from their respective conductance minima to ensure that they were operating at a point of high transconductance.
ISFET array characterization
In the array configuration, the ISFETs drain-source currents Ids were measured simultaneously in real-time using a PalmSens4 potentiostat with one common Ag/AgCl reference electrode, where electrolytic gate potential Vref = −0.2 V, and the transistors were all biased at Vds = 0.1 V.
For the real-time measurements in Fig. 6, the ISFET array was initially placed in a beaker containing 254 mL of DI water. 0.05 mL of 10−2 M NH4Cl, 0.15 mL of 10−2 M K2HPO4, 0.25 mL of 10−2 M KNO3, 0.5 mL of 10−2 M NaCl, and 1 mL of 10−4 M (NH4)2SO4 were added to result in starting concentrations of M, M, M, M, M, M, and M. Once the solution was thoroughly mixed with a stir bar, the currents of the ISFETs were measured in real time and salts were added every few minutes in the following order: 1.5 mL of 10−2 M (NH4)2SO4, 3 mL of 10−2 M KNO3, 0.05 mL of 1 M NH4Cl, 0.15 mL of 1 M K2HPO4, 0.25 mL of 1 M NaCl, 0.5 mL of 1 M KNO3, 1.5 mL of 1 M (NH4)2SO4 and 3 mL of 1 M NaCl. After each mixture was added, the solution was mixed with a stir bar for a few seconds leading to the small transient in current observed. The ion concentrations were obtained by solving the series of Nikolskii–Eisenman equations (Eq. (5)), where the constants were extracted from the calibration tests.
Supplementary information
Acknowledgements
We thank M. Fakih and R. Abdo for technical assistance. We acknowledge financial support from the Natural Sciences and Engineering Research Council, the Canadian Foundation for Innovation, and the McGill William and Rhea Seath award.
Author contributions
I.N., A.C., and A.Z. grew and transferred wafer-scale graphene. I.F. conceived the ISFET structure and performed the wafer-scale processing of the ISFETs. V.Y. identified the ions of concern in water, and best methods for measuring them. I.F., O.D., and F.M. fabricated the individual ISFETs and characterized them. I.F. conducted the multi-analyte experiments and solved for the Nikolskii–Eisenman equations with input from O.D. I.F. and O.D. conducted the aquarium measurements. I.F. and T.S. conceived this work. I.F. and T.S. prepared the paper with contributions from all co-authors.
Data availability
The data that support the findings of this study are available on request from the corresponding authors I.F. and T.S.
Code availability
The code that supports the findings of this study is available on request from the corresponding authors I.F. and T.S.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Ho Won Jong, and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ibrahim Fakih, Email: ibrahim.fakih@mail.mcgill.ca.
Thomas Szkopek, Email: thomas.szkopek@mcgill.ca.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16979-y.
References
- 1.Rothberg JM, et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature. 2011;475:348–352. doi: 10.1038/nature10242. [DOI] [PubMed] [Google Scholar]
- 2.Chapp AD, et al. Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography. Physiol. Rep. 2018;6:e13666. doi: 10.14814/phy2.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Protocols manual for water quality sampling in Canada (Canadian Council of Ministers of the Environment, 2011).
- 4.Developing drinking water quaility regulations and Standards (World Health Organization, 2018).
- 5.Nielsen, S. S. Food Analysis Laboratory Manual (Springer International Publishing, 2017).
