Highlights
-
•
Radiation dose escalation with intraoperative electron beam is feasible in the pelvic region.
-
•
IORT is an option to minimize radiation toxicity to ureters, bladder, prostate, vagina, uterus, small bowel.
-
•
Preoperative therapy, including a re-irradiation component, allows selection of patient candidates for local treatment intensification.
-
•
R0 status IOERT results supports individualized recommendation in expert clinical practice.
-
•
Survival contribution is described in oligo-recurrent patients amenable for radical rescue surgery without previous pelvic radiotherapy.
Keywords: Rectal cancer, Recurrent disease, Oligo-recurrence, Intraoperative radiotherapy, Rescue surgery, Electron beam
Abstract
Multimodal strategies have been implemented for locally recurrent rectal cancer scheduled for complete surgical resection. Irradiation and systemic therapy have been added to improve the oncological outcome, as surgery alone was associated with a poor prognosis. Intraoperative irradiation (IORT) is a component of irradiation intensification. Long-term cancer control and a higher survival rate were consistently reported in patients who had IORT as a component of their multidisciplinary treatment. The experience reported by expert IORT groups is reviewed and recommendations to guide clinical practice are explained in detail.
1. Introduction
The topography of loco-regional recurrences of rectal cancer is heterogeneous after surgical resection (with or without radiotherapy), with a dominant pattern of relapse within the postero-lateral hemipelvis [1], [2]. After TME surgery alone, subsites of relapse in the pelvic area are presacral (32%), lateral (18%), anterior (18%), anastomosis (24%) and perineum (5%) [3]. After adjuvant or neoadjuvant chemoradiation, local recurrences can be classified as 65% in-field, 16% marginal and 19% out-field radiation therapy recurrences. The total rate of presacral in-field recurrences reported is 41%, and the low pelvic region was dominant for both preoperative (60%) and postoperative (43%) irradiation [4], [5].
Haddock has recently reviewed and summarized the results of IOERT investigations for treatment of locally recurrent colorectal cancer in the megavoltage era [6]. Although improvements in surgical technique (total mesorectal excision) and neoadjuvant therapy have significantly reduced the incidence of pelvic recurrence of rectal cancer, management of local recurrence remains problematic. Failure to control pelvic recurrence leads to pain, bleeding, and urinary and rectal obstruction, and can be the cause of death even in the absence of distant metastatic disease. In general, control of locally recurrent cancer requires multimodality therapy, with the only possible exception of early central anastomotic recurrences, which are limited to the bowel wall and can be cured with resection alone [7].
A particularly challenging group of patients with locally recurrent rectal cancer includes those who have received a course of pelvic irradiation for their primary tumour or other pelvic malignancy, such as prostate or cervical cancer [8]. Reirradiation is possible with some limitations regarding dose and volume. In general, reirradiation targets are limited to the gross tumour volume with exclusion of the entire small bowel. Previously irradiated patients are at a higher risk of local relapse (37% vs 22% at 3 years) due to proven biological adversity (radioresistance) and surgical limitations after previous resection [9]. Nevertheless, studies have shown an overall improved oncological outcome in reirradiated patients [10], [11]. These results indicate the feasibility of reirradiation containing an IORT component with electron beam energy technology (IOERT).
2. Evidence review and update
A retrospective review of the literature from 1991 to 2017 [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42] has identified 29 publications (27 journal articles and 2 book chapters) including a total of 2,358 patients with recurrent colorectal cancer treated with intraoperative radiation therapy. The reporting institutions are considered expert centres with regard to multimodality treatment for locally recurrent rectal cancer. Table 1 [9], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24] contains data from selected publications of studies that included more than 30 patients and provided long-term follow-up information. The final number of analysed patients treated between 1978 and 2010 was 1,947. The IORT techniques used were electron beam intraoperative irradiation (1,700, 87%), single-dose high-dose rate brachytherapy (129, 7%), and orthovoltage 250 kV (41, 2%) or 50 kV (32, 2%). A selective analysis focused on the relevance of post-resection disease status at the time of IORT and its impact on cancer outcome is shown in Table 2 [9], [11], [14], [15], [16], [17], [18], [21], [22], [24], which includes a selection of publications containing specific data regarding R0, R1 and R2 status (data modified from reference 6).
Table 1.
Clinical results in locally recurrent rectal cancer: 3 decades review.
