Abstract
The ability to identify odors predicts morbidity, mortality, and quality of life. It varies by age, gender, and race and is used in the vast majority of survey and clinical literature. However, odor identification relies heavily on cognition. Other facets of olfaction, such as odor sensitivity, have a smaller cognitive component. Whether odor sensitivity also varies by these factors has not been definitively answered. We analyzed data from the National Social Life, Health, and Aging Project, a nationally representative study of older US adults (n = 2081). Odor identification was measured using 5 validated odors presented with Sniffin’ Stick pens as was odor sensitivity in a 6-dilution n-butanol constant stimuli detection test. Multivariate ordinal logistic regression modeled relationships between olfaction and age, gender, race, cognition, education, socioeconomic status, social network characteristics, and physical and mental health. Odor sensitivity was worse in older adults (P < 0.01), without gender (P = 0.56) or race (P = 0.79) differences. Odor identification was also worse in older adults, particularly men (both P ≤ 0.01), without differences by race. Decreased cognitive function was associated with worse odor identification (P ≤ 0.01) but this relationship was weaker for odor sensitivity (P = 0.02) in analyses that adjusted for other covariates. Odor sensitivity was less strongly correlated with cognitive ability than odor identification, confirming that it may be a more specific measure of peripheral olfactory processing. Investigators interested in associations between olfaction and health should consider both odor sensitivity and identification when attempting to understand underlying neurosensory mechanisms.
Keywords: aging, cognition, epidemiology, odor identification, odor sensitivity, social network
Introduction
Olfaction is a critical and evolutionarily ancient special sense (Hoskison 2013). The ability to identify odors has been shown to be strongly connected to not only neurological health (Velayudhan et al. 2013; Attems et al. 2015; Yaffe et al. 2017; Tonacci et al. 2017; Quarmley et al. 2017; Adams et al. 2018) but also morbidity and mortality itself (Wilson et al 2011; Gopinath et al. 2012; Pinto et al. 2014b; Schubert et al. 2017b; Somekawa et al. 2017). This facet of olfaction varies by age, gender, and race and is used in the vast majority of survey and clinical literature. However, odor identification is considered to be a secondary level of olfaction processing and relies on intact peripheral and central components of the nervous system—that is, to accurately identify an odor requires both perception and cognition (Martzke et al. 1997; Hedner et al. 2010; Schablitzky and Pause 2014). Therefore, it remains challenging to separate the peripheral and central components of olfaction with regards to their relationships with neurodegenerative disease and other health outcomes.
In contrast, odor sensitivity (also known as threshold) is another facet of olfaction that is thought to be a more direct indicator of the primary level of odor processing that occurs in the olfactory system—namely signaling from the olfactory receptors of the nasal epithelium and, perhaps, basic information processing in the olfactory bulb (Whitcroft et al. 2017). Testing for odor sensitivity gauges an individual’s ability to detect the concentration of a particular odor, independent of recalling and naming it, and, thus, poses a low or minimal cognitive burden (mostly in the working memory domain). This also makes odor sensitivity less culturally specific. Despite these advantages, odor sensitivity is more challenging to measure and there are few large-scale studies of the factors associated with odor sensitivity.
Previous studies reported that odor sensitivity is related to age, gender, nasal diseases, neurodegenerative diseases, and smoking (Hayes and Jinks 2012; Lee et al. 2013). Recently, a study of 832 older adults explored factors related to the odor threshold to n-butanol (Schubert et al. 2017a). Due to the nature of the samples involved in these studies, however, applicability to the general population of older adults and diverse populations of the United States is not clear.
Comparing correlates of odor identification and odor sensitivity may allow us to better understand the relationship between olfaction and health, especially regarding cognition and neurodegeneration (Lötsch et al. 2008). Therefore, we addressed the question of how odor sensitivity is related to demographic, social, and health factors in the National Social Life, Health, and Aging Project (NSHAP), the first study to collect both odor identification and odor sensitivity along with a large amount of health and social information from a representative sample of older US adults.
Materials and methods
Subjects
The NSHAP interviewed a probability sample of home-dwelling older US adults (born 1920–1947) in 2010–2011, assessing demographics, social life, and health conditions, including olfaction. All respondents were living at home and able to participate in the study, consistent with being a representative sample across the spectrum of cognitive function (Kotwal et al. 2016). Further details regarding the design, data collection, and baseline characteristics of NSHAP respondents are available elsewhere (O’Doherty et al. 2014). The institutional review boards of The University of Chicago and NORC approved the study; all respondents provided written, informed consent.
The consent and completion rates for both the n-butanol detection and odor identification tests were high (>95%). The analytic sample includes those respondents born between 1920 and 1947 who had n-butanol sensitivity and race/ethnicity data (n = 2081). Mean age was 72.4 years (standard deviation [SD] = 7.5, range 62–91) and 46.8% self-identified as men. The racial/ethnic composition included 81.5% Whites, 9.9% African Americans, 6.3% Hispanics, and 2.4% Others, including 0.9% Asians and 0.9% American Indians or Alaskan natives (all weighted percentages).
Odor Sensitivity
The sense of smell was evaluated using the Olfactory Function Field Exam (OFFE), a tool designed specifically for field research (Kern et al. 2014b, 2015). The OFFE evaluated odor sensitivity to n-butanol using commercially available Sniffin’ Sticks smell pens (Burghart Medical Technology). Briefly, respondents were first presented with a pen that contained the strongest concentration of n-butanol (8%) to ensure that they understood the stimuli they would be asked to detect. The respondent’s detection abilities were then determined by presenting a series of 3 pens, only one of which contained the target n-butanol odor. After all 3 pens in the series had been presented, the Field Interviewer asked the respondent, “Which of the three pens contains the odor?” and entered the response into the Computer-Assisted Personal Interview before going on to the next pen series. There were 6 series, each with a different concentration of n-butanol (0.13%, 0.25%, 0.50%, 2.00%, 4.00%, and 8.00%). An odor sensitivity score was generated based on the number of correctly identified series (range 0–6). Cronbach’s alpha for this scale was 0.68.
Odor identification
Odor identification (i.e., the ability to recognize and name an odor) was evaluated using a validated 5-item test (Hummel et al. 1997). Odors were presented at suprathreshold levels using odor pens (Sniffin’ Sticks, Burghart Medical Technology), and respondents were asked to identify each odor by choosing from a set of 4 picture or word prompts. Refusals or “don’t knows” were coded as incorrect. The number of correctly identified odors was coded as an odor identification score (range 0–5). Cronbach’s alpha for this scale was 0.63. Further details of these validated tests are available elsewhere (Kern et al. 2014a,b).
