Highlights
-
•
Functional connectivity in the sensorimotor network is altered in children with DCD.
-
•
Connectivity to posterior cingulate cortex and precuneus is impaired in DCD.
-
•
Connectivity to middle posterior temporal gyrus is impaired in DCD.
-
•
Altered connectivity of sensorimotor network may explain motor problems in DCD.
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder; ASD, Autism Spectrum Disorder; Conners 3 AI, Conners 3 ADHD Index; DCD, Developmental Coordination Disorder; DCDQ, Developmental Coordination Disorder Questionnaire; ICA, Independent Component Analysis; MABC-2, Movement Assessment Battery for Children − 2nd edition; pTMG, Posterior Middle Temporal Gyrus; PCC, Posterior Cingulate Cortex; TD, Typically-developing; TFCE, Threshold Free Cluster Enhancement
Keywords: Motor skills disorder, Developmental coordination disorder, Children, Resting state fMRI, Functional connectivity
Abstract
Developmental Coordination Disorder (DCD) is a neurodevelopmental disorder that affects a child’s ability to learn motor skills and participate in self-care, educational, and leisure activities. The cause of DCD is unknown, but evidence suggests that children with DCD have atypical brain structure and function. Resting-state MRI assesses functional connectivity by identifying brain regions that have parallel activation during rest. As only a few studies have examined functional connectivity in this population, our objective was to compare whole-brain resting-state functional connectivity of children with DCD and typically-developing children. Using Independent Component Analysis (ICA), we compared functional connectivity of 8–12 year old children with DCD (N = 35) and typically-developing children (N = 23) across 19 networks, controlling for age and sex. Children with DCD demonstrate altered functional connectivity between the sensorimotor network and the posterior cingulate cortex (PCC), precuneus, and the posterior middle temporal gyrus (pMTG) (p < 0.0001). Previous evidence suggests the PCC acts as a link between functionally distinct networks. Our results indicate that ineffective communication between the sensorimotor network and the PCC might play a role in inefficient motor learning seen in DCD. The pMTG acts as hub for action-related information and processing, and its involvement could explain some of the functional difficulties seen in DCD. This study increases our understanding of the neurological differences that characterize this common motor disorder.
1. Introduction
1.1. Developmental Coordination Disorder
Developmental Coordination Disorder (DCD) is a neurodevelopmental disorder characterized by difficulty in performing and learning coordinated motor skills, which significantly impacts the performance of daily life activities (American Psychiatric Association, 2013). The worldwide prevalence of DCD is approximately 5–6 percent among school-age children (American Psychiatric Association, 2013, Blank, 2019, Harris et al., 2015, Smyth, 1992). DCD is 2 to 7 times more common in males compared to females (Kadesjö and Christopher, 1999, Lingam et al., 2009), as in many other neurodevelopmental disorders (Polyak et al., 2015, Pinares-Garcia et al., 2018). Children with DCD tend to have lower academic achievement, as well as reduced participation in self-care, social, and leisure activities (Izadi-Najafabadi et al., 2019 Jan, Zwicker et al., 2013, Zwicker et al., 2018). These difficulties often persist into adulthood (Cousins and Smyth, 2003), and are related to a more sedentary lifestyle, higher rates of obesity (Rivilis et al., 2011 Feb 9, Vedul-Kjelsås et al., 2012), low self-esteem, social isolation (Zwicker et al., 2013, Chen and Cohn, 2003 Jul 29), anxiety, and depression (Harrowell et al., 2017, Moruzzi et al., 2010, Waszczuk et al., 2016, Pearsall-Jones et al., 2011 Feb 23). Many children with DCD have at least one other diagnosis (Blank et al., 2019). Common comorbidities include Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), learning disorders, and specific language impairment (Kadesjö and Christopher, 1999, Gomez and Sirigu, 2015, Vaivre-Douret, 2014).
ADHD is the most common comorbidity of DCD, with up to 50% of children with DCD having co-occurring ADHD (Kadesjö and Christopher, 1999, Kadesjö and Gillberg, 1998 Dec 21, Kadesjö and Gillberg, 2001), which further intensifies their functional difficulties (Watemberg et al., 2007). With a prevalence of 5% to 9.5% among school-age children, ADHD is characterized by deficits in attention, executive functions, hyperactivity, and impulsivity (American Psychiatric Association, 2013, Polanczyk et al., 2007). Similar to DCD, the prevalence in males is 3–8 times higher compared to prevalence in females, although this difference is thought to be partially due to referral bias (Thapar and Cooper, 2015). It has been suggested that both disorders share the same etiology, due to the similarities in prevalence, onset age, long-term course, and high co-occurrence (Martin et al., 2006, Goulardins et al., 2015). These hypotheses, however, have not been confirmed (Gomez and Sirigu, 2015). Co-occurrence of DCD and ADHD is related to poorer outcomes in adulthood compared to a single diagnosis (Rasmussen and Gillberg, 2000); these may include higher rates of alcohol abuse, criminal offences, antisocial disorder, and low educational level (Rasmussen and Gillberg, 2000, Lange, 2018).
1.2. Neuroimaging studies in DCD
Efforts to identify the neural characteristics of DCD have emerged over the last decade, and although the etiology for DCD appears to be multifactorial (Gomez and Sirigu, 2015, Vaivre-Douret, 2014, Martin et al., 2006), some brain regions have been identified consistently as atypical in children DCD compared to typically-developing children. Structural and functional neuroimaging studies in DCD report on the involvement of the parietal lobe (McLeod et al., 2014, Reynolds et al., 2017, Kashuk et al., 2017, Debrabant et al., 2013, Zwicker et al., 2011, Zwicker et al., 2010, Querne et al., 2008, Reynolds et al., 2015, Kashiwagi et al., 2009, Reynolds et al., 2019, Debrabant et al., 2016). Many of these studies report atypical function of the inferior parietal lobules, precuneus, and parts of the superior parietal lobules (Zwicker et al., 2010, Querne et al., 2008, Reynolds et al., 2015, Kashiwagi et al., 2009, Reynolds et al., 2019). The involvement of the frontal lobe in DCD is also reported frequently (Reynolds et al., 2017, Kashuk et al., 2017, Debrabant et al., 2013, Zwicker et al., 2011, Zwicker et al., 2010, Querne et al., 2008, Caçola et al., 2018, Biotteau et al., 2016, Caeyenberghs et al., 2016, Licari et al., 2015 Mar 11, Mariën et al., 2010, McLeod et al., 2016, Thornton et al., 2018), and includes regions in the prefrontal cortex and the motor cortex. Other regions that have been identified as neural correlates of DCD include the posterior cingulate cortex (PCC) (Reynolds et al., 2017, Zwicker et al., 2010, Reynolds et al., 2015, Reynolds et al., 2019, Biotteau et al., 2016), the basal ganglia (McLeod et al., 2014, Querne et al., 2008) and the cerebellum (Biotteau et al., 2016, Mariën et al., 2010, McLeod et al., 2016, Kashuk et al., 2017, Debrabant et al., 2013, Zwicker et al., 2011, Zwicker et al., 2010, Querne et al., 2008).
Although significant progress has been made in the field of neuroimaging studies of DCD, several common limitations need to be addressed in order to reach a more definite conclusion regarding the neural correlates of DCD. These include a very small sample size (with an average sample size of 10 participants in the DCD group) (Fuelscher et al., 2018), and no control over the effect of age or sex, even in presence of high variance within the sample (McLeod et al., 2014, McLeod et al., 2016). Moreover, most neuroimaging studies in this field are task-based MRI studies. While these studies provide valuable information about neural activation during a specific task, they are rarely replicated due to the high variance in task and study parameters, and the inferences that can be made are limited to the specific task conditions under investigation. A potential solution for these limitations can be found in a well-conducted, large-scale resting-state MRI study.
1.3. Resting-state MRI
Resting-state MRI (rsMRI) assesses brain activity during rest, allowing the study of functional connectivity between spatially-distinct brain regions. In the last two decades, a growing body of evidence reports on the existence of several functional networks that are activated during specific type of tasks (Smith et al., 2009), and are also highly detectable from neural signals at rest (den Heuvel et al., 2010, Smith et al., 2013). Functional networks share a common temporal pattern of low-frequency spontaneous neural activation, that is thought to reflect the functional communication between those brain regions (den Heuvel et al., 2010). The use of rsMRI enables the investigation of functional networks without the constraint of a specific task. Resting-state networks are highly reproducible (Castellanos et al., 2013), and are found consistently across participants and groups, and in different developmental stages (den Heuvel et al., 2010, Smith et al., 2013, Castellanos et al., 2013, Grayson and Fair, 2017). Another advantage of rsMRI is the relative simplicity of data collection due to minimal compliance demands, making it a perfect candidate for investigation of neural differences in pediatric and clinical populations (Castellanos et al., 2013, Biswal, 2012).
