Key Points
Question
What are the risk factors for developing a severe form of coronavirus disease 2019 (COVID-19) in patients with multiple sclerosis (MS)?
Findings
In this cohort study of 347 patients with MS, risk factors for severe forms of COVID-19 were neurological disability, age, and obesity, but no association was found between disease-modifying therapies exposure and COVID-19 severity.
Meaning
The identification of these risk factors could provide a rationale for an individual strategy of clinical management in patients with MS during the COVID-19 pandemic.
This cohort study describes the clinical characteristics and outcomes in patients with multiple sclerosis who contract COVID-19 and identifies factors associated with COVID-19 severity.
Abstract
Importance
Risk factors associated with the severity of coronavirus disease 2019 (COVID-19) in patients with multiple sclerosis (MS) are unknown. Disease-modifying therapies (DMTs) may modify the risk of developing a severe COVID-19 infection, beside identified risk factors such as age and comorbidities.
Objective
To describe the clinical characteristics and outcomes in patients with MS and COVID-19 and identify factors associated with COVID-19 severity.
Design, Setting, and Participants
The Covisep registry is a multicenter, retrospective, observational cohort study conducted in MS expert centers and general hospitals and with neurologists collaborating with MS expert centers and members of the Société Francophone de la Sclérose en Plaques. The study included patients with MS presenting with a confirmed or highly suspected diagnosis of COVID-19 between March 1, 2020, and May 21, 2020.
Exposures
COVID-19 diagnosed with a polymerase chain reaction test on a nasopharyngeal swab, thoracic computed tomography, or typical symptoms.
Main Outcomes and Measures
The main outcome was COVID-19 severity assessed on a 7-point ordinal scale (ranging from 1 [not hospitalized with no limitations on activities] to 7 [death]) with a cutoff at 3 (hospitalized and not requiring supplemental oxygen). We collected demographics, neurological history, Expanded Disability Severity Scale score (EDSS; ranging from 0 to 10, with cutoffs at 3 and 6), comorbidities, COVID-19 characteristics, and outcomes. Univariate and multivariate logistic regression models were used to estimate the association of collected variables with COVID-19 outcomes.
Results
A total of 347 patients (mean [SD] age, 44.6 [12.8] years, 249 women; mean [SD] disease duration, 13.5 [10.0] years) were analyzed. Seventy-three patients (21.0%) had a COVID-19 severity score of 3 or more, and 12 patients (3.5%) died of COVID-19. The median EDSS was 2.0 (range, 0-9.5), and 284 patients (81.8%) were receiving DMT. There was a higher proportion of patients with a COVID-19 severity score of 3 or more among patients with no DMT relative to patients receiving DMTs (46.0% vs 15.5%; P < .001). Multivariate logistic regression models determined that age (odds ratio per 10 years: 1.9 [95% CI, 1.4-2.5]), EDSS (OR for EDSS ≥6, 6.3 [95% CI. 2.8-14.4]), and obesity (OR, 3.0 [95% CI, 1.0-8.7]) were independent risk factors for a COVID-19 severity score of 3 or more (indicating hospitalization or higher severity). The EDSS was associated with the highest variability of COVID-19 severe outcome (R2, 0.2), followed by age (R2, 0.06) and obesity (R2, 0.01).
Conclusions and Relevance
In this registry-based cohort study of patients with MS, age, EDSS, and obesity were independent risk factors for severe COVID-19; there was no association found between DMTs exposure and COVID-19 severity. The identification of these risk factors should provide the rationale for an individual strategy regarding clinical management of patients with MS during the COVID-19 pandemic.
Introduction
In December 2019, the first cases of a new coronavirus infection (coronavirus disease 2019 [COVID-19]) attributable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared in Wuhan, China. The virus has spread rapidly, reaching 225 countries and more than 5 million people as of May 21, 2020. In France, by the same date, 181 000 patients had tested positive for COVID-19 and more than 28 000 patients had died of COVID-19. In the pandemic context, populations at risk of developing severe forms of COVID-19 have begun to be identified.1
Patients with multiple sclerosis (MS) represent a population of particular interest in the pandemic context because most of them are treated with immunosuppressants. On March 13, 2020, the Multiple Sclerosis International Federation issued recommendations regarding the risk of COVID-19 in patients with MS, with a specific statement regarding disease-modifying therapies (DMTs), indicating that “some MS medications might increase the likelihood of developing complications from a COVID-19 infection but this risk needs to be balanced with the risks of stopping treatment.”2 In statements from national MS societies and published editorials,3,4 it is recommended to consider delaying DMT initiation for those treatments with the highest presumed infectious risk or delaying the completion of a cure for sequential DMTs. The attitude toward DMTs in the case of proven COVID-19 infection is to consider temporarily stopping immunosuppressive DMTs. Expert panels have proposed a stratification of the risk of treatments according to known systemic immunosuppression.3,4
In this context, we have initiated a French registry enabling the collection of highly suspect and proven COVID-19 in patients with MS. The purpose of this registry was to determine the characteristics of COVID-19 in this population and identify the risk factors for developing a severe form of this infection.
