Abstract
Experience is an essential factor informing food choice. Eating food generates enduring odor–taste associations that link an odor with a taste’s quality and hedonic value (pleasantness/unpleasantness) and creates the perception of a congruent odor–taste combination. Previous human psychophysical experiments demonstrate that experience with odor–taste mixtures shapes perceptual judgments related to the intensity, familiarity, and pleasantness of chemosensory stimuli. However, how these perceptual judgments inform consummatory choice is less clear. Using rats as a model system and a 2-bottle brief-access task, we investigated how experience with palatable and unpalatable odor–taste mixtures influences consummatory choice related to odor–taste congruence and stimulus familiarity. We found that the association between an odor and a taste, not the odor’s identity or its congruence with a taste, informs consummatory choice for odor–taste mixtures. Furthermore, we showed that the association between an odor and a taste, not odor neophobia, informs consummatory choice for odors dissolved in water. Our results provide further evidence that the association between an odor and a taste, after odor–taste mixture experience, is a fundamental feature guiding consummatory choice.
Keywords: choice, consummatory behavior, flavor, preference, odor–taste association
Introduction
Experience is a key factor guiding future food choice (Sclafani 2001; Verhagen and Engelen 2006). Lacking experience with a novel food often results in avoidance, a behavior known as neophobia (Barnett 1958; Corey 1978; Demattè et al. 2014). A fundamental experiential factor informing consummatory choice is the perception of flavor (Prescott 2015). Although all senses contribute to the perception of flavor, the predominant perceptual qualities are smell and taste (Small 2012). Eating food activates the olfactory and gustatory systems, generating enduring odor–taste associations (Fanselow and Birk 1982; Schul et al. 1996; Sakai and Yamamoto 2001; Gautam and Verhagen 2010; Green et al. 2012). These powerful associations can form after only one pairing (Stevenson et al. 1995, 1998; Prescott et al. 2004; Blankenship et al. 2019), are resistant to extinction (Sakai and Imada 2003; Albertella and Boakes 2006; Yeomans et al. 2006; González et al. 2016), and link odors with the quality and hedonic value (pleasantness/unpleasantness) of tastes (Fanselow and Birk 1982; Holder 1991; Stevenson et al. 1995; Prescott et al. 2004; Gautam and Verhagen 2010; Green et al. 2012). It is these experiences with flavors that inform food choice; foods with pleasant flavors are consumed again, and those with unpleasant flavors are avoided.
Experience with a flavor creates the perception of a congruent odor–taste combination. Congruence is defined as “the extent to which 2 stimuli are appropriate for combination in a food product” (Schifferstein and Verlegh 1996) or, simply, how well an odor and taste “fit together” based on prior experience (Amsellem and Ohla 2016). Many elegant human psychophysical experiments show that experience with a congruent odor–taste mixture increases the detectability of the mixture’s taste or odor components (Dalton et al. 2000; Delwiche and Heffelfinger 2005; White and Prescott 2007; Veldhuizen et al. 2010; Green et al. 2012; Shepard et al. 2015). Furthermore, adding a congruent odor enhances the perceptual intensity of a taste (Frank and Byram 1988). Importantly, congruent odors enhance the perceived hedonic value of tastes. For example, sucrose is rated as more pleasant when mixed with a congruent odor (Schifferstein and Verlegh 1996; Amsellem and Ohla 2016), and an unpleasant concentration of salt is rated as more unpleasant when mixed with a congruent odor (Seo et al. 2013).
Odor–taste incongruence (i.e., mixing of odors and tastes from different congruent odor–taste pairs) impairs perceptual performance, including perturbing intensity (Stevenson et al. 1999; Amsellem and Ohla 2016), disrupting familiarity (Small et al. 2004; Labbe et al. 2006), and decreasing pleasantness (Schifferstein and Verlegh 1996; Amsellem and Ohla 2016). However, these effects are not necessarily maladaptive. For example, mixing a “sweet odor” with citric acid lowers the perceptual “sourness” of the incongruent mixture (Stevenson et al. 1999), suggesting that an incongruent mixture of a pleasant odor and an unpleasant taste may be preferable to a congruent mixture. Together, these studies demonstrate the importance of flavor experience in shaping perceptual judgments related to intensity, familiarity, and pleasantness of chemosensory stimuli. However, how these perceptual judgments relate to the decision to avoid or consume chemosensory stimuli is less well understood.
Two standard paradigms for investigating rodent consummatory behavior are the 2-bottle choice task and the single-bottle brief-access task (Smith 2001; Sclafani 2002). The 2-bottle choice task is a mainstay for investigating chemosensory-dependent associations (Touzani and Sclafani 2005; Bonacchi et al. 2008; Schier et al. 2016). For this task, rats are given a single trial, ranging from a minute to days, to drink from 2 bottles containing different chemosensory stimuli (Flaherty and Mitchell 1999; Glendinning et al. 2005; González et al. 2016; Schier et al. 2016). The differences in consummatory behaviors (e.g., volume or lick number) between the 2 bottles indicate the preferred stimulus. The single-bottle brief-access task involves the pseudorandom presentation of various stimuli using repeated brief-duration trials (typically 5–30 s) to measure taste responsiveness in rodents (Glendinning et al. 2002). The repeated presentation of stimuli over multiple trials, along with limiting the time allowed to initiate a trial, measures immediate consummatory choice and task engagement (Treesukosol et al. 2014). Also, the relatively short sampling time limits postingestive factors such as those associated with satiety or toxicity (Boughter et al. 2002).
To incorporate the strengths of both tasks, we employ a 2-bottle brief-access task (Fredericksen et al. 2019). This paradigm uses a fixed number of trials in which a rat has a limited amount of time to drink from 2 simultaneously presented bottles containing different chemosensory stimuli. The number of times each bottle is sampled (i.e., licks) and the number of trials in which the rat engages are measures of consummatory behavior, whereas the difference between the number of times the 2 bottles are sampled is a measure of stimulus preference. By standardizing the number of trials and sampling time, we investigate consummatory choice for different chemosensory stimuli across sessions.
Using the 2-bottle brief-access task, we investigated how experience with palatable and unpalatable odor–taste mixtures affects decisions to consume congruent and incongruent odor–taste mixtures as well as familiar and novel odors. We found that rats preferred to consume a congruent mixture when both odor–taste mixtures contained a palatable concentration of sucrose but preferred to consume an incongruent mixture when both odor–taste mixtures contained an unpalatable concentration of citric acid. Importantly, consummatory behavior was reduced when both odor–taste mixtures contained an unpalatable concentration of citric acid. Furthermore, rats preferred to consume a novel unpaired odor to an odor previously paired with an unpalatable concentration of citric acid, but preferred to consume an odor previously paired with a palatable concentration of sucrose. Our findings provide further evidence that the association between an odor and a taste after experience with an odor–taste mixture is a fundamental feature guiding consummatory choice.
Materials and methods
Animals
All experimental procedures were performed in accordance with university, state, and federal regulations regarding research animals and were approved by the University of Louisville Institutional Animal Care and Use Committee. Sixteen 3-month-old female Long-Evans rats (275–300 g; Charles Rivers) were single housed and maintained on a 12/12-h light–dark cycle with ad libitum access to food and water unless otherwise specified.
Chemosensory stimuli
Chemical stimuli were selected based on their previous use in chemosensory research involving rats (Gautam and Verhagen 2012; Samuelsen and Fontanini 2017; Bamji-Stocke et al. 2018). The concentrations of sucrose (Bamji-Stocke et al. 2018; Fredericksen et al. 2019) and citric acid (Samuelsen et al. 2013; Samuelsen and Fontanini 2017) were used in our previous studies. Furthermore, the concentrations of sucrose are consumed significantly more than water (Spector et al. 1993; Grobe and Spector 2008; Treesukosol et al. 2014), and the concentrations of citric acid are higher than those shown to be consumed significantly less than water (Grobe and Spector 2008; Treesukosol et al. 2014). Taste stimuli were obtained from VWR (Radnor, PA). Odor stimuli (isoamyl acetate, benzaldehyde, and methyl valerate) were obtained from Sigma–Aldrich (St. Louis, MO). All stimuli were mixed with distilled water.
