Abstract
Serological tests for tuberculosis are inaccurate and WHO has recommended against their use. Although not used by the Revised National TB Control Programme (RNTCP), serodiagnostics are widely used in the private sector in India. A root-cause analysis was undertaken to determine why serological tests are so popular, and seven root causes were identified that can be grouped into three categories: technical/medical, economic, and regulatory. Technical/medical: RNTCP’s current low budget does not allow scale-up of the newer, WHO-endorsed technologies. Thus, under the RNTCP, most patients have access to only smear microscopy, a test that is insensitive and underused in the private sector. Because there is no accurate, validated, point-of-care test for TB, serological tests meet a perceived need among doctors and patients. Economic: While imported molecular or liquid culture tests are too expensive, there are no affordable Indian versions on the market, leaving serological tests as the main alternative. Although serological tests are inaccurate, various players along the value chain profit from their use, and this sustains a market for these tests. Regulatory: TB tests are poorly regulated and a large number of serological kits are on the market. Private healthcare in general is poorly regulated, and doctors in the private sector are outside the scope of RNTCP and do not necessarily follow standard guidelines. A clear understanding of these realities should facilitate market-based strategies that can help replace serological tests with accurate, validated tools.
Keywords: Tuberculosis, Serological tests, Root-cause analysis, India, Private medical sector, Diagnosis
1. Introduction
While serological tests work well for several infectious diseases, existing serological tests for tuberculosis [TB] (both ELISA and rapid lateral flow tests) are not accurate or consistent enough to be useful, as shown by a 2007 meta-analysis [1] and confirmed by an independent evaluation of 19 commercial rapid tests by the World Health Organization (WHO) the following year [2]. Unfortunately, the evidence from these studies was not translated into policy at that time.
While most regulatory bodies have mechanisms to withdraw or ban dangerous drugs and vaccines, there is little awareness about the human and economic consequences of bad diagnostics [3]. Another reason for the initial inaction was possibly the perception that these tests were rarely used. In reality, these tests are widely abused in the private sector in countries such as India, with at least 1.5 million serological tests performed in India every year [4].
Every major private laboratory in India offers TB serological tests, mostly ELISA kits imported from developed countries that do not allow these tests to be used on their own TB patients. The problem extends far beyond India. These tests are available in the private sector in at least 17 of the 22 highest TB-burden countries, from China to South Africa to Afghanistan [4].
In 2010, WHO recognized this widespread problem and convened an Expert Group to formulate a policy. The Expert Group considered an updated meta-analysis of test performance and a cost-effectiveness analysis. The meta-analysis synthesized evidence from 92 studies and concluded that commercial serological tests remain inconsistent and inaccurate, supported only by data of very low quality [5]. The cost-effectiveness study found that serology results in more human suffering, secondary infections, and false-positive diagnoses than sputum smear microscopy, while increasing per-patient costs to the Indian TB control sector [6]. At $10–$30 per test, the costs of testing (often borne by patients who pay out-of-pocket), plus the cost of TB drugs wasted on treating patients with false-positive results, rival the entire Revised National TB Control Programme (RNTCP) annual budget of $65 million.
Based on this evidence, the WHO released a policy on 20 July 2011 concluding that since “the harms/risks [of commercial serodiagnostic tests] far outweigh any potential benefits (strong recommendation)...these tests should not be used on individuals suspected of active pulmonary or extra-pulmonary TB, irrespective of their HIV status [7]”.
While the WHO policy provides global guidance, it is up to high-burden countries to implement this policy. Immediately following the WHO policy announcement, the Indian RNTCP issued an advisory against TB serological tests, endorsing the WHO policy recommendations [8]. The challenge, however, is the Indian private sector, which manages nearly half of all TB cases in India [9].
It is well known that TB management practices in the private sector vary widely, often deviating from national and international standards [10–15]. For example, private providers in India are known to underuse sputum smears, and overuse chest X-rays and serological tests for active TB detection [11,14,16–18]. Prescription studies and TB drug market analyses show use of irrational drug regimens, significant overuse of TB drugs, and easy access to TB drugs over-the-counter, without prescriptions [10,13,19,20].
In this context, the following research will explore why serological tests are so popular in the private sector and what factors have paved the way for their widespread use. The lessons learned from the serology story can be applied to validated, WHO-endorsed technologies that need to be scaled-up.
2. Materials and methods
2.1. Data collection
The research began by reviewing the literature on TB diagnostic and management practices in the Indian private sector, as well as the studies on the quality of private healthcare. The published evidence combined with the insights of the following research was used to formulate the survey and conclusions [10–14,16,17,20–23]. Face-to-face or telephone interviews were conducted with 41 stakeholders in India: private doctors and private hospital laboratory staff (N = 11), private stand-alone laboratories (N = 7), distributors of diagnostic tests (N = 7), manufacturers of diagnostic tests (N = 7), government hospital doctors (N = 4), and NGOs working in TB (N = 5). Over 90% of respondents had a minimum of 10 years’ experience in the diagnostics/healthcare sector in India.
Key informant interviews were performed between March and May 2011, before the formal publication of the WHO serology policy in July 2011; 77% of the interviews were done in person. Respondents were generally from metropolitan areas of Delhi, Bangalore, Mumbai and Chennai. All key informants were assured that their identity would not be revealed in any research reports. Informants were selected based on a prior landscape analysis of TB diagnostics in India [3,18], and new key informants were identified by snowballing from the interviews.