Only two studies to date have used rsMRI to assess the functional connectivity in children with DCD and co-occurring DCD and ADHD (McLeod et al., 2014, McLeod et al., 2016). Both studies analyzed the same data to examine functional connectivity of the sensorimotor system, and found atypical functional connectivity with many regions, including with the right superior temporal gyrus, right frontal operculum cortex, right postcentral gyrus (McLeod et al., 2014), and the basal ganglia and the cerebellum (McLeod et al., 2016). Both studies used a seed-based analysis, in which brain connectivity is investigated as the measure of correlation between a pre-defined seed and all other voxels in the brain. This method requires a priori selection of a seed, which allows the ability to directly answer a specific, pre-defined question (Cole et al., 2010). However, it limits the potential results to a pre-defined network, and disregards all other information in the data. While this method could increase statistical power due to a limited number of comparisons, minor differences in seed selection across participants or studies can lead to high variability in the recognized networks, or to presentation of biased networks (Cole et al., 2010, Buckner et al., 2008). In addition, this method is very sensitive to noise in the rsMRI data, which can lead to false-positive results and to over-estimation of group differences (Cole et al., 2010, Satterthwaite et al., 2012, Bednarz and Kana, 2018). Application of whole-brain independent component analysis (ICA) can overcome these limitations. ICA is a data-driven approach that decompose data into different components, thus allowing the separation of noise from neural signal (Calhoun et al., 2009). This approach is less prone to artifacts due to noise compared to seed-based analysis (Cole et al., 2010), an important consideration in pediatric studies (Satterthwaite et al., 2012, Bednarz and Kana, 2018, Fassbender et al., 2017). ICA does not require a priori assumption about the findings, and since the evidence regarding neural correlates of DCD is limited, such an exploratory method is favorable. The use of ICA allows investigation of whole-brain functional connectivity, while reducing the risk for bias results due to artifacts.
1.4. Purpose of study
In order to overcome the limitations of previous research and bridge the gap in knowledge regarding functional connectivity in children with DCD, the purpose of our study was to determine if whole-brain functional connectivity is altered in DCD. Thus, the specific aim of our study was to assess the differences in functional connectivity at rest between children with DCD (with or without co-occurring ADHD) and typically-developing children.
2. Methods
2.1. Study design
We conducted a cross-sectional study to evaluate differences in functional connectivity between children with DCD and typically-developing children. This study is part of a larger randomized controlled trial in which an intervention effect is being investigated (ClinicalTrials.gov ID: NCT02597751). The research project was approved by UBC Children's and Women's Research Ethics Board, certificate #H14-00397, and was funded by the Canadian Institutes of Health Research (FDN-143258).
2.2. Participants
Using a convenience sample, we recruited children 8- to 12-year old with DCD (with and without co-occurring ADHD) from Dr. Zwicker’s research-integrated DCD Clinic at Sunny Hill Health Centre for Children, BC Children’s Hospital ADHD Clinic, and from the community in the Greater Vancouver area. Typically-developing (TD) children were recruited through advertisements posted at the bulletin boards at BC Children’s Hospital, UBC, Vancouver schools, and the community.
Children were diagnosed with DCD according to the Diagnostic and Statistical Manual – 5th ed. (DSM-5) criteria (American Psychiatric Association, 2013) as follows: (1) score at or below the 16th percentile on the Movement Assessment Battery for Children – 2nd edition (MABC-2) (Henderson et al., 2007); (2) score in the suspected or indicative range on the DCD Questionnaire (DCDQ) (Wilson et al., 2009); (3) parent-reported motor difficulties from a young age; and (4) no other medical condition that could explain motor difficulties as per parent-report, clinical observations, and/or medical exam. The control group included 8- to 12-year old TD children with no history of motor difficulties and a score ≥25th percentile on the MABC-2. Children were excluded from the study if they were: (1) born pre-term (<37 weeks gestational age); or (2) diagnosed with ASD or intellectual disability. Children assigned to the TD group were excluded if they were diagnosed with ADHD.
2.3. Procedure
After screening and recruitment, all parents or legal guardians provided written consent and children assented to participate in the study. Children were assessed by occupational therapists or trained graduate students to ensure that they met inclusion criteria. A research nurse completed an MRI safety screening and informed the children and families about the MRI procedure. Prior to MRI scanning, children participated in an MRI simulator session, to familiarize themselves with the sights and sounds of the MRI environment and to alleviate their anxiety. Children then completed an MRI scanning session of approximately 60 min.
3. Clinical measurement
3.1. Movement Assessment Battery for children – 2nd edition (MABC-2)
The MABC-2 (Henderson et al., 2007) performance test is designed to assess the severity and extent of motor impairments in children 3- to 16-years old; it is one of the most common assessment tools for motor impairments in children, in both research and clinical settings (Blank et al., 2019; Wagner et al., 2011). Raw scores are converted to age-specific normative percentile scores, which were used in this study. The MABC-2 has excellent test–retest reliability (ICC = 0.97), good internal consistency (α = 0.9), and good factorial and construct validity (Wagner et al., 2011, Psotta and Abdollahipour, 2017, Wuang et al., 2012, Schulz et al., 2011). In this study, a cut-off score at or below the 16th percentile on the MABC-2 was used to determine if children met criterion A of the DSM-5 diagnostic criteria, as suggested by international clinical practice recommendations for DCD (Blank et al., 2019). Children who scored at or above the 25th percentile were classified as the control group of TD children.
3.2. Developmental Coordination Disorder questionnaire (DCDQ)
The DCDQ (Wilson et al., 2009, Wilson et al., 2000) is a parent questionnaire designed to be used as a screening tool for identification of motor impairments in children 5- to 15-years old. Parents are asked to compare their child's performance in various every-day tasks to the performance of their TD peers. In this study, we used the age specific cut-off scores as specified in the manual. The DCDQ has high internal consistency (α = 0.94), as well as adequate sensitivity (85%), and good validity and reliability (Wilson et al., 2009, Wilson et al., 2000, Cairney et al., 2008). The DCDQ is the recommended screening tool for DCD according to the international guidelines for identification of children with DCD (Blank et al., 2019).
3.3. Conners 3 ADHD Index (Conners 3 AI)
The Conners 3 AI parent form (Conners, 2008) was used to assess for ADHD symptoms. This short questionnaire can distinguish between children with and without ADHD (Conners, 2008, Conners et al., 2012). A score above 70 is considered clinically significant. The Conners 3 AI has high internal consistency (mean α = 0.90), high predictive value, and mean test–retest reliability of 0.83 (Conners, 2008, Conners et al., 2012). Since children with DCD are more likely to have ADHD compared to TD children (Blank et al., 2019; Kadesjö and Gillberg, 1998 Dec 21), the Conners score was used as a measure of ADHD symptoms.
3.4. Socio-demographic questionnaire
A socio-demographic questionnaire was used to collect information regarding participants’ demographics, such as age, sex, and additional diagnoses.
3.5. MRI data acquisition
All brain imaging was performed on a 3-Tesla General-Electric Discovery MR 750 scanner. Echo-planar imaging was conducted to acquire resting-state functional MR data (TE: 30 ms, TR: 3000 ms, slice thickness: 3 mm, FOV: 288, matrix: 128x128). Resting-state functional data were acquired for six minutes while participants rested in the scanner. A high-resolution 3D T1 anatomical image was collected for co-registration and anatomic localization (3D SPGR, TE: 3.2 ms, TR: 8.1 ms, slice thickness: 1 mm, FOV: 256 mm, matrix: 256 × 256, scan time: 5 min). Anatomical and functional MRI imaging were acquired and reconstructed on the scanner console, and then transferred to an independent workstation for preprocessing and data analysis.
3.6. Preprocessing and denoising
Data were converted from DICOM to Nifti format using the dcm2nii tool from MRIcron (https://www.nitrc.org/projects/mricron) (Rorden and Karnath, 2012). Structural images were visually inspected for motion artifacts, and low-quality scans were excluded from the data (n = 12). Brain extraction was done using FreeSurfer (v5.3.0) (Fischl, 2012). Initial preprocessing of functional data was done using FSL (FMRIB Software Library, 5.0.10, Oxford, UK) (Woolrich et al., 2009, Jenkinson et al., 2012). Preprocessing included motion correction with six parameters using MCFLIRT (Jenkinson et al., 2002) as well as slice timing correction and high-pass filtering (0.01 Hz) using FEAT (Woolrich et al., 2001). We excluded participants from further analysis if they had high levels of head motion during the rsMRI scan, exceeding mean framewise displacement (FD) of 0.5 mm (n = 13) (Power et al., 2015).
Evidence from recent publications indicate the high importance of implementing participant-level denoising methods to alleviate the effect of motion on functional connectivity and to increase reliability of rsMRI (Satterthwaite et al., 2012, Ciric et al., 2017, Parkes et al., 2018). In a recent evaluation and comparison of different denoising strategies, Parkes and colleagues found that ICA-based denoising outperformed other strategies in most benchmarks, especially when combined with global signal regression (Parkes et al., 2018). The use of global signal regression improved most denoising strategies, including ICA-based denoising. Although global signal regression is under debate, its potential negative effects (introducing negative correlation and distance dependence QC-FC), were found to be minimal when combined with ICA-based denoising strategies (Parkes et al., 2018). Global signal regression was useful for removing artifacts from the data that cannot be removed otherwise (Parkes et al., 2018, Power et al., 2017), and is helpful in detecting group differences in functional connectivity (Parkes et al., 2018). We performed single-subject ICA analysis using MELODIC (Beckmann and Smith, 2004). We used FIX (FMRIB's ICA-based Xnoiseifier) (Salimi-Khorshidi et al., 2014, Griffanti et al., 2014) to automate the components classification process. Components of a selective sample (n = 20) were hand-classified according to the guidelines suggested by Griffanti (Griffanti et al., 2017) by two independent assessors (S.R. and S.I.). Level of agreement was high (85%), and a consensus was reached regarding all other components. Then, we used FIX to classify components of all other participants and regress out noisiness components.
