Abstract
Inflammatory cytokines are signaling molecules that regulate numerous physiological processes, from tissue homeostasis to metabolism and food intake. Expression of certain cytokines can be markedly induced in subsets of taste bud cells under acute and chronic inflammation. This may contribute to altered taste perception and preference associated with many diseases. Although the pathways of cytokine induction are well studied in immune cells, they remain poorly characterized in taste cells, in part due to the difficulties of performing biochemical analyses with a limited number of taste cells. The recently developed taste organoid model provides an opportunity to carry out these mechanistic studies in vitro. However, it was unknown whether taste organoids respond to inflammatory stimuli as do in vivo native taste buds. Here we analyze lipopolysaccharide (LPS)-induced expression and secretion of two inflammatory cytokines, tumor necrosis factor (TNF), and interleukin-6 (IL-6). We show that, similarly to native mouse taste epithelia, organoids derived from mouse circumvallate stem cells express several toll-like receptors (TLRs), including TLR4—the primary receptor for LPS. Organoids and native taste epithelia express all five genes in the nuclear factor-κb (Nfkb) family that encode the transcription factor NF-κB, a critical regulator of inflammatory responses. LPS stimulates fast induction of TNF and IL-6 with similar induction kinetics in organoids and native taste epithelia. These results show that taste epithelial cells possess necessary components for inflammatory cytokine induction and secretion and suggest that the organoid model can be a useful tool to dissect the underlying mechanisms.
Keywords: inflammation, IL-6, organoid culture, taste bud, TNF
Introduction
Taste disorders impact negatively on general health and quality of life. A range of diseases and conditions are associated with taste disorders. Upper respiratory viral infections and oral cavity infections are among the most common causes of taste dysfunction (Henkin et al. 1975; Bartoshuk et al. 1987; Goodspeed et al. 1987; Cullen and Leopold 1999; Bromley 2000). AIDS, autoimmune diseases (e.g., Sjögren’s syndrome), cancer, kidney disease, and Parkinson’s disease are known to affect taste function (Gomez et al. 2004; Mansour et al. 2013; Cecchini et al. 2014; Correa et al. 2015; Cohen et al. 2016; Henn et al. 2017; Konstantinova et al. 2017). Inflammation is an underlying condition in many diseases and may play significant roles in taste dysfunction (Rawson and Huang 2009; Feng et al. 2014b).
One of the hallmarks of inflammation is elevated levels of inflammatory cytokines. Triggers of inflammation, such as infection and tissue damage, activate signaling pathways that lead to increased expression, production, and secretion of inflammatory cytokines (Akira et al. 2006; Liu et al. 2017). As signaling molecules, inflammatory cytokines regulate a broad range of biological processes, including tissue homeostasis, metabolic pathways, and food intake. In previous studies, we have found that several inflammation-associated cytokines are preferentially expressed in taste bud cells and affect cell renewal, turnover, and function (Wang et al. 2007; Cohn et al. 2010; Feng et al. 2012, 2014a, 2015; Kim et al. 2012). For example, the inflammatory cytokine tumor necrosis factor (TNF) is predominantly produced by T1R3-positive sweet and umami receptor cells in mice (Feng et al. 2012). In an autoimmune disease model, interferon (IFN)-γ was detected in subsets of phospholipase C-β2 (PLC-β2)-positive and synaptosome-associated protein 25 (SNAP-25)-positive types II and III cells (Kim et al. 2012). The anti-inflammatory cytokine IL-10 is selectively expressed by gustducin-positive bitter receptor cells in mouse circumvallate and foliate taste buds (Feng et al. 2014a). Behavioral tests and gustatory nerve recordings showed that TNF-deficient mice are significantly less sensitive to bitter compounds than wild-type mice (Feng et al. 2015). Kumarhia et al. (2016) found that TNF and IL-1β affect sodium transport in taste bud cells, suggesting that sodium taste function may be regulated by these cytokines (Kumarhia et al. 2016). In a high-fat diet-induced obesity model, the expression of several inflammatory cytokines was increased in taste cells, which correlated with the loss of taste buds (Kaufman et al. 2018). These studies suggest that inflammation-associated cytokines may have various functions in the peripheral taste system. However, the molecular mechanisms of cytokine induction in taste bud cells remain poorly understood, in part due to the difficulties of performing biochemical analyses with a limited number of taste cells.
Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) and its homologs (e.g., Lgr6) mark adult stem cells in multiple tissues. Lgr5 and Lgr6 are also biomarkers for adult taste stem/progenitor cells for both anterior and posterior taste fields (Takeda et al. 2013; Yee et al. 2013; Ren et al. 2014). Isolated Lgr5 or Lgr6-positive stem cells from lingual taste fields can generate continuously expanding 3D structures (i.e., organoids) in vitro (Ren et al. 2014, 2017). Importantly, cells in taste organoids can differentiate and express taste bud cell markers, such as gustducin, carbonic anhydrase 4 (CA4), taste receptor type 1 member 3 (T1R3), nucleoside triphosphate diphosphohydrolase-2, or cytokeratin-8 (Ren et al. 2014, 2017; Aihara et al. 2015). The taste organoid system provides an opportunity to carry out an array of mechanistic studies in vitro. However, in terms of inflammatory responses, it was unknown whether taste organoids respond to inflammatory stimuli as do in vivo native taste buds.
In this study, we compare the induction and secretion of two inflammatory cytokines, TNF and IL-6, in mouse taste tissues versus organoids derived from mouse circumvallate stem cells. We use the LPS-induced inflammation model in which the signaling pathways for cytokines induction are well characterized in a variety of immune cells but are scarcely studied in taste bud cells. Our results show that LPS rapidly induces the expression and secretion of TNF and IL-6 in taste epithelia as well as cultured taste organoids. The induction kinetics of TNF and IL-6 in taste organoids is similar to that in native taste epithelia but is different from that in Raw264.7 macrophage/monocyte cells. Our results indicate that taste buds may utilize unique mechanisms for TLR-mediated inflammatory cytokine expression and the taste organoid model may provide a useful tool to investigate these mechanisms.