Socioeconomic status
Education was defined by the highest degree or certification earned. Education was classified into 4 categories: less than high school degree, high school degree or equivalent, vocational certificate/some college/associate degree, and bachelor’s degree or higher. Respondents also reported their net household assets (house, cars, or rental properties/businesses owned; financial assets including savings accounts, stocks, and pensions minus outstanding debt).
Social network
Social network size was assessed using the network roster. Respondents could name up to 5 individuals with whom they had discussed important matters in the last 12 months. Network density, defined as the proportion of all possible pairs among the relevant set of individuals in which the 2 individuals know each other, ranged from 0 to 1 with higher values indicating a more close-knit social network. Social engagement was evaluated by asking how often the respondent attended social activities (volunteering, religious services, organized group meetings, and socializing with friends and family) in the past year (never, less than once a year, about once or twice a year, several times a year, about once a month, every week, or several times a week; range 0–24). Partner status was defined using the information on whether they were married or had a partner. Social support was assessed by asking subjects how often they can rely on or open up to their spouse, family, or friends (never, hardly ever or rarely, some of the time, or often; range 0–18). Social strain was evaluated by asking how often their spouse, family, and friends make too many demands, criticize, or get on their nerves (never, hardly ever or rarely, some of the time, or often; range 0–27). These measures are described in detail elsewhere (Cornwell et al. 2009).
Mental health
Frequency of depressive symptoms, anxiety symptoms, loneliness, and perceived stressors was measured with standard scales modified for survey use: the 11-item Center for Epidemiologic Studies Depression scale (range 0–22), the Hospital Anxiety and Depression Scale’s anxiety subscale (range 0–21), the Revised UCLA Loneliness Scale (range 0–6), and the Perceived Stress Scale (range 0–8) (Shiovitz-Ezra et al. 2009; Payne et al. 2014).
Cognition
Cognitive function was measured with the survey adaptation of the Montreal Cognitive Assessment (MoCA-SA; range 0–20). The MoCA-SA can be administered reliably in a survey setting while preserving sensitivity to a broad range of cognitive abilities and similar performance across demographic subgroups (Kotwal et al. 2015; Dale et al. 2018). The MoCA-SA can be converted to the full MoCA using the equation MoCA = 6.83 + (1.14 × MoCA-SA) (Payne et al. 2014); a MoCA score >22 has been considered by others to indicate normal cognitive status (Kotwal et al. 2016).
Physical health
Comorbid diseases were measured with the Charlson Index modified for NSHAP (Katz et al. 1996; Vasilopoulos et al. 2014). Self-rated physical health was measured by a standard 5-point scale (poor = 1, fair, good, very good, or excellent = 5). Current smoking status was based on self-report.
Statistical analysis
A series of multivariate ordinal logistic regression models were fit to assess the association between covariates and odor sensitivity or identification using Stata Version 14 (StataCorp LP). Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. Comparison of the strength of the relationship between a covariate and odor sensitivity versus olfactory identification was completed using the seemingly unrelated estimation command (suest) in Stata. Multiple imputation, via chained equations, was used to account for missing data in a number of covariates (Table 1). Twenty sets of imputations were generated. A sensitivity analysis utilizing a structural equation model approach produced similar results (data not shown). Performance on each of the olfactory function tasks was presented by plotting the predicted probability of normosmia (a score of ≥4 for odor identification and ≥5 for odor sensitivity) based on logistic regression models. Analyses were weighted using the person-level weights included in the data set; these weights account for differential nonresponse and differential selection probabilities.
Table 1.
National Social Life Health and Aging Project (NSHAP, Wave 2) sample characteristicsa
| Mean | SD | n | |
|---|---|---|---|
| Age | 72.4 | 7.5 | 2081 |
| Household assets (log10) | 5.4 | 0.7 | 1240 |
| Loneliness | 1.1 | 1.5 | 1782 |
| Perceived stressors | 2.8 | 2.3 | 1745 |
| Depression | 4.5 | 4.2 | 2081 |
| Anxiety | 4.7 | 3.6 | 1745 |
| Social network size | 3.8 | 1.4 | 2081 |
| Social strain | 8.3 | 4.3 | 2080 |
| Social network density | 0.77 | 0.28 | 1909 |
| Social engagement | 12.7 | 6.2 | 2081 |
| Social support | 14.3 | 3.0 | 2080 |
| Cognition (MoCA-SA)b | 14.0 | 3.9 | 2081 |
| Comorbidity index | 1.2 | 1.5 | 2081 |
| % | n | ||
| Odor identification (# correct) | 2076 | ||
| 0 | 2.7 | ||
| 1 | 2.7 | ||
| 2 | 6.1 | ||
| 3 | 10.8 | ||
| 4 | 32.3 | ||
| 5 | 45.4 | ||
| n-Butanol sensitivity (# correct) | 2081 | ||
| 0 | 8.9 | ||
| 1 | 7.8 | ||
| 2 | 12.0 | ||
| 3 | 17.8 | ||
| 4 | 25.0 | ||
| 5 | 20.4 | ||
| 6 | 8.1 | ||
| Gender (% men) | 46.8 | 2081 | |
| Race/ethnicity | 2081 | ||
| White | 81.5 | ||
| African American (AA) | 9.9 | ||
| Hispanic, non-AA | 6.3 | ||
| Other | 2.4 | ||
| Education | 2081 | ||
| Less than high school | 16.0 | ||
| High school graduate | 26.4 | ||
| Some college | 30.5 | ||
| Bachelors or higher | 27.1 | ||
| Partnered | 70.3 | 2081 | |
| Smoking | 12.7 | 2081 | |
| Self-rated physical health | 2078 | ||
| Poor | 5.7 | ||
| Fair | 18.9 | ||
| Good | 31.6 | ||
| Very good | 31.1 | ||
| Excellent | 12.7 |
Estimates were weighted to account for differential selection and nonresponse using the person-level weights provided in the data.
aOnly includes respondents born between 1920 and 1947 who had both n-butanol sensitivity and race/ethnicity data.
bSurvey adapted Montreal Cognitive Assessment.
Results
Odor sensitivity and odor identification by demographic characteristics
The mean odor sensitivity score was 3.4 (SD = 1.7; range 0–6). The mean odor identification score was 4.0 (SD = 1.2; range 0–5). The correlation between odor sensitivity and identification was 0.34, which is in agreement with prior reports (Koskinen et al. 2004).