Two-bottle brief-access task
All experiments employed a computer-controlled 2-bottle brief-access apparatus directed by custom-written LabVIEW scripts (National Instruments, Austin, TX) (Fredericksen et al. 2019). The 2-bottle brief-access apparatus consists of a test chamber, 2 motorized shutters to control port access, and a motorized stage for bottle positioning. The acrylic plastic test chamber (15 cm wide, 30 cm long, and 25 cm tall) has one stainless steel wall with 2 ports (1.25 cm wide, 4 cm tall and 6.75 cm apart) and a stainless steel wire mesh floor. Access to each port is blocked by a shutter controlled by a stepper motor. Small bottles with stainless steel sipper tubes are secured onto a PTFE bar mounted on a linear rail and controlled by a stepper motor. The position of each bottle on the PTFE bar is separated by 2.25 cm. This spacing ensures that simultaneously presented bottles are spaced 6.75 cm apart. For example, when a bottle mounted in position 1 containing stimulus A is at the left port, a bottle mounted in position 4 containing stimulus B is at the right port.
For each 2-bottle brief-access session, rats were first acclimatized to the test chamber for 2–5 min. A given trial began with the opening of the 2 shutters, allowing access at each port to a stainless steel sipper tube (10 cm from the floor). Bottles were presented in a pseudorandom pattern and counterbalanced such that each chemosensory stimulus was presented 10 times at each port (20 trials total). Rats had 15 s to contact either bottle (contact window). If either bottle was contacted during the initial 15 s, the shutters remained open for an additional 15 s (sampling window). The 15-s sampling window afforded the opportunity for switching between ports within a trial. Licks were recorded by a grounded contact circuit. At least 1 of the 2 bottles must have registered a minimum of 3 licks to qualify as an engaged trial. If no contact was made during the 15-s contact window, the shutters closed and a new trial began. Regardless of whether rats chose to engage or not, each trial counted as one of the 20 trials. At the completion of a trial, the port shutters closed, a 30-s intertrial interval began, and the computer moved the next trial’s bottles into position. With a 15-s contact window, a 15-s sampling window, and a 30-s intertrial interval, each 20-trial session could be as short as 15 min (if spout contact was made immediately on shutter opening for each trial) or as long as 20 min (if spout contact occurred at the end of the 15-s contact window for each trial). Each session provided a maximum of 5 min to sample the solutions.
Data are presented as the mean number of licks per engaged trial, mean number of engaged trials, mean total licks per engaged trial, and preference ratio. Preference ratio was calculated as (S1 − S2)/(S1 + S2), where S1 is the total number of licks for bottles containing stimulus 1, and S2 is the total number of licks for bottles containing stimulus 2. Thus, a positive preference ratio indicates a preference for stimulus 1, and a negative preference ratio indicates a preference for stimulus 2.
Experiment 1
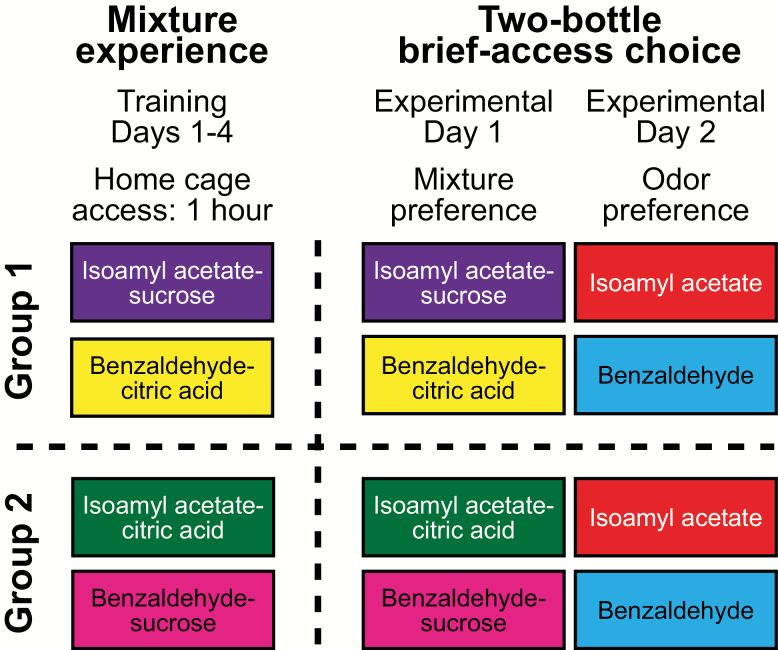
Eight single-housed rats were split into 2 groups and given experience with different odor–taste mixtures for 4 consecutive days. Consecutive days of experience with odor–taste mixtures establish long-term odor–taste associations in rats (Sakai and Yamamoto 2001). Rats in Group 1 (n = 4) received 1 h of home cage access to odor–taste pairings of 0.01% isoamyl acetate–0.1 M sucrose and 0.01% benzaldehyde–0.2 M citric acid. Rats in Group 2 (n = 4) received the opposite odor–taste pairings of 0.01% isoamyl acetate–0.2 M citric acid and 0.01% benzaldehyde–0.1 M sucrose. Following the first day of home cage odor–taste mixture experience, rats were habituated to the 2-bottle brief-access apparatus. For 3 days, rats were placed in the 2-bottle brief-access apparatus and allowed 15 min to drink distilled water from either port. After habituation to the 2-bottle brief-access apparatus, rats were placed on a water regulation schedule in which access to distilled water was allowed for 4 h per day in the home cage after each behavioral session. Over the next 2 training days, rats were allowed to sample odor–taste mixtures and odors dissolved in water (i.e., odorized water) in the 2-bottle brief-access apparatus. During testing, consummatory choice between odor–taste mixtures and odorized water was measured in the 2-bottle brief-access task. Data are presented as mean (± standard error of the mean [SEM]) number of licks for each bottle per 15-s trial, mean (± SEM) total number of licks per 15-s trial, mean (± SEM) number of engaged trials, and preference ratio (± SEM). A positive preference ratio indicates a preference for stimuli containing isoamyl acetate, whereas a negative preference ratio indicates a preference for stimuli containing benzaldehyde.
Experiment 2A
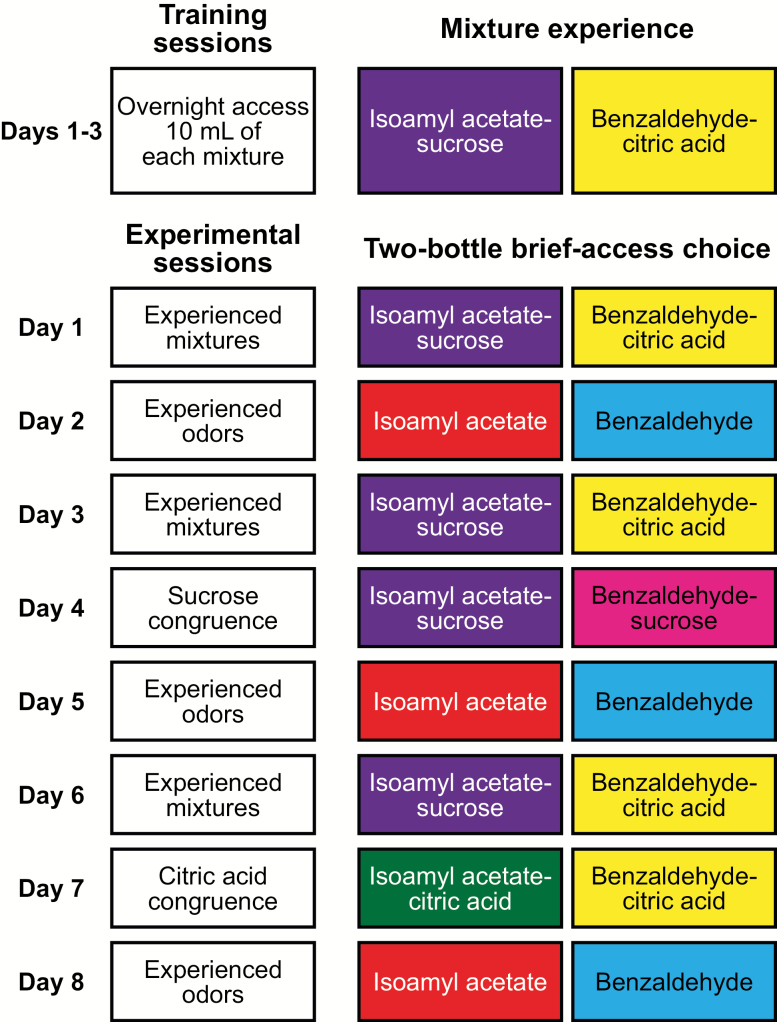
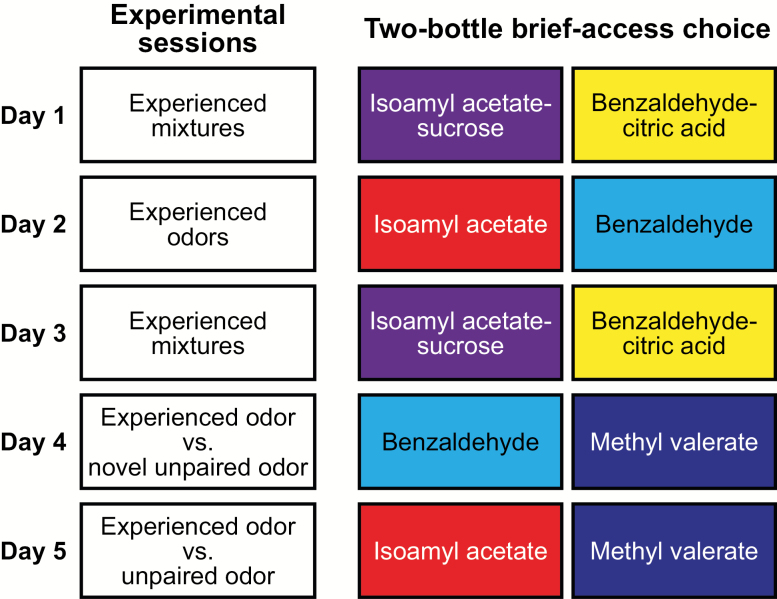
The training paradigm in Experiment 2 differed from Experiment 1. A new group of rats (n = 8) were placed on a water regulation schedule prior to experience with odor–taste mixtures and training in the 2-bottle brief-access apparatus. The day after the removal of water, rats were habituated to the 2-bottle brief-access apparatus and allowed to drink distilled water from either port (15 min) over 3 consecutive days. After each of the 3 training sessions, rats were given experience overnight with 10 mL of 0.01% isoamyl acetate–0.2 M sucrose and 10 mL of 0.01% benzaldehyde–0.3 M citric acid. Following the 3 training sessions and overnight experience with mixtures, rats were placed on a water regulation schedule allowing access to distilled water for 4 h per day in the home cage after each 2-bottle session. After training, preference and consummatory behaviors were measured over 8 experimental days. Day 1 tested consummatory choice between the experienced