Materials and methods
Animals
C57BL/6J mice were purchased from the Jackson Laboratory, housed, and bred in a climate-controlled environment at the animal facility of the Monell Chemical Senses Center. Experiments were performed according to protocols approved by the Monell Center Institutional Animal Care and Use Committee and followed the institutional and national guidelines for the care and use of animals.
Reagents
Rabbit polyclonal antibody against PLC-β2 (sc-206) was purchased from Santa Cruz Biotechnology (Feng et al. 2014a). An affinity-purified goat polyclonal antibody against CA4 (AF2414) was purchased from R&D Systems (Chandrashekar et al. 2009). DyLight 649 (or DyLight 488)-conjugated donkey anti-rabbit or anti-goat antibodies were purchased from Jackson ImmunoResearch Laboratories. Collagenase A and dispase II were from Roche Diagnostics. LPS (from E. coli O111:B4) was purchased from Sigma-Aldrich. Enzyme-linked immunosorbent assay (ELISA) kits for TNF and IL-6 were purchased from eBioscience and BioLegend, respectively. Superscript III Reverse Transcriptase Reagent Kit was purchased from Invitrogen. Protector RNase Inhibitor and recombinant RNase-free DNase I were from Roche Diagnostics. Power UP SYBR Green Master Mix or Power SYBR Green Master Mix for real-time PCR was obtained from Thermo Fisher Scientific. Raw264.7 cell line was purchased from American Type Culture Collection.
3D organoid culture
Culturing of taste organoids was conducted as described previously (Ren et al. 2014, 2017). Briefly, single cell suspension was prepared from circumvallate papillae of 2- to 8-month-old C57BL/6J mice following the published procedure (Ren et al. 2014, 2017). For each batch of taste organoids, five mice were used to generate organoids. Cells were cultured in DMEM/F12 medium (Life Technologies no. 11320033) with 8% Matrigel matrix (Corning) and supplemented with Wnt3a, R-spondin, Noggin, epidermal growth factor, N2, and B27 and maintained in ultra-low attachment tissue culture dishes (Corning). The organoids used for the present study were from the same passage (passage 2).
Isolation of native lingual epithelium
Tongues were immediately excised from euthanized C57BL/6J mice (2- to 8-month-old, both male and female) and placed in calcium-free Tyrode’s solution (in mM: 140 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 10 glucose, 10 sodium pyruvate, and 2 EGTA, pH 7.4). The excised tongues were injected subepithelially with a mixture of dispase II (2 mg/mL) and collagenase A (1mg/mL) in calcium-free Tyrode’s solution and incubated at 37 °C for 20 min. The epithelium was peeled off and washed three times with calcium-containing phosphate-buffered saline (PBS, catalog #14040–117; Invitrogen). The epithelial sections containing circumvallate and foliate taste buds were excised and collected for analysis. Besides, the nontaste epithelium from the region of intermolar eminence was collected as control.
LPS treatments
For in vivo experiments, C57BL/6J mice (2- to 8-month-old, both male and female) were injected with 2.5 mg/kg LPS (i.p.). At 2, 6, 24, and 48 h after LPS injection, mice (three mice per time point) were euthanized and lingual epithelia were isolated as described under the section Isolation of native lingual epithelium and immediately put into the lysis buffer for RNA preparation (see Gene expression analyses by quantitative RT-PCR). A group of control mice were injected with PBS (vehicle) and the epithelial tissues were collected at 2 h after injection.
For in vitro experiments, 1 μg/mL LPS was added directly into the culture medium. Mature taste organoids from early passages that had been cultured for more than 12 days were treated with LPS. Raw264.7 cells were cultured in DMEM with 10% fetal bovine serum (FBS). For gene expression analysis, organoids or Raw264.7 cells were collected at 2, 6, 24, and 48 h after adding LPS. Control samples without LPS treatment were collected at the 48-h time point. RNAs were isolated as described under the section Gene expression analyses by quantitative RT-PCR. For secreted TNF and IL-6 protein analyses, the cell culture medium was collected at 0, 1, 2, 3, 6, 12, 24, and 48 h after adding LPS.
For TNF and IL-6 secretion by native lingual epithelia, tongue epithelium was peeled off as described under the section Isolation of native lingual epithelium. The epithelial sections containing circumvallate and foliate taste buds were cut off and cultured together as the taste epithelium groups, while the nontaste epithelium (from the intermolar eminence section) was cultured as the nontaste epithelium groups. Before LPS treatment, the taste epithelium and nontaste epithelium were cultured in DMEM with 10% FBS for 30 min. After adding LPS (1 μg/mL), the culture medium was collected at 0, 3, 6, 24, and 48 h. A set of control samples without LPS treatment were also collected at 0, 3, 6, 24, and 48 h.
Immunohistochemistry
Taste organoids were fixed in freshly prepared 4% paraformaldehyde in PBS for 2 h at 4 °C. Taste organoids were then blocked in blocking buffer (0.3% TritonX-100 in SuperBlock Blocking Buffer—Thermo Fisher Scientific) at room temperature for 2 h. After that, primary antibodies against PLC-β2 (1:300) and CA4 were incubated with the taste organoids overnight at 4 °C. Secondary antibodies, Dylight-488-conjugated donkey anti-rabbit and Dylight-649-conjugated donkey anti-goat secondary antibodies, were then incubated with the taste organoids in dark for 1 h at room temperature. Fluorescent images were acquired using Leica Sp2 confocal microscope.