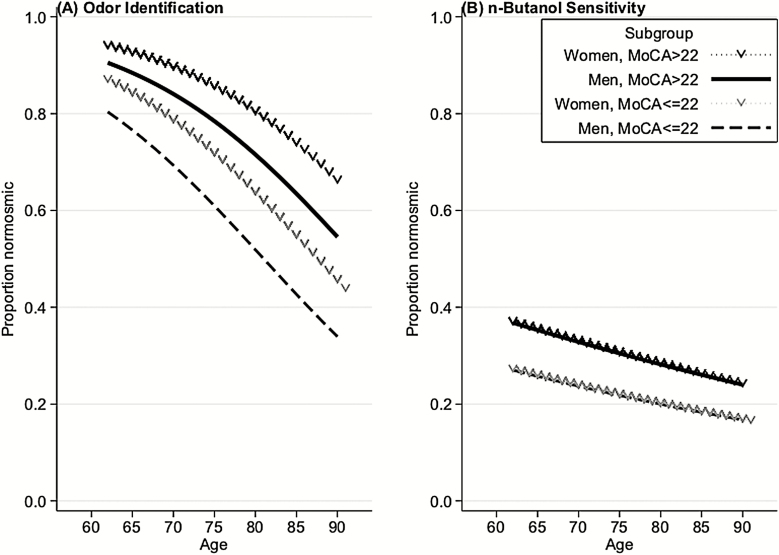
Both odor sensitivity and odor identification were significantly worse at older ages (Table 2; Figure 1). A decade increase in age was associated with a 27% reduction in the odds of meeting a given threshold criterion (OR = 0.73, 95% CI = 0.63–0.85, P < 0.001). For odor identification, the same decade increase in age was associated with a 45% reduction in the odds of meeting a given performance criterion (OR = 0.55, 95% CI = 0.47–0.63, P < 0.001). Although there were no differences by gender for odor sensitivity (OR = 0.94, 95% CI = 0.76–1.17, P = 0.56), male gender was associated with lower odor identification scores (OR = 0.74, 95% CI = 0.58–0.93, P = 0.01).
Table 2.
Odor sensitivity and odor identification, with associations with demographic, social, psychological, and physical traits; ordinal logistic regression models
| OR, (95% CI), and P value | |||
|---|---|---|---|
| n-Butanol sensitivitya (n = 2081) | Odor IDb (n = 2076) | P value, Cross-model comparisons | |
| Demographic characteristics | |||
| Age (decades) | 0.73 | 0.55 | 0.005 |
| (0.63, 0.85) | (0.47, 0.63) | ||
| <0.001 | <0.001 | ||
| Sex (men vs. women) | 0.94 | 0.74 | 0.10 |
| (0.76, 1.17) | (0.58, 0.93) | ||
| 0.56 | 0.01 | ||
| Race/ethnicity (vs. White) | 0.22 | ||
| African American (AA) | 0.99 | 0.83 | 0.53 |
| (0.67, 1.45) | (0.62, 1.12) | ||
| 0.94 | 0.22 | ||
| Hispanic, non-AA | 0.78 | 0.98 | 0.38 |
| (0.37, 1.62) | (0.66, 1.45) | ||
| 0.49 | 0.93 | ||
| Other | 2.63 | 1.13 | 0.13 |
| (1.31, 5.29) | (0.54, 2.36) | ||
| 0.008 | 0.75 | ||
| Educationc | 0.94 | 0.89 | 0.58 |
| (0.81, 1.08) | (0.78, 1.03) | ||
| 0.34 | 0.11 | ||
| Household assets (log10) | 1.13 | 1.04 | 0.21 |
| (1.02,1.26) | (0.95, 1.13) | ||
| 0.02 | 0.41 | ||
| Social network | |||
| Network size | 1.10 | 1.12 | 0.70 |
| (1.01, 1.19) | (1.03, 1.22) | ||
| 0.03 | 0.009 | ||
| Social strain | 1.03 | 1.03 | 0.79 |
| (1.00, 1.05) | (1.00, 1.06) | ||
| 0.08 | 0.047 | ||
| Network density | 1.07 | 0.90 | 0.47 |
| (0.71, 1.59) | (0.60, 1.33) | ||
| 0.75 | 0.58 | ||
| Social engagement | 1.01 | 1.00 | 0.45 |
| (0.98, 1.03) | (0.98, 1.02) | ||
| 0.59 | 0.73 | ||
| Partnered | 0.82 | 0.84 | 0.91 |
| (0.63, 1.06) | (0.66, 1.07) | ||
| 0.13 | 0.15 | ||
| Social support | 0.99 | 0.99 | 0.84 |
| (0.95, 1.03) | (0.95, 1.04) | ||
| 0.58 | 0.76 | ||
| Mental health | |||
| Loneliness score | 0.93 | 0.90 | 0.50 |
| (0.86, 1.01) | (0.82, 0.99) | ||
| 0.09 | 0.03 | ||
| Perceived stress score | 0.95 | 0.99 | 0.23 |
| (0.91, 1.00) | (0.93, 1.05) | ||
| 0.04 | 0.65 | ||
| Depressive symptom score | 1.02 | 1.02 | 0.91 |
| (0.98, 1.05) | (0.98, 1.05) | ||
| 0.36 | 0.30 | ||
| Anxiety symptom score | 0.99 | 0.99 | 0.96 |
| (0.96, 1.03) | (0.96, 1.03) | ||
| 0.75 | 0.78 | ||
| Physical health | |||
| Cognition (MoCA-SA)d | 1.04 | 1.13 | 0.002 |
| (1.01, 1.08) | (1.09, 1.17) | ||
| 0.02 | <0.001 | ||
| Current nonsmoker | 0.82 | 1.06 | 0.29 |
| (0.60, 1.12) | (0.77, 1.46) | ||
| 0.20 | 0.73 | ||
| Self-rated physical healthe | 1.01 | 1.06 | 0.45 |
| (0.90, 1.14) | (0.93, 1.21) | ||
| 0.81 | 0.40 | ||
| Comorbidity index | 1.01 | 0.99 | 0.63 |
| (0.94, 1.08) | (0.92, 1.05) | ||
| 0.81 | 0.67 |
aMeasured as the number of correctly identified n-butanol concentrations.
bMeasured as the number of correctly identified odors.
cTreated as a continuous measure using integer scores for educational level (higher scores = more education).
dMeasured with the survey adaptation of the MoCA-SA.
eTreated as a continuous measure using integer scores for self-rated physical health level (higher scores = better self-rated physical health).
