Summary
Background
An exceptionally high demand for surgical masks and N95 filtering facepiece respirators (FFRs) during the COVID-19 pandemic has considerably exceeded their supply. These disposable devices are generally not approved for routine decontamination and re-use as a standard of care, while this practice has widely occurred in hospitals. The US Centers for Disease Control and Prevention allowed it “as a crisis capacity strategy”. However, limited testing was conducted on the impact of specific decontamination methods on the performance of N95 FFRs and no data was presented for surgical masks.
Aim
We evaluated common surgical masks and N95 respirators with respect to the changes in their performance and integrity resulting from autoclave sterilization and a 70% ethanol treatment; these methods are frequently utilized for re-used filtering facepieces in hospitals.
Methods
The filter collection efficiency and pressure drop were determined for unused masks and N95 FFRs, and for those subjected to the treatments in a variety of ways. The collection efficiency was measured for particles of approximately 0.037–3.2 μm to represent aerosolized single viruses, their agglomerates, bacteria and larger particle carriers.
Findings
The initial collection efficiency and the filter breathability may be compromised by sterilization in an autoclave and ethanol treatment. The effect depends on a protective device, particle size, breathing flow rate, type of treatment and other factors. Additionally, physical damages were observed in N95 respirators after autoclaving.
Conclusion
Strategies advocating decontamination and re-use of filtering facepieces in hospitals should be re-assessed considering the data obtained in this study.
Keywords: Surgical mask, N95 respirator, Collection, COVID-19, Re-use, Disinfection
Introduction
An extraordinary shortage of disposable filtering facepieces such as surgical masks and N95 facepiece respirators (FFRs), which the global healthcare community experienced and continues to experience during the COVID-19 pandemic, led to their widespread re-use and – as a result – numerous attempts to identify appropriate methods for their disinfection between consecutive donnings. Disposable devices are generally not approved for decontamination and subsequent re-use as standard of care. However, decontamination and re-use of FFRs were allowed by the US Centers for Disease Control and Prevention “as a crisis capacity strategy” [1], and surgical masks were de facto re-used and disinfected routinely in an effort to ensure a continuity and effectiveness of respiratory protection programmes in hospitals. On 29 March 2020, the US Food and Drug Administration (FDA) issued the first Emergency Use Authorization (EUA) for a decontamination process, followed by several additional EUAs [2]. Limited testing has been conducted to examine how decontamination may affect the performance of FFRs certified by the National Institute for Occupational Safety and Health (NIOSH) [[3], [4], [5], [6], [7]]. Several specific disinfection treatments applied to FFRs have been studied, e.g., ultraviolet germicidal irradiation, plasma sterilization, microwave oven irradiation, and submersion of FFRs in bleach. However, other physical and chemical methods, which can effectively inactivate viruses and bacteria on FFR filters, have not been evaluated with respect to the post-treatment respirator performance and structural integrity. Essentially no data have been collected for surgical masks on their filtration capability and breathability when re-used after disinfection. The present study addresses this knowledge gap for two decontamination methods, a sterilization in an autoclave and a 70% ethanol treatment, as these methods have been broadly utilized for disinfecting filtering facepieces re-used by hospital personnel during the COVID-19 pandemic.
Materials and methods
Decontamination
The following decontamination methods were implemented. The first was sterilization in an autoclave Tuttnauer Model 5596, (TuttnauerUSA, Hauppauge, NY, USA) under 250°F at 15 psi for 30 min, fast exhaust following by drying for 30 min. This was performed once (× 1) and consecutively five times (× 5). The second decontamination method was a treatment of facepieces by soaking in 70% ethanol for 2 h. A 70% ethanol solution was prepared by diluting a 200 Proof pure ethanol (Decon Laboratories, King of Prussia, PA, USA) with distilled de-ionized (milliQ) water.