Immunohistochemistry with mouse taste tissue sections was performed as described previously (Feng et al. 2014a). Briefly, mouse tongues were fixed in 4% paraformaldehyde/PBS for 1 h on ice and cryoprotected in 20% sucrose/PBS at 4 °C overnight. Circumvallate papillae were sliced parallel to the tongue surface into 10-µm-thick sections using a cryostat (Thermo Scientific Microm). Immunostaining using antibodies to PLC-β2 and CA4 was performed as described in the above paragraph.
Gene expression analyses by quantitative RT-PCR (qRT-PCR)
Total RNA was prepared from mouse tongue taste epithelia, taste organoids, and Raw264.7 cells using Absolutely RNA Microprep Kit (Agilent Technologies) with DNase I (RNase-free) treatment. cDNA was synthesized using Superscript III reverse transcriptase following the manufacturer’s recommended protocol. Real-time PCR with gene-specific primers was set up using Power SYBR Green Master Mix (Thermo Fisher Scientific) and run on StepOnePlus Real-Time PCR System equipment (Applied Biosystems/Life Technologies). The gene-specific primers used for the experiment are listed in Supplementary Table S1. Relative quantification of gene expression was performed based on the 2−ΔΔCt method (Livak and Schmittgen 2001; Wang et al. 2007). β-Actin was used as the endogenous control gene for these analyses.
Measurement of secreted TNF and IL-6 by ELISA
TNF and IL-6 levels in the culture medium were measured using commercially available ELISA kits. For TNF, the Mouse TNF alpha ELISA Ready-SET-Go kit (eBioscience) was used. For IL-6, the Mouse IL-6 ELISA MAX Deluxe Set kit (BioLegend) was used. Culture media from LPS-treated or control samples were either diluted or used directly for measurement. All incubation and washing steps were performed according to the manufacturer’s recommended protocols. The optical density values were measured using a FlexStation 3 instrument (Molecular Devices). Cytokine levels were determined by comparison with the standard curves.
Statistical analysis
qRT-PCR data were first analyzed using StepOne software (Applied Biosystems/Life Technologies). Relative quantification results were then inputted into GraphPad Prism (GraphPad Software) and analyzed by Kruskal–Wallis nonparametric tests. Cytokine levels measured by ELISA were first analyzed in Excel (Microsoft). The results were then inputted into GraphPad Prism (GraphPad Software) and analyzed by repeated measures ANOVA followed by Dunnett’s multiple comparisons test.
Results
Taste organoids express several TLRs and all five genes in the Nfκb family
Taste buds regenerate continuously throughout the life span in mammals. Stem cells isolated from taste papillae have the ability to proliferate and form 3D organoid structures in vitro. Subsets of organoid cells can differentiate into taste receptor cells and respond to taste compounds in manners highly similar to native taste bud cells (Ren et al. 2014, 2017).
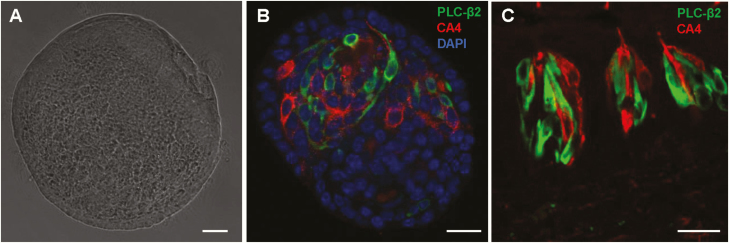
To determine whether the organoid culture system could provide a good model for studying molecular mechanisms of cytokine responses in taste buds, we first confirmed that our circumvallate stem cell-derived organoids contain differentiated taste receptor cells as compared with native taste buds. We performed immunostaining using two well-established taste receptor cell markers, PLC-β2 and CA4, to identify types II and III taste cells, respectively. As shown in Figure 1, subsets of taste organoid cells express either PLC-β2 or CA4, but not both (Figure 1B), as in native taste bud cells (Figure 1C).
Figure 1.
Phospholipase C-β2 (PLC-β2)-positive type II and carbonic anhydrase 4 (CA4)-positive type III taste cells in cultured taste organoids. (A) A bright-field image of an organoid. (B, C) Immunostaining of an organoid (B) and a mouse circumvallate section (C) with antibodies against PLC-β2 (green) and CA4 (red), showing separate populations of types II and III taste cells. Scale bars: 40 µm.