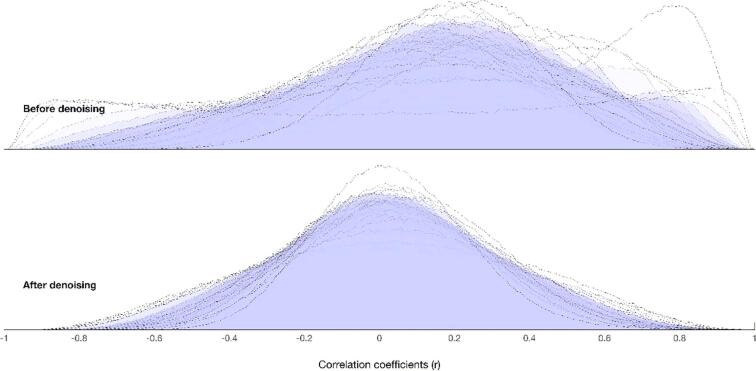
Using CONN functional connectivity toolbox v18a (http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012, Whitfield-Gabrieli and Nieto-Castanon, 2012) and MATLAB (R2018a) (https://www.mathworks.com/products/matlab.html), (The MathWorks) we performed indirect registration and segmentation of functional scans to MNI standard space, spatial smoothing (6 mm full-width at half-maximum [FWHM]), and global signal regression. We regressed out one cerebral spinal fluid component and three WM components (Behzadi et al., 2007), and used QC-FC to estimate the residual relationship between motion and connectivity following denoising (Fig. 1) (Satterthwaite et al., 2012, Whitfield-Gabrieli and Nieto-Castanon, 2012).
Fig. 1.
QC-FC plot before and afterdenoising.
3.7. Identification of resting-state networks
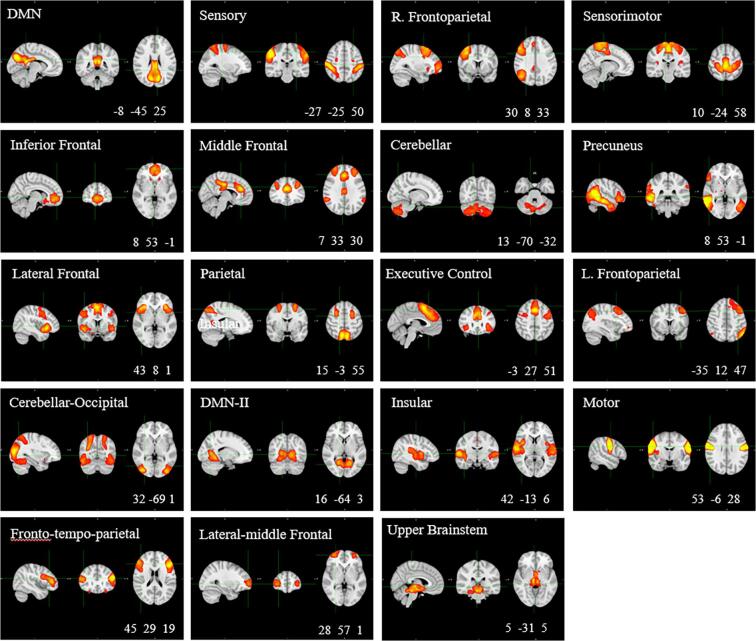
We performed group-level ICA with dimension reduction to 30 components using MELODIC (Beckmann and Smith, 2004, Beckmann et al., 2005), followed by dual regression. In the first phase, each spatial component was regressed out of each individual functional scan and a representative time-series for each component and each participant was determined. In the second phase, these time-series were used as regressors in a second regression to obtain an individual-level spatial map for each group network, which we used for the group comparison. We used a cross-correlation to compare the group-level networks with a pediatric resting-state networks template (Muetzel et al., 2016). Networks with significant spatial correlation (r > 0.3) were carried over to the group comparison. We excluded networks that were classified as visual networks (i.e., visual, anterior visual, and lateral-visual networks) from the group comparison as we were unable to control for participants’ visual stimulus during scans (whether they had their eyes open or closed during scans). Overall, 19 networks were carried over for group comparison (Fig. 2).
Fig. 2.
Identified resting state-networks. Coordinates are in Montreal Neurological Institute (MNI) space; Threshold Z > 5; Network classification based on spatial correlation with pediatric template (Muetzel et al., 2016); DMN, Default Mode Network
3.8. Statistical analysis
We used RStudio (1.1.463) for analysis of behavioural data. To compare the distribution of sex between groups, we used the Chi-square test. To compare group differences in age, ADHD symptoms as measured by Conners 3AI, and motion parameters, we used the student’s t-test, and a Welch’s t to compare MABC-2 scores. All assumptions were met.
We used PALM (Permutation Analysis of Linear Models) for statistical analysis of rsMRI data (Winkler et al., 2014, Winkler et al., 2016). PALM allows statistical inference for neuroimaging data using permutation methods that do not require assumptions regarding data distribution. We used Threshold Free Cluster Enhancement (TFCE), a voxel-wise multiple testing correction method, in which each voxel’s value represents the cluster-like spatial support, and so integrates spatial neighborhood information (Smith and Nichols, 2009). TFCE enhances sensitivity and detectability of neural signal without enforcing assumptions regarding cluster size, thus improving the results’ stability compared to cluster thresholding. To adjust the p-value for family wise error (FWE) rate with multiple testing across networks and contrasts, PALM uses synchronized permutations that account for the non-independence between the tests, and minimizes power loss following correction (Winkler et al., 2016).
To assess group differences in resting-state functional connectivity, we performed t-tests. Since the development during childhood is characterized by substantial neural changes (Grayson and Fair, 2017, Bednarz and Kana, 2018), and there are known sex and age differences in functional connectivity in typically-developing children (Bednarz and Kana, 2018, Muetzel et al., 2016) as well as in children with neurodevelopmental disorders (Subbaraju et al., 2017, Abraham et al., 2017), we included age and sex as covariates in the analysis. Results are presented in threshold of p < 0.05, FWE corrected using TFCE, and a minimum cluster size of 5 voxels, before correction for multiple testing across networks and contrasts.
4. Results
4.1. Cohort characteristics
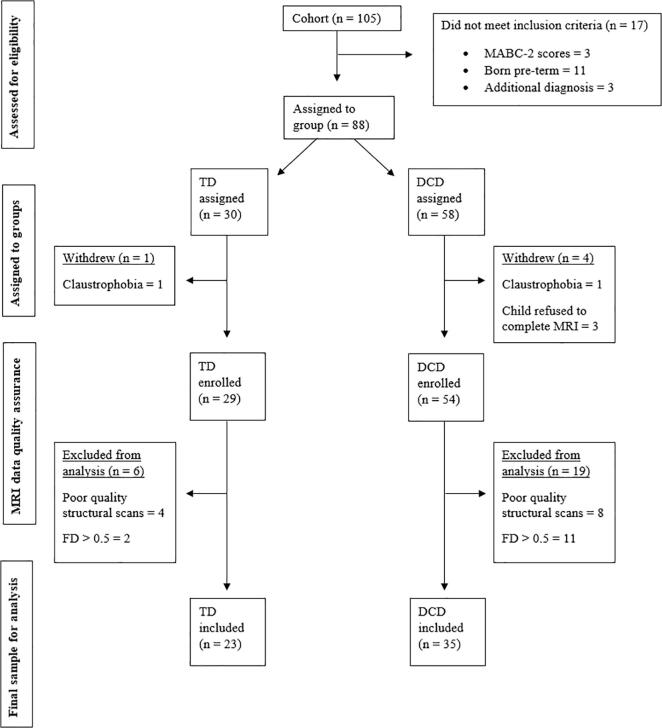
The overall cohort included 105 children recruited between September 2014 and January 2019, from which 88 participants met the inclusion criteria, and 58 had good quality structural and functional scans to be included in this study (Fig. 3). While we initially planned to compare functional connectivity between three groups (DCD, DCD + ADHD, TD), we had limited power due to a smaller sample size than anticipated. Therefore, we combined both DCD and DCD + ADHD groups for the analysis. The TD group included 23 children, and the DCD group included 35 children, 17 of whom were diagnosed with co-occurring ADHD (48%), which is similar to the co-occurrence rate reported in the literature. (Blank et al., 2019; Kadesjö and Christopher, 1999). Motor function as measured by MABC-2 scores and ADHD symptoms as measured by Conners 3 AI scores showed high correlation (Spearman’s r = −0.62, p < 0.001). Due to the high correlation between motor and ADHD symptoms, we did not include ADHD symptoms as a covariate in our analysis. There were no group differences in age or sex (Table 1), or in head motion between the groups in any of the motion parameters (Table 2).
Fig. 3.
Participant enrolment and exclusion chart. DCD, Developmental Coordination Disorder; FD, Framewise displacement; MABC-2, Movement Assessment Battery for Children – 2nd ed; TD, typically-developing children.
Table 1.