Protective devices
Initially, two surgical masks and two NIOSH-certified N95 FFRs commonly used in healthcare settings were selected to examine whether these devices could maintain their integrity after being subjected to a single or multiple autoclave sterilizations or ethanol treatments. The masks tested for integrity included Lsp M-301 (Life Science Products, Chestertown, MD, USA) and 3M-1818 (3M Corp. St. Paul, MN, USA). The FFRs were both from 3M Corp.: Model 8210 and Model 1870.
Neither sterilization in an autoclave nor ethanol treatment caused visible damage to the surgical masks. The 3M 8210 respirator revealed physical damage after implementing a single autoclave disinfection such as partial disintegration of the soft sealing material around the nose clip, and, importantly, loss of strap elasticity, which made this respiratory protection device non-reusable. Consequently, the further testing of this respirator was discontinued. The treatments produced notable, but moderate damages to the 3M 1870 FFR, e.g., some detachment and a minor deformation of the nose foam after a single and multiple autoclaving. Accordingly, after eliminating the 3M 8210 FFR, the three remaining devices were selected for the performance evaluation – for collection efficiency and pressure drop.
Experimental design
A protective device being tested was mounted on a frame designed to utilize the entire effective filtration area. An air flow rate through the system was chosen to achieve the same face velocity as calculated for the filter of the tested device under an inhalation flow of 30 L/min (breathing under moderate workload) and 85 L/min (breathing under strenuous work load, also used in NIOSH respirator certification testing). The challenge aerosol, NaCl particles aerosolized from a particle generator (model 8026, TSI Inc Shoreview, MN, USA), mixed with a dry air in a 23-m3 stabilization chamber at a concentration of about 50,000 particles/cm3, entered the testing system and was carried by the air flow towards the filter assembly. The aerosol particle concentration was measured upstream (Cup) and downstream (Cdown) of the tested filter with an Electrical Low-Pressure Impactor (ELPI, Classic+Environmental version, Kangasala, Finland), and the collection efficiency was calculated as
| (1) |
and expressed as a percentage. The ELPI provides the particle size specific measurement in 12 channels approximately from 0.037 to 8.1 μm. To represent aerosolized single viruses, including SARS-CoV-2 of ∼0.1 μm [[8], [9], [10]], their agglomerates, bacteria as well as larger particles carrying viruses or bacteria, we recorded the aerosol concentrations in 10 size fractions within a range of 0.037–3.2 μm. The filter collection efficiency was thus determined as a function of particle size. Also, the size-integrated collection efficiency calculated from the total particle concentrations upstream and downstream was determined for this entire size range. Additionally, the air pressure drop, Δp, was measured for the filters using Differential pressure Magnehelics (Models 2001 and 2002, Dwyer Instruments Inc., Mich.City, IN, USA). Based on the size-integrated filter collection efficiency and the measured air pressure drop through the filter, the quality factor, qf, was calculated for each protective device, breathing flow rate and decontamination method as follows:
Here, Ec is expressed as a fraction.
To simulate contamination of facepieces between donnings, they were soiled with protein (egg whites) and prepared as described in Khodoun et al. [11]. Briefly, egg whites derived from commercial eggs were dialysed against de-ionized distilled (milliQ) water. Protein solution containing 1 mg of egg whites in 3 mL of water was sprayed on masks/respirators before their sterilization in an autoclave. When multiple sterilizations were applied, facepieces were soiled before each autoclave treatment to mimic the device usage in air environments contaminated with protein that may be associated with emission of pathogenic virions by infected persons. We choose to perform five soil + sterilization cycles to simulate a daily re-use of the filtering facepiece over a period of 5 days (working week). With three protective devices, two breathing flow rates, six conditions of the mask/respirator (new, autoclaved once, autoclaved five times, soiled and autoclaved once, soiled and autoclaved five times, and treated with ethanol), and three replicates, we conducted 3 ×2 × 6 × 3 = 108 test runs. Each run produced the filter collection efficiency curve (a function of particle size) and the pressure drop value.