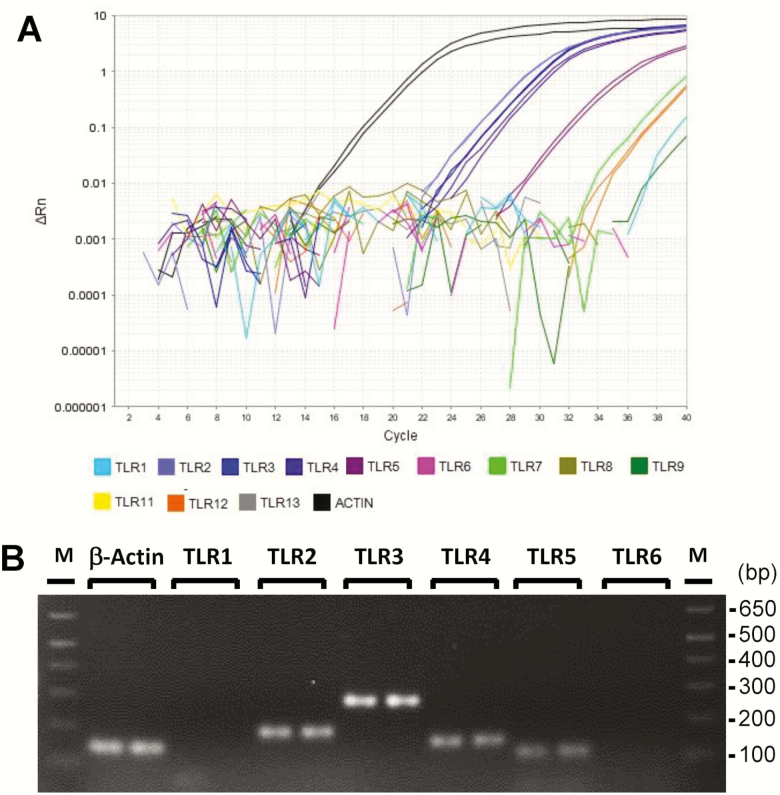
Next, we performed RT-PCR analyses of 12 mouse TLRs. TLRs are pattern recognition receptors that detect pathogen-derived molecules to initiate defense responses to pathogens. Activation of TLR pathways induces expression and secretion of inflammatory cytokines (Janeway and Medzhitov 1999; Akira et al. 2006). Previously, we and others have shown that taste buds express several TLRs (Wang et al. 2009; Camandola and Mattson 2017). RT-PCR analysis showed that taste organoids express at least 6 of the 12 mouse TLRs (Figure 2A). The most prominently expressed TLRs are TLR2, 3, 4, and 5 (Figure 2A,B). These TLRs are involved in the detection of a variety of microbial molecules (Janeway and Medzhitov 1999; Akira et al. 2006). These results suggest that, similar to taste buds, cultured taste organoids express receptors for the recognition of pathogen-derived molecules.
Figure 2.
TLR mRNA expression in organoids derived from mouse circumvallate stem cells. (A) Amplification curves from qRT-PCR of mouse TLRs and β-actin, showing readily detectable expression of several TLRs. (B) Agarose gel image of DNA products from RT-PCR of TLRs 1–6 and β-actin. M: 1 Kb Plus DNA Ladder (Invitrogen). Technical repeats from one of two independent experiments are shown.
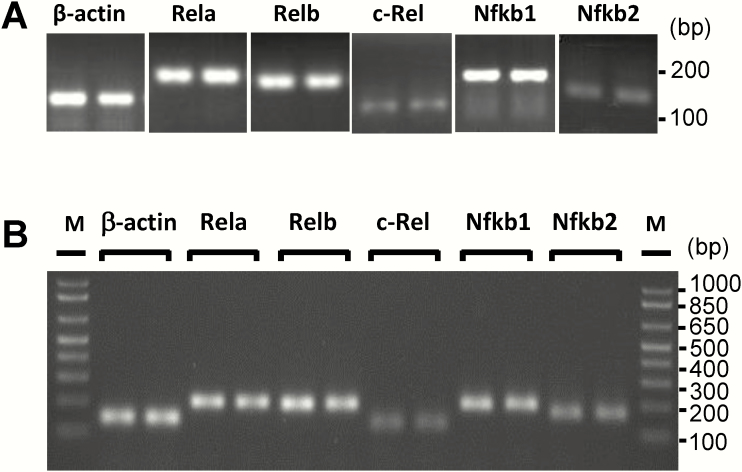
The NF-κB family of transcription factors are major regulators of inflammatory cytokine expression. The five NF-κB family members, RelA, RelB, c-Rel, NF-κB1, and NF-κB2, form various homo- and heterodimers that can activate or suppress the transcription of numerous inflammation-related genes (Hayden and Ghosh 2012). NF-κB proteins are normally inactive and sequestered in the cytoplasm. NF-κB activation occurs via two major pathways, the canonical and the noncanonical pathways (Sun 2017). To investigate whether and which NF-κB family members are expressed in native taste epithelium and cultured taste organoids, we performed qRT-PCR experiments using gene-specific primers (Supplementary Table S1) and confirmed the PCR product length by gel electrophoresis. The expression of all five Nfkb genes was detected in mouse taste epithelium and taste organoids, and Rela, Relb, and Nfkb1 are abundantly expressed (Figure 3).
Figure 3.
Expression of Nfκb genes in taste epithelia and cultured organoids derived from mouse circumvallate stem cells. DNA products from RT-PCR of five Nfκb genes and β-actin. Technical repeats from one of two independent experiments are shown. (A) RT-PCR analysis of Nfκb mRNA expression in lingual epithelia containing circumvallate and foliate taste buds. (B) RT-PCR analysis of Nfκb mRNA expression in organoids derived from mouse circumvallate stem cells. M: 1 Kb Plus DNA Ladder (Invitrogen).
LPS-induced gene expression and secretion of TNF in native taste epithelia, taste organoids, and Raw264.7 cells
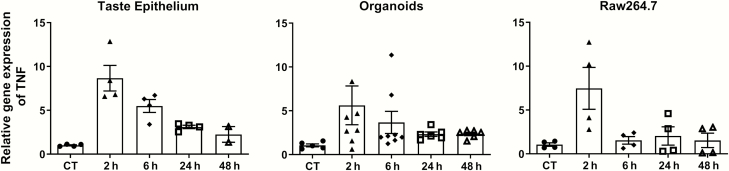
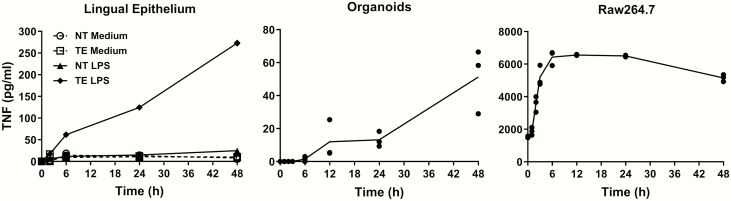
To investigate inflammatory cytokine responses, we used the bacterial endotoxin LPS to induce inflammation. LPS is recognized by TLR4 and activates NF-κB transcription factors to induce the expression of many cytokines. As shown in Figure 4, the levels of TNF mRNA were rapidly induced by LPS in the mouse taste epithelia (P = 0.004, Kruskal–Wallis test), cultured taste organoids (P = 0.026, Kruskal–Wallis test), and Raw264.7 cells (P = 0.14, Kruskal–Wallis test). In all three samples, the levels of TNF mRNA peaked at 2 h and downregulated at 6, 24, and 48 h. One interesting observation is the relatively slow decrease in TNF levels in taste epithelia and organoids as compared with that in Raw264.7 cells. At 6 h after LPS stimulation, the levels of TNF mRNA in taste epithelia and organoids persisted higher than in the controls, whereas TNF levels fell to the control levels in Raw264.7 cells.
