Abstract
Background: The 3 ml volume currently used as the hand hygiene (HH) measure has been explored as the pertinent dose for an indirect indicator of HH compliance. A multicenter study was conducted in order to ascertain the required dose using different products.
Method: The average contact duration before drying was measured and compared with references. Effective hand coverage had to include the whole hand and the wrist. Two durations were chosen as points of reference: 30 s, as given by guidelines, and the duration validated by the European standard EN 1500. Each product was to be tested, using standardized procedures, by three nosocomial infection prevention teams, for three different doses (3, 2 and 1.5 ml).
Results: Data from 27 products and 1706 tests were analyzed. Depending on the product, the dose needed to ensure a 30-s contact duration in 75% of tests ranging from 2 ml to more than 3 ml, and to ensure a contact duration exceeding the EN 1500 times in 75% of tests ranging from 1.5 ml to more than 3 ml. The aftermath interpretation is the following: if different products are used, the volume utilized does not give an unbiased estimation of the HH compliance. Other compliance evaluation methods remain necessary for efficient benchmarking.
Keywords: Hand hygiene, Compliance, Hand rub, Benchmarking
1. Introduction
With the development of benchmarking and public reporting for infection control, indirect hand hygiene (HH) indicators have been developed, with the aim of giving repeated data more frequently than was possible with classic compliance studies [1]. The most common HH indicator translates a quantity of consumed hand rubs into a number of HH actions and calculates the number of HH actions by patients/days. This method has been successfully used for time series analysis on the evaluation of prevention policies or for comparison between units within a hospital or within a hospital group using the same products [2–6], but some studies have highlighted the limited use of this tool for wider comparisons [7–9] with an exception in the case of a very large difference [7]. The necessity to validate each component of such an indicator has arisen (i.e., dose, activity reference) for effective reproducibility and unbiased historic comparisons. The European historic choice of a 3 ml dose as reference is based on the EN 1500 standard, which required the comparison of tested products to two applications of 3 ml of propanol [10]. At the present time, this dose is challenged because hand rub suppliers do recommend different doses on their instructions and have also validated the standard EN 1500 with different doses. Furthermore, there is no systematic link between the suppliers’ recommended dose and the one needed to validate the EN 1500. The actual dose distributed by cheap dispensers is frequently variable as well. The initial dose ranges from 1 to 3 ml, but after a short period of use, the actual dose is very frequently smaller as demonstrated by the Kohan study [11]. The aim of this multicenter prospective open trial was to define the required dose for correct HH while using different rubs.
2. Methods
2.1. Objective
For a dose and a product, the evaluation criteria were: proper coverage of the whole hand, average contact duration before drying, and accuracy of this duration according to available references. Three doses were tested (3, 2 and 1.5 ml).
2.2. Definition of correct duration of hand hygiene
For the study, the duration of HH was considered correct for a dose and a product if:
-
•
According to the 2002 French recommendations (http://www.sf2h.net/publications-SF2H/SF2H_recommandations_hygiene-des-mains-2009.pdf), at least 75% of tests allowed for contact duration longer than or equal to 30 s.
-
•
According to the EN 1500, at least 75% of tests allowed for contact duration longer than or equal to that of the contact time validated by the product by means of EN 1500 [10].
Duration was counted in seconds from the spreading of the product to the very moment when any treated part of the hand is considered dry. A horizontal light was used for better evaluation. The dose was measured with a syringe (one syringe was used for every product). Duration was measured by the operator with a stopwatch.
2.3. Definition of common hand hygiene procedure
The selected procedure included the six steps which were described in EN 1500, completed by a wrist rubbing. While this step is not included in EN 1500, as there are no microbiological references to prove its effectiveness, it is still recommended in French health care facilities, and it should be taken into account for consumption measurement. This selected procedure was used in all test centers. A training course was organized in order to harmonize the procedures of this study.