odor–taste mixtures (isoamyl acetate–sucrose and benzaldehyde–citric acid). Day 2 tested the consummatory choice between the experienced odors (isoamyl acetate and benzaldehyde). This was the first-day rats were given a choice between bottles containing only odorized water. Day 3 tested consummatory choice between the experienced odor–taste mixtures, reinforcing mixture experience. On Day 4 (sucrose congruence), rats chose between a congruent mixture of isoamyl acetate–sucrose and an incongruent mixture of benzaldehyde–sucrose. Day 5 tested the consummatory choice between the experienced odors after the sucrose congruence test. Day 6 tested consummatory choice between the experienced odor–taste mixtures, reinforcing mixture experience. On Day 7 (citric acid congruence), rats chose between a congruent mixture of benzaldehyde–citric acid and an incongruent mixture of isoamyl acetate–citric acid. Day 8 tested the consummatory choice between the experienced odors after the citric acid congruence test. This design ensured that days featuring incongruent mixtures (Day 4 and Day 7) followed days featuring experienced congruent mixtures and preceded days featuring experienced odors. Bottles were counterbalanced such that chemosensory stimuli were presented 10 times at each port (20 trials total). Rats were placed back on water ad libitum after the final experimental session. Data were collected and analyzed as above.
Experiment 2B
The same group of rats (n = 8) from Experiment 2A were used in Experiment 2B. After 1 week of water ad libitum, rats were placed back on the same water regulation schedule as above and retrained to drink water in the 2-bottle brief-access apparatus for 3 days. Preference and consummatory behaviors were measured over 5 experimental days. Day 1 tested consummatory choice between the experienced odor–taste mixtures (isoamyl acetate–sucrose and benzaldehyde–citric acid). Day 2 tested the consummatory choice between the experienced odors (isoamyl acetate and benzaldehyde). Day 3 tested consummatory choice between the experienced odor–taste mixtures, reinforcing mixture experience. On Day 4, rats chose between the odor previously paired with citric acid (benzaldehyde) and a novel unpaired odor (0.01% methyl valerate). On Day 5, rats chose between the odor previously paired with sucrose (isoamyl acetate) and methyl valerate. Bottles were counterbalanced so that each pairing was presented 10 times at each port (20 trials total). Data were collected and analyzed as above.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Two-way repeated-measures analysis of variance (ANOVA) was used to test whether the number of licks for odor–taste mixtures or odors differed across days. Differences in number of licks between pairs of chemosensory stimuli were tested using 2-tailed paired t-tests. Differences in total number of licks across days were tested using a 1-way repeated-measures ANOVA. Differences between preference ratios across days were tested using one-way repeated-measures ANOVA. In Experiment 2B, one rat’s lick data (Day 2) were lost due to a computer malfunction. Therefore, repeated-measures comparisons were performed using a mixed-effects model analysis. Post hoc analyses were performed using Holm–Sidak tests to correct for familywise errors. χ 2 tests were used to test whether the proportion of trials in which both bottles were contacted differed across days. Post hoc comparisons between specific days were performed using Fisher’s exact tests with Dunn–Sidak correction for familywise errors.
Results
Experiment 1: odor–taste mixture experience, not odor identity, informs consummatory choice
To demonstrate that experience with odor–taste mixtures, not the odor’s chemical identity, is a key factor informing consummatory choice (Schul et al. 1996; Slotnick et al. 1997; Gautam and Verhagen 2010), 2 groups of rats were given experience with different pairings of the same odor and taste components (Figure 1). Rats in Group 1 had experience with mixtures of 0.01% isoamyl acetate–0.1 M sucrose and 0.01% benzaldehyde–0.2 M citric acid. Rats in Group 2 had experience with mixtures of 0.01% isoamyl acetate–0.2 M citric acid and 0.01% benzaldehyde–0.1 M sucrose. During the 1 h access to odor–taste mixtures, rats consumed significantly more of the odor–taste mixture containing sucrose (8.28 ± 0.76 mL) than the odor–taste mixture containing citric acid (0.28 ± 0.09 mL; t(31) = 10.54, P < 0.001). After training, we used a 2-bottle brief-access task to determine how experience affects consummatory choice between odor–taste mixtures and odors dissolved in water.
Figure 1.
Schematic outline of Experiment 1. Rats in Group 1 (n = 4) received 1 h of home cage access to odor–taste pairings of 0.01% isoamyl acetate–0.1 M sucrose and 0.01% benzaldehyde–0.2 M citric acid for 4 consecutive days. Rats in Group 2 (n = 4) received 1 h of home cage access to the opposite odor–taste pairings, 0.01% isoamyl acetate–0.2 M citric acid and 0.01% benzaldehyde–0.1 M sucrose, for 4 consecutive days. Following training, consummatory choice between odor–taste mixtures (Experimental Day 1) and odorized water (Experimental Day 2) was measured in the 2-bottle brief-access task for both groups.
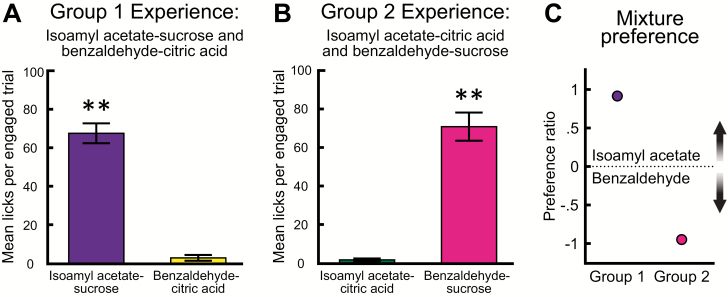
Rats in Group 1 sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid (t(3) = 10.32, P = 0.002) (Figure 2A). Rats in Group 2 sampled significantly more benzaldehyde–sucrose than isoamyl acetate–citric acid (t(3) = 8.87, P = 0.003) (Figure 2B). The preference ratio indicates which of the 2 odorized stimuli were sampled more during each 2-bottle choice; a positive preference ratio indicates a preference for stimuli containing isoamyl acetate, and a negative ratio indicates a preference for stimuli containing benzaldehyde (Figure 2C). Preference ratios for odor–taste mixtures significantly differed between groups (t(3) = 37.55, P < 0.001). Both groups of rats preferred to consume the odor–taste mixture containing sucrose.