Figure 4.
LPS-induced TNF mRNA expression. qRT-PCR analysis of TNF mRNA levels in mouse circumvallate and foliate epithelium (left), cultured taste organoids (middle), and cultured Raw264.7 cells (right) at various time points after LPS treatments (2.5 mg/kg i.p. for mice and 1 µg/mL in culture medium for organoids and Raw264.7 cells). For LPS treatment in vivo (left), control (CT) mice were injected with PBS (vehicle) and taste epithelia were collected 2 h after PBS injection. Control samples (CT, without LPS treatment) for organoids and Raw264.7 cells (middle and right) were cultured under the same condition as the LPS treatment groups and were collected at the last time point (48 h). Relative quantification was performed using β-actin as the endogenous control. TNF mRNA levels in control samples were set to 1. Data are mean ± SEM. N = 2–8 per time point.
Figure 5 shows the secretion of TNF proteins by lingual epithelia, organoids, and Raw264.7 cells after LPS stimulation. Without LPS stimulation, TNF was barely detectable in the culture medium containing isolated taste or nontaste lingual epithelia for up to 48 h (Figure 5; left, P = 0.21 for taste epithelia; P = 0.07 for nontaste epithelia, repeated measures ANOVA). LPS induced significant TNF secretion by taste epithelia (up to 272.9 pg/mL, P = 0.0013, repeated measures ANOVA), whereas nontaste lingual epithelia only produced a small amount of TNF (the highest level was 24.8 pg/mL at 48 h after LPS, P = 0.06, repeated measures ANOVA). These results are consistent with our previous observations (Feng et al. 2012). Organoids also secreted TNF after LPS stimulation (up to 50.4 pg/mL, Figure 5, middle, P = 0.0026, repeated measures ANOVA). Raw264.7 cells secreted a large amount of TNF after LPS induction (up to 6556 pg/mL, Figure 5, right, P = 0.0074, repeated measures ANOVA). The levels of TNF in the culture medium of Raw264.7 cells peaked at 12 h and then slowly declined, whereas TNF levels in the culture medium of taste epithelia and organoids were the highest at the 48-h time point. These results suggest that the mechanisms of TNF induction, secretion, or degradation in taste tissues are different from those in macrophages and that taste organoids showed similar kinetics of TNF expression and secretion compared with the native taste epithelia.
Figure 5.
LPS-induced TNF protein secretion. Levels of secreted TNF proteins in the culture medium were measured by ELISA. Isolated lingual epithelia in culture medium (left), cultured taste organoids (middle), and cultured Raw264.7 cells were treated with LPS (1 µg/mL in culture medium) for indicated time periods. NT, nontaste lingual epithelia. TE, taste epithelia containing circumvallate and foliate taste buds. For the lingual epithelium experiment (left), samples of lingual epithelia without LPS treatment (NT Medium and TE Medium) were also included for comparison. N = 2–3 per time point.
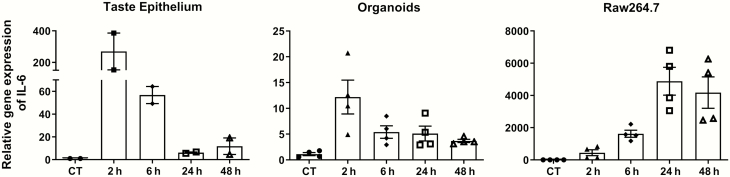
LPS-induced gene expression and secretion of IL-6 in native taste epithelia, taste organoids, and Raw264.7 cells
IL-6 is an important regulator of food intake and metabolism (Flint et al. 2016). Previously we showed that LPS can strongly induce the expression of IL-6 in taste buds (Cohn et al. 2010). Here, we measured IL-6 expression at several time points after LPS stimulation in taste epithelia, organoids, and Raw264.7 cells. Figure 6 shows that LPS induced the expression of IL-6 mRNA in all three types of samples (P = 0.0095, 0.01, and 0.0015 for taste epithelia, organoids, and Raw264.7 cells, respectively, Kruskal–Wallis test). LPS injection in mice rapidly induces the expression of IL-6 in taste epithelia, and the levels of IL-6 mRNA peaked at 2 h after LPS injection and declined thereafter (Figure 6, left panel). Similarly, in taste organoids, the levels of IL-6 mRNA peaked at 2 h after LPS stimulation and declined thereafter (Figure 6, middle panel). In contrast, the levels of IL-6 mRNA in Raw264.7 cells peaked at 24 h and then declined (Figure 6, right panel). The difference in IL-6 induction between taste epithelial cells and Raw264.7 macrophages suggests that the underlying mechanisms of IL-6 induction are different between these two very different cell types.
Figure 6.