2.4. Selection of products
As products had to meet the French requirements, 59 products were selected and their suppliers contacted in order to supply free samples. They were chosen from the 2008 “Liste Positive Désinfectants (LPD),” a publication of the French Society of Hospital Hygiene (http://www.sf2h.net/publications-SF2H/SF2H_LPD-2009.pdf), which presented the disinfectants conforming to a list of efficacy tests, and from the “Liste Positive Désinfectants Dentaires,” which was the equivalent publication for use by dentists (http://sf2h.net/publications-SF2H/SF2H-ADF_LPDdentaire-2009.pdf). Similar products that were marketed too recently to be accepted by the LPD group were also included in the selection. All free available products were tested. The order between products was randomly assigned in each center.
2.5. Test centers
Five test centers were set up: Bordeaux, Lille, Lyon, Rennes and Strasbourg. Each person taking part in the test was an infection control worker and each product had to be tested for each dosage (3, 2 and 1.5 ml) in three centers and at least seven times per center and per dosage.
2.6. Organization and analysis
The tests were carried out between May and July 2008. For each dose, if a product had not been tested 10 times, it was then taken out of the analysis. The data were analyzed with Epi Info 2002 and SPSS V12. The duration of hand rubbing was considered correct according to the 2002 French guidelines, if this value is longer than or equal to 30 s, and effective according to the EN 1500 and if this value is longer than or equal to that of the contact time validated by the product by means of EN 1500.
3. Results
During the study, 1800 tests were performed. Between 9 and 111 tests were carried out per product. The number of professionals participating in the testing process was 71 (21 men and 50 women). Only 27 products and 1706 tests were included in the analysis, as four scheduled products were not available in three test centers and were tested less than 10 times. The included products were Actisène alcogel (Werner and Mertz), Alco Aloe solution and gel (Ansell), Alcocide, Alcoogel H and Septigel (Prodene Klint), Anios gel 85 NPC and Manugel 85 NPC (Anios), Assanis pro (Blue Skin SA), Dermalkan (Alkapharm), Desderman gel and Desderman N (Schülke), Elusept gel and Elusept solution (Elusept), GHA (Cellande), Manupure (Elis), Manurub and Manurub gel (Steridine), Nosocomia gel (Prodene Klint), Procide (IPC), Purell and Purell 85 (Gojo), Softalind hand sanitizer (BBraun), Spirigel and Spitacid (Ecolab), Sterilium (Rivadis), and Stoko progel and Stokosept (Stoko).
3.1. Results per dose and per product
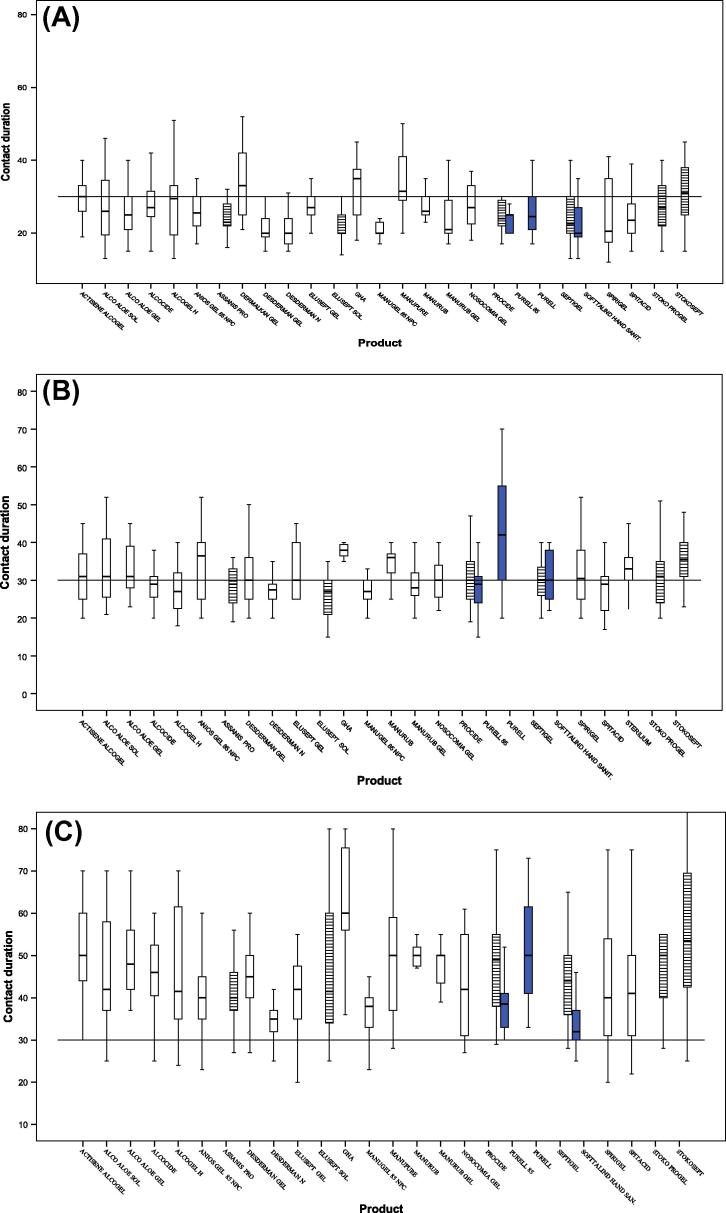