Changes in the filter collection efficiency, pressure drop and quality factor, which occurred due to the decontamination treatments investigated in this study, were quantified for each mask/respirator and breathing flow rate and presented as a function of the particle size as well as the entire tested size range (size integrated). The data were analysed using descriptive statistics. A t-test and analysis of variance were utilized for data comparison; P-value <0.05 signified a significant difference. The autoclave sterilization and ethanol treatment data were analysed separately.
Results
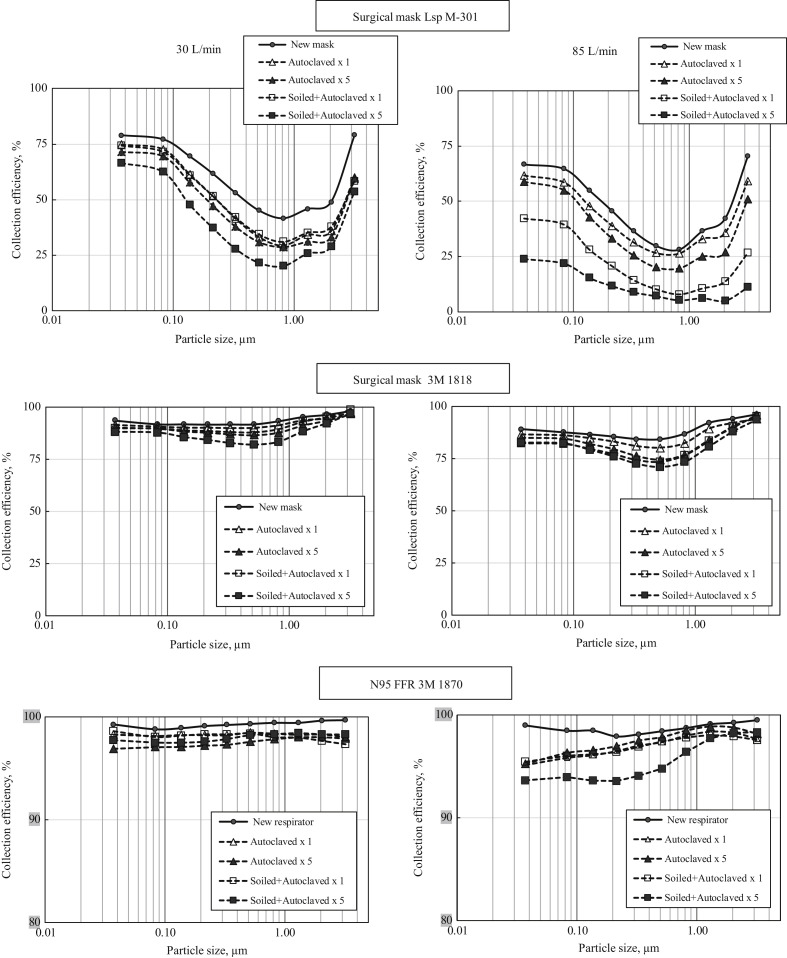
Figure 1 presents the filter collection efficiency for two surgical masks and one N95 FFR as a function of particle size at two breathing flow rates. Each point represents an arithmetic average value; the standard deviations were calculated but not depicted in the graphs to keep the graphs visually comprehesible. The curves in Figure 1 represent a new (unused) protective device as well as the devices sterilized in an autoclave once or five times, with and without soiling before sterilization. The first surgical mask, Lsp M-301, demonstrated a relatively low initial collection efficiency – even before any decontamination procedure was implemented. At 30 L/min, the best collection (slightly above 75%) was achieved for the lowest and the greatest tested sizes; particles of the most penetrating size (∼0.8 μm for this filter) were barely collected at Ec≈ 42%. At 85 L/min, the same trend was observed but the collection efficiency was even lower, ranging approximately from 28% to 73% in the tested particle size interval. It is seen that sterilization in an autoclave decreased the size-specific collection efficiency of the mask filter with the highest difference observed for the filters that were soiled and autoclaved five times. The autoclaving effect on the filter ability to capture particles became more pronounced for the filters soiled with protein before each autoclave treatment. It was seen that conducting five soiling and autoclave sterilization cycles significantly compromised the mask collection characteristics. At 30 L/min, this most damaging treatment decreased the collection efficiency of an unused mask from 78.9% to 66.5% for ∼0.04-μm particles and from about 70% to 50% (interpolated) for ∼0.1-μm particles (the size of a single SARS-CoV-2 virus). The highest relative decrease, from 41.7% to 20.2%, was observed for the most penetrating particle size (MPPS) of ∼0.8 μm identified for this surgical mask at 30 L/min.