Methods
Data Collection
We conducted a multicenter, retrospective, observational cohort study of patients with MS with confirmed or highly suspected COVID-19 diagnosis (Covisep registry). All French MS expert centers (Centre de Ressources et de Compétences–Sclérose en Plaques), part of the French Clinical Research Infrastructure Network for Multiple Sclerosis (which is part of Institut National de la Santé et de la Recherche Médicale), and general hospitals and neurologists collaborating with the Centre de Ressources et de Compétences–Sclérose en Plaques or members of the French MS society (Société Francophone de la Sclérose en Plaques) participated in the collection of data.
The study followed the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study received approval from the ethics committee of Sorbonne University and is registered at the Institut National des Données de Santé for the use of confidential electronically processed patient data. The study protocol is registered on ClinicalTrials.gov (NCT04355611). Patients included were informed about the objective of the study, and the collection of nonopposition (consent) to the use of medical data was carried out according to French law, good clinical practice, and the General Data Protection Regulation. Using an electronic clinical record form on RedCap (https://www.project-redcap.org/), study data were collected from medical records of patients who were diagnosed with COVID-19 between March 1, 2020, and May 21, 2020.
Population of Interest
Inclusion criteria were MS and at least 1 of the following 4 criteria: (1) a biologically confirmed COVID-19 diagnosis based on a positive result of a SARS-CoV-2 polymerase chain reaction (PCR) test on a nasopharyngeal swab; (2) typical thoracic computed tomography (CT) abnormalities (ground-glass opacities) in epidemic areas; (3) anosmia or ageusia of sudden onset in the absence of rhinitis or nasal obstruction; or (4) COVID-19–typical symptoms (triad of cough, fever, and asthenia) in an epidemic zone of COVID-19. The noninclusion criterion was the patient’s opposition to the use of his or her medical data.
Definition of Study End Points
We collected patient demographics, current DMT use, Expanded Disability Severity Scale score (EDSS) before COVID-19, and comorbidities and the presence of known lymphopenia before infection. Regarding COVID-19, we collected symptoms and diagnostic data (PCR for SARS-CoV-2 or chest CT). The primary end point was the participant’s clinical status at the most severe point of their COVID-19 course (on a 7-point ordinal scale5), referred as the COVID-19 severity score, where 1 indicated the patient was not hospitalized and had no limitations on activities; 2, the patient was not hospitalized and had limitation on activities; 3, the patient was hospitalized and did not require supplemental oxygen; 4, the patient was hospitalized and required supplemental oxygen; 5, the patient was hospitalized and receiving noninvasive ventilation or high-flow oxygen; 6, the patient was hospitalized and receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; or 7, death.
Statistical Analysis
Descriptive statistics were performed on demographic and clinical variables. Demographic data were compared between patients with COVID-19 severity scores of 1 or 2 and patients with COVID-19 severity scores of 3 or more (ie, hospitalization or death from COVID-19). Group comparisons were performed using the Mann-Whitney U test for numerical and ordinal variables and the Fisher test or χ2 test when appropriate for categorical variables. Any 2-sided P < .05 was considered statistically significant.
Univariate logistic regression models were performed on identified variables to assess their association with COVID-19 outcome (severity scores, 1-2 vs ≥3). Age was treated as a continuous variable, and EDSS was segmented into 3 bins (<3, 3-5.5, and ≥6). Disease-modifying therapies were categorized according to systemic infection risk (no risk: interferon beta and glatiramer; low risk: teriflunomide, dimethylfumarate, natalizumab, and other drugs [mycophenolate mofetil, methotrexate, and cyclophosphamide]; intermediate or high risk: fingolimod, anti-CD20 therapies, cladribine, and alemtuzumab) as proposed in a previous study.4 Subsequently, a multivariate logistic regression model was performed to determine which variables are independently associated with severe COVID-19 (severity score ≥3). Variable selection was done through backward elimination and forward selection by minimizing Akaike information criterion. Results are expressed as odds ratios (ORs) and 95% CIs. McFadden pseudo-R2 values were calculated for each variable to quantify the proportion of the total variability of the outcome that is accounted for by the model. Data analyses were performed in Python (Python Software Foundation) using Pandas package version 1.0.3, Scipy version 1.4.1, and Statsmodels version 0.11.1.
Results
Study Population
As of May 21, 2020, 348 patients with MS were in the Covisep registry. Among 46 patients with a negative SARS-CoV-2 PCR result, 10 patients had a chest CT showing typical lesions for COVID-19 and 1 patient had further SARS-CoV-2–positive serology results. For the remaining patients with negative SARS-CoV-2 PCR results, 34 of 35 were confirmed to have a highly suspicious COVID-19 with a typical medical history. One patient had an uncertain COVID-19 diagnosis and was excluded from the analysis. Ultimately, 347 patients with MS were included.
Demographics and Clinical Characteristics
Demographics and clinical characteristics of the cohort are presented in the Table. Briefly, their mean (SD) age was 44.6 (12.8) years, 249 of 347 were women, and the mean (SD) disease duration was 13.5 (10.0) years.