Participant characteristics.
| DCD (N = 35) N (%) or Mean (SD) |
TD (N = 23) N (%) or Mean (SD) |
p | |
|---|---|---|---|
| Male | 27 (77) | 15 (65) | 0.32 |
| Age (years) | 9.78 (1.6) | 9.9 (1.4) | 0.73 |
| MABC-2 (percentile) | 3.8 (4.5) | 64.8 (22.2) | 0.001* |
| DCDQ | 30.5 (10.1) | 65.4 (9) | 0.001* |
| Conner’s 3 AI (t-scores) | 81.9 (13.0) | 54.3 (12.3) | 0.001* |
AI, ADHD (Attention Deficit Hyperactivity Disorder) Index; DCD, Developmental Coordination Disorder; DCDQ, Developmental Coordination Disorder Questionnaire; MABC-2, Movement Assessment Battery for Children – 2nd ed; TD, typically-developing children.
Table 2.
Motion parameters.
| DCD Mean (SD) |
TD Mean (SD) |
t | p | |
|---|---|---|---|---|
| Framewise displacement (mm) | 0.20 (0.10) | 0.18 (0.13) | 0.56 | 0.57 |
| Root mean square | 0.34 (0.25) | 0.30 (0.27) | 0.62 | 0.53 |
| Relative displacement (mm) | 0.11 (0.06) | 0.10 (0.08) | 0.31 | 0.75 |
| Absolute displacement (mm) | 0.46 (0.37) | 0.59 (0.73) | 0.82 | 0.42 |
DCD, Developmental Coordination Disorder; TD, typically-developing children.
4.2. Group differences in functional connectivity
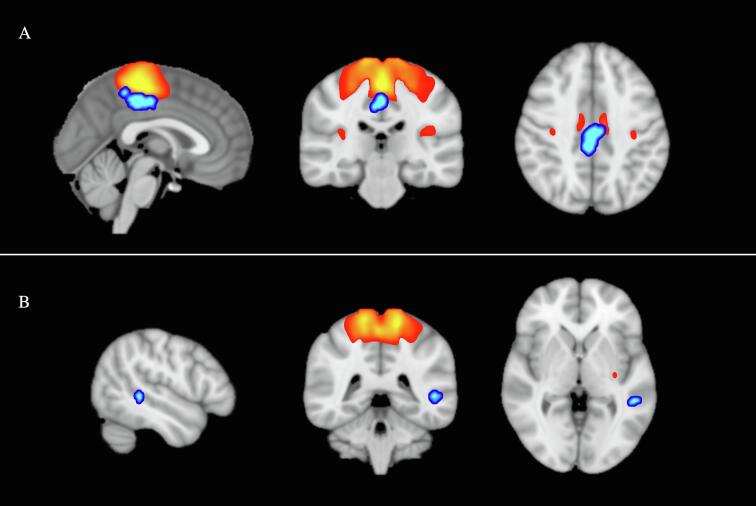
Significant group differences between the groups were found in functional connectivity of the sensorimotor network (Table 3; Fig. 4). The DCD group showed significantly less functional connectivity between the sensorimotor network and a cluster located at the PCC and precuneus bilaterally. A second cluster was found in the left posterior middle temporal gyrus (pMTG). Group differences identified in right PCC remained significant even following correction to adjust the p-value for multiple comparisons across all functional networks and contrasts (p = 0.006). We found no significant group differences in other functional networks.
Table 3.
Group differences in functional connectivity.a
| Network | Region (Harvard-Oxford Atlas) | MNI-space |
t | Cluster p | Cluster sizeb | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Sensorimotor | R + L PCC | 2 | −32 | 44 | 4.9 | <0.0001 | 317 | 1.31 |
| Sensorimotor | L middle temporal gyrus | −48 | −40 | 2 | 4.53 | <0.0001 | 32 | 1.21 |
PCC, posterior cingulate cortex.
Effects are shown at a threshold of p < 0.05 (FWE corrected, with TFCE), before correction for multiple comparisons across networks, and a minimum cluster size of 5 voxels. Effects in bold survived correction for multiple comparisons.
Number of voxels (voxel size = 2 mm).
Fig. 4.
Group differences in functional connectivity. A. Posterior cingulate cortex and precuneus (in blue) show significantly less functional connectivity with the sensorimotor network (in red) in children with DCD compared to TD children (p < 0.0001). B. Posterior middle temporal gyrus (in blue) show significantly less functional connectivity with the sensorimotor network (in red) in children with DCD compared to TD children (p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Discussion
5.1. Functional connectivity in DCD
This is the first study to investigate a whole-brain resting-state functional connectivity in children with DCD. Our results suggest that the neural impairment seen in DCD is predominantly in the sensorimotor network, which consists of the primary motor cortex, supplementary motor area, premotor cortex, primary somatosensory cortex, and the somatosensory association cortex. The disrupted functional connectivity between the sensorimotor network, the PCC, and precuneus could indicate impaired coordination in activation of the sensorimotor network and other functional networks, or a deficit in allocation of neural and attentional resources to the sensorimotor system (Hagmann et al., 2008, Jin and Jeong, 2013). Involvement of the pMTG could indicate a disruption between the semantic system that holds the mental representation of meaningful actions (Noonan et al., 2013, Papeo et al., 2015), and the sensorimotor network that produce these actions. These results contribute to our understanding of the underlying neural deficit in DCD.
5.2. PCC and precuneus in DCD
The results of our study indicate there is a disruption in functional connectivity between the PCC and precuneus and the sensorimotor network in DCD. These results are in line with several neuroimaging studies that showed atypical activation of the PCC and precuneus in DCD (Zwicker et al., 2010, Reynolds et al., 2015, Reynolds et al., 2019, Biotteau et al., 2015 Jul, Zwicker, 2010). In a study by Zwicker et al. (Zwicker et al., 2010, Zwicker, 2010), children with DCD showed significantly more activation of the PCC during fine motor task performance compared to the control group, while the control group showed increased activation in the precuneus. Following practice, the control group showed a significant reduction in PCC activation, but no such change was observed in the DCD group. In addition, activation of the PCC was negatively correlated with task performance in the control group. Two studies by Reynolds and colleagues (Reynolds et al., 2015, Reynolds et al., 2019) reported decreased activation of the PCC and precuneus in the DCD group compared to controls during a finger sequencing task, and a negative correlation between PCC and precuneus activation and performance of a praxis imitation task (Reynolds et al., 2015). The different activation patterns in Zwicker study compared to Reynolds’ studies could be different involvement of the PCC with varying levels of task complexity: Zwicker’s study evaluated novel and complex fine motor task, whereas Reynolds’ studies involved finger sequencing tasks, which likely required different cognitive resources. In a structural study, Reynolds et al. (2017) found a positive correlation between PCC and precuneus grey-matter volume and motor performance in the DCD and the control groups. Lastly, another study reported differences in PCC activation in children with DCD compared to children with developmental dyslexia. However, the lack of a TD control group prevents a comparison to typical activation patterns (Biotteau et al., 2015 Jul).
The weak functional connectivity between the PCC, precuneus, and the sensorimotor network could potentially be a key to understanding the neural nature of DCD. The PCC [Brodmann area (BA) 23 and 31] and the precuneus (BA 7 and 31) are components of several functional networks, including the default mode network, dorsal attention network, and fronto-parietal networks (Leech and Sharp, 2014, Cavanna and Trimble, 2006). The PCC is involved in many cognitive functions, including visual processing (Field et al., 2015, Hinkley et al., 2009), visuospatial navigation (Bzdok et al., 2015), decision-making (Heilbronner et al., 2011), working memory involving images (Baker et al., 2018), memory retrieval and emotion processing (Bzdok et al., 2015, Baker et al., 2018), and in motor performance (Field et al., 2015, Amiez and Petrides, 2014). The precuneus is active in self-related processes, such as during autobiographical (Addis et al., 2004) and episodic memory retrieval (Vilberg and Rugg, 2008, Dörfel et al., 2009), and during visuospatial processing (Cavanna and Trimble, 2006, Schott et al., 2019, Byrne et al., 2007, Brodt et al., 2016), navigation (Brodt et al., 2016), and motor imagery (Hétu et al., 2013). Many studies indicate precuneus involvement in different aspects of visuospatial processing. Visuospatial abilities were reported to be implicated in DCD (Tsai et al., 2009, Tsai et al., 2012, Wang et al., 2017). While this is a potential explanation to the precuneus involvement in DCD, other studies suggest that the precuneus subregion located within BA 31 is associated with self-relating processing, and propose it is a transitional zone between medial parietal regions and the PCC (Zhang and Li, 2012, Cavanna, 2007). Most of the neuroimaging studies that found precuneus involvement in DCD report similar involvement of the PCC (Reynolds et al., 2017, Reynolds et al., 2015, Reynolds et al., 2019, den Heuvel et al., 2010). Thus, the accumulating evidence to date seems to suggest that the activity of the precuneus and PCC is inter-dependent.
The characteristics of the PCC - including high and complex structural and functional connectivity, (Hagmann et al., 2008, Baker et al., 2018, Leech et al., 2012) very high metabolic rate, (Pfefferbaum et al., 2011) and involvement in variety of tasks - have supported the assumption that the PCC has a key role in cognitive function (Hagmann et al., 2008, Jin and Jeong, 2013, Leech and Sharp, 2014, Leech et al., 2012, Lord et al., 2017, Pearson et al., 2011). The PCC has been suggested to act as a hub for information processing, integrating information flow across the brain, coordinating activation of different functional networks, and regulating changes in foci of attention. [97,103,123,125,126] The involvement of the PCC in many other neurodevelopmental, neurological and psychiatric disorders, also support its central role as a functional hub. Moreover, changes to functional connectivity of the PCC were often related to severity of clinical symptoms, or, on the other hand, to treatment effect (Li et al., 2018, Abdallah et al., 2017, Li et al., 2017). Several studies show that abnormal connectivity of the PCC in ASD (Cherkassky et al., 2006, Kleinhans et al., 2008, Hull et al., 2017), which was correlated with social function and the severity of clinical symptoms of ASD (Li et al., 2017, Cherkassky et al., 2006). In ADHD, abnormal connectivity between PCC and the default mode network was reported (Uddin et al., 2009, Sripada et al., 2014). Disruption to functional connectivity of the PCC was evident in children with learning disabilities as well (Jäncke et al., 2019).