Figure 1.
Particle-size-specific filter collection efficiency for two surgical masks and one N95 FFR, which have undergone an autoclave sterilization under different conditions. Breathing flow rates = 30 and 85 L/min.
At a breathing flow of 85 L/min, the negative impact of autoclave disinfection on the collection efficiency was even more evident for both soiled and non-soiled filters with the presence of protein significantly worsening the outcome. It is acknowledged that the Ec data variability increased with the flow rate rising from 30 to 85 L/min– the effect reflecting an increase in between-tests particle concentration variation. From the practical perspective, autoclaving the Lsp M-301 surgical mask, which demonstrated a very low initial protection in the first place, essentially diminished any protective qualities for a wearer of the mask under breathing at 85 L/min.
The second surgical mask, 3M 1818, when tested unused, featured much better filter collection characteristics. For all tested particle sizes, the size-specific Ec values were above 91.6% at 30 L/min and above 84.3% at 85 L/min. As seen in Figure 1, the collection efficiency curves for sub-micrometre particles are almost flat at both flow rates with the MPPS being between 0.33 and 0.52 μm, depending on the flow rate. The autoclave sterilization reduced the filter collection efficiency and the repeated autoclaving enhanced the effect at both flow rates, especially for soiled filters. As the decontamination methods were applied to the mask, its collection efficiency became more particle size dependent with the MPPS shifting slightly towards larger particles. While the drop in size-specific Ec was significant, the re-used and decontaminated mask in the worst-case scenario was still able to filter out >82% of particles of MPPS at 30 L/min and >70% of particles of MPPS at 85 L/min.
As expected, the N95 FFR offered a significantly greater collection. Even the most penetrating particles were collected at 99.1% at 30 L/min and 97.1% at 85 L/min (note that a different scale was chosen for the y-axis of the graphs presenting the N95 FFR data to achieve an appropriate visual resolution). Similar to surgical masks, re-use and decontamination of the tested N95 respirator was found to reduce the size-specific filter efficiency (for a single and multiple sterilizations and for soiled and non-soiled filters). However, even with this reduction, the N95 filter offered a 97.5% efficiency or higher at 30 L/min. At the higher flow rate (85 L/min), the filter collected more than 95% of particles of all tested sizes under all tested scenarios with one exception: (soiled + autoclaved) × 5, when Ec decreased to 93.6−94.8% for particles of 0.037−0.52 μm, just a little below the 95% benchmark. The Ec value was still above 95% for larger particles. It is noted that the above particle size range includes the single coronavirus as well as particle-carriers that are up to five-times bigger in size.