Figure 2.
Rats prefer to consume odor–taste mixtures containing sucrose. Rats in Group 1 were given experience with mixtures of isoamyl acetate–sucrose and benzaldehyde–citric acid. Rats in Group 2 received the opposite pairings, isoamyl acetate–citric acid and benzaldehyde–sucrose. (A) Mean number of licks per engaged trial (± SEM) during the odor–taste mixture 2-bottle brief-access task. Rats in Group 1 sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid. (B) Rats in Group 2 sampled significantly more benzaldehyde–sucrose than isoamyl acetate–citric acid. (C) Preference ratios (± SEM) for each 2-bottle choice between mixtures. Rats in both groups preferred to consume mixtures containing sucrose. **P < 0.01. These data are from Experiment 1.
Next, we tested consummatory choice between odorized water containing isoamyl acetate or benzaldehyde. Rats in Group 1 sampled significantly more isoamyl acetate than benzaldehyde (t(3) = 22.68, P < 0.001) (Figure 3A). Rats in Group 2 sampled significantly more benzaldehyde than isoamyl acetate (t(3) = 8.50, P = 0.003) (Figure 3B). Similar to odor–taste mixtures, preference ratios for odorized water significantly differed between groups (t(3) = 775.30, P < 0.001) (Figure 3C). These results are consistent with previous findings that the association between the odor and taste after odor–taste mixture experience, not the identity of the odor, informs consummatory choice (Schul et al. 1996; Slotnick et al. 1997; Gautam and Verhagen 2010).
Figure 3.
Experience with odor–taste mixtures informs consummatory choice for odorized water. Rats in Group 1 were given experience with mixtures of isoamyl acetate–sucrose and benzaldehyde–citric acid. Rats in Group 2 received the opposite pairings, isoamyl acetate–citric acid and benzaldehyde–sucrose. (A) Mean number of licks per engaged trial (± SEM) during the odorized water 2-bottle brief-access task. Rats in Group 1 sampled significantly more isoamyl acetate than benzaldehyde. (B) Rats in Group 2 sampled significantly more benzaldehyde than isoamyl acetate. (C) Preference ratios (± SEM) for each 2-bottle choice. Rats in both groups preferred to consume odorized water containing an odor previously paired with sucrose. *P < 0.001; **P < 0.01. These data are from Experiment 1.
Experiment 2A: odor–taste mixture experience, not congruence, informs consummatory choice
Although rats in Experiment 1 sampled both odor–taste mixtures during the 1-h training sessions, they consumed little of the mixtures containing citric acid (see above). To ensure that rats consumed both odor–taste mixtures, training was modified for Experiment 2. Water-restricted rats were given overnight access to 10 mL of 0.01% isoamyl acetate–0.2 M sucrose and 10 mL of 0.01% benzaldehyde–0.3 M citric acid. By limiting the volume of each mixture to 10 mL, thirsty rats had the option to consume the less preferred mixture if they consumed all of the preferred mixture. During these overnight sessions, rats consumed significantly more isoamyl acetate–sucrose (8.54 ± 0.23 mL) than benzaldehyde–citric acid (5.67 ± 0.31 mL; t(31) = 10.54, P < 0.001). After training, we used a 2-bottle brief-access task to determine how experience with odor–taste mixtures informs consummatory choice for experienced mixtures, experienced odors, as well as congruent and incongruent mixtures (Figure 4).
Figure 4.
Schematic outline of Experiment 2A. A new group of rats (n = 8) was water regulated and given overnight access to odor–taste pairings of 0.01% isoamyl acetate–0.2 M sucrose and 0.01% benzaldehyde–0.3 M citric acid for 3 consecutive days. Following training, rats consummatory choice was measured across 8 experimental days. Rats chose between experienced odor–taste mixtures on Days 1, 3, and 6; between experienced odors on Days 2, 5, and 8; between a congruent mixture of isoamyl acetate–sucrose and an incongruent mixture of benzaldehyde–sucrose on Day 4 (sucrose congruence); and between a congruent mixture of benzaldehyde–citric acid and an incongruent mixture of isoamyl acetate–citric acid on Day 7 (citric acid congruence).
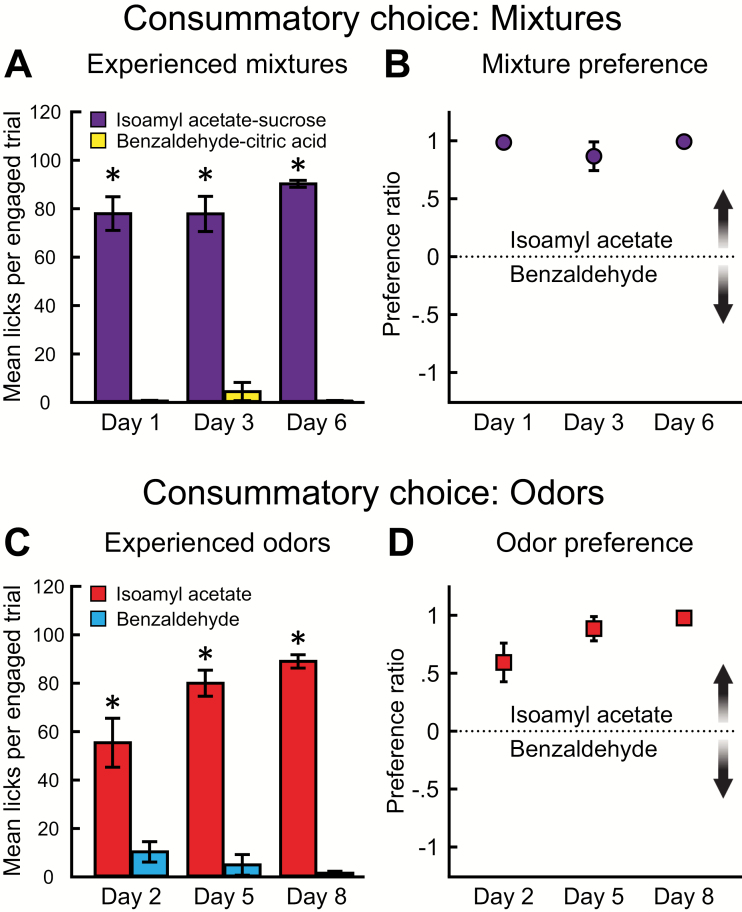
Just as in Experiment 1 when rats were trained with isoamyl acetate–sucrose (Figure 2A), the rats in this experiment sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid (F (1, 14) = 416.9, P < 0.001). There was no significant difference across days (F (2, 28) = 1.115, P = 0.342) and no significant interaction between stimulus and day (F (2, 28) = 2.02, P = 0.152) (Figure 5A). Post hoc analyses of the number of licks on each day showed that rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid (Day 1: t(42) = 12.36, P < 0.001; Day 3: t(42) = 11.73, P < 0.001; Day 6: t(42) = 14.33, P < 0.001). Preference ratios did not differ across days (F (2, 14) = 1.020, P = 0.386) (Figure 5B). Rats also sampled significantly more isoamyl acetate than benzaldehyde (F (1, 14) = 210.2, P < 0.001). There was no significant difference across days (F (2, 28) = 3.126, P = 0.060), but there was a significant interaction between stimulus and day (F (2, 28) = 8.954, P = 0.001) (Figure 5C). Post hoc analyses of the number of licks on each day showed that rats sampled significantly more isoamyl acetate than benzaldehyde (Day 2: t(42) = 5.890, P < 0.001; Day 5: t(42) = 9.805, P < 0.001; Day 8: t(42) = 11.46, P < 0.001). Preference ratios did not differ across days (F (2, 14) = 3.546, P = 0.067) (Figure 5D).
Figure 5.
Experience informs consistent consummatory choice. (A) Mean number of licks per engaged trial (± SEM) across 3 odor–taste mixture 2-bottle brief-access task sessions. During each session, rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid. (B) Preference ratios (± SEM) did not significantly differ across sessions. (C) Mean number of licks per engaged trial (± SEM) across 3 odorized water 2-bottle brief-access task sessions. During each session, rats sampled significantly more isoamyl acetate than benzaldehyde. (D) Preference ratios (± SEM) did not significantly differ across sessions (P = 0.067). *P < 0.001. These data are from Experiment 2A.