LPS-induced IL-6 mRNA expression. qRT-PCR analysis of IL-6 mRNA levels in mouse circumvallate and foliate epithelium (left), cultured taste organoids (middle), and cultured Raw264.7 cells (right) at various time points after LPS treatments (2.5 mg/kg i.p. for mice and 1 µg/mL in culture medium for organoids and Raw264.7 cells). For LPS treatment in vivo (left), control (CT) mice were injected with PBS (vehicle) and taste epithelia were collected 2 h after PBS injection. Control samples (CT, without LPS treatment) for organoids and Raw264.7 cells (middle and right) were cultured under the same condition as the LPS treatment groups and were collected at the last time point (48 h). Relative quantification was performed using β-actin as the endogenous control. IL-6 mRNA levels in control samples were set to 1. Data are mean ± SEM. N = 2–4 per time point.
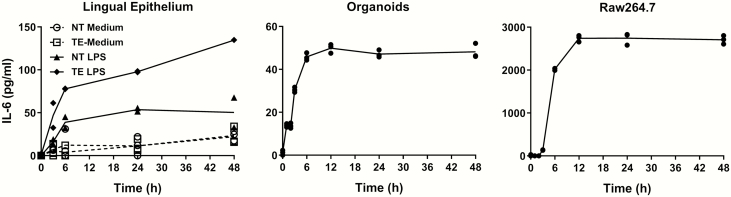
Taste epithelia and organoids also possess the ability to secrete IL-6 after LPS stimulation (Figure 7). Isolated taste epithelia secreted a significant amount of IL-6 after LPS induction (up to 134.5 pg/mL, P = 0.0002, ANOVA). Nontaste lingual epithelia produced much less IL-6 (up to 53.53 pg/mL, P = 0.024, ANOVA). Minimal amounts of IL-6 were detectable in culture medium without LPS induction (up to 22.1 pg/mL for taste epithelia and 23.5 pg/mL for nontaste lingual epithelia, P = 0.09 and 0.32 for taste epithelia and nontaste epithelia, respectively, ANOVA). Cultured organoids secreted up to 49.9 pg/mL of IL-6 after LPS stimulation (P < 0.0001, ANOVA). In line with the rapid induction of IL-6 mRNA in taste epithelia and organoids, IL-6 protein levels in their culture medium quickly increased at 3 and 6 h after adding LPS (Figure 7, left and middle panels). IL-6 levels in the culture medium did not significantly decrease up to 48 h after adding LPS. In Raw264.7 cells (Figure 7, right panel), IL-6 protein levels in culture medium followed a slower kinetics than those in taste epithelia or organoids but were induced to very high levels (up to 2743 pg/mL, P = 0.0001, ANOVA). These results again suggest that the mechanism of IL-6 induction in taste epithelial cells is different from that in immune cells.
Figure 7.
LPS-induced IL-6 protein secretion. Levels of secreted IL-6 proteins in the culture medium were measured by ELISA. Isolated lingual epithelia in culture medium (left), cultured taste organoids (middle), and cultured Raw264.7 cells were treated with LPS (1 µg/mL in culture medium) for indicated time periods. NT, nontaste lingual epithelia. TE, taste epithelia containing circumvallate and foliate taste buds. For the lingual epithelium experiment (left), samples of lingual epithelia without LPS treatment (NT Medium and TE Medium) were also included for comparison. N = 2–3 per time point.
Discussion
In this study, we compared TNF and IL-6 expression and secretion induced by bacterial endotoxin LPS in native taste epithelia, taste organoids, and Raw264.7 cells. Our results show that taste epithelial cells, as well as cultured taste organoids, express several pattern recognition receptors and all five members of the Nfkb gene family (Figures 2 and 3). Taste epithelial cells and organoids can quickly respond to LPS stimulation and increase the level of TNF and IL-6 expression and secretion (Figures 4–7). Unexpectedly, we found that the induction kinetics of TNF and IL-6 in the taste epithelia and organoids differs from that in Raw264.7 macrophages. Our results suggest that some aspects of cytokine induction pathways or feedback mechanisms are different in taste cells compared with those in macrophages. Further investigation of these underlying pathways may lead to a better understanding of the roles of inflammatory cytokines in taste disorders associated with inflammatory diseases.
Taste organoid culture is a recently established model for investigating taste stem cell function, taste cell differentiation, and renewal. Transcriptome analysis, immunostaining, and calcium imaging experiments have shown that taste organoids conform to a similar differentiation program as native taste buds (Ren et al. 2014, 2017; Aihara et al. 2015). The pathways for a number of well-characterized taste morphogenic factors, such as Wnt, bone morphogenetic proteins, Notch, and Hedgehog, are expressed in taste organoids. Stem cells in organoid cultures proliferate continuously, similar to endogenous taste stem cells. All three types of mature taste bud cells (i.e., types I–III) are found in taste organoids (Ren et al. 2014). In this study, we confirmed that subsets of organoid cells express PLC-β2 and CA4, markers for types II and III taste receptor cells. Further, PLC-β2+ and CA4+ organoid cells are nonoverlapping and are morphologically similar to PLC-β2+ and CA4+ taste bud cells (Figure 1B,C). These results support that the cellular properties of taste organoids are similar to native taste buds. Nonetheless, taste organoids lack neuronal connections, whereas native taste buds are innervated by gustatory nerves. In adult rodents, innervation is required for taste bud maintenance, and denervation leads to rapid loss of taste bud cells (Takeda et al. 1996; St John et al. 2003; He et al. 2012). Considering the fact that organoids, without innervation, continue to grow and accumulate differentiated taste-marker-positive cells, the requirement of innervation for cell survival is at least partially evaded in organoid cultures. It is possible that the factors present in the culture medium (e.g., various added growth factors) play similar roles as of innervation to support taste cell survival and/or differentiation. However, the exact mechanism remains unknown. It is also unclear whether these added factors affect organoid responses to inflammatory stimuli.