Table. Demographic and Disease Characteristics of Patients With Multiple Sclerosis Diagnosed With Coronavirus Disease 2019 in the Covisep Registry, as of May 21, 2020.
| Characteristic | No. (%) |
|---|---|
| No. of patients with MS | 347 |
| Demographics | |
| Age, mean (SD), y | 44.6 (12.8) |
| Sex ratio, female:male | 249:98 |
| Disease duration, mean (SD), y | 13.5 (10.0) |
| Disease course, No. | |
| Clinically isolated syndrome | 6 |
| Remitting-relapsing multiple sclerosis | 276 |
| Secondary progressive multiple sclerosis | 48 |
| Primary progressive multiple sclerosis | 17 |
| Expanded Disability Severity Score, median (range) | 2.0 (0.0-9.5) |
| Disease-modifying therapies | |
| Interferon beta | 20 (5.8) |
| Glatiramer | 33 (9.5) |
| Teriflunomide | 33 (9.5) |
| Dimethylfumarate | 35 (10.1) |
| Natalizumab | 57 (16.4) |
| Fingolimod | 42 (12.1) |
| Ocrelizumab | 38 (11.0) |
| Rituximab | 17 (4.9) |
| Cladribine | 3 (0.9) |
| Alemtuzumab | 1 (0.3) |
| Othera | 5 (1.4) |
| None | 63 (18.2) |
| Lymphopenia before COVID-19 onset, No./No. tested (%) | |
| No lymphopenia | 128/187 (68.4) |
| Lymphopenia | 59/187 (31.6) |
| Lymphopenia, excluding patients receiving fingolimod | 34/157 (21.6) |
| Lymphopenia grade, No. | |
| 1 | 10 |
| 2 | 21 |
| 3 | 23 |
| 4 | 2 |
| Comorbid conditions | |
| Cardiovascular disease | 23 (6.6) |
| Pulmonary disease | 15 (4.3) |
| Diabetes | 16 (4.6) |
| Obesity (BMI>30) | 24 (6.9) |
| Smoking | 33 (9.5) |
| COVID-19 diagnosis, No./No. tested (%) | |
| Positive SARS-CoV-2 polymerase chain reaction result | 146/191 (76.4) |
| Ground-glass opacity on thoracic computed tomography scan | 62/93 (66.7) |
| COVID-19 symptoms | |
| Asthenia | 290 (83.6) |
| Fever | 260 (74.9) |
| Cough | 266 (76.7) |
| Anosmia/ageusia | 150 (43.2) |
| Headache | 180 (51.9) |
| Dyspnea | 162 (46.7) |
| Digestive disorders | 88 (25.4) |
| Dizziness | 54 (15.6) |
| Inclusion criteria | |
| Positive SARS-CoV-2 polymerase chain reaction | 146 (42.1) |
| Ground-glass opacity on thoracic computed tomography scan, without fulfilling above criteria | 18 (5.2) |
| Anosmia/ageusia, without fulfilling above criteria | 91 (26.2) |
| Typical COVID-19 symptoms, without fulfilling above criteria | 92 (26.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Other treatments for patients with multiple sclerosis: mycophenolate mofetil (n = 3), cyclophosphamide (n = 1), and methotrexate (n = 1).
Seventy-three patients had a COVID-19 severity score of 3 or more. Patients presented with typical symptoms for COVID-19. Notably, fever (temperature more than 38 °C) and dyspnea were more frequent in patients with severe COVID-19 (67 of 73 [91.8%] and 54 of 73 [74.0%], respectively) than patients who were not hospitalized (193 of 274 [70.4%] and 108 of 274 [39.4%] respectively; P < .001), while anosmia/ageusia and headache were more common in patients who were not hospitalized (135 of 274 [49.3%] and 164 of 274 [59.8%], respectively) vs patients who were hospitalized (15 of 73 [20.5%] and 16 of 73 [21.9%], respectively; P < .001).
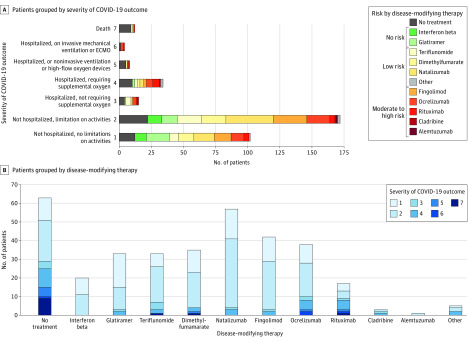
The distribution of clinical severity for COVID-19 according to the different DMTs is presented in Figure 1. The ages and EDSSs of patients in each DMT group are shown in eFigure 1 in the Supplement.
Figure 1. Clinical Condition Rating at Nadir and Disease-Modifying Therapies in Patients With Multiple Sclerosis.