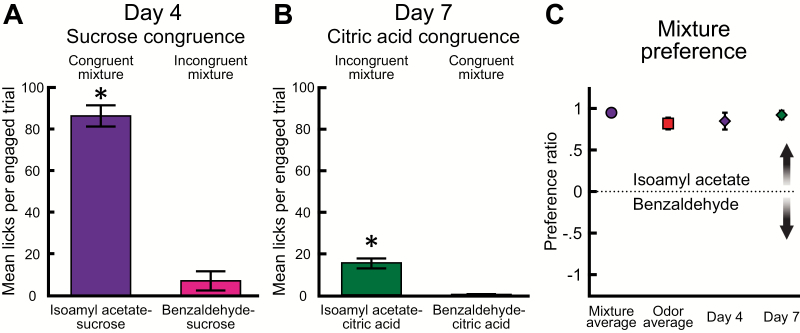
Next, we tested how experience impacts consummatory choice between congruent and incongruent odor–taste mixtures. First, we investigated which odor–taste mixture rats preferred to consume, a congruent mixture containing sucrose (isoamyl acetate–sucrose) or an incongruent mixture containing sucrose (benzaldehyde–sucrose). Rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–sucrose (t(7) = 8.211, P < 0.001) (Figure 6A). Second, we investigated which odor–taste mixture rats preferred to consume, a congruent mixture containing citric acid (benzaldehyde–citric acid) or an incongruent mixture containing citric acid (isoamyl acetate–citric acid). Rats sampled significantly more isoamyl acetate–citric acid than benzaldehyde–citric acid (t(7) = 5.991, P < 0.001) (Figure 6B). Next, we examined whether preference ratios for an odor previously paired with sucrose differed across the four 2-bottle brief-access choices. As there was no significant difference across days, preference ratios were averaged for isoamyl acetate–sucrose/benzaldehyde–citric acid on Days 1, 3, and 6 (Figure 5B) and for isoamyl acetate/benzaldehyde on Days 2, 5, and 8 (Figure 5D). There was no significant difference in preference ratios among the four 2-bottle brief-access choices (F (3, 21) = 1.192, P = 0.337) (Figure 6C). These findings show that regardless of the mixture taste component or the congruence of the odor–taste mixture, rats preferred to consume stimuli containing an odor previously paired with sucrose.
Figure 6.
After mixture experience, rats prefer to consume mixtures containing an odor previously paired with sucrose, regardless of odor–taste congruence. (A) Mean number of licks per engaged trial (± SEM) during the sucrose congruence 2-bottle brief-access task (Day 4). Rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–sucrose. (B) Mean number of licks per engaged trial (± SEM) during the citric acid congruence 2-bottle brief-access task (Day 7). Rats sampled significantly more isoamyl acetate–citric acid than benzaldehyde–citric acid. (C) Preference ratios (± SEM) were averaged for isoamyl acetate–sucrose/benzaldehyde–citric acid sessions (mixture average: Days 1, 3, and 6) and isoamyl acetate/benzaldehyde sessions (odor average: Days 2, 5, and 8). Preference ratios (± SEM) did not significantly differ across sessions. Rats preferred to consume stimuli containing an odor (isoamyl acetate) previously paired with sucrose. *P < 0.001. These data are from Experiment 2A.
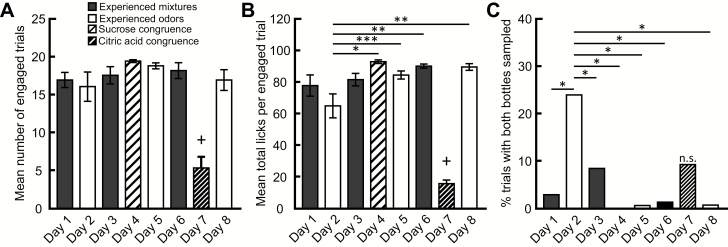
However, preference is only one measure of consummatory behavior. The number of trials that the rats chose to engage significantly differed across days (F (7, 49) = 16.92, P < 0.001) (Figure 7A) with significantly fewer trials on Day 7 than on all other days (Day 1: t(7) = 11.63, P < 0.001; Day 2: t(7) = 10.75, P < 0.001; Day 3: t(7) = 12.25, P < 0.001; Day 4: t(7) = 14.13, P < 0.001; Day 5: t(7) = 13.50, P < 0.001; Day 6: t(7) = 12.88, P < 0.001; Day 8: t(7) = 11.63, P < 0.001). In addition, the total licks to both bottles significantly differed across days (F (7, 49) = 39.11, P < 0.001) (Figure 7B). Post hoc analyses revealed that rats sampled from bottles significantly less on Day 7, when bottles contained congruent and incongruent odor–citric acid mixtures, compared with all other days (Day 1: t(7) = 10.81, P < 0.001; Day 2: t(7) = 8.575, P < 0.001; Day 3: t(7) = 11.46, P < 0.001; Day 4: t(7) = 13.44, P < 0.001; Day 5: t(7) = 11.97, P < 0.001; Day 6: t(7) = 12.95, P < 0.001; Day 8: t(7) = 12.86, P < 0.001). These results show that although rats preferred to consume an incongruent isoamyl acetate–citric acid mixture to a congruent benzaldehyde–citric acid mixture, consummatory behavior was reduced when both mixtures contained an unpalatable concentration of citric acid.
Figure 7.
Consummatory behavior is perturbed when both the congruent and incongruent mixtures contain citric acid. (A) Rats engaged in significantly fewer trials when the bottles contained congruent and incongruent odor–citric acid mixtures (Day 7) compared with all other days. +P < 0.001 (B) Mean total licks per engaged trial (± SEM) during each 2-bottle session. The total number of licks to both bottles per trial was significantly lower when the bottles contained congruent and incongruent odor–citric acid mixtures (Day 7). +P < 0.001. Also, the total number of licks to both bottles per trial the first-day rats chose between bottles containing only odorized water (Day 2) was significantly less than on Days 4, 5, 6, and 8. (C) The first day the choice was between bottles containing only odorized water (Day 2), rats switched between bottles for a significantly greater proportion of trials compared with most other days. Day 7 was the only session that did not differ from Day 2. During this session, the bottles contained congruent and incongruent odor–citric acid mixtures (Day 7; n.s.). *P < 0.001; **P < 0.01; ***P < 0.05. These data are from Experiment 2A.
Although consummatory choice between experienced odors did not significantly differ across days (Figure 5C,D), the results suggest a modulation in behavior. Interestingly, post hoc analyses of the total licks to both bottles revealed that rats sampled significantly less on Day 2, which was the first-day rats were given a choice between bottles containing only odorized water, compared with Day 4 (t(7) = 4.863, P < 0.001), Day 5 (t(7) = 3.396, P < 0.05), Day 6 (t(7) = 4.379, P < 0.01), and Day 8 (t(7) = 4.286, P < 0.01) (Figure 7B). However, rats engaged in a similar number of trials across days (Figure 7A). These results indicate a perturbation of consummatory behavior; rats decreased their sampling overall, but engaged in a similar number of trials. Although the 2-bottle brief-access task requires rats to decide between 2 simultaneously presented bottles within a set amount of time, the 15-s presentation window allows rats to switch between bottles. To investigate whether the reduction in the total number of licks to both bottles on Day 2 was related to rats trying to sample from both bottles within the same trial, we quantified the percentage of engaged trials in which both bottles were sampled (Figure 7C). We found that the proportion of trials in which both bottles were sampled significantly differed across days (χ 2 (7, N = 1038) = 121.3, P < 0.001). The greatest proportion of engaged trials in which rats sampled from both bottles was on Day 2 (24.03%, 31/129), the first-day rats chose between bottles containing only odorized water. Post hoc analyses revealed that the proportion of trials in which rats sampled from both bottles was significantly higher on Day 2 than on all other days (Day 1: 2.94%, 4/136, P < 0.001; Day 3: 8.51%, 12/141, P < 0.001; Day 4: 0%, 0/156, P < 0.001; Day 5: 0.66%, 1/151, P < 0.001; Day 6: 1.37%, 2/146, P < 0.001; Day 8: 0.74%, 1/136, P < 0.001), except when rats were presented with bottles containing congruent and incongruent odor–citric acid mixtures (Day 7: 9.30%, 4/43, P > 0.05). These results show that when rats were first given a choice between bottles containing only odorized water, they spent a greater proportion of trials switching between bottles.