Figure 1.
Olfactory function, as assessed by odor identification (A) and odor sensitivity (B), among older US adults versus age, stratified by gender and cognitive function. Normosmia is defined as 4 or 5 correct for odor identification and 5 or 6 correct for odor sensitivity. For plotting purposes only, cognition was categorized using a MoCA cutpoint of 22.
For both odor sensitivity and odor identification, no significant differences were detected between African Americans and White Americans (P > 0.05) or between Hispanics and White Americans (P > 0.05). However, participants who identified their race as Other had better sensitivity to n-butanol compared to White Americans (OR = 2.63, 95% CI = 1.31–5.29, P = 0.008), which was primarily driven by the American Indian/Alaskan Native group (OR = 3.74, 95% CI = 2.07–6.76; n = 48).
We employed education and net household assets in our models as measures of socioeconomic status (Murphy et al. 2002). There was no evidence of an association between education and odor sensitivity or identification (P > 0.05). Having higher household assets was associated with better odor sensitivity (OR = 1.13, 95% CI = 1.02–1.26, P = 0.02) but not odor identification (OR = 1.04, 95% CI = 0.95–1.13, P = 0.41).
Social network
To evaluate whether social factors were associated with olfaction, we examined social network size, network density, social engagement, having a partner, social support, and social strain. Larger social network size was associated both with better odor sensitivity (OR = 1.10, 95% CI = 1.01–1.19, P = 0.03) and higher odor identification scores (OR = 1.12, 95% CI = 1.03–1.22, P = 0.009). Interestingly, increased self-reported social strain was associated with higher odor identification scores (OR = 1.03, 95% CI = 1.00–1.06, P = 0.047), with a similar trend toward better odor sensitivity (OR = 1.03, 95% CI = 1.00–1.05, P = 0.08). However, there was no statistically significant association between sensitivity or identification and the remaining social variables (P > 0.05, all) (Table 2).
Mental health
Higher perceived stress was associated with worse odor sensitivity (OR = 0.95, 95% CI = 0.91–1.00, P = 0.04) but not lower odor identification scores (OR = 0.99, 95% CI = 0.93–1.05, P = 0.65). Increased loneliness was associated with both worse odor identification (OR = 0.90, 95% CI = 0.82–0.99, P = 0.03) and a trend toward worse odor sensitivity (OR = 0.93, 95% CI = 0.86–1.01, P = 0.09). There was no evidence that depression or anxiety symptoms were associated with poor odor sensitivity or lower odor identification scores (P > 0.05, all; Table 2).
Cognition
Better cognition was associated with better odor sensitivity (OR = 1.04, 95% CI = 1.01–1.08, P = 0.02), as well as higher odor identification scores (OR = 1.13, 95% CI = 1.09–1.17, P < 0.001). However, the relationship of cognition with odor identification was significantly stronger than the association between cognition with odor sensitivity (P = 0.002; Figure 1).
Physical health/smoking
Self-rated physical health and comorbidity index showed no associations with odor sensitivity or odor identification (P > 0.05, for both). Current smoking status also showed no associations with olfaction (P > 0.05, for both; Table 2).
Discussion
We found that odor sensitivity and odor identification have many of the same correlates in older US adults. However, important differences were observed in the age and cognition domains, potentially reflecting dysfunction in different parts of the olfactory pathway.
Both odor sensitivity and identification are worse with increasing age, a relationship that has been well corroborated in the literature (Doty et al. 1984; Ship and Weiffenbach 1993; Ship et al. 1996; Murphy et al. 2002; Mackay-Sim et al. 2006; Menon et al. 2013; Kern et al. 2014a; Devanand et al. 2015; Schubert et al. 2017a; Zhang and Wang 2017; Oleszkiewicz et al. 2019; Palmquist et al. 2019) The age-related development of dysfunction in both sensitivity and identification suggests a shared mechanism and has been linked to a number of age-related changes within the nose, olfactory epithelium, and olfactory bulb, as well as higher brain structures (Murphy et al. 1994, 2000; Hummel et al 1998; Kovács 2004; Doty et al. 2011; Doty and Kamath 2014). A recent large study has noted a more pronounced age-related loss in odor sensitivity as opposed to other measures of olfaction (Oleszkiewicz et al. 2019). However, we found the relationship between age and odor identification to be stronger than that of age and odor sensitivity (P = 0.005).
Women were found to have better odor identification than men as has been found in prior studies (Doty et al. 1984; Ship and Weiffenbach 1993; Ship et al. 1996; Murphy et al. 2002; Landis et al. 2004; Doty and Kamath 2014; Devanand et al. 2015; Oleszkiewicz et al. 2019). The relationship between gender and odor sensitivity is less clear in the literature. Depending on the odorant used, some studies have reported greater sensitivity among women (Koelega 1994; Cometto-Muñiz and Abraham 2008), whereas others have reported no difference (Koelega 1970; Koelega and Köster 1974; Schubert et al. 2017a). Our findings are consistent with the latter—namely that odor sensitivity to n-butanol, as opposed to odor identification, does not differ between men and women. However, the neuronal basis for gender differences in olfaction is complex and, to date, the underlying mechanisms remain unclear (Brand and Millot 2001; Sundermann et al. 2008; Doty et al. 2008; Doty and Cameron 2009; Oliveira-Pinto et al. 2014; Kollndorfer et al. 2016). Notably, gender differences in olfaction have been recorded among prepubescent children (Gellrich et al. 2019), as well as postmenopausal women (Doty and Cameron 2009). Hormone replacement therapy in postmenopausal women has not been found to enhance olfactory performance (Hughes et al. 2002), making it likely that gender differences in olfaction cannot be attributed to the influence of concurrent gonadal hormones alone. Instead, it may be that endocrine factors affect the development of neural circuits relevant to olfaction as has been shown in mice (Pierman et al. 2008)—if this is the case, the gender difference in odor identification may be due to its larger cognitive component as compared with odor sensitivity. However, imaging studies on the sex differences in odor-induced brain activation have not been uniform (Yousem et al. 1999; Bengtsson et al. 2001; Morrot et al. 2013; Melero et al. 2019) and additional study of gender differences in olfaction is needed to address these questions.
With regards to racial/ethnic disparities, neither African Americans nor Hispanics differed significantly in either odor sensitivity or identification when compared to their White counterparts, though a disparity was present prior to the addition of cognition as a covariate in the odor identification analysis (Supplementary Appendix A) but not odor sensitivity (Supplementary Appendix B). This highlights the importance of adjusting for cognition in studies of odor identification.