| AUTHOR YEAR (ref.) | # PATIENTS | PERIOD | RADIOTHERAPY |
R1/2 | IORT type | LC | OS | |
|---|---|---|---|---|---|---|---|---|
| IORT Gy | EBRT Gy(delivered) | |||||||
| Suzuki et al. [12] 1995 | 42 | 1981–1988 | 10–30 | 45 (94%) | 80% | electrons | 62% | 40% |
| Bussieres et al. [13] 1996 | 73 | less than 1995 | 10–25 | 39 (49%) | 57% | electrons | 31% | 20% |
| Eble et al. [14] 1998 | 31 | 1991–1995 | 12–20 | 41.4 (45%) | 58% | electrons | 71% | 58% |
| Aletkiar et al. [15] 2000 | 74 | 1992–1998 | 10–18 | 50.4 (39%) | 28% | IOHDRB | 39% | 23% |
| Lyndel et al. [16] 2001 | 49 | 1978–1997 | 15–20 | 19.8–50 (100%) | 31% | electrons | 35% | 27% |
| Wiig et al. [17] 2002 | 59 | 1990–1999 | 15–20 | 40–56 (100%) | 65% | electrons | 55% | 28% |
| Dresen et al. [18] 2008 | 147 | 1994–2006 | 10–17.5 | 30–50.4 (84%) | 43% | electrons | 54% | 31% |
| Haddock et al. [9] 2011 | 607 | 1981–2008 | 7.5–30 | 36–50 (96%) | 63% | electrons | 72% | 30% |
| Daly et al. [19] 2012 | 41 | 1990–2009 | 7.5–20 | 30–54 (52%) | 85% | 250 Kv | 51% | 32% |
| Guo et al. [20] 2012 | 32 | 2000–2009 | 5 (@1cm) | 50.4 (82%) | 45% | Kv | 68% | 20% |
| Roeder et al. [21] 2012 | 97 | 1991–2006 | 10–20 | 41.4 (52%) | 47% | electrons | 54% | 30% |
| Calvo et al. [22] 2013 | 60 | 1995–2011 | 10–20 | 45–50 (47%) | 37% | electrons | 44% | 43% |
| Alberda et al. [23] 2014 | 59 | 1996–2012 | 10 | 27–52 (100%) | 68% | IOHDRB | 37% | 33% |
| Hyngtorm et al. [24] 2014 | 70 | 2001–2010 | 10–15 | 39–50.4 (82%) | 46% | IOHDRB | 56% | 56% |
| Holman et al. [11] 2017 | 565 | 1981–2010 | 10–20 | 36–50 (90%) | 56% | electrons | 55% | 33% |
IORT: IntraOperative Radiation Therapy, EBRT: External Beam Radiation Therapy, Gray: Gy, LC: Local Control, OS: Overall Survival, R1: microscopic positive resection margins, R2: gross residual tumor.
Table 2.
Outcome results by resection status.
| RESECTION STATUS (ref.) | PERIOD | #PTS | EBRT (Gy) | IORT (Gy) | 3-5y LC (mean value) | 3-5y OS (mean value) |
|---|---|---|---|---|---|---|
| R0 [8,11,14–18,21,22] | 1978–2010 | 793 | 41,4 – 50,4 | 10–20 | 43%–82% (72%) | 37%–80% (56%) |
| R1 [8,11,14–18,21,22] | 1981–2010 | 599 | 41,4 – 50,4 | 10–20 | 19%–67% (41%) | (11%–44%) (37%) |
| R2 [8,11,14,16,18,21] | 1978–2010 | 362 | 41,4 – 50,4 | 10–20 | 18%–75% (37%) | 13%–33% (22%) |
#PTS: number of patients, IORT: IntraOperative Radiation Therapy, EBRT: External Beam Radiation Therapy, Gray: Gy, LC: Local Control, OS: Overall Survival, R0: free margins, R1: microscopic positive resection margins, R2: gross residual tumor.
3. Pre-treatment investigations
3.1. Patient selection for IORT
Patients diagnosed with locally recurrent rectal cancer should be discussed and re-evaluated by a specialized tumour board. Before any multimodality treatment decision can be made, a workup regarding imaging should be available.
Table 3 shows patient selection for IORT
Table 3.
Patient selection for IORT: disease, treatment sequence and radiation dose recommendations.
| DISEASE STATUS | |
|---|---|
| Clinical setting | locally recurrent rectal cancer |
| Indications | Potentially Resectable, debulking surgery, oligometastatic |
| Dominant sites of involvement | Postero-lateral pelvic space |
| TREATMENT | |
| Preoperative chemo/RT followed by resection + IOERT boost | |
| RADIOTHERAPY DOSE | |
| IORT boost | 12.5–15 Gy for negative resection margins (R0) 15–20 Gy for microscopic positive resection margins (R1) 15 to 20 Gy for macroscopic or gross residual tumor (R2) |
| External Beam Radiation Therapy (EBRT) full course | 45–50 Gy (in 25–28 fractions) |
| External Beam Radiation Therapy re-irradiation | 25–35 Gy (12–15 fractions) |
Studies required for candidate selection include the following:
-
-
Patient factors:
-
•
Data primary tumour treatment
-
•
Comorbidity/ASA
-
•
Physical exam
-
•
Conventional blood test
-
-
Tumour factors:
-
•
Biopsy if possible
-
•
CEA
-
•
MRI
-
•
(PET)CT abdomen (liver) and thorax
Potential supportive actions to be considered preoperatively include the following:
-
-
Deviating colostomy
-
-
Self-expanding stent (anastomotic relapse)
-
-
Pain management
Additional studies in patients at high risk for synchronic systemic disease or peritoneal carcinomatosis may include the following:
-
-
Diagnostic Laparoscopy
Neoadjuvant strategies will be recommended in a significant number of locally recurrent patients and these strategies will be followed by a waiting period before surgery. Due to this prolonged treatment time, patients should be restaged before the definitive surgical procedure.
3.2. Pre-treatment imaging clinical staging
Clinical assessment of patients is performed by means of abdomino-pelvic computed tomography (CT) and magnetic resonance imaging (MRI), which demonstrate the extension of the recurrent mass and the topographical relationship to critical organs or structures. In addition, PET-CT may help to exclude distant metastatic disease.