Experiment 2B: odor–taste mixture experience, not neophobia, informs consummatory choice
The findings above show that rats avoided consuming stimuli containing an odor previously paired with an unpalatable concentration of citric acid. Rats are also neophobic and avoid novel odorized stimuli (Miller et al. 1986; Lin et al. 2009; Fredericksen et al. 2019). Using the same group of rats from Experiment 2A, we first confirmed that consummatory choice between experienced chemosensory stimuli remained unchanged. Next, we tested consummatory choice between a novel unpaired odor (methyl valerate) and an odor previously paired with an unpalatable concentration of citric acid (benzaldehyde) (Figure 8).
Figure 8.
Schematic outline of Experiment 2B. The same group of rats from Experiment 2A was place back on water restriction and retrained in the 2-bottle brief-access apparatus. Following training, consummatory choice was measured across 5 experimental days. Rats chose between experienced congruent odor–taste mixtures on Days 1 and 3, between experienced odors on Day 2, between the odor previously paired with citric acid (benzaldehyde) and a novel unpaired odor (0.01% methyl valerate) on Day 4, and between methyl valerate and the odor previously paired with sucrose (isoamyl acetate) on Day 5.
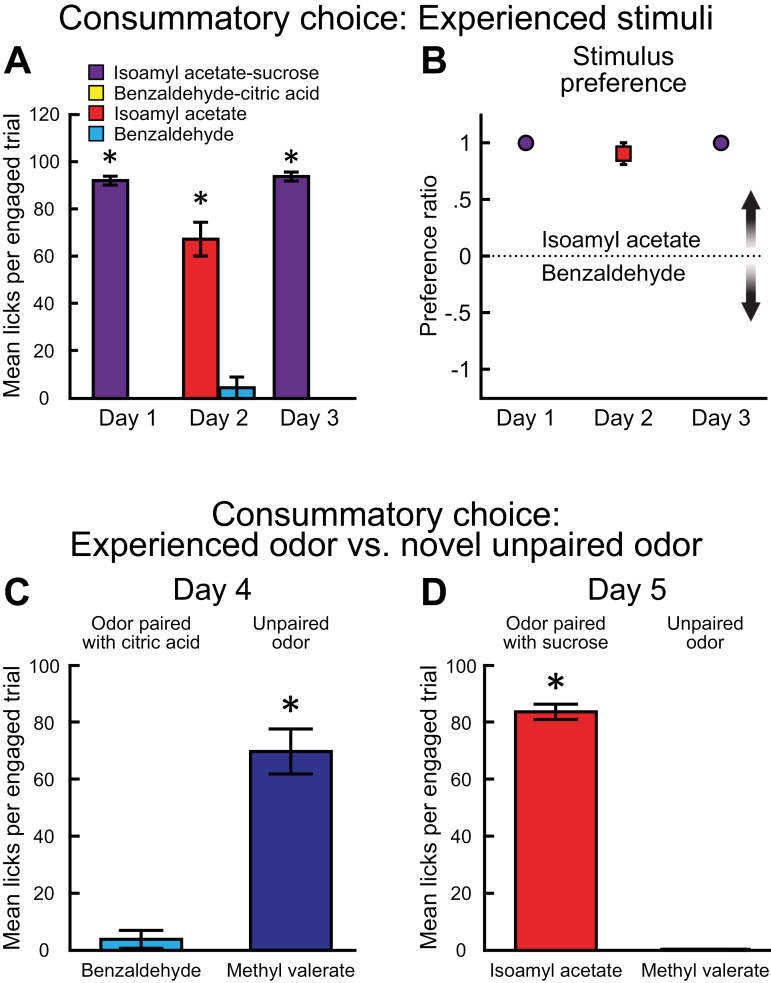
Just as in Experiments 1 and 2A, rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid (F (1, 14) = 4307, P < 0.001). There was no difference between Day 1 and Day 3 (F (1, 14) = 0.4805, P = 0.4995) and no significant interaction between stimulus and day (F (1, 14) = 4805, P = 0.4995) (Figure 9A). Post hoc analyses of the number of licks on each day showed that rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–citric acid (Day 1: t(28) = 48.76, P < 0.001; Day 3: t(28) = 49.67, P < 0.001). In addition, rats sampled significantly more isoamyl acetate than benzaldehyde (Day 2: t(6) = 7.125, P < 0.001). Preference ratios did not significantly differ across Days 1–3 (F (2, 13) = 1.154, P = 0.346) (Figure 9B). Furthermore, preference ratios for the choice between isoamyl acetate–sucrose/benzaldehyde–citric acid (F (4,28) = 1.088, P = 0.3816) and between isoamyl acetate/benzaldehyde (F (3, 20) = 2.672, P = 0.0751) were not significantly different from those in Experiment 2A.
Figure 9.
A novel unpaired odor is preferred over an odor previously paired with citric acid, but not over an odor previously paired with sucrose. (A) Mean number of licks per engaged trial (± SEM) across the 2-bottle brief-access task sessions involving previously experienced chemosensory stimuli. Rats sampled significantly more isoamyl acetate–sucrose than benzaldehyde–sucrose and significantly more isoamyl acetate than benzaldehyde. (B) Preference ratios (± SEM) did not significantly differ across sessions. (C) Mean number of licks per engaged trial (± SEM) during the experienced odor versus novel odor 2-bottle brief-access task. Rats sampled significantly more of the novel unpaired odor (methyl valerate) than an odor previously paired with citric acid (benzaldehyde). (D) Rats sampled significantly more of an odor previously paired with sucrose (isoamyl acetate) than methyl valerate *P < 0.001. These data are from Experiment 2B.
Next, we investigated whether rats would choose to consume odorized water containing a novel unpaired odor (methyl valerate) or odorized water containing an odor previously paired with an unpalatable concentration of citric acid (benzaldehyde) (Figure 9C). Rats sampled significantly more methyl valerate than benzaldehyde (t(7) = 7.177, P < 0.001). One possible explanation for this result is that rats confused methyl valerate with the odor previously paired with sucrose (isoamyl acetate). To test this, we performed a 2-bottle brief-access task in which one bottle contained methyl valerate and the other contained isoamyl acetate (Figure 9D). Rats sampled significantly more isoamyl acetate than methyl valerate (t(7) = 30.14, P < 0.001). Importantly, there were no significant differences in the number of licks (t(7) = 0.1.166, P = 0.2819), number of engaged trials (t(7) = 0.6519, P = 0.5352), or proportion of trials in which both bottles were sampled (Day 4: 1.74%, 2/115 vs. Day 5: 4.65%, 6/129; P = 0.288). These results indicate that rats preferred to consume a stimulus containing a novel odor to a stimulus containing an odor previously paired with an unpalatable concentration of citric acid; however, they preferred a stimulus containing an odor previously experienced with a palatable concentration of sucrose.
Discussion
In this study, we investigated how experience with hedonically dissimilar odor–taste mixtures informs consummatory choice between congruent and incongruent odor–taste mixtures as well as familiar and novel odors. A 2-bottle brief-access task employs a fixed number of trials and a limited sampling period to measure instantaneous consummatory choice between 2 simultaneously presented chemosensory stimuli. Using this task, our results demonstrate that the association between an odor and a taste after odor–taste mixture experience is a principal factor guiding consummatory choices. These findings are consistent with human studies demonstrating that experience-dependent odor–taste associations inform perceptual judgments of chemosensory stimuli (Stevenson et al. 1995; White and Prescott 2007; Green et al. 2012).
Previous experiments show that rats do not exhibit preferences between 2 orthonasally presented odors paired with water (Torquet et al. 2014). Although we did not test whether rats preferred isoamyl acetate or benzaldehyde prior to mixture experience, the results of Experiment 1 demonstrate that experience with odor–taste mixtures guides preferences for experienced odors. Rats that experienced mixtures of isoamyl acetate–sucrose and benzaldehyde–citric acid preferred to consume isoamyl acetate odorized water, whereas rats that experienced mixtures of isoamyl acetate–citric acid and benzaldehyde–sucrose preferred to consume benzaldehyde. These results are consistent with classical conditioning studies showing that consummatory choice depends on the association between an odor and a taste, not the odor’s identity (Schul et al. 1996; Slotnick et al. 1997; Gautam and Verhagen 2010; Slotnick and Coppola 2015).
The variability of people’s food histories makes controlling for mixture experience difficult. Therefore, most studies of odor–taste congruence in humans utilize chemosensory stimuli representing familiar and pleasant foods with characteristic olfactory and gustatory components (De Araujo et al. 2003; Small et al. 2004; Amsellem and Ohla 2016; Fondberg et al. 2018). Studies examining the relationship between odor–taste congruence and perceptual judgments demonstrate that the most and least congruent odor–taste pairings are judged to have similar intensities, whereas both familiarity and pleasantness ratings increase with perceived congruence (Amsellem and Ohla 2016; Fondberg et al. 2018). Although the relationship between perceptual judgments and consummatory choice was not directly tested, these findings suggest that congruent mixtures are preferable to incongruent mixtures. It is important to reiterate that these studies used chemosensory stimuli from familiar and pleasant foods; begging the question, would a congruent mixture be preferred if one of the experienced odor–taste mixtures was unpleasant?