Moreover, our experience shows the importance of using a highly sensitive measure for cognitive dysfunction. A prior NSHAP study from our lab observed a racial/ethnic disparity in olfaction (Pinto et al. 2014a) despite having adjusted for cognition. However, cognition was measured at the time using the Short Portable Mental Status Questionnaire, a screening measure for dementia. In comparison, the current study uses the MoCA-SA, a superior measure that is capable of capturing cognitive dysfunction with greater sensitivity and detail (Roccaforte et al. 1994; Nasreddine et al. 2005; Gagnon et al. 2013; Moyer 2014; Shega et al. 2014; Kotwal et al. 2015; Dale et al. 2018) and does not demonstrate a racial disparity.
In this study, greater household assets were associated with better odor sensitivity. There was no significant association found with odor identification similar to what has been previously described by our group (Pinto et al. 2014a). This may reflect a difference in environmental exposures to olfactory toxins as those with more household assets may be less likely to have occupational chemical exposures or to live near major sources of air pollution, both of which have been associated with worse odor sensitivity (Greenberg et al. 2013).
Previous research has also described a connection between social life and both odor sensitivity and odor identification (Croy et al. 2014; Zou et al. 2016; Leschak and Eisenberger 2018). Our results similarly show that increased social network size is associated with better odor sensitivity. It is possible that participants with higher odor sensitivity may be able to obtain more social chemical signals, which aid communication and facilitate relationships. Among women, odor identification has previously been correlated with social network size, with a similar trend among men (Boesveldt et al. 2017). Our study extends these findings by demonstrating that, even after controlling for gender, larger network size is associated with improved odor identification.
Although a socially engaged lifestyle has positive effects through social support, it is also thought to have negative effects through social strain (Rook 1984; Tun et al. 2013). Interestingly, despite this expectation, increased social strain was found to be associated with slightly better odor identification and, to a lesser extent, odor sensitivity. Although no studies to date have investigated social strain with olfaction, prior research has suggested that the effects of social strain may not be entirely negative—for example, it has also been found to be associated with higher global cognitive and executive function (Ge et al. 2017). Thus, the current methodology may be capturing not only social strain but also a more socially engaged lifestyle. Furthermore, it may be that certain individuals are perceived by their social network members as less capable and, therefore, have fewer social demands placed on them (Seeman et al. 2011).
Research has also established the importance of looking at an individual’s perception of social isolation otherwise conceptualized as loneliness (Ong et al. 2016). Loneliness has been recognized as a major risk factor for morbidity and mortality and reflects the difference between an individual’s desired and actual relationships, regardless of objective social network measures (Holt-Lunstad et al. 2010; Hawkley and Cacioppo 2010; Perissinotto et al. 2012; Cacioppo et al. 2014). We found increased loneliness to be associated with worse odor identification and sensitivity (Gopinath et al. 2011; Schablitzky and Pause 2014; Sivam et al. 2016). This likely reflects the impact of loneliness on 2 key areas of the central nervous system: the prefrontal cortex and the hippocampus. In animals, loneliness is linked to increased cortisone levels in the prefrontal cortex, which is also the site of extensive cognitive processing of odors in the orbitofrontal cortex (Lundström et al. 2010). The hippocampus is also implicated in the identification of familiar odors (Kareken et al. 2003; Kjelvik et al. 2012) and, in animal models, social isolation has been found to decrease hippocampal neurogenesis (Lieberwirth and Wang 2012; Cacioppo et al. 2014). Therefore, increased loneliness may be directly impairing an individual’s ability to process olfactory signals in the brain, though additional studies with human subjects are needed.
Although the relationship between stress and olfaction has not been well studied, research suggests that sensitivity to malodors is improved following acute stressors (Pacharra et al. 2016) but worsened following prolonged chronic stress (Yuan and Arias-Carrion 2015; Raynaud et al. 2015; Vaz et al. 2018). We found that increased perceived stress was associated with worse odor sensitivity, which suggests that our perceived stress score more accurately reflects chronic rather than acute stress. Given that prior studies have reported associations between decreased olfactory function and depression (Sivam et al. 2016; Croy and Hummel 2017; Pabel et al. 2018; Churnin et al. 2019; Qazi et al. 2020), the lack thereof demonstrated in the current study suggests that the relationship between olfaction and depression may be partially mediated by the psychosocial and cognition measures accounted for in the current model.
The observed differences in the strength of the association between cognition and odor sensitivity versus identification warrant further mention. Given that odor identification is thought to be a higher order of odor processing, cognition is one of the most important measures for elucidating this relationship. Cognition has been found to be closely related to odor identification (Larsson et al. 2005; Velayudhan et al. 2013; Attems et al. 2015; Yaffe et al. 2017; Tonacci et al. 2017; Quarmley et al. 2017; Adams et al. 2018). In contrast to a prior study that did not observe a difference related to odor sensitivity (Hedner et al. 2010), we found that better cognition was associated with both better odor sensitivity and odor identification. Further testing demonstrated that the relationship between cognition and odor identification is significantly stronger than that of odor sensitivity. This relationship between odor identification and cognition is hypothesized to rely on executive function, language processing, and verbal episodic memory (Larsson et al. 2005; Wehling et al. 2016). This has been supported by studies demonstrating associations between odor identification and measures of verbal episodic memory (Wehling et al. 2016), as well as volumes of the left inferior frontal gyrus (Wu et al. 2019), an area associated with verbal fluency (Costafreda et al. 2006). Taken together, these results support the hypothesis that odor sensitivity has a lower cognitive burden than odor identification (Hedner et al. 2010). It also affirms the utility of odor sensitivity as a relatively more specific measure of peripheral olfactory system function. Given the cooccurrence of cognitive decline during aging, odor sensitivity may also be a more useful test to isolate the effect of aging of the olfactory epithelium function/peripheral olfactory system.
Although the current study surveyed a representative sample of US older adults, there were few numbers of American Indians/Alaskan Natives and Asian American/Pacific Islanders. Further research will be needed to determine if the correlates identified for odor sensitivity and identification remain consistent across different races/ethnicities and other demographic subgroups. Moreover, additional longitudinal studies will be necessary to explore the causal relationships between different measures of olfaction, cognition, and other factors.