3.3. External beam radiation therapy (EBRT)
The use of EBRT combined with IORT as a part of the intensive treatment for locally recurrent rectal cancer has been reported over the past four decades [43]. Fluoropyrimidine -sensitized full-dose preoperative EBRT using multiple-field techniques, including 3D conformal or rotational intensity-modulated irradiation (3D CRT, IMRT, VMAT), have been used based on institutional protocols and available technology. Primary radiation volumes usually include the dorsal pelvis (i.e. at least mesorectum, presacral area, internal iliac lymphnodes, and depending on the location of the tumour recurrence the obturator region and/ or anal region. Reirradiation volumes usualy include the tumour or gross target volume (GTV)) with a margin of 1 cm to the clinical target volume (CTV) and another 1 cm margin to the planning target volume (PTV). The CTV should not be adjusted to anatomical structures since the normal anatomy is distorted by previous surgery and recurrent tumours often grow into the pelvic wall. Pelvic volumes should reach a total cumulative dose of 45 Gy to 50.4–54 Gy [44]. IMRT is useful in decreasing small-bowel volumes when reirradiation strategies are used. The total dose of reirradiation should be individualized after evaluation of possible dose-limiting factors. In most reports, 30 Gy in conventional fractionated schemes was considered acceptable [9], [10], [11]. Image-guided radiotherapy approaches may be used to reduce PTV margins if possible.
4. Surgical procedures
4.1. Surgical factors
After a full course of preoperative chemoradiation or reirradiation, surgical exploration is undertaken following a waiting period of 6–12 weeks, which allows for tumour downsizing. The delay also permits the resolution of treatment-induced acute side effects. Accurate preoperative staging is important because IOERT primarily benefits those patients who can undergo a grossly complete tumour resection. Ideal patients are those with a high Karnofsky score who are willing to undergo major surgery that may include stoma creation and possible pelvic exenteration. Resectable oligometastatic disease is not a contraindication for treatment of local recurrence. Invasion of pelvic nerves or the sciatic notch (i.e. no sciatica or sacral/buttock pain) or invasion of the S1 and S2 bodies or foramina are considered a contraindication for resection by most pelvic surgeons. If a deviating colostomy is necessary, preoperative evaluation by a stoma therapist should be considered.
Surgery usually requires a midline incision, which allows extension as necessary and permits the construction of a single-sided or even double stoma after bladder resection. Adhesiolysis and abdomen evaluation for liver and peritoneal metastases is mandatory. If metastases are found that are not resectable with curative intent (i.e. extended peritoneal carcinomatosis), resection can only be palliative and may need to be reconsidered, and intraoperative irradiation is not performed. If no metastases are evident or are limited and can be resected for cure, the patient undergoes abdominoperineal resection, low anterior resection or pelvic exenteration, depending upon the extent and location of the tumour. En bloc wide resection is the best option: grossly complete resection of the tumour is desirable. Haemostasis after resection is important because of fluid accumulation in the tumour bed, which may decrease the IOERT dose at a certain depth (bolus effect). If anastomosis can be performed, it will be done after the intraoperative radiation dose delivery. In order to minimize the potential for complications, tension-free anastomosis is pivotal and, in most cases, it is preferable to mobilize the entire left colon and use the unirradiated bowel (descending colon) for the proximal end of the anastomosis.
4.2. IORT factors
As well as the pre-treatment evaluation, the final decision to treat with IORT is also based on perioperative findings such as the pathologic margin assessment. It is important that both the radiation oncologist and surgeon make a collaborative judgment in order to define the area at highest risk for subsequent local relapse and to determine the optimal position of the IORT target. Margins of resection are determined by means of frozen-section analysis of the surgical specimen and sometimes the tumour bed.
5. IORT Procedure: Post resection
5.1. IORT: treatment methods and techniques
IORT for locally recurrent rectal cancer has predominantly been delivered with megavoltage electrons produced by a medical linear accelerator. There are not sufficient scientific data to support the use of brachytherapy or orthovoltage delivery systems for IORT and for the remaining of this report the term IOERT will be used referring to megavolt electron-based IORT. The electron beam energy and dose of IORT are determined by the resection status and geometry of the treated target. For most applicators, dose distribution does not require the use of bolus. Accumulation of intra surgical fluid could influence radiation penetration in an unpredictable way and should be avoided. Shielding lead inside the tumour bed radiation target is not recommended due to the dosimetric uncertainties that this involves. The best possible protection is mechanical retraction to displace dose-sensitive structures outside the IORT field. Surgical retractors for the displacement of remaining uninvolved movable structures such as the rectal stump, bladder, prostate, uterus, vagina, small bowel, descending colon and ureters are most helpful for exposing the radiation target properly (presacral area or posterior and lateral pelvic space) and expediting positioning of the IORT applicator. Displacement of normal tissue should be documented with photos, drawings or written reports.
5.2. Radiation target definition
Persisting tumour adherence after preoperative chemoradiotherapy, inadequate radial margins (close <1 mm) and high risk of relapse, including enlarged nodes or adherence to surrounding structures, are considered indications for an IOERT boost. Patients with potentially positive margins are candidates for IOERT. The tumour bed can be marked to facilitate positioning of the IOERT applicator and the IOERT beam collimation. An IOERT applicator is selected according to the location and size of the area to be irradiated. The internal diameters of circular applicators typically range from 4 to 10 cm in diameter. Applicator size is selected to allow full coverage of the residual disease in a high-risk area, which is generally located in the presacral area or pelvic sidewall. Usually, the largest applicator that will fit into the area is preferred. The applicator shape is chosen so that the geometry fits the specific situation. The applicator must abut the site being treated. Most applicators have bevelled ends up to 45°, enabling good position of the applicator to sloping surfaces in the pelvis and maximize dose homogeneity. Sensitive normal tissue should not be included in the beam and fluid buildup in the treatment area should also be avoided. The applicator accurately collimates the electron beam to the target area and also serves to retract sensitive normal tissue, especially small bowel or ureters (Fig. 1). Visceral retraction and packing are also usually necessary. A distal rectal stump, which will be used to create an anastomosis, should be excluded from the IOERT field either by retraction outside the applicator or with the use of lead to shield sensitive normal tissue. During treatment, suction catheters should be positioned to minimize fluid buildup within the applicator. Most IOERT treatments in rectal cancer are applied via a transabdominal approach, as the area of concern is usually the posterior presacrum or posterolateral pelvic sidewall. A perineal port is occasionally used after abdominoperineal resection to treat the coccyx or distal presacrum, distal pelvic sidewall or portions of the prostate, and base of the bladder when exenteration is not performed.