- 6.Gros N, Camòes MF, Oliveira C, Silva MCR. Ionic composition of seawaters and derived saline solutions determined by ion chromatography and its relation to other water quality parameters. J. Chromatogr. A. 2008;1210:92–98. doi: 10.1016/j.chroma.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Aßmann S, Frank C, Kortzinger A. Spectrophotometric high-precision seawater pH determination for use in underway measuring systems. Ocean Sci. 2011;7:597–607. [Google Scholar]
- 8.Bergveld P. Thirty years of ISFETOLOGY what happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators, B. 2003;88:1–20. [Google Scholar]
- 9.Nikolskii BP. Theory of the glass electrode. I. theoretical. J. Phys. Chem. 1937;10:495–503. [Google Scholar]
- 10.Eisenman G, Rudin DO, Casby JU. Glass electrode for measuring sodium ion. Science. 1957;126:831–834. doi: 10.1126/science.126.3278.831. [DOI] [PubMed] [Google Scholar]
- 11.Bobacka J, Ivaska A, Lewenstam A. Potentiometric ion sensors. Chem. Rev. 2008;108:329–351. doi: 10.1021/cr068100w. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi I, Ito T, Taguchi T, Tabata O, Inagaki H. Multiple ion sensor array. Sens. Actuators B. 1990;1:8–11. [Google Scholar]
- 13.Forster RJ, Regan F, Diamond D. Modeling of potentiometric electrode arrays for multicomponent analysis. Anal. Chem. 1991;63:876–882. [Google Scholar]
- 14.Forster RJ, Diamond D. Nonlinear calibration of ion-selective electrode arrays for flow injection analysis. Anal. Chem. 1992;64:1721–1728. [Google Scholar]
- 15.Huang X, Yu H, Liu X, Jiang Y, Yan M, Wu D. A dual-mode large-arrayed CMOS ISFET sensor for accurate and high-throughput pH sensing in biomedical diagnosis. IEEE Trans. Biomed. Eng. 2015;63:2224–2233. doi: 10.1109/TBME.2015.2419233. [DOI] [PubMed] [Google Scholar]
- 16.Milgrew M, Cumming D. Matching the transconductance characteristics of CMOS ISFET arrays by removing trapped charge. IEEE Trans. Electron Devices. 2008;55:1074–1079. [Google Scholar]
- 17.Moser N, Lande TS, Toumazou C, Georgiou P. ISFETs in CMOS and emergent trends in instrumentation: a review. IEEE Sens. J. 2016;16:6496–6514. [Google Scholar]
- 18.Fakih I, Mahvash F, Siaj M, Szkopek T. Sensitive precise pH measurement with large-area graphene feld-effect transistors at the quantum-capacitance limit. Phys. Rev. Appl. 2017;8:044022. [Google Scholar]
- 19.Knopfmacher O, Tarasov A, Fu W, Wipf M, Niesen B, Calame M, Schönenberger C. Nernst limit in dual-gated si-nanowire fet sensors. Nano Lett. 2010;10:2268–2274. doi: 10.1021/nl100892y. [DOI] [PubMed] [Google Scholar]
- 20.Nishiguchi K, Clement N, Yamaguchi T, Fujiwara A. Si nanowire ion sensitive field-effect transistors with a shared floating gate. Appl. Phys. Lett. 2009;94:163106. [Google Scholar]
- 21.Lee JS, et al. Silicon nanowire ion sensitive field effect transistor with integrated Ag/AgCl electrode: pH sensing and noise characteristics. Analyst. 2011;136:5012–5016. doi: 10.1039/c1an15568g. [DOI] [PubMed] [Google Scholar]
- 22.Chang S-R, Chen H. A CMOS-Compatible, Low-Noise ISFET Based on High Efficiency Ion-Modulated Lateral-Bipolar Conduction. Sensors. 2009;9:8336-8348. doi: 10.3390/s91008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgakilas A, et al. AlGaN/GaN high electron mobility transistor sensor sensitive to ammonium ions. Phys. Stat. Sol. 2007;204:2059–2063. [Google Scholar]
- 24.Parish G, et al. Nitrate ion detection using AlGaN/GaN heterostructure-based devices without a reference electrode. Sens. Actuators B. 2013;181:301–305. [Google Scholar]
- 25.Hu W, et al. High-stability pH sensing with a few-layer MoS2 field-effect transistor. Nanotechnology. 2019;30:375203. doi: 10.1088/1361-6528/ab277b. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee K, et al. MoS2 field-effect transistor for next generation label-free biosensors. ACS Nano. 2014;8:3992–4003. doi: 10.1021/nn5009148. [DOI] [PubMed] [Google Scholar]
- 27.Yeh JA, et al. Highly sensitive pH sensing using an indium nitride ion-sensitive field-effect transistor. Sens. Actuators B. 2011;11:1157–1161. [Google Scholar]
- 28.Cid CC, Riu J, Maroto A, Rius FX. Ion-sensitive field effect transistors using carbon nanotubes as the transducing layer. Analyst. 2008;133:1001–1004. doi: 10.1039/b800862k. [DOI] [PubMed] [Google Scholar]
- 29.Inczedy, J., Lengyel, T. & Ure, A. Compendium of Analytical Nomenclature: Definitive Rules (International Union of Pure and Applied Chemistry, 1997).