In conclusion, odor sensitivity was less dependent on cognitive ability than odor identification, confirming that it is a more pure measure of peripheral and/or central olfactory processing. Age was also more strongly associated with odor identification performance. These differences may yield insights into the physiology of the olfactory and nervous systems. Investigators interested in the associations between olfaction and health outcomes should consider both olfactory sensitivity and identification rather than the total discriminatory score alone when attempting to understand underlying neurosensory mechanisms.
Funding
This work was supported by the National Institute on Aging (AG030481, AG043538, AG048511, AG000243, and AG029795) and the National Institute of Environmental Health Sciences (ES026718), the Department of Surgery at The University of Chicago, and the Pritzker School of Medicine.
Conflict of interests
The authors have no conflicts of interest to disclose.
Supplementary Material
Acknowledgments
We thank National Social Life, Health, and Aging Project respondents for their generous participation. Members of the Olfactory Research Group and colleagues at National Social Life, Health, and Aging Project freely provided constructive feedback for which we are grateful.
References
- Adams DR, Kern DW, Wroblewski KE, McClintock MK, Dale W, Pinto JM. 2018. Olfactory dysfunction predicts subsequent dementia in older U.S. adults. J Am Geriatr Soc. 66(1):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA. 2015. Olfaction and aging: a mini-review. Gerontology. 61(6):485–490. [DOI] [PubMed] [Google Scholar]
- Bengtsson S, Berglund H, Gulyas B, Cohen E, Savic I. 2001. Brain activation during odor perception in males and females. Neuroreport. 12(9):2027–2033. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Yee JR, McClintock MK, Lundström JN. 2017. Olfactory function and the social lives of older adults: a matter of sex. Sci Rep. 7:45118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand G, Millot JL. 2001. Sex differences in human olfaction: between evidence and enigma. Q J Exp Psychol B. 54(3):259–270. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Capitanio JP, Cacioppo JT. 2014. Toward a neurology of loneliness. Psychol Bull. 140(6):1464–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churnin I, Qazi J, Fermin CR, Wilson JH, Payne SC, Mattos JL. 2019. Association between olfactory and gustatory dysfunction and cognition in older adults. Am J Rhinol Allergy. 33(2):170–177. doi: 10.1177/1945892418824451. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Abraham MH. 2008. Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pharmacol Biochem Behav. 89(3):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B, Schumm LP, Laumann EO, Graber J. 2009. Social networks in the NSHAP study: rationale, measurement, and preliminary findings. J Gerontol B Psychol Sci Soc Sci. 64B(Suppl 1):i47–i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. 2006. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 27(10):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Hummel T. 2017. Olfaction as a marker for depression. J Neurol. 264(4):631–638. [DOI] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life—an updated review. Chem Senses. 39(3):185–194. [DOI] [PubMed] [Google Scholar]
- Dale W, Kotwal AA, Shega JW, Schumm LP, Kern DW, Pinto JM, Pudelek KM, Waite LJ, McClintock MK. 2018. Cognitive function and its risk factors among older US adults living at home. Alzheimer Dis Assoc Disord. 32(3):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, Stern Y, Mayeux R, Doty RL. 2015. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 78(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Cameron EL. 2009. Sex differences and reproductive hormone influences on human odor perception. Physiol Behav. 97(2):213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Kamath V. 2014. The influences of age on olfaction: a review. Front Psychol. 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Kisat M, Tourbier I. 2008. Estrogen replacement therapy induces functional asymmetry on an odor memory/discrimination test. Brain Res. 1214:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Petersen I, Mensah N, Christensen K. 2011. Genetic and environmental influences on odor identification ability in the very old. Psychol Aging. 26(4):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. 1984. Smell identification ability: changes with age. Science. 226(4681):1441–1443. [DOI] [PubMed] [Google Scholar]
- Gagnon G, Hansen KT, Woolmore-Goodwin S, Gutmanis I, Wells J, Borrie M, Fogarty J. 2013. Correcting the MoCA for education: effect on sensitivity. Can J Neurol Sci. 40(5):678–683. [DOI] [PubMed] [Google Scholar]
- Ge S, Wu B, Bailey DE, Dong X. 2017. Social support, social strain, and cognitive function among community-dwelling U.S. Chinese older adults. J Gerontol A Biol Sci Med Sci. 72(Suppl 1):S16–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellrich J, Sparing-Paschke LM, Thieme T, Schwabe K, Dworschak A, Hummel T, Schriever VA. 2019. Normative data for olfactory threshold and odor identification in children and adolescents. Int J Pediatr Otorhinolaryngol. 123:5–9. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Anstey KJ, Sue CM, Kifley A, Mitchell P. 2011. Olfactory impairment in older adults is associated with depressive symptoms and poorer quality of life scores. Am J Geriatr Psychiatry. 19(9):830–834. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Sue CM, Kifley A, Mitchell P. 2012. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. 67(2):204–209. [DOI] [PubMed] [Google Scholar]
- Greenberg MI, Curtis JA, Vearrier D. 2013. The perception of odor is not a surrogate marker for chemical exposure: a review of factors influencing human odor perception. Clin Toxicol. 51(2):70–76. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. 2010. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 40(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Jinks AL. 2012. Evaluation of smoking on olfactory thresholds of phenyl ethyl alcohol and n-butanol. Physiol Behav. 107(2):177–180. [DOI] [PubMed] [Google Scholar]
- Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. 2010. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 32(10):1062–1067. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7(7):e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskison EE. 2013. Olfaction, pheromones and life. J Laryngol Otol. 127(12):1156–1159. [DOI] [PubMed] [Google Scholar]
- Hughes LF, McAsey ME, Donathan CL, Smith T, Coney P, Struble RG. 2002. Effects of hormone replacement therapy on olfactory sensitivity: cross-sectional and longitudinal studies. Climacteric. 5(2):140–150. [PubMed] [Google Scholar]
- Hummel T, Barz S, Pauli E, Kobal G. 1998. Chemosensory event-related potentials change with age. Electroencephalogr Clin Neurophysiol. 108(2):208–217. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. “Sniffin’ sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22(1):39–52. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. 2003. Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology. 17(3):482–495. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. 1996. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 34(1):73–84. [DOI] [PubMed] [Google Scholar]