Considering the multi-dimensional role of the PCC (Leech and Sharp, 2014), we assume that the disrupted connectivity between the PCC and the sensorimotor network may indicate an inability to allocate the appropriate attentional resources for sensorimotor tasks, which in turn leads to a deficit in motor learning and motor performance. Such an interpretation suits the high level of inattention symptoms in DCD, both with and without co-occurring ADHD, and their association with level of motor impairment (Asonitou et al., 2012, Wilmut et al., 2007, Chen et al., 2012). If we consider the role of PCC as a functional hub for information processing that links and coordinates the activation of functional networks across the brain, altered functional connectivity between the PCC and the sensorimotor network might indicate an inability to integrate information from other networks to sensorimotor processing at the neural level.
5.3. Temporal involvement and praxis in DCD
We found altered functional connectivity between pMTG and the sensorimotor network in DCD compared to TD children. The pMTG was suggested to act as a semantic hub, an interface between the lingual and semantic representations of tools and actions, and the sensorimotor representation to which these refer (Xu et al., 2016, Tomasello et al., 2017). These results are in agreement with several other studies that have identified abnormalities in temporal regions (Reynolds et al., 2015, Debrabant et al., 2013, Zwicker et al., 2011, Zwicker et al., 2010, Caeyenberghs et al., 2016). In a task-based fMRI study by Reynolds et al. (2015) children with DCD had significantly less activation of left pMTG compared to the control group during action observation phase of a finger sequencing task. These results are in line with other work that investigated imitation, gestures, and tool use in DCD (Reynolds et al., 2017, Sinani et al., 2011, Costini et al., 2017, Hill et al., 1998). Children with DCD show slower, less accurate, and more variable patterns in gesture production tasks (Reynolds et al., 2017, Sinani et al., 2011, Costini et al., 2017). The few studies that evaluated action- or tool-related knowledge in DCD found no significant group differences (Sinani et al., 2011, Costini et al., 2017, Hill et al., 1998), and concluded that DCD does not involve a semantic knowledge problem in relation to action, gestures, or tool use (Costini et al., 2017). The pMTG is associated with different action-related functions, such as semantic action recognition, (Kalénine et al., 2010) action representation, (Wallentin et al., 2011, Davey et al., 2015) action monitoring during performance, (van Kemenade et al., 2019) and comparison of sensory input to sensory prediction (Yomogida et al., 2010, Wen et al., 2017, Aue et al., 2018). In a recent study, van Kemenade and colleagues (van Kemenade et al., 2019) suggested that the role of the pMTG is to detect the mismatch between predicted and actual sensory feedback, or the presence of conflicted inter-sensory input, in agreement with past results (Yomogida et al., 2010, Wen et al., 2017, Aue et al., 2018). Combining the current evidence regarding its functions makes it reasonable to conclude that the pMTG has a key role in organization and interpretation of action-related knowledge, linking together semantic and sensorimotor knowledge about meaningful actions (Noonan et al., 2013, Papeo et al., 2015).
Models of apraxia often distinguish between the conceptual knowledge system and the production system (Osiurak and Gall, 2012, Stamenova et al., 2012 Oct 01, Roy and Square, 1985). The conceptual system holds knowledge that supports the internal mental representation of an action (such as semantic knowledge about relevant tools, the actions for which they are used, and the relevant context); in some models, the conceptual system includes the body movements that are associated with the action as well (Stamenova et al., 2012 Oct 01, Roy and Square, 1985). The production system makes the necessary adaptations that allow appropriate execution of an action in a given context and environment (Roy and Square, 1985, Buxbaum, 2001 Feb 01). Our results indicate that disrupted connectivity between regions associated with action-related knowledge and the sensorimotor network is present in DCD. Such a disruption could explain the praxis problem associated with DCD – while the conceptual action-related knowledge exists, and even while the sensorimotor knowledge is intact (Yomogida et al., 2010), the communication of this knowledge to the production system (i.e., the sensorimotor network) is disrupted, potentially preventing efficient use of this knowledge and impairing motor learning.
5.4. Discrepancy with past results
The results of our study do not agree with previous studies investigating functional connectivity in DCD (McLeod et al., 2014, McLeod et al., 2016). There are several potential explanations for this disagreement, mainly significant methodological differences. McLeod and colleagues used seed-based analysis, a method that is prone to false-positive results, especially in presence of motion in the rsMRI data (Cole et al., 2010). Moreover, they did not perform denoising steps to alleviate the effect of motion on rsMRI data, which is widely acceptable as a necessary step (Satterthwaite et al., 2012, Power et al., 2015, Parkes et al., 2018). In addition, no correction for multiple comparisons was done in one study (McLeod et al., 2014) or was done only at the cluster level in the other (McLeod et al., 2016). While this is not uncommon practice in neuroimaging studies, this results in inflation of alpha level, which can dramatically increases the risk for false positive results (Eklund et al., 2016, Woo et al., 2014). Some of the reported clusters are outside of the brain, and the exact p values or the effect sizes for each cluster were not reported. Other methodological weaknesses include the wide age range of participants (8–17 years) and no control for age and sex in their analyses, and a relatively small sample size (7 participants with DCD; 18 participants with DCD + ADHD). In contrast, the results of our study show large effect size (Cohen’s d > 1 for both clusters) and high statistical significance (p < 0.0001) for the group differences following correction for multiple comparisons using TFCE. The group differences identified in the PCC and precuneus remain significant even following adjusted p-value to account for multiple testing across all functional networks and contrasts. We have included relatively large sample, used rigorous denoising steps, and an analysis method that is less sensitive to motion.
5.5. Clinical implications
The results of this study join the growing body of evidence that indicate the neural impairments that underpin the motor deficit in DCD. Our results further support the understanding that children with DCD cannot learn and perform motor tasks in the same way as TD children. These results may help therapists explain to parents why children with DCD struggle to learn motor skills. These findings can guide future research and development of new interventions.
5.6. Limitations and future directions
The final sample size in our study was smaller than anticipated. The reduced power prevented the planned comparison between children with DCD and children with co-occurring DCD and ADHD. In addition, the high correlation between motor function and ADHD symptoms prevented us from including ADHD symptoms as a covariate in our analysis. This should be considered when interpreting our findings. While the rate of co-occurrence DCD and ADHD in our sample is similar to rates reported in the literature, and therefore is representative of children with DCD, future studies should examine functional connectivity in DCD with and without co-occurring ADHD, and investigate differences between the two groups, as we initially planned to do. In addition, it is possible that other, more subtle differences in functional connectivity exist in DCD, but require more statistical power to detect. This smaller sample size is partially due to high rate of participants’ exclusion from the study to ensure we had good quality data. While this limitation should be acknowledged, similar exclusion rates are quite common in resting-state MRI studies in pediatric populations, especially those with neurodevelopmental disorders (Wilson et al., 2009, Yerys et al., 2009). Another limitation is due to our inability to control for participants visual stimulus during scans (whether their eyes were open or closed), which prevented analysis of visual networks. Future studies should address this unanswered question. Finally, the results of our study do not agree with previous studies on functional connectivity in DCD. Methodological differences, as discussed above, could be the reason for this discrepancy. Our study included a well phenotyped sample, extensive denoising steps in our analysis, and large group differences (Cohen’s d > 1) for both clusters. The group differences identified in PCC and precuneus remain significant even following the most stringent correction for multiple testing. These results are robust compared to most neuroimaging studies in the field, and improved our understanding of the neuro-deficit associated with DCD. Future research should build upon our study results and extend our understanding of neural impairment seen in DCD, as well as the effect of treatment on functional connectivity in this population.
6. Conclusions
We used rsMRI to study functional connectivity across the brain in children with DCD compared to TD peers. Our results indicate a disruption in functional connectivity in DCD in two main regions connected to the sensorimotor network: one is located at the PCC, extending to the precuneus, and the other is located in pMTG. These results suggest that the neural impairment seen in DCD is predominantly in the sensorimotor network. Disrupted functional connectivity between the PCC and the sensorimotor network could indicate a deficit in allocation of attentional and neural resources to the sensorimotor system, or impaired coordination in activation between the sensorimotor network and other functional networks. The involvement of the pMTG could indicate a disruption between the semantic system that holds the mental representation of meaningful actions, and the sensorimotor network that produce these actions. The results of our study increase our understanding of the neural impairment seen in DCD, and provide potential explanation as to why children with DCD struggle to learn motor skills.
CRediT authorship contribution statement
Shie Rinat: Methodology, Investigation, Formal analysis, Data curation, Writing - original draft. Sara Izadi-Najafabadi: Methodology, Investigation, Formal analysis, Writing - review & editing. Jill G. Zwicker: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank Kamaldeep Gill, Meisan Brown-Lum, Gisela Gosse, and Janet Rigney for their assistance with data collection and data management. We thank all the families and children who participated in our study – we are truly grateful for your contribution. We would like to thank Dr. Liisa Holsti and Dr. Lara Boyd for their guidance.