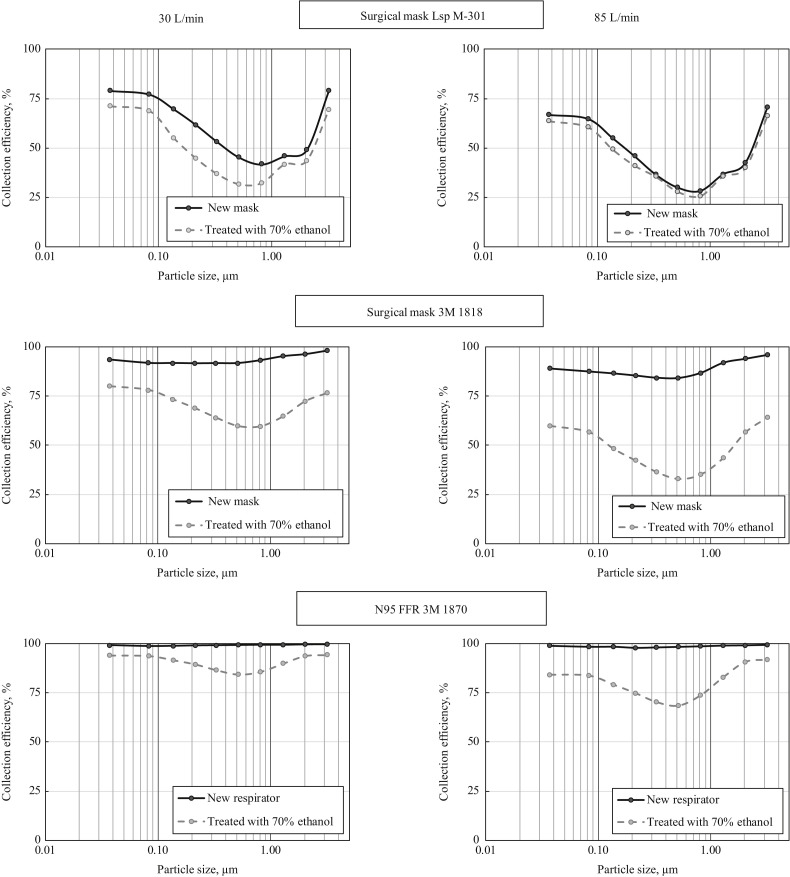
Figure 2 presents the size-specific collection efficiency data for the tested protective devices, which were treated with 70% ethanol before being re-used. The data obtained with new (unused) devices are presented for comparison. The ethanol treatment significantly reduced the size-specific collection efficiency across the particle size range for all three filters, except for the mask Lsp M-301 at a breathing flow rate of 85 L/min. From the practical standpoint, the most notable ethanol-caused damage resulting in the filter efficiency decrease occurred in the high-performance surgical mask (3M 1818) and the N95 respirator. The N95 filter that demonstrated a superior initial performance, Ec ≥99%, did not even meet the 95% efficiency requirement after the treatment. It was observed for any tested particle size and flow rate. Furthermore, its efficiency dropped below 90% at 30 L/min and below 70% for the MPPS, which, in turn, shifted from ∼0.2–0.3 μm (pre-treatment) to ∼0.5 μm (post-treatment).
Figure 2.
Particle size specific filter collection efficiency for two surgical masks and one N95 FFR which have undergone decontamination treatment with 70% ethanol. Breathing flow rates = 30 and 85 L/min.
The size integrated Ec values (calculated for the entire particle size range) are presented in Table I as arithmetic average values with standard deviations. Also in this table are the average pressure drop values, Δp, and the average filter quality factors, qf. The size integrated collection efficiency of the Lsp M−301 mask significantly decreased due to its autoclave sterilization and this effect was more pronounced for soiled filters. Multiple soiling and autoclaving generated a greater decrease, which showed a strong significance for 30 L/min. This was consistent with the findings of the particle size selective testing presented above for the autoclave sterilization. The ethanol treatment always significantly affected the particle size integrated particle collection efficiency.
Table I.