- Kern DW, Schumm LP, Wroblewski KE, Pinto JM, Hummel T, McClintock MK. 2015. Olfactory thresholds of the U.S. population of home-dwelling older adults: development and validation of a short, reliable measure. PLoS One. 10(3):e0118589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. 2014aOlfactory function in wave 2 of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S134–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DW, Wroblewski KE, Schumm LP, Pinto JM, McClintock MK. 2014b. Field survey measures of olfaction: the Olfactory Function Field Exam (OFFE). Field Methods. 26(4):421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelvik G, Evensmoen HR, Brezova V, Håberg AK. 2012. The human brain representation of odor identification. J Neurophysiol. 108(2):645–657. [DOI] [PubMed] [Google Scholar]
- Koelega HS, Köster EP. 1974. Some experiments on sex differences in odor perception. Ann N Y Acad Sci. 237:234–246. [DOI] [PubMed] [Google Scholar]
- Koelega HS. 1970. Extraversion, sex, arousal and olfactory sensitivity. Acta Psychol (Amst). 34(1):51–66. [DOI] [PubMed] [Google Scholar]
- Koelega HS. 1994. Sex differences in olfactory sensitivity and the problem of the generality of smell acuity. Percept Mot Skills. 78(1):203–213. [DOI] [PubMed] [Google Scholar]
- Kollndorfer K, Ohrenberger I, Schöpf V. 2016. Contraceptive use affects overall olfactory performance: investigation of estradiol dosage and duration of intake. PLoS One. 11(12):e0167520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen S, Vento S, Malmberg H, Tuorila H. 2004. Correspondence between three olfactory tests and suprathreshold odor intensity ratings. Acta Otolaryngol. 124(9):1072–1077. [DOI] [PubMed] [Google Scholar]
- Kotwal AA, Kim J, Waite L, Dale W. 2016. Social function and cognitive status: results from a US nationally representative survey of older adults. J Gen Intern Med. 31(8):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal AA, Schumm P, Kern DW, McClintock MK, Waite LJ, Shega JW, Huisingh-Scheetz MJ, Dale W. 2015. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 29(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács T. 2004. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 3(2):215–232. [DOI] [PubMed] [Google Scholar]
- Landis BN, Konnerth CG, Hummel T. 2004. A study on the frequency of olfactory dysfunction. Laryngoscope. 114(10):1764–1769. [DOI] [PubMed] [Google Scholar]
- Larsson M, Öberg C, Bäckman L. 2005. Odor identification in old age: demographic, sensory and cognitive correlates. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 12(3):231–244. [DOI] [PubMed] [Google Scholar]
- Lee WH, Wee JH, Kim DK, Rhee CS, Lee CH, Ahn S, Lee JH, Cho YS, Lee KH, Kim KS, et al. 2013. Prevalence of subjective olfactory dysfunction and its risk factors: Korean National Health and Nutrition Examination Survey. PLoS One. 8(5):e62725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschak CJ, Eisenberger NI. 2018. The role of social relationships in the link between olfactory dysfunction and mortality. PLoS One. 13(5):e0196708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. 2012. The social environment and neurogenesis in the adult Mammalian brain. Front Hum Neurosci. 6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Reichmann H, Hummel T. 2008. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 33(1):17–21. [DOI] [PubMed] [Google Scholar]
- Lundström JN, Boesveldt S, Albrecht J. 2010. Central processing of the chemical senses: an overview. ACS Chem Neurosci. 2(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Johnston AN, Owen C, Burne TH. 2006. Olfactory ability in the healthy population: reassessing presbyosmia. Chem Senses. 31(8):763–771. [DOI] [PubMed] [Google Scholar]
- Martzke JS, Kopala LC, Good KP. 1997. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry. 42(8):721–732. [DOI] [PubMed] [Google Scholar]
- Melero H, Borromeo S, Cristobal-Huerta A, Manzanedo E, Luna G, Toledano A, Hernández-Tamames JA. 2019. Sex differences in the olfactory system: a functional MRI study. Chemosens Percept. 12:50–58. doi: 10.1007/s12078-018-9250-1. [DOI] [Google Scholar]
- Menon C, Westervelt HJ, Jahn DR, Dressel JA, O’Bryant SE. 2013. Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol. 27(6):946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrot G, Bonny JM, Lehallier B, Zanca M. 2013. fMRI of human olfaction at the individual level: interindividual variability. J Magn Reson Imaging. 37(1):92–100. [DOI] [PubMed] [Google Scholar]
- Moyer VA. 2014. U.S. Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 160(11):791–797. [DOI] [PubMed] [Google Scholar]
- Murphy C, Morgan CD, Geisler MW, Wetter S, Covington JW, Madowitz MD, Nordina S, Polich JM. 2000. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 36(2):133–145. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, de Wijk RA, Cain WS, Polich J. 1994. Olfactory-evoked potentials: assessment of young and elderly, and comparison to psychophysical threshold. Chem Senses. 19(1):47–56. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. 2002. Prevalence of olfactory impairment in older adults. JAMA. 288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. 2005. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53(4):695–699. [DOI] [PubMed] [Google Scholar]
- O’Doherty K, Jaszczak A, Hoffmann JN, You HM, Kern DW, Pagel K, McPhillips J, Schumm LP, Dale W, Huang ES, et al. 2014. Survey field methods for expanded biospecimen and biomeasure collection in NSHAP wave 2. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S27–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. 2019. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Santos RM, Coutinho RA, Oliveira LM, Santos GB, Alho AT, Leite RE, Farfel JM, Suemoto CK, Grinberg LT, et al. 2014. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PLoS One. 9(11):e111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Uchino BN, Wethington E. 2016. Loneliness and health in older adults: a mini-review and synthesis. Gerontology. 62(4):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabel LD, Hummel T, Weidner K, Croy I. 2018. The impact of severity, course and duration of depression on olfactory function. J Affect Disord. 238:194–203. [DOI] [PubMed] [Google Scholar]
- Pacharra M, Schäper M, Kleinbeck S, Blaszkewicz M, Wolf OT, van Thriel C. 2016. Stress lowers the detection threshold for foul-smelling 2-mercaptoethanol. Stress. 19(1):18–27. [DOI] [PubMed] [Google Scholar]