A, Patients were grouped according to coronavirus disease 2019 (COVID-19) severity score (from 1 [not hospitalized with no limitations on activities] to 7 [death]). B, Patients were grouped according to disease-modifying therapy. Systemic immunosuppression risk was adapted from Brownlee et al.3 Other treatments used by patients with multiple sclerosis included mycophenolate mofetil (n = 3), cyclophosphamide (n = 1), and methotrexate (n = 1).
Variables Associated With COVID-19 Severity
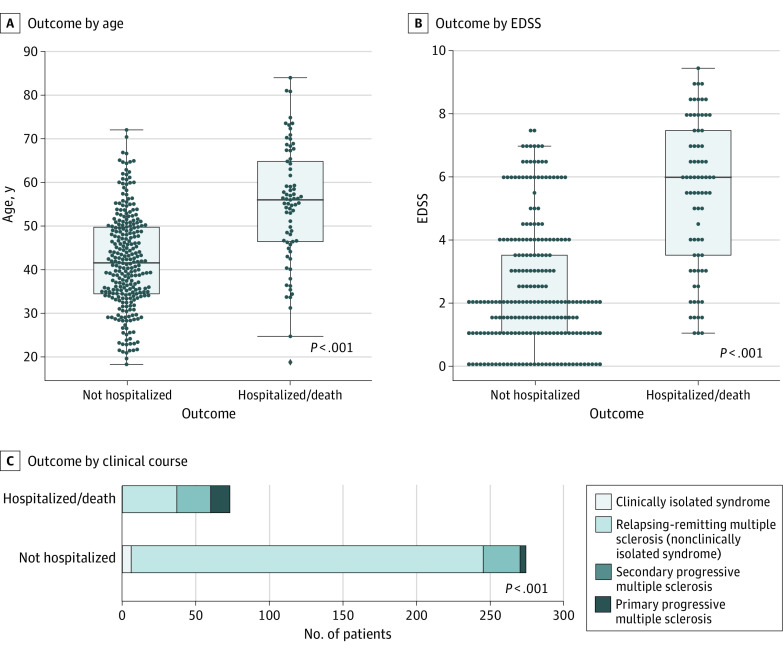
Patients with COVID-19 severity scores of 3 or more were more frequently male compared with patients with COVID-19 who were not hospitalized (female:male sex ratio: 1.4 vs 3.0; P = .008). Age and EDSS differences between groups and clinical course distribution are represented in Figure 2. Patients with COVID-19 severity scores of 3 or more were older (mean [SD] age, 55.0 [13.7] years vs 41.9 [11.0] years; P < .001), had higher EDSS (median EDSS, 6.0 vs 2.0; P < .001), and more frequently had a progressive course (36 of 73 [49.3%] vs 29 of 274 [10.6%]; P < .001) compared with patients with COVID-19 who were not hospitalized.
Figure 2. Characteristics of Patients Not Hospitalized for Coronavirus Disease 2019 (COVID-19) and Patients Who Were Hospitalized With or Died of COVID-19.
A, Age. B, Expanded Disability Severity Scale (EDSS) score. C, Clinical course. The P values were obtained from Mann-Whitney U tests for age and EDSS group differences and χ2 tests for clinical course distribution differences.
The presence of at least 1 comorbidity was associated with an increased risk of severe COVID-19. The proportion of patients with at least 1 comorbidity was lower in the nonhospitalized group vs the severe COVID-19 group (75 of 274 [27.4%] vs 38 of 73 [52.1%], respectively; P < .001). Comorbidities associated with a COVID-19 severity score of 3 or more were diabetes (9 of 73 [12.3%] vs 7 of 274 [2.5%]; P = .002), cardiovascular comorbidity (13 of 73 [17.8%] vs 10 of 274 [3.6%]; P < .001), pulmonary comorbidity (7 of 73 [9.6%] vs 8 of 274 [2.9%]; P = .02), and obesity (10 of 73 [13.7%] vs 14 of 274 [5.1%]; P = .02).
There was a higher proportion of patients with severe COVID-19 among patients with no DMTs relative to patients who were receiving DMTs (29 of 63 [46.0%] vs 44 of 284 [15.5%]; P < .001). Among patients with DMTs (n = 284), there was a difference in COVID-19 outcome between DMT groups, with a higher proportion of patients with severe COVID-19 in the moderate-risk or high-risk DMT group (24 of 101 [23.8%] vs 17 of 130 [13.1%] in the low-risk DMT group and 3 of 53 [5.7%] in the no-risk DMT group; P = .007). Disease-modifying treatments were stopped or postponed (in the case of sequential treatment) in 77 patients (27.1%), but DMT modification was not associated with COVID-19 outcome.
Among 187 patients for whom lymphocyte counts were available before COVID-19 onset, we did not find an association between lymphopenia and severe COVID-19. However, if we excluded patients taking fingolimod because of this drug’s mechanism of action, lymphopenia was more frequent in patients with COVID-19 severity scores of 3 or more (22 of 57 [38.6%] vs 12 of 100 [12.0%]; P < .001).