For a 1.5 ml dose, the dose was sufficient to cover both hands and wrists in 551/575 cases (95.8%). Variability between the average contact duration before drying of each product was very important (p < 10−3) (Fig. 1A and Table 1). According to the 30-s duration, there was not one single product for which 75% of tests showed a drying duration longer than or equal to the reference, but, according to EN 1500 there were 3/27 tested products for which 75% of tests showed a drying duration longer than or equal to the validated standard duration: Purell, Purell 85 and Softalind hand sanitizer, which are validated for the 15-s duration.
Figure 1.
Contact hand rubbing duration in seconds; distribution by product and by dose. EN 1500 validation:  15 s; □ 30 s;
15 s; □ 30 s;  60 s. (A) For a 1.5 ml dose; (B) for a 2 ml dose; and (C) for a 3 ml dose.
60 s. (A) For a 1.5 ml dose; (B) for a 2 ml dose; and (C) for a 3 ml dose.
Table 1.
Drying durations by dose and product: value of percentile 25 of duration of tests made for each product and each duration (i.e. 75% of tests have been longer than this value).
| EN 1500 contact time validateda | Products | Dose 1.5 ml | Dose 2 ml | Dose 3 ml | |||
|---|---|---|---|---|---|---|---|
| Tests number | Percentile 25b (s) | Tests number | Percentile 25b (s) | Tests number | Percentile 25b (s) | ||
| 15 s | Purell | 28 | 21 | 33 | 30 | 28 | 41 |
| Purell 85 | 14 | 20 | 21 | 24 | 14 | 32 | |
| Softalind hand sanitizer | 14 | 19 | 14 | 25 | 14 | 28 | |
| 30 s | Actisène alcoogel | 25 | 26 | 14 | 25 | 30 | 44 |
| Alcocide | 19 | 24 | 19 | 25 | 19 | 40 | |
| Alcogel H | 24 | 19 | 24 | 22 | 24 | 35 | |
| Anios gel 85 NPC | 22 | 22 | 26 | 25 | 29 | 35 | |
| Dermalkan | 14 | 25 | – | – | – | – | |
| Desderman gel | 41 | 19 | 28 | 25 | 42 | 40 | |
| Desderman N | 32 | 17 | 30 | 25 | 34 | 32 | |
| Elusept gel | 11 | 25 | 13 | 25 | 11 | 30 | |
| GHA | 12 | 25 | 11 | 35 | 12 | 56 | |
| Manugel 85 NPC | 21 | 20 | 31 | 25 | 21 | 33 | |
| Manupure | 14 | 28 | – | – | 14 | 37 | |
| Manurub | 18 | 25 | 17 | 31 | 19 | 47 | |
| Manurub gel | 19 | 20 | 7 | 28 | 27 | 43 | |
| Nosocomia gel | 19 | 22 | 19 | 25 | 19 | 31 | |
| Spitacid | 14 | 20 | 14 | 22 | 14 | 30 | |
| Spirigel | 28 | 17 | 28 | 25 | 28 | 31 | |
| Sterilium | – | – | 15 | 30 | – | – | |
| Alco Aloe gel | 26 | 21 | 33 | 28 | 26 | 42 | |
| Alco Aloe solution | 27 | 19 | 14 | 19 | 25 | 21 | |
| 2 × 30 s | Assanis pro | 21 | 22 | 21 | 24 | 21 | 37 |
| Elusept solution | 25 | 18 | 19 | 20 | 26 | 30 | |
| Procide | 14 | 22 | 14 | 25 | 15 | 38 | |
| Septigel | 36 | 20 | 24 | 26 | 34 | 36 | |
| Stoko progel | 17 | 22 | 20 | 24 | 14 | 39 | |
| Stokosept | 20 | 25 | 24 | 31 | 32 | 41 | |
Suppliers data.