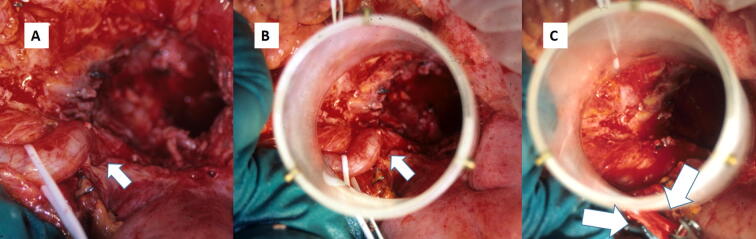
Fig. 1.
IORT electron procedure view of a recurrent rectal cancer with associated uretero-hidronefrosis: A. Pelvic cavity exposed and right ureteral dilatation. B. Simulated IOERT applicator positioning to treat recurrent tumor bed. The involved ureter is included in the treatment volume. C. Post-resection recurrent tumor bed area to be treated by IOERT. The right ureter has been sectioned and mobilized out of the electron beam collimator. After irradiation an uretero-ureteral anastomosis to the left ureter will be performed for urinary tract reconstruction.
The goal of IOERT delivery is to avoid re-recurrences in patients who have close or microscopically positive margins after surgical resections. However, clear margins should always be the primary goal of resection. In these cases, doses in the range of 10 to 12.5 Gy are applied. Unintended macroscopically involved margins may be treated with higher doses: 15.0 Gy or even 20 Gy as a single boost. There is little evidence that IOERT has value in unresected cases and should be performed only in the framework of a trial. A library of predefined isodose-curve distributions for a range of IORT applicator diameter, bevelled-end shapes and electron beam energies should be available for intraoperative consultation. An electron energy should be chosen to adequately encompass the target tissues at risk within the 90% isodose curves. The prescribed dose is specified at the 90% isodose.
The whole process can be summarized as follows:
-
1.
Definition of radiation target volume: tumour bed assessment; surgical margin status (inspection of the surgical field and the posterior aspect of the surgical specimen).
-
2.
Normal uninvolved tissue to be excluded (mobilized out) from the IOERT radiation volume: rectal remnant (if present); uninvolved intrapelvic organs (bladder, prostate, uterus, vagina, ovaries); distal colon; small bowel; ureters.
-
3.
Normal tissue at risk to be included in the radiation target volume: postero-lateral pelvic area; vascular and lymphatic structures (iliac regions).
5.3. Applicator selection
IORT applicator adaptation to the postoperative bed at risk:
-
1.
Size selection (diameter): able to encompass the presacral region and/or lateral pelvic walls at risk (the largest able to fit in the pelvic cavity). The availability of diameters of between 4 and 10 cm is a safe range for applicator sizes.
-
2.
Bevelled end selection: bevelled ends of between 30° and 45° adapted to the anatomical configuration of the presacral region in the pelvic cavity are recommended for small pelvis sites to appropriately encompass the surgical bed with residual disease or at risk of recurrence. The same criteria apply in the case of dominant lateral pelvic wall involvement.
5.4. IOERT irradiation
To guarantee the sterile field throughout the procedure, before irradiation, sterile drapes should cover the surgical bed and the part of the collimator inside and near the surgical bed. An aspiration drain may be useful to prevent fluid accumulation and avoid a bolus effect.
IOERT irradiation consists of:
-
1.
Electron energy selection: 90% isodose should encompass in depth the tissue content involved or at risk with a safety dosimetric margin (0.1–0.5 cm). Fluid stability is key for appropriate energy selection. This depth can be estimated by real-time intraoperative measurements, together with data obtained from the preoperative CT scan. Fluid accumulation in the IORT field should be avoided. When bone tissue is part of the target volume, a higher electron energy could be selected. If the surface dose of the chosen applicator is less than 90%, bolus with an appropriate thickness should be applied. Dose selection (single fraction boost component): doses of 10 to 12.5 Gy are recommended for resection specimens at low risk after a favourable dissection procedure. In specimens with close or suspected/confirmed cancer-involved margins, the recommended dose ranges from 12.5 to 15 Gy. After laborious vascular and/or soft-tissue dissection with suspected residual cancer, a dose of 12.5 to 15 Gy should be considered. A medical physicist should be involved pre- or intra-operatively in the choice of bolus, or corrections for special situations (e.g. residual air gap, additional screening, bone tissue density correction). In vivo dosimetry is strongly recommended as a quality-assurance procedure.
-
2.
In the case of multiple IOERT target volumes, overlapping of the corresponding irradiation volumes should be avoided. Enlarging the irradiated volume by using abutting fields should be avoided, as significant hot & cold spots are likely.