Funding
The research project was funded by the Canadian Institutes of Health Research (FDN-143258). Rinat was funded by the Jenny Panitch Beckow Memorial Scholarship from the Jewish Community Foundation of Montreal. Izadi-Najafabadi is funded by a Brain Canada/NeuroDevNet Research Training Award and a Four Year Fellowship (4YF) from the University of British Columbia. Dr. Zwicker is funded by the Michael Smith Foundation for Health Research, Canadian Child Health Clinician Scientist Program, BC Children’s Hospital Research Institute, Sunny Hill Foundation, and Canadian Institutes of Health Research.
References
- Abdallah C.G., Averill L.A., Collins K.A., Geha P., Schwartz J., Averill C., DeWilde K.E., Wong E., Anticevic A., Tang C.Y., Iosifescu D.V. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A., Milham M.P., Di Martino A., Craddock R.C., Samaras D., Thirion B., Varoquaux G. Deriving reproducible biomarkers from multi-site resting-state data: An Autism-based example. Neuroimage. 2017;15(147):736–745. doi: 10.1016/j.neuroimage.2016.10.045. [DOI] [PubMed] [Google Scholar]
- Addis D.R., McIntosh A.R., Moscovitch M., Crawley A.P., McAndrews M.P. Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage. 2004;23(4):1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Fifth edition ed. Washington, DC: American Psychiatric Publishing; 2013.
- Amiez C., Petrides M. Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb. Cortex. 2014;24(3):563–578. doi: 10.1093/cercor/bhs329. [DOI] [PubMed] [Google Scholar]
- Asonitou K., Koutsouki D., Kourtessis T., Charitou S. Motor and cognitive performance differences between children with and without developmental coordination disorder (DCD) Res. Dev. Disabil. 2012;33(4):996–1005. doi: 10.1016/j.ridd.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Aue T., Guex R., Chauvigné L.A.S., Okon-Singer H., Vuilleumier P. Expectancies influence attention to neutral but not necessarily to threatening stimuli: An fMRI study. Emotion. 2018 doi: 10.1037/emo0000496. [DOI] [PubMed] [Google Scholar]
- Baker C.M., Burks J.D., Briggs R.G., Conner A.K., Glenn C.A., Manohar K. A connectomic atlas of the human cerebrum-chapter 8: The posterior cingulate cortex, medial parietal lobe, and parieto-occipital sulcus. Oper Neurosurg. 2018;15(suppl_1):S371. doi: 10.1093/ons/opy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarz H.M., Kana R.K. Advances, challenges, and promises in pediatric neuroimaging of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2018;31(90):50–69. doi: 10.1016/j.neubiorev.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biotteau M., Chaix Y., Albaret J. Procedural learning and automatization process in children with developmental coordination disorder and/or developmental dyslexia. Hum. Mov. Sci. 2015 Jul;21(43):78–89. doi: 10.1016/j.humov.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Biotteau M., Péran P., Vayssière N., Tallet J., Albaret J., Chaix Y. Neural changes associated to procedural learning and automatization process in developmental coordination disorder and/or developmental dyslexia. Eur. J. Paediatr. Neurol. 2016;21(2):286–299. doi: 10.1016/j.ejpn.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Biswal B.B. Resting state fMRI: A personal history. Neuroimage. 2012;62(2):938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- Blank R. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019;61(3):242–285. doi: 10.1111/dmcn.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt S., Poehlchen D., Gais S., Schönauer M. Rapid and independent memory formation in the parietal cortex. PNAS. 2016;113(46):3251–13256. doi: 10.1073/pnas.1605719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J. Ideomotor apraxia: a call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Byrne P., Becker S., Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol. Rev. 2007;114(2):340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Heeger A., Langner R., Laird A.R., Fox P.T., Palomero-Gallagher N. Subspecialization in the human posterior medial cortex. Neuroimage. 2015;01(106):55–71. doi: 10.1016/j.neuroimage.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caçola P., Getchell N., Srinivasan D., Alexandrakis G., Liu H. Cortical activity in fine-motor tasks in children with Developmental Coordination Disorder: A preliminary fNIRS study. Int. J. Dev. Neurosci. 2018;03(65):83–90. doi: 10.1016/j.ijdevneu.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Taymans T., Wilson P.H., Vanderstraeten G., Hosseini H., Waelvelde H. Neural signature of developmental coordination disorder in the structural connectome independent of comorbid autism. Dev. Sci. 2016;19(4):599–612. doi: 10.1111/desc.12424. [DOI] [PubMed] [Google Scholar]
- Cairney J., Missiuna C., Veldhuizen S., Wilson B. Evaluation of the psychometric properties of the developmental coordination disorder questionnaire for parents (DCD-Q): Results from a community based study of school-aged children. Hum. Mov. Sci. 2008;27(6):932–940. doi: 10.1016/j.humov.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1):S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Di Martino A., Craddock R.C., Mehta A.D., Milham M.P. Clinical applications of the functional connectome. Neuroimage. 2013;15(80):527–540. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E. The precuneus and consciousness. CNS Spectr. 2007;12(7):545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006 1 Mar;129(3):564-583. [DOI] [PubMed]
- Chen H., Cohn E.S. Social participation for children with developmental coordination disorder: conceptual, evaluation and intervention considerations. Phys. Occup. Ther. Pediatr. 2003;23(4):61–78. [PubMed] [Google Scholar]
- Chen W., Wilson P.H., Wu S.K. Deficits in the covert orienting of attention in children with developmental coordination disorder: Does severity of DCD count? Res. Dev. Disabil. 2012;33(5):1516–1522. doi: 10.1016/j.ridd.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154(1):174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4(8):8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners, C.K., Pitkanen, J., Rzepa, S.R. Conners 3rd Edition. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology: Springer, New York, NY; 2012. p. 675-678.
- Conners, C.K. Conners 3rd edition manual. Toronto, Ontario, Canada: Multi-Health Systems; 2008.
- Costini O., Roy A., Remigereau C., Faure S., Fossoud C., Le Gall D. Nature and specificity of gestural disorder in children with developmental coordination disorder: a multiple case study. Front. Psychol. 2017;04(8):995. doi: 10.3389/fpsyg.2017.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins M., Smyth M.M. Developmental coordination impairments in adulthood. Hum. Mov. Sci. 2003;22(4–5):433–459. doi: 10.1016/j.humov.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Davey J., Cornelissen P.L., Thompson H.E., Sonkusare S., Hallam G., Smallwood J. Automatic and controlled semantic retrieval: tms reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J. Neurosci. 2015;35(46):15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrabant J., Gheysen F., Caeyenberghs K., Van Waelvelde H., Vingerhoets G. Neural underpinnings of impaired predictive motor timing in children with developmental coordination disorder. Res. Dev. Disabil. 2013;34(5):1478–1487. doi: 10.1016/j.ridd.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Debrabant J., Vingerhoets G., Van Waelvelde H., Leemans A., Taymans T., Caeyenberghs K. Brain connectomics of visual-motor deficits in children with developmental coordination disorder. J. Pediatr. 2016;02(169):21–27. doi: 10.1016/j.jpeds.2015.09.069. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel, Martijn P., Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol, 2010 May 14;20(8):519-534. [DOI] [PubMed]
- Dörfel D., Werner A., Schaefer M., von Kummer R., Karl A. Distinct brain networks in recognition memory share a defined region in the precuneus. Eur. J. Neurosci. 2009;30(10):1947. doi: 10.1111/j.1460-9568.2009.06973.x. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PANS. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Mukherjee P., Schweitzer J.B. Minimizing noise in pediatric task-based functional MRI; Adolescents with developmental disabilities and typical development. Neuroimage. 2017;1(149):338–347. doi: 10.1016/j.neuroimage.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D.T., Inman L.A., Li L. Visual processing of optic flow and motor control in the human posterior cingulate sulcus. Cortex. 2015;31(71):377–389. doi: 10.1016/j.cortex.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage. 2012 Jan 10;62(2):774-781. [DOI] [PMC free article] [PubMed]
- Fuelscher I., Caeyenberghs K., Enticott P.G., Williams J., Lum J., Hyde C. Differential activation of brain areas in children with developmental coordination disorder during tasks of manual dexterity: an ALE meta-analysis. Neurosci. Biobehav. Rev. 2018;12(86):77–84. doi: 10.1016/j.neubiorev.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Gomez A., Sirigu A. Developmental coordination disorder: Core sensori-motor deficits, neurobiology and etiology. Neuropsychologia. 2015;79(Pt B):272–287. doi: 10.1016/j.neuropsychologia.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Goulardins J.B., Rigoli D., Licari M., Piek J.P., Hasue R.H., Oosterlaan J. Attention deficit hyperactivity disorder and developmental coordination disorder: Two separate disorders or do they share a common etiology. Behav. Brain Res. 2015;01(292):484–492. doi: 10.1016/j.bbr.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. Neuroimage. 2017;15(160):15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Salimi-Khorshidi G., Beckmann C.F., Auerbach E.J., Douaud G., Sexton C.E. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;15(95):232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M.F. Hand classification of fMRI ICA noise components. Neuroimage. 2017;1(154):188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C.J., Wedeen V.J. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7) doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.R., Mickelson E.C.R., Zwicker J.G. Diagnosis and management of developmental coordination disorder. CMAJ. 2015;187(9):659–665. doi: 10.1503/cmaj.140994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrowell I., Hollén L., Lingam R., Emond A. Mental health outcomes of developmental coordination disorder in late adolescence. Dev. Med. Child Neurol. 2017;59(9):973–979. doi: 10.1111/dmcn.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner S.R., Hayden B.Y., Platt M.L. Decision salience signals in posterior cingulate cortex. Front. Neurosci. 2011;5(55):55. doi: 10.3389/fnins.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, S., Sugden, D., Barnett, A. Movement assessment battery for children-2 (MABC-2). 2007.