Value of percentile 25: the duration of hand rubbing was considered effective, according to the 2002 French guidelines, if this value is longer than or equal to 30 s, and effective according to the EN 1500 if this value is longer than or equal to that of the contact time validated by the product by means of EN 1500.
For a 2 ml dose, the dose was sufficient to cover both hands and wrists in 530/538 cases (98.5%). Variability between the average contact duration before drying of each product was very important (p < 10−3) (Fig. 1B and Table 1). According to the 30-s duration, there were 5/26 tested products for which 75% of tests showed a drying duration longer than or equal to the reference: GHA, Manurub, Purell, Sterillium and Stokosept gel. According to EN 1500, there were six products for which 75% of tests showed a drying duration longer than or equal to the validated standard duration: all products which were validated for the 15-s duration (Purell, Purell 85, Softalind hand sanitizer), and 3 products which were validated for the 30-s duration (GHA, Manurub and Sterillium).
For a 3 ml dose, the dose was sufficient to cover both hands and wrists in 592/593 cases (99.8%). Variability between the average contact duration before drying of each product was very important (p < 10−3) (Fig. 1C and Table 1). According to the 30-s duration, all 26 tested products, except Softalind hand sanitizer, showed a drying duration longer than the reference. According to EN 1500, for 18 products, 75% of tests showed a drying duration longer than the expected duration defined by EN 1500. This criterion was not validated for all products that passed the standard 30 s twice, repeatedly.
4. Discussion
In this study, correct coverage of hands and wrists was achieved for all tested products and all doses. Depending on the product, the dose needed to ensure a contact of 30 s, in 75% of the tests, varied from 2 ml to more than 3 ml. The dose needed to ensure the requisite contact duration for the validation of the standard EN 1500 in 75% of the tests ranged from 1.5 ml to more than 3 ml. For some products, the dose needed was higher than 3 ml (the maximum dose tested), consequently it was not checked.
This result is consistent with previous observations during local tests which showed that, within the same unit and for the same test duration, required doses varied between products [12]. Consequently, an estimation of the compliance (number of HH made divided by the number of necessary HH) by the quantity of product used (in ml per patient day, for example) is not accurate, and a comparison between hospitals could be biased, independent regardless of the limits of the prediction of the necessary HH by the type of service and per patient days. It is pertinent within a hospital where units use the same product and over a period of time if this product remained unchanged, but seems biased for a national score [4].