Particle size integrated filter collection efficiency, air pressure drop, and quality factor for two surgical masks and one N95 FFR, which have undergone an autoclave sterilization under different conditions and decontamination treatment with a 70% ethanol
| Protective device | Condition | 30 L/min |
85 L/min |
||||
|---|---|---|---|---|---|---|---|
| Ec % | Δp mm wg | qf per mm wg |
Ec % | Δp mm wg | qf per mm wg |
||
| Surgical mask Lsp M-301 | New | 67.9 ± 2.2 | 0.8 | 1.42 | 53.9 ± 4.8 | 2.0 | 0.39 |
| Autoclaved × 1 | 60.8 ± 2.3 | 0.7 | 1.34 | 48.3 ± 6.4 | 1.7 | 0.39 | |
| Autoclaved × 5 | 57.2 ± 2.7 | 1.0 | 0.85 | 43.7 ± 6.2 | 1.8 | 0.32 | |
| Soiled+Autoclaved × 1 | 60.4 ± 3.1 | 0.7 | 1.32 | 29.6 ± 5.9 | 1.5 | 0.23 | |
| Soiled+Autoclaved × 5 | 49.4 ± 2.9 | 0.9 | 0.76 | 19.7 ± 6.2 | 1.5 | 0.12 | |
| Treated with 70% ethanol | 56.2 ± 4.2 | 1.6 | 0.52 | 51.1 ± 5.2 | 3.0 | 0.24 | |
| Surgical mask 3M 1818 | New | 92.3 ± 1.3 | 0.6 | 4.28 | 86.9 ± 2.3 | 1.5 | 1.36 |
| Autoclaved × 1 | 90.7 ± 0.8 | 0.6 | 3.96 | 84.8 ± 1.2 | 1.3 | 1.45 | |
| Autoclaved × 5 | 88.9 ± 0.7 | 0.7 | 3.38 | 81.9 ± 1.1 | 1.7 | 1.01 | |
| Soiled+Autoclaved × 1 | 89.1 ± 0.6 | 0.5 | 4.43 | 79.6 ± 1.2 | 1.6 | 0.99 | |
| Soiled+Autoclaved × 5 | 85.9 ± 0.8 | 0.6 | 3.27 | 79.0 ± 1.4 | 1.4 | 1.12 | |
| Treated with 70% ethanol | 74.5 ± 1.3 | 0.5 | 2.73 | 51.8 ± 2.4 | 2.0 | 0.36 | |
| N95 FFR 3M 1870 | New | 99.5 ± 0.8 | 3.1 | 1.74 | 96.9 ± 1.8 | 12.7 | 0.28 |
| Autoclaved × 1 | 98.2 ± 0.9 | 2.7 | 1.49 | 95.5 ± 1.1 | 9.6 | 0.32 | |
| Autoclaved × 5 | 97.1 ± 0.9 | 2.5 | 1.42 | 96.0 ± 2.1 | 8.4 | 0.38 | |
| Soiled+Autoclaved × 1 | 98.3 ± 1.1 | 2.4 | 1.69 | 96.0 ± 1.9 | 8.2 | 0.39 | |
| Soiled+Autoclaved × 5 | 97.6 ± 1.2 | 2.5 | 1.50 | 93.0 ± 1.8 | 8.8 | 0.32 | |
| Treated with 70% ethanol | 91.9 ± 1.1 | 2.7 | 0.93 | 80.2 ± 1.7 | 9.3 | 0.17 | |
Breathing flow rates = 30 L/min and 85 L/min.
The three devices tested in this study differ by the initial air pressure drop through their filters; it is relatively low (0.80 and 0.60 mm wg at 30 L/min, and 2.0 and 1.5 mm wg at 85 L/min) for the surgical masks and moderate (3.1 mm wg at 30 L/min and 12.7 mm wg at 85 L/min) for the N95 FFR. For the soiled and non-soiled surgical masks, neither a single nor multiple sterilization in an autoclave affected the filter pressure drop in a major way (interestingly, the breathability showed an improvement post-treatments); the only exception was identified for Lsp M-301 treated with ethanol: here the pressure drop notably increased. For the N95 FFR, the post-treatment pressure drop decreased by 13–35%, depending on the flow rate and decontamination method applied. Overall, we concluded that there was no major impact of decontamination on the filter breathability within the range of conditions tested in this study.