- Hétu S., Grégoire M., Saimpont A., Coll M.P., Eugène F., Michon P.E. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013;37(5):930–949. doi: 10.1016/j.neubiorev.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Hill E.L., Bishop D.V.M., Nimmo-Smith I. Representational gestures in Developmental Coordination Disorder and specific language impairment: error-types and the reliability of ratings. Hum. Mov. Sci. 1998;17(4):655–678. [Google Scholar]
- Leighton, B.N., Hinkley, Leah A. Krubitzer, Jeff Padberg, Elizabeth A. Disbrow. Visual-manual exploration and posterior parietal cortex in humans. J. Neurophysiol. 2009 Dec 1;102(6):3433-3446. [DOI] [PMC free article] [PubMed]
- Hull J.V., Dokovna L.B., Jacokes Z.J., Torgerson C.M., Irimia A., Van Horn J.D. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry. 2017;4(7):205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi-Najafabadi S., Ryan N., Ghafooripoor G., Gill K., Zwicker J.G. Participation of children with developmental coordination disorder. Res. Dev. Disabil. 2019;1(84):75–84. doi: 10.1016/j.ridd.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Jäncke L., Saka M.Y., Badawood O., Alhamadi N. Resting-state electroencephalogram in learning-disabled children: power and connectivity analyses. NeuroReport. 2019;30(2):95–101. doi: 10.1097/WNR.0000000000001166. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Seung-Hyun Jin, Woorim Jeong, Jaeho Seol, Jiyeon Kwon, Chun Kee Chung. Functional cortical hubs in the eyes-closed resting human brain from an electrophysiological perspective using magnetoencephalography. PLoS One. 2013 Jul 09;8(7):e68192. [DOI] [PMC free article] [PubMed]
- Kadesjö B., Christopher G. Developmental coordination disorder in Swedish 7-year-old children. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(7):820–828. doi: 10.1097/00004583-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Kadesjö B., Gillberg C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev. Med. Child Neurol. 1998 Dec 21;40(12):796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x. [DOI] [PubMed] [Google Scholar]
- Kadesjö B., Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. J. Child Psychol. Psychiatry. 2001;42(4):487–492. [PubMed] [Google Scholar]
- Kalénine S., Buxbaum L.J., Coslett H.B. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi M., Iwaki S., Narumi Y., Tamai H., Suzuki S. Parietal dysfunction in developmental coordination disorder: a functional MRI study. NeuroReport. 2009;20(15):1319–1324. doi: 10.1097/WNR.0b013e32832f4d87. [DOI] [PubMed] [Google Scholar]
- Kashuk S.R., Williams J., Thorpe G., Wilson P.H., Egan G.F. Diminished motor imagery capability in adults with motor impairment: an fMRI mental rotation study. Behav. Brain Res. 2017;334(15):86–96. doi: 10.1016/j.bbr.2017.06.042. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Lange S. ADHD and comorbid developmental coordination disorder: Implications and recommendations for school psychologists. Contemp. School Psychol. 2018;22(1):30–39. [Google Scholar]
- Leech R., Braga R., Sharp D.J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012;32(1):215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.J., Friston K., Mody M., Wang H.N., Lu H.B., Hu D.W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018;24(11):1004–1019. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wang Q., Zhang J., Rolls E.T., Yang W., Palaniyappan L., Zhang L., Cheng W., Yao Y., Liu Z., Gong X. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr. Bull. 2017;43(2):436–448. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari M., Billington J., Reid S., Wann J., Elliott C., Winsor A. Cortical functioning in children with developmental coordination disorder: a motor overflow study. Exp. Brain Res. 2015;233(6):1703–1710. doi: 10.1007/s00221-015-4243-7. [DOI] [PubMed] [Google Scholar]
- Lingam R., Hunt L., Golding J., Jongmans M., Emond A. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: A UK population-based study. Pediatrics. 2009;123(4) doi: 10.1542/peds.2008-1770. [DOI] [PubMed] [Google Scholar]
- Lord A.R., Li M., Demenescu L.R., Van der Meer J., Borchardt V., Krause A.L. Richness in functional connectivity depends on the neuronal integrity within the posterior cingulate cortex. Front. Neurosci. 2017;7(11):184. doi: 10.3389/fnins.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P., Wackenier P., De Surgeloose D., De Deyn P.P., Verhoeven J. Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum. 2010;9(3):405–410. doi: 10.1007/s12311-010-0177-6. [DOI] [PubMed] [Google Scholar]
- Martin N.C., Piek J.P., Hay D. DCD and ADHD: A genetic study of their shared etiology. Hum. Mov. Sci. 2006;25(1):110–124. doi: 10.1016/j.humov.2005.10.006. [DOI] [PubMed] [Google Scholar]
- McLeod K.R., Langevin L.M., Goodyear B.G., Dewey D. Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin. 2014;26(4):566–575. doi: 10.1016/j.nicl.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod K.R., Langevin L.M., Dewey D., Goodyear B.G. Atypical within- and between-hemisphere motor network functional connections in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin. 2016;28(12):157–164. doi: 10.1016/j.nicl.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi S., Pesenti-Gritti P., Brescianini S., Salemi M., Battaglia M., Ogliari A. Clumsiness and psychopathology: Causation or shared etiology? A twin study with the CBCL 6–18 questionnaire in a general school-age population sample. Hum. Mov. Sci. 2010;29(2):326–338. doi: 10.1016/j.humov.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Muetzel R.L., Blanken L.M.E., Thijssen S., van der Lugt A., Jaddoe V.W.V., Verhulst F.C. Resting-state networks in 6-to-10 year old children. Hum. Brain Mapp. 2016;37(12):4286–4300. doi: 10.1002/hbm.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Lambon Ralph M.A. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Osiurak, F., Gall, D.L. Apraxia: Clinical Types, Theoretical models, and evaluation. In: Heinbockel T, editor. Neuroscience: IntechOpen; 2012.
- Papeo L., Lingnau A., Agosta S., Pascual-Leone A., Battelli L., Caramazza A. The origin of word-related motor activity. Cereb. Cortex. 2015;25(6):1668–1675. doi: 10.1093/cercor/bht423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;1(171):415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Pearsall-Jones J.G., Piek J.P., Rigoli D., Martin N.C., Levy F. Motor disorder and anxious and depressive symptomatology: a monozygotic co-twin control approach. Res. Dev. Disabil. 2011;32(4):1245–1252. doi: 10.1016/j.ridd.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Pearson J.M., Heilbronner S.R., Barack D.L., Hayden B.Y., Platt M.L. Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Chanraud S., Pitel A., Müller-Oehring E., Shankaranarayanan A., Alsop D.C. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb. Cortex. 2011;21(1):233–244. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinares-Garcia P., Stratikopoulos M., Zagato A., Loke H., Lee J. Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 2018;8(8):154. doi: 10.3390/brainsci8080154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polyak A., Rosenfeld J.A., Girirajan S. An assessment of sex bias in neurodevelopmental disorders. Genome Med. 2015;7(1):94. doi: 10.1186/s13073-015-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;15(105):536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Plitt M., Laumann T.O., Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;1(146):609–625. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psotta R., Abdollahipour R. Factorial validity of the Movement Assessment Battery for Children—2nd Edition (MABC-2) in 7–16-year-olds. Percept. Mot. Skills. 2017;124(6):1051–1068. doi: 10.1177/0031512517729951. [DOI] [PubMed] [Google Scholar]
- Querne L., Berquin P., Vernier-Hauvette M.P., Fall S., Deltour L., Meyer M.E. Dysfunction of the attentional brain network in children with developmental coordination disorder: A fMRI study. Brain Res. 2008;09(1244):89–102. doi: 10.1016/j.brainres.2008.07.066. [DOI] [PubMed] [Google Scholar]
- Rasmussen P., Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(11):1424–1431. doi: 10.1097/00004583-200011000-00017. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Licari M.K., Billington J., Chen Y., Aziz-Zadeh L., Werner J. Mirror neuron activation in children with developmental coordination disorder: a functional MRI study. Int. J. Dev. Neurosci. 2015;47(Pt B):309–319. doi: 10.1016/j.ijdevneu.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Licari M.K., Reid S.L., Elliott C., Winsor A.M., Bynevelt M. Reduced relative volume in motor and attention regions in developmental coordination disorder: a voxel-based morphometry study. Int. J. Dev. Neurosci. 2017;21(58):59–64. doi: 10.1016/j.ijdevneu.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Kerrigan S., Elliott C., Lay B.S., Licari M.K. Poor imitative performance of unlearned gestures in children with probable developmental coordination disorder. J. Mot. Behav. 2017;49(4):378–387. doi: 10.1080/00222895.2016.1219305. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Billington J., Kerrigan S., Williams J., Elliott C., Winsor A.M. Mirror neuron system activation in children with developmental coordination disorder: A replication functional MRI study. Res. Dev. Disabil. 2019;3(84):16–27. doi: 10.1016/j.ridd.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Rivilis I., Hay J., Cairney J., Klentrou P., Liu J., Faught B.E. Physical activity and fitness in children with developmental coordination disorder: A systematic review. Res. Dev. Disabil. 2011;32(3):894–910. doi: 10.1016/j.ridd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. MRIcron dicom to nifti converter. 2012.