5.5. Treatment delivery
Before dose delivery, the appropriate physical and technical parameters (electron energy, tube size, length and bevel angle, monitor units, bolus choice, use of additional shielding inside the irradiated area) are checked by a medical physicist as well as by the physician (radiation oncologist) in a four-eyes principle. Usually, a medical physicist or a radiation therapist (RTT) aligns the gantry to the applicator for soft-docking system and enters the data in the control console. During irradiation (approximately 1–2 min, depending on dose and dose rate), nobody is allowed to stay in the operation room except the patient for radiation protection purposes. Patients should be carefully monitored by camera during the irradiation process and vital parameters should be monitored and be visible from outside the operating room. In event of an emergency, irradiation should be stopped immediately and nurses, anaesthesiologists and surgeons should be prepared to enter the operating room at any time immediately after cessation of irradiation.
5.6. Applicator removal
After treatment has been completed, special attention should be paid to applicator removal in order to avoid any trauma to surrounding tissues and possible bleeding. In the event of bleeding during the irradiation time, it is advisable to aspirate the blood first in order to clearly visualize the end of the collimator in contact with the patient's tissue and allow for a safe manoeuvre. Removal of the applicator may be performed by the surgeon or by the radiation oncologist.
5.7. Recording and reporting
Clinical and dosimetry forms should be completed with all relevant patient, tumour and treatment parameters. Information should include demographics, performance status, symptoms and serum tests, including CEA, comorbidities and Charlson comorbidity index. Tumour-related data should include imaging studies, biopsy report, clinical and pathological stage, grading and possible biomolecular characteristics. Treatment data should include neoadjuvant treatments (including reirradiation), the surgical report (size, location and frozen sections of margins) and main characteristics of the IORT procedure, such as collimator diameter, bevel angle, bolus, beam energy, dose prescription and duration of the procedure. In vivo dosimetry is strongly recommended as a quality-assurance procedure. Radiation target contents should be described: organs and structures included in the IOERT radiation beam. Radiation protection of normal uninvolved tissue should be described: the manoeuvres for temporary mobilization or intra-field customized protection (in particular rectal stump, small bowel, bladder and ureters) (Fig. 1). Documentation of the radiation target, adjacent - if necessary mobilized - normal tissue and used shielding should ideally include drawings or photos.
No fully reliable treatment planning systems currently exist for intraoperative irradiation; however, the availability of preoperative images may help to identify at-risk anatomical structures involved and to guide the positioning of the collimator. All these imaging data should be included in the patient's final documentation, if available. Photographs of final applicator positioning and surface anatomy in the IOERT target is recommended.
Intraoperative ultrasound may also be helpful in some cases for verifying residual tumour thickness in depth and the location of critical structures such as ureters and major vessels.
The final documentation of the IORT procedure should also include the surgical notes and the anaesthesiology report.
Table 4 shows parameters for the IORT electron beam procedure in locally recurrent rectal cancer.
Table 4.
Reporting parameters for IORT electron beam procedures in recurrent rectal cancer.
| IORT PARAMETERS | |
|---|---|
| Target volumen description | Tumour residue (R0, R1, R2)Normal tissues exposedNormal tissues protected/mobilizedSpecial conditions:Vascular manipulationOthers (extended resections; pelvectomies) |
| IORT factors |
|
| Integrated pre-IORT treatment factors |
|
6. Recommendations on patient care
6.1. Care during the course of IORT
The sterile field should be guaranteed throughout the IORT procedure. Before irradiation, sterile drapes should cover the surgical bed and the part of the collimator inside and near the surgical bed. Patients should be carefully monitored by camera during the irradiation process and vital parameters should be monitored and be visible from outside the operating room. In event of an emergency, irradiation should be stopped immediately and nurses, anaesthesiologists and surgeons should be prepared to enter the operating room at any time immediately after cessation of irradiation.
6.2. Post-treatment patient care and follow-up
Patients treated with IORT for locally recurrent rectal cancer require thorough care. All vital and clinical parameters should be monitored in the days following the procedure, and special attention should be paid to blood tests, including renal and liver functions, bowel movements and onset of new symptoms and signs.
After the IORT procedure combined with surgical resection, the patient may receive further treatment, including postoperative adjuvant systemic treatment. The follow-up schedule starts after treatment has been completed and the patient is discharged from hospital. During imaging studies, special attention should be paid to any tissue potentially included in the IOERT volume, such as the sacrum, rectal remnant and ureters. Systemic treatment has recently been reported as a part of neoadjuvant therapy to further improve oncological outcome [46].
6.3. QA recommendations
Specific IORT QA actions include:
-
-
MDT evaluation and scheduling
-
-
Pre-treatment beam calibration: dose per monitor unit for each radiation energy
-
-
Equipment and applicators performance
-
-
Safety interlocks check-up
-
-
QA report completion, inter-specialty signature and registration
-
-
Clinical report of technical parameters in the electronic chart
6.4. Treatment tolerance and adverse effects
Patients with locally recurrent rectal cancer often experience significant tumour-related and treatment-related toxicity. Most treatment-induced effects are multifactorial and it is often difficult to attribute toxicity to a single modality. In a systematic review of 29 published studies including 3,003 patients with locally advanced primary or recurrent colorectal cancer, IOERT was associated with significant improvement in local control and survival without an increase in total, urologic or anastomotic complications [43]. Increased risk of wound complications following IOERT was also noticed. Wound infections and pelvic abscess are the most commonly reported complications, occurring in 25% or more of IOERT patients in several series [40], [28], [46].
Expert reports have registered a total incidence of severe, life-threatening or fatal deep infection or abscesses in 13% of cases, with 7% potentially attributable to IOERT [21], [45].