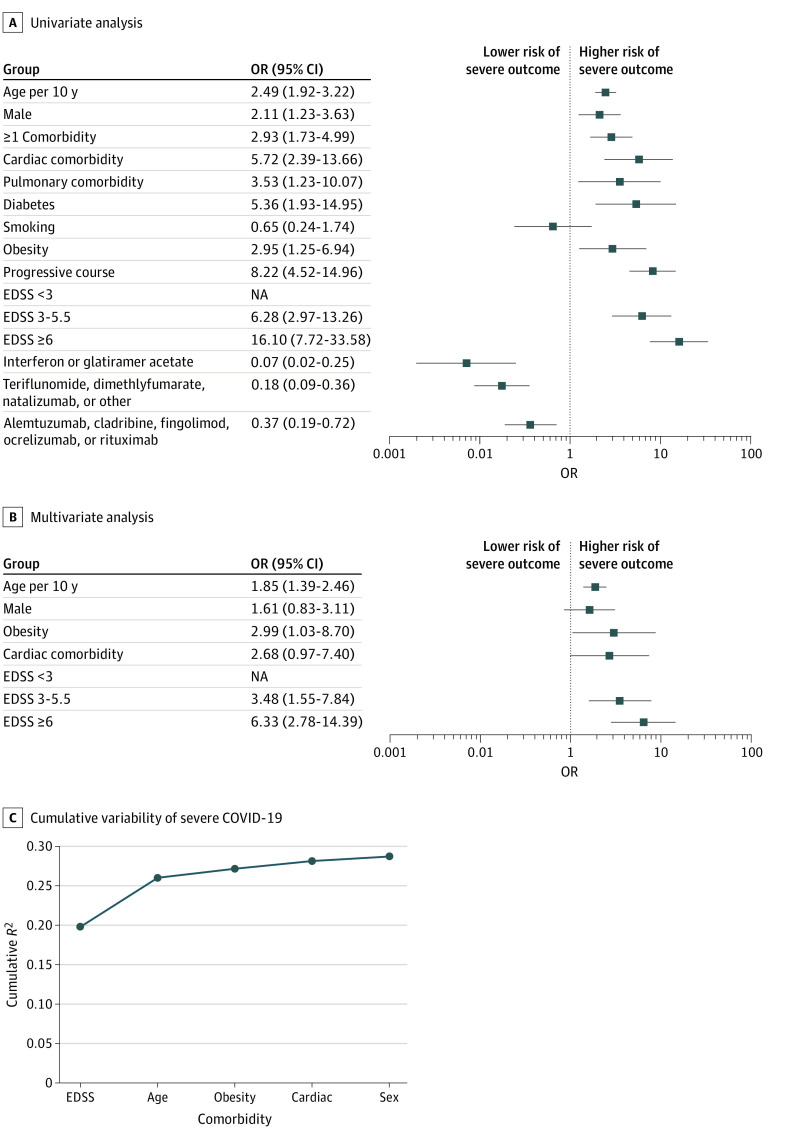
Univariate and Multivariate Logistic Regression Models of Severe COVID-19
To estimate the risk factors associated with COVID-19 severity scores of 3 or more, we calculated the odds ratios (ORs) from univariate logistic regression models (Figure 3A). Greater age (OR per 10 years, 2.49 [95% CI, 1.92-3.22]), male sex (OR, 2.11 [95% CI, 1.23-3.63]), comorbidities (OR, 2.93 [95% CI, 1.73-4.99]), progressive disease (OR, 8.22 [95% CI, 4.52-14.96]), and higher EDSS (ORs, EDSS 3-5.5, 6.28 [95% CI, 2.97-13.26]; EDSS ≥6, 16.10 [95% CI, 7.72-33.58]) were associated with increased risk of severe COVID-19, while DMTs were associated with lower risks of severe COVID-19 (interferon or glatiramer acetate: OR, 0.07 [95% CI, 0.02-0.25]; teriflunomide, dimethylfumarate, natalizumab, or other drugs: OR, 0.18 [95% CI, 0.09-0.36]; alemtuzumab, cladribine, fingolimod, ocrelizumab, or rituximab: OR, 0.37 [95% CI, 0.19-0.72]). In the multivariate logistic regression model, age, male, obesity, cardiac comorbidity, and EDSS were retained in the model as independent variables associated with severe COVID-19 (Figure 3B). Among these, higher age (OR per 10 years, 1.9 [95% CI, 1.4-2.5]), an EDSS of 6 or more (OR, 6.3 [95% CI, 2.8-14.4]), and obesity (OR, 3.0 [95% CI, 1.03-8.7]) were independent variables associated with severe COVID-19 (severity score ≥3). The EDSS showed the highest variability of COVID-19 severe outcome (R2, 0.20), followed by age (additional R2, 0.06) and obesity (additional R2, 0.01) (Figure 3C).
Figure 3. Risk Factors of Severe Coronavirus Disease 2019 (COVID-19).