- Roy, E.A., Square, P.A. Common considerations in the study of limb, verbal and oral apraxia. Advances in Psychology. North-Holland: Elsevier Science & Technology; 1985. p. 111-161.
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;15(90):449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott B.H., Wüstenberg T., Lücke E., Pohl I., Richter A., Seidenbecher C.I. Gradual acquisition of visuospatial associative memory representations via the dorsal precuneus. Hum. Brain Mapp. 2019;40(5):1554–1570. doi: 10.1002/hbm.24467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J., Henderson S.E., Sugden D.A., Barnett A.L. Structural validity of the Movement ABC-2 test: Factor structure comparisons across three age groups. Res. Dev. Disabil. 2011;32(4):1361–1369. doi: 10.1016/j.ridd.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Sinani, C.., Sugden, D.A., Hill, E.L. Gesture production in school vs. clinical samples of children with Developmental Coordination Disorder (DCD) and typically developing children. Res. Dev. Disabil. 2011 Feb 24;32(4):1270-1282. [DOI] [PubMed]
- Smith S., Laird A., Glahn D., Fox P., Fox P., Mackay C. FMRI resting state networks match BrainMap activation networks. Neuroimage. 2009;47(1):S147. [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vidaurre D., Beckmann C.F., Glasser M.F., Jenkinson M., Miller K. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;12(17):668–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth T.R. Impaired motor skill (clumsiness) in otherwise normal children: a review. Child Care Health Dev. 1992;18(5):283–300. doi: 10.1111/j.1365-2214.1992.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35(9):4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenova V., Black S.E., Roy E.A. An update on the Conceptual-Production Systems model of apraxia: evidence from stroke. Brain Cogn. 2012 Oct 01;80(1):53–63. doi: 10.1016/j.bandc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Subbaraju V., Suresh M.B., Sundaram S., Narasimhan S. Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Med. Image Anal. 2017;1(35):375–389. doi: 10.1016/j.media.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Thapar A., Cooper M. Attention deficit hyperactivity disorder. Lancet. 2015;387(10024):1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- The MathWorks I. MATLAB and statistics toolbox release. R2017a (9.2.0).
- Thornton S., Bray S., Langevin L.M., Dewey D. Functional brain correlates of motor response inhibition in children with developmental coordination disorder and attention deficit/hyperactivity disorder. Hum. Mov. Sci. 2018;11(59):134–142. doi: 10.1016/j.humov.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Tomasello R., Garagnani M., Wennekers T., Pulvermüller F. Brain connections of words, perceptions and actions: a neurobiological model of spatio-temporal semantic activation in the human cortex. Neuropsychologia. 2017;1(98):111–129. doi: 10.1016/j.neuropsychologia.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Tsai C., Chang Y., Hung T., Tseng Y., Chen T. The neurophysiological performance of visuospatial working memory in children with developmental coordination disorder. Dev. Med. Child Neurol. 2012;54(12):1114–1120. doi: 10.1111/j.1469-8749.2012.04408.x. [DOI] [PubMed] [Google Scholar]
- Tsai C.L., Pan C.Y., Cherng R.J., Hsu Y.W., Chiu H.H. Mechanisms of deficit of visuospatial attention shift in children with developmental coordination disorder: a neurophysiological measure of the endogenous Posner paradigm. Brain Cogn. 2009;71(3):246–258. doi: 10.1016/j.bandc.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Clare Kelly A.M., Biswal B.B., Xavier Castellanos F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaivre-Douret L. Developmental coordination disorders: State of art. Neurophysiol. Clin. 2014;44(1):13–23. doi: 10.1016/j.neucli.2013.10.133. [DOI] [PubMed] [Google Scholar]
- van Kemenade B.M., Arikan B.E., Podranski K., Steinsträter O., Kircher T., Straube B. Distinct roles for the cerebellum, angular gyrus, and middle temporal gyrus in action-feedback monitoring. Cereb. Cortex. 2019;29(4):1520–1531. doi: 10.1093/cercor/bhy048. [DOI] [PubMed] [Google Scholar]
- Vedul‐Kjelsås, V., Sigmundsson, H., Stensdotter, A., Haga, M., The relationship between motor competence, physical fitness and self‐perception in children. Child Care Health Dev. 2012 May 01;38(3):394-402. [DOI] [PubMed]
- Vilberg, K.L., Rugg, M.D. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008 Jan 117;46(7):1787-1799. [DOI] [PMC free article] [PubMed]
- Wagner M.O., Kastner J., Petermann F., Bös K. Factorial validity of the Movement Assessment Battery for Children-2 (age band 2) Res. Dev. Disabil. 2011;32(2):674–680. doi: 10.1016/j.ridd.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Wallentin M., Nielsen A.H., Vuust P., Dohn A., Roepstorff A., Lund T.E. BOLD response to motion verbs in left posterior middle temporal gyrus during story comprehension. Brain Lang. 2011;119(3):221–225. doi: 10.1016/j.bandl.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Wang C., Tseng Y., Liu D., Tsai C. Neural oscillation reveals deficits in visuospatial working memory in children with developmental coordination disorder. Child Dev. 2017;88(5):1716–1726. doi: 10.1111/cdev.12708. [DOI] [PubMed] [Google Scholar]
- Waszczuk M.A., Leonard H.C., Hill E.L., Rowe R., Gregory A.M. Coordination difficulty and internalizing symptoms in adults: a twin/sibling study. Psychiatry Res. 2016;30(239):1–8. doi: 10.1016/j.psychres.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Watemberg N., Waiserberg N., Zuk L., Lerman-Sagie T. Developmental coordination disorder in children with attention-deficit–hyperactivity disorder and physical therapy intervention. Dev. Med. Child Neurol. 2007;49(12):920–925. doi: 10.1111/j.1469-8749.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Wen X., Xiang Y., Cant J., Wang T., Cupchik G., Huang R. The neural correlates of internal and external comparisons: An fMRI study. Brain Struct. Funct. 2017;222(1):563–575. doi: 10.1007/s00429-016-1234-9. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. CONN toolbox. 2012;v18. [DOI] [PubMed]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilmut K., Brown J.H., Wann J.P. Attention disengagement in children with developmental coordination disorder. Disabil. Rehabil. 2007;29(1):47–55. doi: 10.1080/09638280600947765. [DOI] [PubMed] [Google Scholar]
- Wilson B.N., Kaplan B.J., Crawford S.G., Campbell A., Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. Am. J. Occup. Ther. 2000;54(5):484–493. doi: 10.5014/ajot.54.5.484. [DOI] [PubMed] [Google Scholar]
- Wilson B.N., Crawford S.G., Green D., Roberts G., Aylott A., Kaplan B.J. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys. Occup. Ther. Pediatr. 2009;29(2):182–202. doi: 10.1080/01942630902784761. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92(100):381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Webster M.A., Brooks J.C., Tracey I., Smith S.M., Nichols T.E. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp. 2016;37(4):1486–1511. doi: 10.1002/hbm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;1(91):412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1):S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Wuang Y.P., Su J.H., Su C.Y. Reliability and responsiveness of the Movement Assessment Battery for Children-Second edition test in children with developmental coordination disorder. Dev. Med. Child Neurol. 2012;54(2):160–165. doi: 10.1111/j.1469-8749.2011.04177.x. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lin Q., Han Z., He Y., Bi Y. Intrinsic functional network architecture of human semantic processing: Modules and hubs. Neuroimage. 2016;15(132):542–555. doi: 10.1016/j.neuroimage.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Yerys, B.E., Jankowski, K.F., Shook, D., Rosenberger, L.R., Barnes, K.A., Berl, M.M., et al. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp. 2009 1 Oct;30(10):3426-3435. [DOI] [PMC free article] [PubMed]
- Yomogida Y., Sugiura M., Sassa Y., Wakusawa K., Sekiguchi A., Fukushima A. The neural basis of agency: an fMRI study. Neuroimage. 2010;50(1):198–207. doi: 10.1016/j.neuroimage.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.R. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics. 2010;126(3) doi: 10.1542/peds.2010-0059. [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation associated with motor skill practice in children with developmental coordination disorder: An fMRI study. Int. J. Dev. Neurosci. 2011;29(2):145–152. doi: 10.1016/j.ijdevneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Harris S.R., Klassen A.F. Quality of life domains affected in children with developmental coordination disorder: a systematic review. Child Care Health Dev. 2013;39(4):562–580. doi: 10.1111/j.1365-2214.2012.01379.x. [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Suto M., Harris S.R., Vlasakova N., Missiuna C. Developmental coordination disorder is more than a motor problem: Children describe the impact of daily struggles on their quality of life. Br. J. Occup. Ther. 2018;81(2):65–73. [Google Scholar]
- Zwicker, J.G. Neural and behavioural correlates of motor performance in children with developmental coordination disorder. Vancouver: The University of British Columbia; 2010.