The quality factor ranged widely. This reflects the relative contributions of the collection efficiency and pressure drop to the overall performance of the filters. A higher qf represents a better overall performance with respect to combined impact of the particle removal by the filter and the filter breathability. A relatively low collection efficiency of Lsp M-301 was somewhat compensated by its high breathability (low Δp) producing the quality factor ranging from 0.52 per mm wg (post ethanol treatment) to 1.42 per mm wg (new mask) at 30 L/min and from 0.12 per mm wg (soiled + autoclaved × 5) to 0.39 per mm wg (new or once-autoclaved mask) at 85 L/min. Surgical mask 3M 1818 with relatively high collection efficiency and better breathability, showed a much improved quality factor that ranged from 2.73 to 4.43 per mm wg at 30 L/min and from 0.36 to 1.45 per mm wg at 85 L/min. Finally, the tested N95 FFR was characterized by a very good particle collection but relatively high pressure drop. As their contributions compensated each other, the qf ranges, 0.93–1.74 per mm wg at 30 L/min and 0.17–0.39 per mm wg at 85 L/min, were found to be similar to those obtained for the least-efficient device (the Lsp M-301 mask). The N95's quality factor was not dramatically affected by autoclaving but significantly decreased after the respirator was treated with ethanol.
Discussion
The non-monotonic function of the collection efficiency on the particle size identified for unused protective devices and the MPPS values are consistent with the tested filter materials. The observed post-treatment shift of the MPPS towards larger particles is explainable. Exposure to moist heat in an autoclave as well as the ethanol treatment generally remove electric charges from the filter media. This has been shown to degrade the so called ‘electret’ filters, which rely on the electrostatic charge, causing a significant decrease in the collection efficiency of the filter media and some increase in the MPPS [[12], [13], [14]] – the effects which we noted in this study (two of the three tested protective devices had electret filters). Structural degradation of the filter media may occur also with non-electret materials if they are exposed to heat and chemicals, which can damage the integrity of the fibers.
A single and multiple autoclave sterilization of a non-soiled filter may produce comparable damages on some media. For other media, the damaging effects can increase with every cycle and accumulate. Filters of a relatively simple structure may sustain major damages during the first sterilization cycle such that the subsequent autoclaving cycles would add no significant destruction. This is exemplified by the data collected in the present study for devices featuring higher efficiency, especially the N95 FFR. A more complicated situation occurs for a ‘loaded’ filter, which is re-used with its outer surface loaded with the previously collected particles (the soiled filters tested in this study). Multiple soiling and autoclaving cycles can potentially (i) remove electrical charges from the filter media and (ii) affect the media integrity through repetitive heating the protein inside the filter being autoclaved. Both phenomena are likely to decrease the filter performance, perhaps differently for different filters and face air velocities (flow rates). Some data obtained with surgical mask filters operating at the lower flow rate represented the case when a multiple-cycle autoclave sterilization produced a greater effect on the particle size integrated Ec values.
A significant decrease in the filter collection efficiency, which was found after the 3M 1818 surgical mask and the N95 respirator were treated with ethanol, occurred also because of degradation of their electret filters.
Martin and Moyers [12] reported a significant decrease in collection efficiency and increase in MPPS after the filters were dipped in isopropanol for 15 s and dried overnight. In our study, the effect was found to be less pronounced for Lsp M-301, especially for the higher flow rate of 85 L/min, because this mask offers such a poor initial particle collection (it probably uses a non-electret filter) that the ethanol-caused structural degradation cannot make it much worse. A greater flow rate decreases the particle residence time and, consequently, makes the particle–media interaction less effective. This, in turn, diminishes the impact of the degradation caused by ethanol.
It is also noted that an autoclave sterilization may have a different quantitative impact on particle size selective versus size integrated Ec. Since the latter derives from the total aerosol concentration measured upstream and downstream of the filter, the difference can be attributed to the particle size distribution of the challenge aerosol, which has a considerable fraction of ultrafine particles (up to 0.1 μm) and rather few particles above 1 μm.