One of the objectives of this study is to evaluate whether taste organoids respond to inflammatory stimuli in similar ways as do in vivo native taste buds. Our results show that LPS stimulates fast induction of TNF and IL-6 in both organoids and native taste epithelia. The induction kinetics of TNF and IL-6 in organoids is similar to that in mouse taste epithelia. These results indicate that the taste organoid model can be a useful tool to dissect mechanisms of inflammatory responses in taste cells.
The molecular mechanisms of LPS induction of cytokine release are well studied in immune cells. TLR4 on the immune cell membrane recognizes LPS, hence leading to the activation of NF-κB that regulates expression of proinflammatory cytokines, such as TNF, IL-6, and IL-1β (Akira et al. 2006; Liu et al. 2017). In contrast, the signaling pathways of TLR-induced cytokine expression in taste buds have not been characterized. The present study found that the taste organoids and native taste epithelia express several TLRs and all five genes in the Nfκb family that encode the transcription factor NF-κB (Figures 2 and 3). Interestingly, the induction kinetics of TNF and IL-6 in organoids and native taste epithelia are different from that in Raw264.7 cells (Figures 4–7). For example, the levels of TNF mRNA in Raw264.7 cells were sharply reduced at 6 h after LPS treatment, while the levels of TNF mRNA in taste epithelia and taste organoid cultures remained elevated even 48 h after adding LPS (Figures 4 and 5). These results indicate that taste buds may utilize unique induction or feedback mechanisms for TLR-mediated inflammatory cytokine expression.
We previously reported that taste bud cells express the two known TNF receptors TNFR1 and TNFR2 (Feng et al. 2015). Further tests suggest that TNF signaling preferentially modulates bitter taste responses (Feng et al. 2015). The slow-off rate of TNF induction in taste buds indicates that the effects of TNF on taste may last much longer than the acute TNF response in the blood circulation, where the main source of TNF is from immune cells. On the other hand, IL-6 induction by LPS is much faster in taste epithelia and organoids than in Raw264.7 cells (Figures 6 and 7), again suggesting differences in the underlying mechanisms. IL-6 plays important role in regulating food intake and metabolism (Sakic et al. 2001; Flint et al. 2016). However, its roles in taste regulation remain unclear. Besides LPS, many other inflammatory triggers can induce TNF and IL-6 through the NF-κB pathways (Liu et al. 2017). Cancer patients often have elevated levels of TNF and IL-6 and often develop taste problems (Sherry 2002; Schalk et al. 2018). It is possible that inflammatory cytokine production in taste buds contributes to taste abnormalities associated with various diseases.
We have shown previously that LPS administration transiently inhibits taste progenitor/stem cell proliferation and shortens the life span of taste bud cells (Cohn et al. 2010). Both TNF and IL-6 can strongly affect cell proliferation and survival (Gaur and Aggarwal 2003; Monje et al. 2003; Iosif et al. 2006) and could potentially play parts in the LPS effects on taste buds. In addition, Zhu et al. (2014) have shown that LPS ingestion decreases taste sensitivity to sucrose and lowers the lingual expression of Tas1r2 and Tas1r3 (Zhu et al. 2014). A recent study also reports that TLR4 knockout mice show a reduced preference for lipids, sugars, and umami compounds in two-bottle preference tests compared with wild-type mice (Camandola and Mattson 2017). These studies revealed the complex interactions between the pathways that detect microbial molecules and the sense of taste, although the underlying mechanisms remain to be further elucidated.
Conclusion
The present study used a taste organoid model to evaluate whether taste organoids respond to inflammatory stimuli as do in vivo native taste buds. The results show that, similar to taste epithelia, organoids cultured from circumvallate stem cells express several TLRs and all five genes in the Nfκb family. The induction kinetics of TNF and IL-6 in organoids is similar to that in mouse taste epithelia but different from that in Raw264.7 monocyte/macrophage cells. These results indicate that taste buds may utilize unique mechanisms for TLR-mediated inflammatory cytokine expression. The taste organoid model can be a useful tool to dissect these mechanisms.
Funding
This work was supported in part by National Institutes of Health (NIH)/National Institute on Deafness and Other Communication Disorders (NIDCD) [R56DC015819 and DC016921 to H.W., R01DC013807 to P.J., R01 DC014105 to R.F.M., and P30 DC011735] and a National Institute of Health (NIH) grant G20 OD020296. S.F. was supported by grants from National Natural Science Foundation of China (NSFC) [NSFC-81903817 to S.F.], Fundamental Research Funds for the Central Universities [XDJK2019C113 to S.F.], China Postdoctoral Science Foundation [2016M602641to S.F.), and Postdoctoral Foundation of Southwest University [102060-20750902 to S.F.].
Supplementary Material
Acknowledgment
We thank Drs. Hans Clevers and Jeffery Whitsett for providing Wnt3a- and R-spondin-producing cell lines.
Conflict of interest
The authors declare no conflicts of interest.