With the addition of IOERT to EBRT, the dose-limiting normal tissue is typically peripheral nerve and neuropathy is the most commonly reported toxicity attributed to IOERT in the pelvis. IOERT-related neuropathy most commonly manifests as pain without weakness or sensory loss. When it occurs, the pain is often chronic and may be severe, but is often manageable with gabapentin or pregabalin. Both the incidence and severity of IOERT-related neuropathy appear to be related to the IOERT dose. Even in previously irradiated patients, the incidence of neuropathy is related to the IOERT dose and not to the total cumulative dose including EBRT. In locally recurrent disease, IOERT doses of 12.5 Gy or less were associated with a 5% incidence of grade 2–3 neuropathy compared to 14% for IORT doses of 15 Gy or higher [24].
IOERT dose-sensitive structures in the pelvis anatomy are the peripheral nerves, ureters, bladder, small intestine, rectal stump, ovaries, urethra and vagina. The uterus and prostate are considered relatively resistant to escalated doses, including IOERT boost. Neuropathy is dose-dependent: 3% for 12 Gy boost and 23% for 15 Gy boost. Ureter dysfunction is reported in 56% of ureters included in the IOERT field (any dose) and in less than 15% of ureters excluded [16].
Late toxicities described included small-bowel obstruction in 14% of patients, wound infection/breakdown in 9%, fistula with abscess in 8%, bladder dysfunction in 7%, sexual dysfunction in 6%, enteritis/proctitis in 3%, and abdominopelvic abscess in 3% [21].
7. Conclusions and future directions
Treatment of locally recurrent rectal cancer that includes reirradiation strategies has evolved over the past 30 years. The current international consensus recognizes the value of preoperative strategies with chemoradiation and the potential of IOERT boost in recurrent patients (NCCN guidelines).
IOERT is a feasible, tolerable and efficient radiation-boosting technique that can be explored in tailored treatment for patients with locally recurrent rectal cancer [47]. Recommendations available to guide tailoring IORT in recurrent disease in terms of local tumour control promotion include implementing strategies with short-course preoperative pelvic irradiation, hypofractionated radiation or reirradiation [48], including an IORT component. In-vivo-dosimetry and intra-operative imaging could improve the accuracy, reproducibility and documentation and provide data for evaluation and tailoring of IOERT.
This requires further defining correlations between biological equivalent dose (BED) calculations, topographic patterns of recurrence, and prognostic features for local effects. This information is not available at present. It will open the clinical scope for using single-dose IORT alone, with field-within-a-field dosimetric modulations, and in combination with systemic therapy in the oligometastatic model.
The pioneering work of expert institutions has been validated internationally and with long-term follow-up analysis [6], [11], [49].
Acknowledgments
Acknowledgements
Intraoperative radiotherapy (IORT) is a multidisciplinary oncological activity requiring a demanding the integration of individual technical quality and team work coordination. The authors of this guideline acknowledge the remarkable contribution of all the health professionals involved in the care of patient candidates to IORT procedures.
Authors are grateful to reviewers Robert Krempien, Philipp Scherer, Alexandra Stewart, Dirk Verellen for their useful and constructive comments and to Eralda Azizaj (ESTRO staff member responsible for the work of ACROP) for facilitating the review and journal submission process.
References
- 1.Sole C.V., Calvo F.A., Serrano J. Post-chemoradiation intraoperative electron-beam radiation therapy boost in resected locally advanced rectal cancer: Long term results focused on topographic pattern of locoregional relapse. Radiother Oncol. 2014;112:52–58. doi: 10.1016/j.radonc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Ogura A., Konishi T., Cunningham C., Garcia-Aguilar J., Iversen H., Toda S. Lateral node study consortium. Neoadjuvant (Chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low ct3/4 rectal cancer. J Clin Oncol. 2019;37:33–43. doi: 10.1200/JCO.18.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusters M., Marijnen C.A., Van de Velde C.J. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Kusters M., van de Velde C.J., Beets-Tan R.G. Patterns of local recurrence in rectal cancer: a single center experience. Ann Surg Oncol. 2009;6:289–296. doi: 10.1245/s10434-008-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu T.K., Bhosale P.R., Crane C.H., Yyier R.B. Patterns of locoregional recurrence after surgery and radiotherapy or chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;71:1175–1180. doi: 10.1016/j.ijrobp.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddock M.J. Intraoperative radiation therapy for colon and rectal cancers: a clinical review. Radiat Oncol. 2017;12:11–19. doi: 10.1186/s13014-016-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salo J.C., Paty P.B., Guillem J. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171–177. doi: 10.1007/s10434-999-0171-8. [DOI] [PubMed] [Google Scholar]