Severe COVID-19 was defined as a severity score of 3 or more. A, Univariate analysis. B, Multivariate analysis. Results are expressed as odds ratios (ORs) with 95% CIs. C, Cumulative variability (McFadden R2) of severe COVID-19 accounted for each variable retained in the multiple logistic regression model. Numbers of patients per category: men, 98; with 1 or more comorbidity, 113; with obesity, 24; with cardiac comorbidity, 23; with an Expanded Disability Severity Status (EDSS) score less than 3, 194; with an EDSS of 3 to 5.5, 75; with an EDSS of 6 or more, 69; without disease-modifying therapy, 63; receiving interferon or glatiramer acetate, 53; receiving teriflunomide, dimethylfumarate, natalizumab, or other drugs, 130; and receiving fingolimod, ocrelizumab, rituximab, cladribine, or alemtuzumab, 101. NA indicates not applicable.
A cutoff score of 7 for EDSS provided similar results (eFigure 2 in the Supplement). As a sensitivity analysis, when including only patients with positive SARS-CoV-2 PCR results (n = 146), a higher age (OR per 10 years, 1.6 [95% CI, 1.1-2.3]) and an EDSS of 6 or more (OR, 7.0 [95% CI, 2.3-21.2]) remained independent variables associated with severe COVID-19.
We performed a post hoc analysis with a threshold of 4 for COVID-19 severity score (hospitalized, requiring supplemental oxygen, or higher severity). We obtained similar results at univariate analysis (eFigure 3 in the Supplement). In the multivariate logistic regression model, age, male sex, obesity, cardiac comorbidity, and EDSSs were retained as independent variables associated with severe COVID-19 (eFigure 3 in the Supplement). A higher age (OR per 10 years, 2.1 [95% CI, 1.5-3.0]), EDSS of 6 or more (OR, 8.3 [95% CI, 3.1-22.3]), male sex (OR, 2.6 [95% CI, 1.2-5.3]), and obesity (OR, 5.2 [95% CI, 1.6-16.5]) were the independent variables associated with severe COVID-19 (severity score ≥4). The EDSS showed the highest variability of COVID-19 severe outcome (R2, 0.23), followed by age (additional R2, 0.08), obesity (R2, 0.02), and male sex (R2, 0.01) (eFigure 3 in the Supplement).
Clinical Description of Patients With Highest COVID-19 Severity
Twelve patients in the cohort (3.5%) died of COVID-19, with most of these patients having a progressive form of the disease and a high EDSS. Four patients were hospitalized in intensive care units. One relatively young patient was in intensive care under mechanical ventilation for 3 weeks and is now in rehabilitation with a tracheotomy. Another patient was admitted to an intensive care unit 10 days after symptom onset. Both patients were receiving ocrelizumab and had obesity as a comorbidity. A third patient with primary progressive MS was in intensive care under mechanical ventilation for 8 days and has since been discharged from the hospital. This person was taking rituximab, had an EDSS of 5.5, and had no known comorbidities. A fourth patient, who had relapsing-remitting MS with no DMT, was admitted in intensive care under mechanical ventilation.
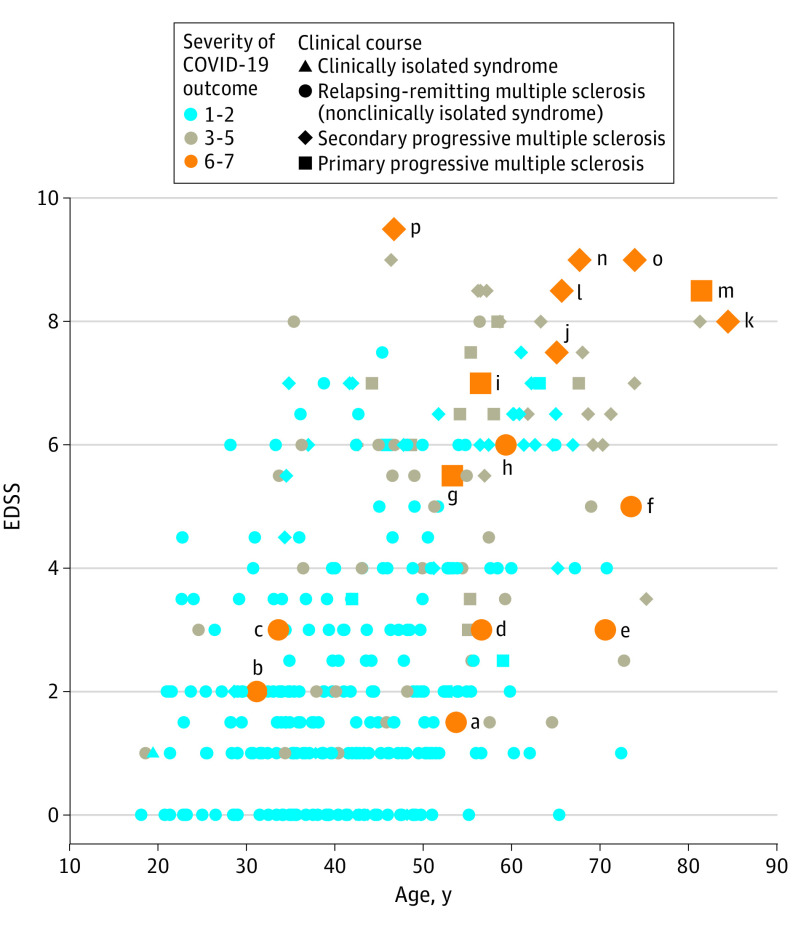
Figure 4 displays an associative plot between age, EDSS, and COVID-19 severity of individuals from the cohort. A description of demographics and clinical characteristics of patients with the most severe COVID-19 outcome (score 6: under mechanical ventilation; score 7: death) is provided in eTable 1 in the Supplement.