References
- Aihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, Noah TK, Matsu-ura T, Moore SR, Hong CI, et al. 2015. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci Rep. 5:17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L, Desnoyers S, Hudson C, Marks L, O’Brien M. 1987. Tasting on localized areas. Ann NY Acad Sci. 510: 166–168. [Google Scholar]
- Bromley SM. 2000. Smell and taste disorders: a primary care approach. Am Fam Physician. 61:427–36, 438. [PubMed] [Google Scholar]
- Camandola S, Mattson MP. 2017. Toll-like receptor 4 mediates fat, sugar, and umami taste preference and food intake and body weight regulation. Obesity (Silver Spring). 25:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini MP, Osculati F, Ottaviani S, Boschi F, Fasano A, Tinazzi M. 2014. Taste performance in Parkinson’s disease. J Neural Transm (Vienna). 121:119–122. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. 2009. The taste of carbonation. Science. 326:443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Wakefield CE, Laing DG. 2016. Smell and taste disorders resulting from cancer and chemotherapy. Curr Pharm Des. 22:2253–2263. [DOI] [PubMed] [Google Scholar]
- Cohn ZJ, Kim A, Huang L, Brand J, Wang H. 2010. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Laing DG, Hutchinson I, Jinks AL, Armstrong JE, Kainer G. 2015. Reduced taste function and taste papillae density in children with chronic kidney disease. Pediatr Nephrol. 30:2003–2010. [DOI] [PubMed] [Google Scholar]
- Cullen MM, Leopold DA. 1999. Disorders of smell and taste. Med Clin North Am. 83:57–74. [DOI] [PubMed] [Google Scholar]
- Feng P, Chai J, Zhou M, Simon N, Huang L, Wang H. 2014a. Interleukin-10 is produced by a specific subset of taste receptor cells and critical for maintaining structural integrity of mouse taste buds. J Neurosci. 34:2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Huang L, Wang H. 2014b. Taste bud homeostasis in health, disease, and aging. Chem Senses. 39:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, Bachmanov AA, Huang L, Wang H. 2015. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun. 49:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Zhao H, Chai J, Huang L, Wang H. 2012. Expression and secretion of TNF-α in mouse taste buds: a novel function of a specific subset of type II taste cells. PLoS One. 7:e43140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. 2016. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur U, Aggarwal BB. 2003. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 66:1403–1408. [DOI] [PubMed] [Google Scholar]
- Gomez FE, Cassís-Nosthas L, Morales-de-León JC, Bourges H. 2004. Detection and recognition thresholds to the 4 basic tastes in Mexican patients with primary Sjögren’s syndrome. Eur J Clin Nutr. 58:629–636. [DOI] [PubMed] [Google Scholar]
- Goodspeed RB, Gent JF, Catalanotto FA. 1987. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad Med. 81:251–7, 260. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. 2012. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26:203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Yadgarov A, Sharif S, McCluskey LP. 2012. Aging profoundly delays functional recovery from gustatory nerve injury. Neuroscience. 209:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin RI, Larson AL, Powell RD. 1975. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Otol Rhinol Laryngol. 84:672–682. [DOI] [PubMed] [Google Scholar]
- Henn IW, da Silva RO, Chaiben CL, Fernandes Â, Naval Machado MÂ, de Lima AA. 2017. Perception of taste in HIV-positive individuals in treatment antiretroviral: results of a case-control study. Spec Care Dentist. 37:3–9. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. 2006. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 26:9703–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R. 1999. Lipoproteins take their toll on the host. Curr Biol. 9:R879–R882. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Choo E, Koh A, Dando R. 2018. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 16:e2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Feng P, Ohkuri T, Sauers D, Cohn ZJ, Chai J, Nelson T, Bachmanov AA, Huang L, Wang H. 2012. Defects in the peripheral taste structure and function in the MRL/lpr mouse model of autoimmune disease. PLoS One. 7:e35588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova D, Nenova-Nogalcheva A, Pancheva R, Alexandrova Y, Pechalova P. 2017. Taste disorders in patients with end-stage chronic kidney disease. G Ital Nefrol. 34:54–60. [PubMed] [Google Scholar]
- Kumarhia D, He L, McCluskey LP. 2016. Inflammatory stimuli acutely modulate peripheral taste function. J Neurophysiol. 115:2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC. 2017. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2:e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- Mansour MJ, He C, Al-Farra ST, Khuder SA, Wright JM, Kessler HP, Hinton RJ, Al-Hashimi I. 2013. Sarcoidosis and Sjögren’s syndrome: clinical and salivary evaluation. J Oral Pathol Med. 42:594–599. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. 2003. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 302:1760–1765. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Huang L. 2009. Symposium overview: impact of oronasal inflammation on taste and smell. Ann N Y Acad Sci. 1170:581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Aihara E, Lei W, Gheewala N, Uchiyama H, Margolskee RF, Iwatsuki K, Jiang P. 2017. Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci Rep. 7:4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. 2014. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci U S A. 111:16401–16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakić B, Gauldie J, Denburg JA, Szechtman H. 2001. Behavioral effects of infection with IL-6 adenovector. Brain Behav Immun. 15:25–42. [DOI] [PubMed] [Google Scholar]
- Schalk P, Kohl M, Herrmann HJ, Schwappacher R, Rimmele ME, Buettner A, Siebler J, Neurath MF, Zopf Y. 2018. Influence of cancer and acute inflammatory disease on taste perception: a clinical pilot study. Support Care Cancer. 26:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry VW. 2002. Taste alterations among patients with cancer. Clin J Oncol Nurs. 6:73–77. [DOI] [PubMed] [Google Scholar]
- St John SJ, Garcea M, Spector AC. 2003. The time course of taste bud regeneration after glossopharyngeal or greater superficial petrosal nerve transection in rats. Chem Senses. 28:33–43. [DOI] [PubMed] [Google Scholar]
- Sun SC. 2017. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 17:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, Li D, Li L, Lu MM, Epstein JA. 2013. Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One. 8:e66314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Suzuki Y, Obara N, Nagai Y. 1996. Apoptosis in mouse taste buds after denervation. Cell Tissue Res. 286:55–62. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. 2007. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 27:10703–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. 2009. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 1170:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. 2013. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 31:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, He L, McCluskey LP. 2014. Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience. 258:47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.