Finally, different decontamination methods produced different effects on the device breathability. In some cases, the pressure drop increased after treatment, but in most cases it decreases. These changes, however, are relatively minor from the practical perspective. They may reflect different structure damages resulting from different treatments.
The main limitation of this study was that it is focused primarily on the filter performance and did not consider the fit issue. It is acknowledged that decontamination may affect not only the filter but the whole protective device. The physical damages observed in N95 FFRs after autoclaving challenged the respirator integrity.
Among them are the partial detachment and minor deformation of the nose foam, partial disintegration of the soft sealing material around the nose clip, and – for one model – a critical loss of strap elasticity. The severity of these damages varied from one device to the other, e.g., the impact of sterilization on the integrity of the N95 FFRs was greater for Model 8210 as compared with Model 1870. Even if a certain decontamination method does not significantly affect the filter performance, it may negatively impact the performance of the whole respirator, e.g., its ability to fit.
The initial particle filtration characteristics and the filter breathability of surgical masks and N95 filtering facepieces may be significantly compromised by either single or multiple sterilizations in an autoclave or by a 70% ethanol treatment. The changes depend on a type and model of a protective device, particle size, breathing flow rate, type of treatment and other factors. Additionally, autoclaving of FFRs affected their integrity suffering from physical damages of the devices. The study findings underline the limitations of strategies advocating re-use of filtering facepieces in hospitals involving their decontamination via sterilization in an autoclave or treatment with ethanol.
Conflict of interest statement
None declared.
Funding sources
This study did not benefit from any specifically assigned funding source.
References
- 1.Centers for Disease Control and Prevention (CDC) Decontamination and Reuse of Filtering Facepiece Respirators. Atlanta, GA. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed May 2020]
- 2.Food and Drug Administration (FDA) Emergency Use Authorizations. Silver Spring, MD. 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations Available at: [last accessed May 2020]
- 3.Bergman M.S., Viscusi D.J., Heimbuch B.K. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Eng Fiber Fabr. 2010;5(4):33–41. [Google Scholar]
- 4.Bergman M., Viscusi D.J., Palmiero A.J., Powell J., Shaffer R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J Int Soc Respir Prot. 2011;28(1):48–59. [Google Scholar]
- 5.Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12(8):509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viscusi D.J., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53(8):815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A.J., Powell J. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J. Occup. Environ Hygiene. 2011;8(7):426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 8.Encyclopedia Britannica: Coronavirus. 2020. https://www.britannica.com/science/coronavirus-virus-group Available at: [last accessed April 2020]
- 9.Goldsmith C.S., Tatti K.M., Ksiazek T.G., Rollin P.E., Comer J.A., Lee W.W. Ultrastructural Characterization of SARS coronavirus. Emerg. Infect. Dis. 2004;10(2):320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman B.W., Adair B.D., Yoshioka C., Quispe J.D., Orca G., Kuhn P. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006;80(16):7918–7928. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodoun M.V., Morris S.C., Angerman E., Potter C., Schuman R., Wunderlich M. Rapid desensitization of humanized mice with anti-human FcεRIα monoclonal antibodies. J Allergy Clin Immunol. 2020;145(3):907–921. doi: 10.1016/j.jaci.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S.B., Jr., Moyer E.S. Electrostatic respirator filter media: Filter efficiency and most penetrating particle size effects. Appl. Occup Environ. Hyg. 2000;15(8):609–617. doi: 10.1080/10473220050075617. [DOI] [PubMed] [Google Scholar]
- 13.Balazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses and how adequate are surgical masks? Amer. J. Infect. Control. 2006;34(2):51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Balazy A., Toivola M., Reponen T., Podgorski A., Zimmer A., Grinshpun S.A. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann. Occup. Hyg. 2006;50(3):259–269. doi: 10.1093/annhyg/mei058. [DOI] [PubMed] [Google Scholar]