Figure 4. Plot Associating Expanded Disability Severity Scale (EDSS) Score, Age, and Coronavirus Disease 2019 (COVID-19) Severity Score.
Letters a to p refer to case reports of patients in need of mechanical ventilation or extracorporeal membrane oxygenation, or dead of COVID-19, as detailed in eTable 1 in the Supplement.
Fatality Rate in Patients With Multiple Sclerosis Hospitalized for COVID-19
We provide in eTable 2 in the Supplement the numbers of patients, stratified by age and sex, and the death rate among patients who were hospitalized. As a reference, we have included the death rate among patients hospitalized for COVID-19 in the overall population in France.6 Although no statistical analysis can be performed because of the low number of patients who died in this cohort, we observed a higher fatality rate among patients who were hospitalized for most age groups in patients with MS compared with reference estimates.
Discussion
We found COVID-19 symptom profiles to be consistent with that described in the general population. In this cohort, fever and dyspnea were more common in patients hospitalized for COVID-19. Similarly, in a series of 201 patients with COVID-19 pneumonia, high fever was associated with the development of acute respiratory distress syndrome.7 Headache and anosmia were more frequently associated with ambulatory forms in our cohort, in line with a recent article8 reporting that anosmia was strongly and independently associated with outpatient care.
Importantly, we identified in univariate analyses risk factors for COVID-19 severity: age, EDSS, progressive course of MS, and male sex. Comorbidities were also identified as risk factors, including cardiovascular and pulmonary diseases, diabetes, and obesity. These comorbidities were most frequently found in a large series of patients hospitalized in an intensive care unit for COVID-19 in New York, New York.9
The presence of DMT was associated with a lower risk of hospitalization in univariate analysis. Among patients who were hospitalized or died, there was an increased proportion of patients without treatment compared with the patients who were not hospitalized. Given the interactions between age, disease course, EDSS, and exposure to DMTs, it is crucial to carry out a multivariate analysis to estimate the independent outcome of each of these variables. Indeed, DMTs are more frequently prescribed in younger patients, those with a relapsing form of MS, and those with a lower level of disability. In multivariate analyses, only the following variables were independent risk factors of severe form of COVID-19: age, EDSS, and obesity. Additionally, the multivariate analysis carried out with a threshold of severity of COVID-19 of 4 or more found an independent outcome of male sex as risk factor for severe COVID-19, beside age, obesity, and EDSS.
In this cohort, the exposure to DMTs and the level of immunosuppression were not factors that independently modified the risk of developing a severe form of COVID-19. Caution should be exercised when interpreting this result, because it is possible that a larger cohort could identify a subgroup of patients with 1 or more DMTs that modify the risk of COVID-19. Therefore, the pooling of registries and/or replication of results in other cohorts from several countries will be important to reinforce this result. In our population, 2 patients with relapsing-remitting MS who were receiving ocrelizumab have developed acute respiratory distress syndrome. Although these 2 patients also had obesity, it cannot be excluded that their DMTs may have increased their susceptibility to COVID-19 severity. In the literature, cases of patients with MS and COVID-19 who were receiving ocrelizumab were reported, with 1 being asymptomatic10 and the other having a favorable outcome.11 Serological studies following COVID-19 infection in these patients will be crucial to determine the characteristics of the immune response to COVID-19, depending on the different DMTs and in particular on anti-CD20 therapies, for which lower immune response to vaccination has been reported.12
It is not surprising to identify age and obesity as independent risk factors for COVID-19 severity. Age was identified in the earliest published study13 as a major risk factor for the severity of COVID-19 infection. More recently, obesity has also been identified as an independent risk factor.14
The association between COVID-19 severity and the level of neurological disability assessed by EDSS in this cohort has not been previously reported, because there are (to our knowledge) currently no data available on the severity of COVID-19 in patients with preexisting neurological disabilities. Respiratory dysfunction is common in patients with MS and EDSS of 7 or more and is linked to expiratory muscles and cough impairment.15 With the EDSS being the independent variable with the highest explained variability on COVID-19 severity, this finding should lead to strengthening and sustaining precautions and barrier measures to limit the risk of COVID-19 infection in patients with high disability.