This study considers it important to assess the representativeness and reproducibility of the study. A large number of marketed products were tested in five different centers, but without randomization or exhaustiveness, it is impossible to extend results to all marketed products or to all hospitals for this matter. The tests were carried out over the same period, and it does not seem relevant to take into account any seasonal effect, which is a well-known confounding factor in tolerance studies and professional conduct. The variation between required doses according to the product may be associated with the consistency (liquid or gel, alcohol concentration, various additives), but no systematic association was to be found in this study, and this point is debated [13,14]. This study does not give correcting factors, and other measures are necessary with this in mind. The bias of the large difference, in the field, on habits of rubbing techniques, on the actual dose and of hand size have been eliminated by the standardization of the method, the use of a measured dose and the repartition of the test between the different centers.
If one wanted to define the ideal dose needed for accurate proper hand rub disinfection, the protocol should take into account all confounding factors. The choice of a pertinent reference is also necessary. This study used the EN 1500 standard, as required in Europe for all marketed products, as proof of a real efficacy on bacteria in the case of hygienic hand disinfection, but the duration of 30 s was equally used, as the required duration for a minimal activity on viruses. When the ISO system includes similar standards, it will be more adapted for international comparison, some products being available in many countries. It is important, too, to perform a study on a sample of sufficient size, in a representative selection of healthcare facilities and climates. But such a test could give more data on products, with the aim of eliminating the effect of the product in comparisons based on consumption measures.
The range of available hydro-alcoholic solutions, gels or foams has considerably increased over the last few years. Hand rub is now the main HH method, and indirect monitoring of HH compliance, easier than observational studies, is frequently used. But it is only useful if complete data are available on consumption and activity by unit, aggregated data at an entire hospital being of little use in the field.
Indeed, in the field of HH evaluation, it is necessary to use indicators for benchmarking or for evaluation of campaigns [15]. All indicators could be biased: HH compliance measured by observation could also be biased if different methods of observation or analysis are used [16]. Local customs can also interfere: interpretation of five moments of the WHO guidelines seems to differ between services, for example, or encouraging the use of products by patients and visitors can increase consumption [17]. The only safeguards against all these biases are the continuous use of the same method or the introduction of a correction in comparison, and the association of different media: HH observation, hand rub consumption or new techniques, such as electronic devices [18,19].
In conclusion, the old idea of an exact relationship between the volume of hand rub consumed and HH compliance remains false, and necessary comparisons should be made with care. Further studies appear necessary, in order to develop tools for modulating this relationship, while remaining circumspect about the limits of the use of the volume of product used as an indicator of good compliance.
Acknowledgements
To Marie Yvonne Dixon and Ian Russell for English text revision, and to product suppliers for products.
No grant was obtained for this study.
No conflict of interest to declare.
References
- [1].Haustein T, Gastmeier P, Holmes A, Lucet JC, Shannon RP, Pittet D, et al. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011;11(6):471–81. doi: 10.1016/s1473-3099(10)70315-7. [DOI] [PubMed] [Google Scholar]