- 8.Valentini V., Morganti A.G., Gambacorta M.A. preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Haddock M.G., Miller R.C., Nelson H. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143–150. doi: 10.1016/j.ijrobp.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Bosman S.J., Holman F.A., Nieuwenhuijzen G.A., Martijn H., Creemers G.J., Rutten H.J. Feasibility of reirradiation in the treatment of locally recurrent rectal cancer. Br J Surg. 2014;101:1280–1289. doi: 10.1002/bjs.9569. [DOI] [PubMed] [Google Scholar]
- 11.Holman F.A., Bosman S.J., Haddock M.G., Gunderson L.L., Kusters M., Nieuwenhuijzen G.A. Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: results of 565 patients of two major treatment centres. Eur J Surg Oncol. 2017;43:107–117. doi: 10.1016/j.ejso.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K., Gunderson L.L., Devine R.M. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939–952. doi: 10.1002/1097-0142(19950215)75:4<939::aid-cncr2820750408>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Bussieres E., Gilly F.N., Rouanet P. Recurrences of rectal cancers: results of a multimodal approach with intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1996;34:49–56. doi: 10.1016/0360-3016(95)02048-9. [DOI] [PubMed] [Google Scholar]
- 14.Eble M.J., Lehnert T., Treiber M., Latz D., Herfarth C., Mannenmacher M. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal cancer. Radiother Oncol. 1998;49:169–174. doi: 10.1016/s0167-8140(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 15.Alektiar K.M., Zelefsky M.J., Paty P.B. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2000;48:219–226. doi: 10.1016/s0360-3016(00)00634-9. [DOI] [PubMed] [Google Scholar]
- 16.Lindel K., Willett C.G., Shellito P.C., Ott M.J., Clark J., Grossbard M. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83–87. doi: 10.1016/s0167-8140(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 17.Wiig J.N., Tveit K.M., Poulsen J.P. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? A prospective study. Radiother Oncol. 2002;62:207–213. doi: 10.1016/s0167-8140(01)00486-8. [DOI] [PubMed] [Google Scholar]
- 18.Dresen R.C., Gosens M.J., Martijn H., Nieuwenhuijzen G.A., Creemers G.J., Daniels-Gooszen A.W. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol. 2008;15:1937–1947. doi: 10.1245/s10434-008-9896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly M.E., Kapp D.S., Maxim P.G., Welton M.L., Tran P.T., Koong A.C. Orthovoltage intraoperative radiotherapy for locally advanced and recurrent colorectal cancer. Dis Colon Rectum. 2012;55:695–702. doi: 10.1097/DCR.0b013e31824d464c. [DOI] [PubMed] [Google Scholar]
- 20.Guo S., Reddy C.A., Kolar M., Woody N., Mahadevan A., Deibel F.C. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol. 2012;7:110. doi: 10.1186/1748-717X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeder F., Goetz J.M., Habl G., Bischof M., Krempien R., Buechler M.W. Intraoperative Electron Radiation Therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer. 2012;12:592. doi: 10.1186/1471-2407-12-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo F.A., Sole C.V., Alvarez de Sierra P., Gómez-Espí M., Blanco J., Lozano M.A. Prognostic impact of external beam radiation therapy in patients treated with and without extended surgery and intraoperative electrons for locally recurrent rectal cancer: 16-year experience in a single institution. Int J Radiat Oncol Biol Phys. 2013;86:892–900. doi: 10.1016/j.ijrobp.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Alberda W.J., Verhoef C., Nuyttens J.J., Rothbarth J., van Meerten E., de Wilt J.H. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann Surg Oncol. 2014;21:520–526. doi: 10.1245/s10434-013-3306-x. [DOI] [PubMed] [Google Scholar]
- 24.Hyngstrom J.R., Tzeng C.W., Beddar S., Das P., Krishnan S., Delclos M.E. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: ten-year institutional experience. J Surg Oncol. 2014;109:652–658. doi: 10.1002/jso.23570. [DOI] [PubMed] [Google Scholar]
- 25.Abuchaibe O., Calvo F.A., Azinovic I., Aristu J., Pardo F., Alvarez-Cienfuegos J. Intraoperative radiotherapy in locally advanced recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 1993;26:859–867. doi: 10.1016/0360-3016(93)90502-m. [DOI] [PubMed] [Google Scholar]
- 26.Hashiguchi Y., Sekine T., Kato S. Indicators for surgical resection and intraoperative radiation therapy for pelvic recurrence of colorectal cancer. Dis Colon Rectum. 2003;46:31–39. doi: 10.1007/s10350-004-6493-5. [DOI] [PubMed] [Google Scholar]
- 27.Nuyttens J.J., Kolkman-Deurloo I.K., Vermaas M., Ferenschild F.T., Graveland W.J., De Wilt J.H. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:106–112. doi: 10.1016/s0360-3016(03)01494-9. [DOI] [PubMed] [Google Scholar]
- 28.Guiney M.J., Smith J.G., Worotniuk V., Ngan S., Blakey D. Radiotherapy treatment for isolated loco-regional recurrence of rectosigmoid cancer following definitive surgery: Peter MacCullum Cancer Institute Experience, 1981–1990. Int J Radiat Oncol Biol Phys. 1997;38:1019–1025. doi: 10.1016/s0360-3016(97)00315-5. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Monge R., Nag S., Martin E.W. Three different intraoperative radiation modalities (electron beam, high-dose-rate brachytherapy, and iodine-125 brachytherapy) in the adjuvant treatment of patients with recurrent colorectal adenocarcinoma. Cancer. 1999;86:236–247. doi: 10.1002/(sici)1097-0142(19990715)86:2<236::aid-cncr7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kramer T, Share R, Kiel K, Rosman D. Intraoperative radiation therapy of colorectal cancer. In: Abe M, editors. Intraoperative radiation therapy. New York: Pergamon Press; 308. p. 10–1991.