- 30.Wang X, Tabakman SM, Dai H. Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 2008;130:8152–8153. doi: 10.1021/ja8023059. [DOI] [PubMed] [Google Scholar]
- 31.Fu W, et al. Graphene transistors are insensitive to pH changes in solution. Nano Lett. 2011;11:3597–3600. doi: 10.1021/nl201332c. [DOI] [PubMed] [Google Scholar]
- 32.Fu W, et al. High mobility graphene ion-sensitive field-effect transistors by noncovalent functionalization. Nanoscale. 2013;5:12104. doi: 10.1039/c3nr03940d. [DOI] [PubMed] [Google Scholar]
- 33.Koley G, et al. Graphene field effect transistors for highly sensitive and selective detection of K+ ions. Sens. Actuators B. 2017;253:759–765. [Google Scholar]
- 34.Fakih I, et al. High resolution potassium sensing with large-area graphene field-effect transistors. Sens. Actuators B. 2019;291:89–95. [Google Scholar]
- 35.Jørgensen, S., Löffler, H., Rast, W. & Straskraba, M. Lake and Reservoir Management (Elsevier, 2005).
- 36.Jørgensen, S. & Fath, B. D. Encyclopedia of Ecology (Elsevier, 2008).
- 37.Sabri, S. S. et al. Graphene field effect transistors with parylene gate dielectric. Appl. Phys. Lett.95, 242104 (2009).
- 38.Ferrari AC, Basko DM. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013;8:235–246. doi: 10.1038/nnano.2013.46. [DOI] [PubMed] [Google Scholar]
- 39.Rao R, Tishler D, Katoch J, Ishigami M. Multiphonon Raman scattering in graphene. Phys. Rev. B. 2011;84:113406. [Google Scholar]
- 40.Huang PY, et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature. 2011;469:389–393. doi: 10.1038/nature09718. [DOI] [PubMed] [Google Scholar]
- 41.van Hal R, Eijkel J, Bergveld P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators, B. 1995;24:201–205. [Google Scholar]
- 42.Telting-Diaz M, Bakker E. Effect of lipophilic ion-exchanger leaching on the detection limit of carrier-based ion-selective electrodes. Anal. Chem. 2001;73:5582–5589. doi: 10.1021/ac010526h. [DOI] [PubMed] [Google Scholar]
- 43.Powell KJ, et al. Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+, Cl−, OH−, CO, SO, and PO in aquatic systems. Pure Appl. Chem. 2005;77:739–800. [Google Scholar]
- 44.Guilbault GG, et al. Recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem. 1976;48:127–132. [Google Scholar]
- 45.Umezawa Y, Bühlmann P, Umezawa K, Tohda K, Amemiya S. Potentiometric selectivity coefficients of ion-selective eletrodes. Pure Appl. Chem. 2000;72:1851–2082. [Google Scholar]
- 46.Bakker E, Bühlmann P, Pretsch E. Carrier-based ion-selective electrodes and bulk optodes. 1. general characteristics. Chem. Rev. 1997;97:3083–3132. doi: 10.1021/cr940394a. [DOI] [PubMed] [Google Scholar]
- 47.Diamond D, et al. All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly (vinyl chloride) membrane with a polypyrrole solid contact. Anal. Chem. 1992;64:2496–2501. [Google Scholar]
- 48.Kukorelli T, et al. In vivo measurements with a potassium ion-selective microelectrode based on a new bis (crown ether) Analytica Chim. Acta. 1985;178:231–237. [Google Scholar]
- 49.Töke L, et al. Novel bis (crown ether)-based potassium sensor for biological applications. Microchimica Acta. 1990;100:157–169. [Google Scholar]
- 50.Launay J, et al. Multimodal probe based on isfet electrochemical microsensors for in-situ monitoring of soil nutrients in agriculture. Multidiscip. Digital Publ. Inst. Proc. 2017;1:420. [Google Scholar]
- 51.Ohba S, et al. Design and synthesis of a more highly selective ammonium ionophore than nonactin and its application as an ion-sensing component for an ion-selective electrode. Anal. Chem. 2000;72:2200–2205. doi: 10.1021/ac9911241. [DOI] [PubMed] [Google Scholar]
- 52.Duckweed: A tiny aquatic plant with enormous potential for agriculture and environment (World Watch, 1997).
- 53.Porath D, Pollock J. Ammonia stripping by duckweed and its feasibility in circulating aquaculture. Aquat. Bot. 1982;13:125–131. [Google Scholar]
- 54.Gibbons WS, Patel HM, Kusy RP. Effects of plasticizers on the mechanical properties of poly(vinyl chloride) membranes for electrodes and biosensors. Polymer. 1997;38:2633–2642. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors I.F. and T.S.
The code that supports the findings of this study is available on request from the corresponding authors I.F. and T.S.