The overall mortality rate of COVID-19 in the cohort was 3.5%. The case-fatality ratio was estimated at 0.44% in mainland China in the age group of 40 to 49 years.16 A recent study estimated the infection fatality ratio in France as 0.7% (95% CI, 0.4%-1.0%), including asymptomatic cases.6 In our cohort, only 2 patients without symptoms were diagnosed as positive for SARS-CoV-2 by PCR swab, and most cases were reported because of typical symptoms for COVID-19. Therefore, we cannot infer an infection fatality ratio from this cohort. Instead, the fatality rate among patients who were hospitalized is a relevant measure at this stage of the outbreak. The fatality rate among patients who were hospitalized was higher for most age groups in patients with MS compared with the general population in France, suggesting an increased risk of death in patients with MS compared with the general population, which needs to be confirmed with large epidemiological studies.
Few data are available on the risk of COVID-19 in other autoimmune diseases in which patients are frequently treated by immunosuppressants. The preliminary results of the Global Rheumatology Alliance registry show a hospitalization rate of 35% (vs 21% in our population) and a death rate of 5% (vs 3.5% in our cohort) in 110 patients with various autoimmune pathologies.17 The identification of risk factors associated with COVID-19 severity in this population will be particularly relevant compared with our cohort.
Limitations
Limitations may include a potential referral bias toward severe COVID-19 or patients receiving DMTs who may have been more concerned about COVID-19 symptoms than patients who are untreated. It is also important to note that some immunosuppressive treatments, notably cladribine and alemtuzumab, are not commonly used in France, so it is not possible to analyze specifically whether these treatments could increase the risk associated with COVID-19.
Conclusions
COVID-19 has significantly changed medical practice for several weeks, and it is likely to remain an issue for months. In patients with MS, we identified disability, age, and obesity as the main risk factors for COVID-19 severity. Our data do not support an increased risk of severe outcome associated with DMTs, which should reinforce the recommendation of not stopping current DMTs and not delaying treatment initiation in patients who have higher disease inflammatory activity, risk for relapses, or subsequent disability. Conversely, patients with high EDSS and older age are at highest risk of severe COVID-19. Given the importance of these 2 variables on COVID-19 outcomes, the identification of a potential outcome of certain DMTs on COVID-19 risk would require a large international effort to collect data and a specific focus on patients with less advanced disease that also considers known comorbidities for COVID-19.
eFigure 1. Age and EDSS per disease modifying therapy group
eFigure 2. Risk factors of severe COVID-19 (severity score ≥ 3, hospitalized or higher severity) in multivariate logistic regression model
eFigure 3. Risk factors of severe COVID-19 (severity score ≥ 4, hospitalized, requiring supplemental oxygen or higher severity)
eTable 1. eTable 1. Demographic and clinical characteristics of patients with MS who were on mechanical ventilation due to COVID-19 (score 6) or died from COVID-19 (score 7)
eTable 2. Number of patients with multiple sclerosis and COVID-19, stratified by age and sex: i) in the cohort; ii) hospitalized, not deceased at follow-up; iii) deceased from COVID-19
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multiple Sclerosis International Federation The coronavirus and MS—updated global advice. Published April 13, 2020. Updated June 18, 2020. Accessed June 19, 2020. https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/.
- 3.Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949-952. doi: 10.1212/WNL.0000000000009507 [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G, Hawkes C, Lechner-Scott J, Levy M, Waubant E, Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39:102073. doi: 10.1016/j.msard.2020.102073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787-1799. doi: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;eabc3517. doi: 10.1126/science.abc3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. Published online March 13, 2020. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020. doi: 10.1002/alr.22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. ; and the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louapre C, Maillart E, Roux T, et al. Patients with MS treated with immunosuppressive agents: across the COVID-19 spectrum. Rev Neurol (Paris). 2020;176(6):523-525. doi: 10.1016/j.neurol.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novi G, Mikulska M, Briano F, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020;42:102120. doi: 10.1016/j.msard.2020.102120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokmaier D, Winthrop K, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology. 2018;90(15 supplement). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng KI, Gao F, Wang XB, et al. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy J, Bensmail D, Brotier-Chomienne A, et al. Respiratory impairment in multiple sclerosis: a study of respiratory function in wheelchair-bound patients. Eur J Neurol. 2017;24(3):497-502. doi: 10.1111/ene.13231 [DOI] [PubMed] [Google Scholar]
- 16.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669-677. doi: 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianfrancesco MA, Hyrich KL, Gossec L, et al. ; COVID-19 Global Rheumatology Alliance Steering Committee . Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2(5):E250-E253. doi: 10.1016/S2665-9913(20)30095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Age and EDSS per disease modifying therapy group
eFigure 2. Risk factors of severe COVID-19 (severity score ≥ 3, hospitalized or higher severity) in multivariate logistic regression model
eFigure 3. Risk factors of severe COVID-19 (severity score ≥ 4, hospitalized, requiring supplemental oxygen or higher severity)
eTable 1. eTable 1. Demographic and clinical characteristics of patients with MS who were on mechanical ventilation due to COVID-19 (score 6) or died from COVID-19 (score 7)
eTable 2. Number of patients with multiple sclerosis and COVID-19, stratified by age and sex: i) in the cohort; ii) hospitalized, not deceased at follow-up; iii) deceased from COVID-19