To determine how experience with hedonically dissimilar odor–taste mixtures informs consummatory choice for congruent and incongruent mixtures, rats were given experience with 2 odor–taste mixtures: one palatable (isoamyl acetate–sucrose) and one unpalatable (benzaldehyde–citric acid). We found that when both mixtures contained a palatable taste (sucrose), rats preferred to consume the congruent odor–taste mixture. When both mixtures contained an unpalatable taste (citric acid), rats preferred to consume the incongruent odor–taste mixture but exhibited reduced consummatory behaviors, engaging in fewer trials and decreasing their overall sampling. These findings indicate that odor–taste congruence has different effects on consummatory behavior depending on the palatability of the mixture. Previous studies in humans show that an odor judged to be sweet decreases the perceptual sourness of citric acid (Stevenson et al. 1999). The preferred consumption of the incongruent isoamyl acetate–citric acid mixture (Figure 6B) could be due to a shift in the intensity/pleasantness of the incongruent mixture, a change in its incentive value (Berridge 2004), or due to avoidance of the congruent benzaldehyde–citric acid mixture. Future studies examining taste reactivity (Myers and Sclafani 2003) and lick pattern analyses (Dwyer 2012) are necessary to determine whether mixture experience shifts the hedonic value of odorized stimuli. Regardless, our results show that an incongruent mixture, one that is comprised of an unpalatable taste coupled with an odor previously paired with a palatable taste, is preferred to an unpalatable congruent mixture.
A second experiential factor informing consummatory choice is lack of experience. Both humans and rodents tend to avoid novel foods, a behavior known as neophobia (Barnett 1958; Corey 1978; Demattè et al. 2014). Rats are particularly hesitant to consume novel odorized stimuli (Miller et al. 1986; Lin et al. 2009; Fredericksen et al. 2019). Although we did not test preferences for experienced odors compared with water in this study, in a previous 2-bottle brief-access experiment, we found that rats preferred to consume water to novel odorized water. However, a single session of mixture experience, where the odor was paired with sucrose, changed the consummatory choice, eliminating the preference for water (Fredericksen et al. 2019). A limitation of investigating consummatory choice related to neophobia is the requirement that rats had no experience with one of the odors. Since a stimulus is only novel once, we could not test whether an individual subject had an “original bias” for one odor over another and still assume the odors to be novel. However, even if an original bias was present, the results of Experiment 1 demonstrate that experience with odor–taste mixtures guides preferences for experienced odors. Because consummatory behaviors are perturbed by both a novel odor or an odor previously paired with an unpalatable taste, we sought to determine how experience with hedonically dissimilar odor–taste mixtures informs consummatory choice between orally consumed familiar and novel odors. Our findings demonstrate that rats preferred to consume a novel unpaired odor (methyl valerate) to an odor previously paired with citric acid. One possible explanation for this outcome is that rats confused methyl valerate with the odor previously paired with sucrose. This was not the case, as rats preferred the odor previously paired with sucrose to methyl valerate. Furthermore, consummatory behavior did not differ between the other 2-bottle choices. Together, these results show that the experience-dependent association between an odor and an unpalatable taste is less preferred than a novel unpaired odor.
Although rats had no prior experience with methyl valerate, they had much experience sampling odorized stimuli in the context of the 2-bottle brief-access task. Studies show that stimulus neophobia is less pronounced in familiar contexts (Mitchell 1976). Therefore, it is possible that rats’ experience with the 2-bottle brief-access task reduced the negative aspects of the novel odor. During training, rats only had experience with odor–taste mixtures. On the first day that rats experienced odorized water alone (Experiment 2A, Day 2), they preferred to sample the odor previously paired with sucrose, but switched between bottles in a significant proportion of trials (Figure 7C). Rats did not switch between bottles during subsequent sessions. The switching behavior most likely explains the lower sampling on the first day that rats experienced odorized water alone, suggesting that the unfamiliar context influenced consummatory behavior. Combining an unfamiliar context with the choice between familiar and novel odors may result in different consummatory choices. Another means of challenging context familiarity would be to incorporate a taste-potentiated odor aversion experience (Lin et al. 2009). Pairing a novel-odor taste mixture with gastrointestinal malaise within the context of the 2-bottle brief-access task may cause avoidance of other novel odors. Further studies are needed to investigate how manipulating context familiarity influences consummatory choice.
It is important to recognize that experience-dependent odor–taste associations are only one of a complex range of properties underlying consummatory choice. Consummatory behaviors are influenced by psychological factors, such as cue-potentiated feeding (Reppucci and Petrovich 2012) and physiological factors, such as hunger (Hoefling and Strack 2010), salt depletion (Lundy et al. 2003), and postingestive effects (Bonacchi et al. 2008). In the present study, rats were on a fixed water regulation schedule where access to water was limited to 4 h after each 2-bottle session. It is possible that different water access protocols could alter how experience-dependent odor–taste associations inform consummatory choice. For instance, rats with free access to water may choose to engage in fewer trials and sample less frequently, whereas a strict water regulation paradigm may lead to a sampling rate ceiling effect or drive indiscriminate sampling. Future studies are needed to further investigate how interactions between psychological and physiological factors affect consummatory choice. Furthermore, we specifically limited experience to 2 odor–taste mixtures with opposite hedonic values. However, experience with only 2 odor–taste mixtures comprises an artificially small stimulus space. Future studies that increase stimulus space complexity by adding concentration ranges, mixtures with similar qualities and hedonic values, and foods will provide a more complete understanding of how experience and odor–taste congruence interact to guide consummatory choice.
In conclusion, our findings indicate that the taste associated with an odor after odor–taste mixture experience is a fundamental factor guiding consummatory choice. Our results show that rats prefer to consume a congruent mixture when both mixtures contain a palatable taste but prefer to consume an incongruent mixture when both mixtures contain an unpalatable taste. Furthermore, an unpaired novel odor is preferred to an odor previously paired with an unpalatable taste, but it is not preferred to an odor previously paired with a palatable taste. Future research will investigate how the specific neural mechanisms underlying odor–taste integration affects decisions driving consummatory behavior.
Funding
This work was supported by the National Institute of Deafness and Other Communication Disorders at the National Institutes of Health (R03 DC014319).