- 31.Lanciano R, Calkins A, Wolkov H, et al. A phase I, II study of intraoperative radiotherapy in advanced unresectable or recurrent carcinoma of the rectum: a RTOG study. In: Abe M, editors. Intraoperative radiation therapy. New York: Pergamon Press; 311. p. 3–1991. [DOI] [PubMed]
- 32.Lybeert M.L.M., Martijn H., DeNeve W., Crommelin M.A., Ribot J.G. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery: definite but limited influence of relapse free survival and survival. Int J Radiat Oncol Biol Phys. 1992;24:241–246. doi: 10.1016/0360-3016(92)90678-b. [DOI] [PubMed] [Google Scholar]
- 33.Kishan A.U., Voog J.C., Wiseman J., Cook R.R., Ancukiewicz M., Lee P. Standard fractionation external beam radiotherapy with and without intraoperative radiotherapy for locally recurrent rectal cancer: the role of local therapy in patients with a high competing risk of death from distant disease. Br J Radiol. 2017;90(1076):20170134. doi: 10.1259/bjr.20170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Susko M., Lee J., Salama J., Thomas S., Uronis H., Hsu D. The use of re-irradiation in locally recurrent. Non-metastatic rectal cancer. Ann Surg Oncol. 2016;23:3609–3615. doi: 10.1245/s10434-016-5250-z. [DOI] [PubMed] [Google Scholar]
- 35.Brady J.T., Crawshaw B.P., Murrell B., Dosokey E.M., Jabir M.A., Steele S.R. Influence of intraoperative radiation therapy on locally advanced and recurrent colorectal tumors: a 16-year experience. Am J Surg. 2017;213:586–589. doi: 10.1016/j.amjsurg.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Klink C.D., Binnebösel M., Holy R., Neumann U.P., Junge K. Influence of intraoperative radiotherapy (IORT) on perioperative outcome after surgical resection of rectal cancer. World J Surg. 2014;38:992–996. doi: 10.1007/s00268-013-2313-1. [DOI] [PubMed] [Google Scholar]
- 37.Tan J., Heriot A.G., Mackay J., Van Dyk S., Bressel M.A., Fox C.D. Prospective single-arm study of intraoperative radiotherapy for locally advanced or recurrent rectal cancer. J Med Imaging Radiat Oncol. 2013;57:617–625. doi: 10.1111/1754-9485.12059. [DOI] [PubMed] [Google Scholar]
- 38.Klaver Y.L., Lemmens V.E., Nienhuijs S.W., Nieuwenhuijzen G.A., Rutten H.J., de Hingh I.H. Intraoperative radiotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Five consecutive case reports of locally advanced rectal cancer with synchronous peritoneal carcinomatosis. Strahlenther Onkol. 2013;189:256–260. doi: 10.1007/s00066-012-0282-1. [DOI] [PubMed] [Google Scholar]
- 39.Turley R.S., Czito B.G., Haney J.C., Tyler D.S., Mantyh C.R., Migaly J. Intraoperative pelvic brachytherapy for treatment of locally advanced or recurrent colorectal cancer. Tech Coloproctol. 2013;17:95–100. doi: 10.1007/s10151-012-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelfatah E., Page A., Sacks J., Pierorazio P., Bivalacqua T., Efron J. Postoperative complications following intraoperative radiotherapy in abdominopelvic malignancy: a single institution analysis of 113 consecutive patients. J Surg Oncol. 2017;115:883–890. doi: 10.1002/jso.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezner R.D., Chu D.Z., Ellenhorn J.D. Intraoperative radiation therapy for patients with recurrent rectal and sigmoid colon cancer in previously irradiated fields. Radiother Oncol. 2002;64:47–52. doi: 10.1016/s0167-8140(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 42.Kolkman-Deurloo I.K., Nuyttens J.J., Hanssens P.E., Levendag P.C. Intraoperative HDR brachytherapy for rectal cancer using a flexible intraoperative template: standard plans versus individual planning. Radiother Oncol. 2004;70:75–79. doi: 10.1016/j.radonc.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Mirnezami R., Chang G.J., Das P. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol. 2013;22:22–35. doi: 10.1016/j.suronc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minsky B.D., Rödel C.M., Valentini V. Rectal cancer. In: Gunderson L.L., Tepper J.E., editors. Clinical radiation onocology. Elsevier; 2016. pp. 992–1018. [Google Scholar]
- 45.Vermeer T.A., Orsini R.G., Daams F., Nieuwenhuijzen G.A., Rutten H.J. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: incidence, risk factors and treatment. Eur J Surg Oncol. 2014;40:1502–1509. doi: 10.1016/j.ejso.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 46.van Zoggel D.M.G.I., Bosman S.J., Kusters M., Nieuwenhuijzen G.A.P., Cnossen J.S., Creemers G.J. Preliminary results of a cohort study of induction chemotherapy-based treatment for locally recurrent rectal cancer. Br J Surg. 2018;105:447–452. doi: 10.1002/bjs.10694. [DOI] [PubMed] [Google Scholar]
- 47.Wiig J.N., Giercksky K.E., Tveit K.M. Intraoperative radiotherapy for locally advanced or locally recurrent rectal cancer: Does it work at all? Acta Oncol. 2014;53:865–876. doi: 10.3109/0284186X.2014.895037. [DOI] [PubMed] [Google Scholar]
- 48.Mohiuddin M., Marks G.M., Lingareddy V., Marks J. Curative surgical resection following re-irradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;39:643–649. doi: 10.1016/s0360-3016(97)00340-4. [DOI] [PubMed] [Google Scholar]
- 49.Rominger C.J., Gelber R.D., Gunderson L.L. Radiation therapy alone or in combinationwith chemotherapy in the treatment of residual or inoperable carcinoma of the rectum and rectosigmoid or pelvic recurrence following colorectal surgery. Am J Clin Oncol. 1985;8:118–127. doi: 10.1097/00000421-198504000-00003. [DOI] [PubMed] [Google Scholar]