- Palmquist E, Larsson M, Olofsson JK, Seubert J, Bäckman L, Laukka EJ. 2019. A prospective study on risk factors for olfactory dysfunction in aging. J Gerontol A Biol Sci Med Sci. 75(3), 603–610. doi: 10.1093/gerona/glz265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Hedberg EC, Kozloski M, Dale W, McClintock MK. 2014. Using and interpreting mental health measures in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S99–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, Covinsky KE. 2012. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med. 172(14):1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Bakker J. 2008. Evidence for a role of early oestrogens in the central processing of sexually relevant olfactory cues in female mice. Eur J Neurosci. 27(2):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. 2014a. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 69(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. 2014b. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi JJ, Wilson JH, Payne SC, Mattos JL.January 2020. Association between smell, taste, and depression in nationally representative sample of older adults in the United States. Am J Rhinol Allergy. doi: 10.1177/1945892419897217. [DOI] [PubMed] [Google Scholar]
- Quarmley M, Moberg PJ, Mechanic-Hamilton D, Kabadi S, Arnold SE, Wolk DA, Roalf DR. 2017. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 55(4):1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud A, Meunier N, Acquistapace A, Bombail V. 2015. Chronic variable stress exposure in male Wistar rats affects the first step of olfactory detection. Behav Brain Res. 291:36–45. [DOI] [PubMed] [Google Scholar]
- Roccaforte WH, Burke WJ, Bayer BL, Wengel SP. 1994. Reliability and validity of the Short Portable Mental Status Questionnaire administered by telephone. J Geriatr Psychiatry Neurol. 7(1):33–38. [PubMed] [Google Scholar]
- Rook KS. 1984. The negative side of social interaction: impact on psychological well-being. J Pers Soc Psychol. 46(5):1097–1108. [DOI] [PubMed] [Google Scholar]
- Schablitzky S, Pause BM. 2014. Sadness might isolate you in a non-smelling world: olfactory perception and depression. Front Psychol. 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Cruickshanks KJ. 2017. Odor detection thresholds in a population of older adults. Laryngoscope. 127(6):1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Tweed TS, Cruickshanks KJ. 2017. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 72(5):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. 2011. Histories of social engagement and adult cognition: midlife in the U.S. study. J Gerontol B Psychol Sci Soc Sci. 66(Suppl 1):i141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shega JW, Sunkara PD, Kotwal A, Kern DW, Henning SL, McClintock MK, Schumm P, Waite LJ, Dale W. 2014. Measuring cognition: the Chicago cognitive function measure in the national social life, health and aging project, wave 2. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S166–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiovitz-Ezra S, Leitsch S, Graber J, Karraker A. 2009. Quality of life and psychological health indicators in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 64(Suppl 1):i30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ship JA, Pearson JD, Cruise LJ, Brant LJ, Metter EJ. 1996. Longitudinal changes in smell identification. J Gerontol A Biol Sci Med Sci. 51(2):M86–M91. [DOI] [PubMed] [Google Scholar]
- Ship JA, Weiffenbach JM. 1993. Age, gender, medical treatment, and medication effects on smell identification. J Gerontol. 48(1):M26–M32. [DOI] [PubMed] [Google Scholar]
- Sivam A, Wroblewski KE, Alkorta-Aranburu G, Barnes LL, Wilson RS, Bennett DA, Pinto JM. 2016. Olfactory dysfunction in older adults is associated with feelings of depression and loneliness. Chem Senses. 41(4):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekawa S, Mine T, Ono K, Hayashi N, Obuchi S, Yoshida H, Kawai H, Fujiwara Y, Hirano H, Kojima M, et al. 2017. Relationship between sensory perception and frailty in a community-dwelling elderly population. J Nutr Health Aging. 21(6):710–714. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Gilbert PE, Murphy C. 2008. The effect of hormone therapy on olfactory sensitivity is dependent on apolipoprotein E genotype. Horm Behav. 54(4):528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonacci A, Bruno RM, Ghiadoni L, Pratali L, Berardi N, Tognoni G, Cintoli S, Volpi L, Bonuccelli U, Sicari R, et al. 2017. Olfactory evaluation in mild cognitive impairment: correlation with neurocognitive performance and endothelial function. Eur J Neurosci. 45(10):1279–1288. [DOI] [PubMed] [Google Scholar]
- Tun PA, Miller-Martinez D, Lachman ME, Seeman T. 2013. Social strain and executive function across the lifespan: the dark (and light) sides of social engagement. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 20(3):320–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilopoulos T, Kotwal A, Huisingh-Scheetz MJ, Waite LJ, McClintock MK, Dale W. 2014. Comorbidity and chronic conditions in the National Social Life, Health and Aging Project (NSHAP), Wave 2. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz RP, Cardoso A, Serrão P, Pereira PA, Madeira MD. 2018. Chronic stress leads to long-lasting deficits in olfactory-guided behaviors, and to neuroplastic changes in the nucleus of the lateral olfactory tract. Horm Behav. 98:130–144. [DOI] [PubMed] [Google Scholar]
- Velayudhan L, Pritchard M, Powell JF, Proitsi P, Lovestone S. 2013. Smell identification function as a severity and progression marker in Alzheimer’s disease. Int Psychogeriatr. 25(7):1157–1166. [DOI] [PubMed] [Google Scholar]
- Wehling EI, Wollschlaeger D, Nordin S, Lundervold AJ. 2016. Longitudinal changes in odor identification performance and neuropsychological measures in aging individuals. Neuropsychology. 30(1):87–97. [DOI] [PubMed] [Google Scholar]
- Whitcroft KL, Cuevas M, Haehner A, Hummel T. 2017. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope. 127(2):291–295. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Bennett DA. 2011. Odor identification and mortality in old age. Chem Senses. 36(1):63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Geng Z, Zhou S, Bai T, Wei L, Ji GJ, Zhu W, Yu Y, Tian Y, Wang K. 2019. Brain structural correlates of odor identification in mild cognitive impairment and Alzheimer’s disease revealed by magnetic resonance imaging and a Chinese olfactory identification test. Front Neurosci. 13:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Freimer D, Chen H, Asao K, Rosso A, Rubin S, Tranah G, Cummings S, Simonsick E. 2017. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 88(5):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousem DM, Maldjian JA, Siddiqi F, Hummel T, Alsop DC, Geckle RJ, Bilker WB, Doty RL. 1999. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 818(2):480–487. [DOI] [PubMed] [Google Scholar]
- Yuan T-F, Arias-Carrion O. 2015. Chronic stress impacts on olfactory system. CNS Neurol Disord Drug Targets; 14(4):486–491. Available from: http://www.eurekaselect.com/130811/article. Accessed October 3, 2018. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang X. 2017. Initiation of the age-related decline of odor identification in humans: a meta-analysis. Ageing Res Rev. 40:45–50. [DOI] [PubMed] [Google Scholar]
- Zou L, Yang Z, Wang Y, Lui SSY, Chen A, Cheung EFC, Chan RCK. 2016. What does the nose know? Olfactory function predicts social network size in human. Sci Rep. 6:25026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.