Author contributions
K.A.M. and K.E.F. contributed equally to this work. C.L.S. designed the research. K.A.M. and K.E.F. performed the research. K.A.M., K.E.F., and C.L.S. analyzed the data. K.A.M., K.E.F., and C.L.S. wrote the paper. All authors reviewed and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Albertella L, Boakes RA. 2006. Persistence of conditioned flavor preferences is not due to inadvertent food reinforcement. J Exp Psychol Anim Behav Process. 32(4):386–395. [DOI] [PubMed] [Google Scholar]
- Amsellem S, Ohla K. 2016. Perceived odor-taste congruence influences intensity and pleasantness differently. Chem Senses. 41(8):677–684. [DOI] [PubMed] [Google Scholar]
- Bamji-Stocke S, Biggs BT, Samuelsen CL. 2018. Experience-dependent c-Fos expression in the primary chemosensory cortices of the rat. Brain Res. 1701:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. 1958. Experiments on neophobia in wild and laboratory rats. Br J Psychol. 49(3):195–201. [DOI] [PubMed] [Google Scholar]
- Berridge KC. 2004. Motivation concepts in behavioral neuroscience. Physiol Behav. 81(2):179–209. [DOI] [PubMed] [Google Scholar]
- Blankenship ML, Grigorova M, Katz DB, Maier JX. 2019. Retronasal odor perception requires taste cortex, but orthonasal does not. Curr Biol. 29(1):62–69.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacchi KB, Ackroff K, Sclafani A. 2008. Sucrose taste but not Polycose taste conditions flavor preferences in rats. Physiol Behav. 95(1–2):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. 2002. A brief-access test for bitter taste in mice. Chem Senses. 27(2):133–142. [DOI] [PubMed] [Google Scholar]
- Corey DT. 1978. The determinants of exploration and neophobia I exploration: the measurement problem. Neurosci Biobehav Rev. 2:235–253. [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PA. 2000. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci. 3(5):431–432. [DOI] [PubMed] [Google Scholar]
- De Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N. 2003. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 18:2059–2068. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Heffelfinger AL. 2005. Cross-modal additivity of taste and smell. J Sens Stud. 20:512–525. [Google Scholar]
- Demattè ML, Endrizzi I, Gasperi F. 2014. Food neophobia and its relation with olfaction. Front Psychol. 5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DM. 2012. Licking and liking: the assessment of hedonic responses in rodents. Q J Exp Psychol. 65:371–394. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Birk J. 1982. Flavor-flavor associations induce hedonic shifts in taste preference. Anim Learn Behav. 10:223–228. [Google Scholar]
- Flaherty CF, Mitchell C. 1999. Absolute and relative rewarding properties of fructose, glucose, and saccharin mixtures as reflected in anticipatory contrast. Physiol Behav. 66(5):841–853. [DOI] [PubMed] [Google Scholar]
- Fondberg R, Lundström JN, Blöchl M, Olsson MJ, Seubert J. 2018. Multisensory flavor perception: the relationship between congruency, pleasantness, and odor referral to the mouth. Appetite. 125:244–252. [DOI] [PubMed] [Google Scholar]
- Frank RA, Byram J. 1988. Taste-smell interactions are tastant and odorant dependent. Chem Senses. 13:445–455. [Google Scholar]
- Fredericksen KE, McQueen KA, Samuelsen CL. 2019. Experience-dependent c-fos expression in the mediodorsal thalamus varies with chemosensory modality. Chem Senses. 44(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam SH, Verhagen JV. 2010. Evidence that the sweetness of odors depends on experience in rats. Chem Senses. 35(9):767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam SH, Verhagen JV. 2012. Direct behavioral evidence for retronasal olfaction in rats. PLoS One. 7(9):e44781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. 2005. Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chem Senses. 30(7):601–614. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. 2002. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 27(5):461–474. [DOI] [PubMed] [Google Scholar]
- González F, Morillas E, Hall G. 2016. The extinction procedure modifies a conditioned flavor preference in nonhungry rats only after revaluation of the unconditioned stimulus. J Exp Psychol Anim Learn Cogn. 42(4):380–390. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe CL, Spector AC. 2008. Constructing quality profiles for taste compounds in rats: a novel paradigm. Physiol Behav. 95(3):413–424. [DOI] [PubMed] [Google Scholar]
- Hoefling A, Strack F. 2010. Hunger induced changes in food choice. When beggars cannot be choosers even if they are allowed to choose. Appetite. 54(3):603–606. [DOI] [PubMed] [Google Scholar]
- Holder MD. 1991. Conditioned preferences for the taste and odor components of flavors: blocking but not overshadowing. Appetite. 17(1):29–45. [DOI] [PubMed] [Google Scholar]
- Labbe D, Damevin L, Vaccher C, Morgenegg C, Martin N. 2006. Modulation of perceived taste by olfaction in familiar and unfamiliar beverages. Food Qual Prefer. 17:582–589. [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S. 2009. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 1251:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF Jr, Blair M, Horvath N, Norgren R. 2003. Furosemide, sodium appetite, and ingestive behavior. Physiol Behav. 78(3):449–458. [DOI] [PubMed] [Google Scholar]
- Miller JS, Nonneman AJ, Kelly KS, Neisewander JL, Isaac WL. 1986. Disruption of neophobia, conditioned odor aversion, and conditioned taste aversion in rats with hippocampal lesions. Behav Neural Biol. 45(2):240–253. [DOI] [PubMed] [Google Scholar]
- Mitchell D. 1976. Experiments on neophobia in wild and laboratory rats: a reevaluation. J Comp Physiol Psychol. 90(2):190–197. [DOI] [PubMed] [Google Scholar]
- Myers KP, Sclafani A. 2003. Conditioned acceptance and preference but not altered taste reactivity responses to bitter and sour flavors paired with intragastric glucose infusion. Physiol Behav. 78(2):173–183. [DOI] [PubMed] [Google Scholar]
- Prescott J. 2015. Multisensory processes in flavour perception and their influence on food choice. Curr Opin Food Sci. 3:47–52. [Google Scholar]
- Prescott J, Johnstone V, Francis J. 2004. Odor-taste interactions: effects of attentional strategies during exposure. Chem Senses. 29(4):331–340. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. 2012. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 59(2):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Imada S. 2003. Bilateral lesions of the insular cortex or of the prefrontal cortex block the association between taste and odor in the rat. Neurobiol Learn Mem. 80(1):24–31. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. 2001. Effects of excitotoxic brain lesions on taste-mediated odor learning in the rat. Neurobiol Learn Mem. 75(2):128–139. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Fontanini A. 2017. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci. 37(2):244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, Fontanini A. 2013. Thalamic contribution to cortical processing of taste and expectation. J Neurosci. 33(5):1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Blonde GD, Spector AC. 2016. Bilateral lesions in a specific subregion of posterior insular cortex impair conditioned taste aversion expression in rats. J Comp Neurol. 524(1):54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferstein HN, Verlegh PW. 1996. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol (Amst). 94(1):87–105. [DOI] [PubMed] [Google Scholar]
- Schul R, Slotnick BM, Dudai Y. 1996. Flavor and the frontal cortex. Behav Neurosci. 110(4):760–765. [PubMed] [Google Scholar]
- Sclafani A. 2001. Psychobiology of food preferences. Int J Obes Relat Metab Disord. 25 (Suppl 5):S13–S16. [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2002. Flavor preferences conditioned by sucrose depend upon training and testing methods: two-bottle tests revisited. Physiol Behav. 76(4–5):633–644. [DOI] [PubMed] [Google Scholar]
- Seo HS, Iannilli E, Hummel C, Okazaki Y, Buschhüter D, Gerber J, Krammer GE, van Lengerich B, Hummel T. 2013. A salty-congruent odor enhances saltiness: functional magnetic resonance imaging study. Hum Brain Mapp. 34(1):62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TG, Veldhuizen MG, Marks LE. 2015. Response times to gustatory-olfactory flavor mixtures: role of congruence. Chem Senses. 40(8):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B, Coppola DM. 2015. Odor-cued taste avoidance: a simple and robust test of mouse olfaction. Chem Senses. 40(4):269–278. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Westbrook F, Darling FMC. 1997. What the rat’s nose tells the rat’s mouth: long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim Learn Behav. 25:357–369. [Google Scholar]
- Small DM. 2012. Flavor is in the brain. Physiol Behav. 107(4):540–552. [DOI] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. 2004. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 92(3):1892–1903. [DOI] [PubMed] [Google Scholar]
- Smith JC. 2001. The history of the “Davis Rig”. Appetite. 36(1):93–98. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP, Norgren R. 1993. Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behav Neurosci. 107(4):694–702. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Boakes RA, Prescott J. 1998. Changes in odor sweetness resulting from implicit learning of a simultaneous odor-sweetness association: an example of learned synesthesia. Learn Motiv. 29:113–132. [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. 1995. The acquisition of taste properties by odors. Learn Motiv. 26:433–455. [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. 1999. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem Senses. 24(6):627–635. [DOI] [PubMed] [Google Scholar]
- Torquet N, Aimé P, Messaoudi B, Garcia S, Ey E, Gervais R, Julliard AK, Ravel N. 2014. Olfactory preference conditioning changes the reward value of reinforced and non-reinforced odors. Front Behav Neurosci. 8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. 2005. Critical role of amygdala in flavor but not taste preference learning in rats. Eur J Neurosci. 22(7):1767–1774. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Boersma GJ, Oros H, Choi P, Tamashiro KL, Moran TH. 2014. Similarities and differences between “proactive” and “passive” stress-coping rats in responses to sucrose, NaCl, citric acid, and quinine. Chem Senses. 39(4):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Shepard TG, Wang MF, Marks LE. 2010. Coactivation of gustatory and olfactory signals in flavor perception. Chem Senses. 35(2):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Engelen L. 2006. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev. 30(5):613–650. [DOI] [PubMed] [Google Scholar]
- White TL, Prescott J. 2007. Chemosensory cross-modal stroop effects: congruent odors facilitate taste identification. Chem Senses. 32(4):337–341. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Mobini S, Elliman TD, Walker HC, Stevenson RJ. 2006. Hedonic and sensory characteristics of odors conditioned by pairing with tastants in humans. J Exp Psychol Anim Behav Process. 32(3):215–228. [DOI] [PubMed] [Google Scholar]