- [2].Herud T, Nilsen RM, Svendheim K, Harthug S. Association between use of hand hygiene products and rates of health care-associated infections in a large university hospital in Norway. Am J Infect Control. 2009;37(4):311–7. doi: 10.1016/j.ajic.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [3].Conrad C. Increase in hand-alcohol consumption among medical staff in a general hospital as a result of introducing a training program and a visualization test. Infect Control Hosp Epidemiol. 2001;22(1):41–2. doi: 10.1086/501823. [DOI] [PubMed] [Google Scholar]
- [4].Benet T, Treny-Juhen D, Chemorin C, Morandat L, Vanhems P. Relationship between hand rub consumption and nosocomial infection rates in intensive care units. J Hosp Infect. 2007;65(2):182–4. doi: 10.1016/j.jhin.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [5].Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection control programme. Lancet. 2000;356(9238):1307–12. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- [6].Pessoa-Silva CL, Hugonnet S, Pfister R, Touveneau S, Dharan S, Posfay-Barbe K, et al. Reduction of health care associated infection risk in neonates by successful hand hygiene promotion. Pediatrics. 2007;120(2):e382–390. doi: 10.1542/peds.2006-3712. [DOI] [PubMed] [Google Scholar]
- [7].Eckmanns T, Schwab F, Bessert J, Wettstein R, Behnke M, Grundmann H, et al. Hand rub consumption and hand hygiene compliance are not indicators of pathogen transmission in intensive care units. J Hosp Infect. 2006;63(4):406–11. doi: 10.1016/j.jhin.2006.03.015. [DOI] [PubMed] [Google Scholar]
- [8].Muller A, Denizot V, Mouillet S, Blanchot C, Bertrand X, Bailly P, et al. Lack of correlation between consumption of alcohol-based solutions and adherence to guidelines for hand hygiene. J Hosp Infect. 2005;59(2):163–4. doi: 10.1016/j.jhin.2004.09.010. [DOI] [PubMed] [Google Scholar]
- [9].Slekovec C, Gbaguidi-Haore H, Coignard B, Bertrand X, Talon D. Relationship between prevalence of device-associated infections and alcohol-based hand-rub consumption: a multi-level approach. J Hosp Infect. 2011;78(2):133–7. doi: 10.1016/j.jhin.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [10].Rotter ML. European norms in hand hygiene. J Hosp Infect. 2004;56(Suppl. 2):S6–9. doi: 10.1016/j.jhin.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [11].Kohan C, Ligi C, Dumigan DG, Boyce JM. The importance of evaluating product dispensers when selecting alcohol-based handrubs. Am J Infect Control. 2002;30:373–5. doi: 10.1067/mic.2002.125586. [DOI] [PubMed] [Google Scholar]
- [12].Girard R, Bousquet E, Carre E, Bert C, Coyault C, Coudrais S, et al. Tolerance and acceptability of 14 surgical and hygienic alcohol-based hand rubs. J Hosp Infect. 2006;63(3):281–8. doi: 10.1016/j.jhin.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [13].Larson E, Girard R, Pessoa-Silva CL, Boyce J, Donaldson L, Pittet D. Skin reactions related to hand hygiene and selection of hand hygiene products. Am J Infect Control. 2006;34(10):627–35. doi: 10.1016/j.ajic.2006.05.289. [DOI] [PubMed] [Google Scholar]
- [14].Traore O, Hugonnet S, Lubbe J, Griffiths W, Pittet D. Liquid versus gel handrub formulation: a prospective intervention study. Crit Care. 2007;11(3):R52. doi: 10.1186/cc5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stone S, Gallagher R, Storr J, Tanner G. Campaigns and continuity. Lancet Infect Dis. 2011;11(5):340–1. doi: 10.1016/s1473-3099(11)70095-0. [DOI] [PubMed] [Google Scholar]
- [16].Stewardson A, Sax H, Longet-Di Pietro S, Pittet D. Impact of observation and analysis methodology when reporting hand hygiene data. J Hosp Infect. 2011;77(4):358–9. doi: 10.1016/j.jhin.2010.12.008. [DOI] [PubMed] [Google Scholar]
- [17].McGuckin M, Shubin A, Hujcs M. Interventional patient hygiene model: infection control and nursing share responsibility for patient safety. Am J Infect Control. 2008;36(1):59–62. doi: 10.1016/j.ajic.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [18].Marra AR, Moura DF, Jr., Paes AT, dos Santos OF, Edmond MB. Measuring rates of hand hygiene adherence in the intensive care setting: a comparative study of direct observation, product usage, and electronic counting devices. Infect Control Hosp Epidemiol. 2010;31(8):796–801. doi: 10.1086/653999. [DOI] [PubMed] [Google Scholar]
- [19].Edmond MB, Goodell A, Zuelzer W, Sanogo K, Elam K, Bearman G. Successful use of alcohol sensor technology to monitor and report hand hygiene compliance. J Hosp Infect. 2010;76(4):364–5. doi: 10.1016/j.jhin.2010.07.006. [DOI] [PubMed] [Google Scholar]