2.2. Semi-structured interviews
All respondents were asked the following questions: “Why is ELISA (TB serological tests) the major test for TB in the private sector? Could you list all possible reasons?” Each answer was followed by asking why each of the reasons existed. They were also asked: “What are the interests of the stakeholders (e.g. economic, clinical, etc.) of TB diagnostics in India?” Interviewees were prompted to speak about their personal experience with TB diagnostics (rather than hearsay). Data were also collected on cost of various TB tests from diagnostic companies, distributors and private laboratories.
2.3. Data analysis
2.3.1. Root-cause analysis (RCA)
RCA is a tool designed to help identify not only what and how an event occurred, but also why it happened [24]. RCA prevents the most visible causal factor from getting all the attention. It charts out the causes and their effects for a particular problem. Root causes are [24]: (a) specific underlying causes; (b) those that can reasonably be identified; (c) those that can be fixed; and (d) those for which effective recommendations for preventing recurrences can be generated. The process of formulating the RCA involves data collection, cause charting, root-cause identification, recommendation generation and implementation [24]. The following proposed recommendations are either directly from the authors or were suggested by interviewees.
Current Reality Tree (CRT) is a type of RCA [25]. CRT allows incorporating multiple problems into a single diagram. It also enables identifying causal interdependency, intermediate factors and feedback loops [25]. Doggett further explains the CRT as follows: “The CRT was designed to show the current state of reality as it exists in a system. Typically, arrows in the CRT signify a sufficiency relationship between the entities. Sufficiency implies that the cause is, in fact, enough to create the effect. Entities that do not meet the sufficiency criteria are not connected. The relationship between two entities is read as an “if-then” statement such as, “If [cause statement entity], then [effect statement entity]” [25].
In addition, the CRT uses a unique symbol, the oval or ellipse, to show relationships between interdependent causes. These relationships were constructed by the authors, based on the interviews and published literature. Because the CRT is based on sufficiency, there may be cases where one cause is not sufficient by itself to create the proposed effect. Thus, the ellipse shows that multiple causes are required for the produced effect.
3. Results
3.1. Quantitative analysis of interviews
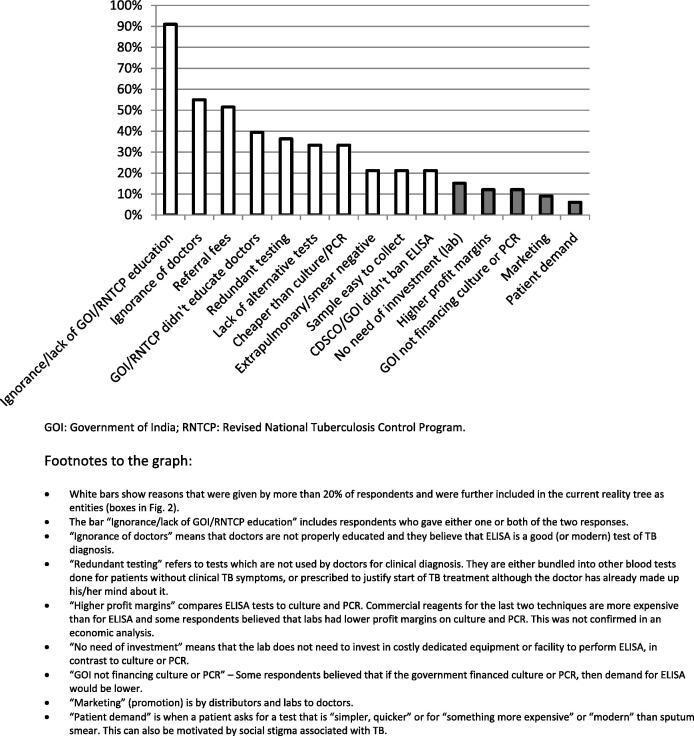
Fig. 1 summarizes the responses to the question: “Why is ELISA (TB serological test) the major test for TB in the private sector? Could you list all possible reasons?” Overall, a lack of awareness among doctors regarding TB diagnosis was the most frequently given reason. This was formulated either as “doctors’ ignorance” about the lack of accuracy of serological tests, or by pointing out that RNTCP and the government had failed to educate the private doctors. This reason was followed by referral fees (incentives) which encourage the use of serodiagnostics. Doctors who request serological tests are often offered by the private laboratories about 20–50% of the price (i.e., between 150 and 300 rupees) paid by the patient. Redundant testing where serology is one component in a battery of lab tests, a lack of accurate rapid tests with sensitivity higher than sputum smear (preferably also blood-based) and patient’s affordability were some other leading reasons.
Figure 1.
Bar chart showing the frequency of all replies to the question: “Why is ELISA (and other serological tests) the major test for TB in the private sector?” (N = 33 respondents with direct experience in TB diagnostics).
3.2. Economics of TB diagnostics from the laboratory perspective
This analysis was undertaken to understand why private-for-profit laboratories prefer serological tests. As shown in Table 1 (based on data collected from laboratories), there are striking disparities in prices of TB tests across private labs (100% variation) and the informants were unable to disaggregate the margins and specific non-reagent costs. In fact, when pricing a new test, the informants indicated that laboratories would typically consider 4–7-fold increase versus the reagent price as well as patient’s willingness to pay for a given test. Private laboratories also factor in the referral/incentive fees that they would need to pay back to referring doctors. These factors collectively result in inflation of the price of diagnostics for patients.
Table 1.
Economics of TB diagnostics in private sector laboratories in India.
| Characteristics per single test | Sputum Smears (Z-N and fluorescent) | ELISA (serological tests for TB) | Automated liquid culture | PCR-based molecular tests |
|---|---|---|---|---|
| Cost of reagents and consumables (Rupees) | 15 | 90 for TB IgG 90 for TB IgM |
200 (detection) 500 (detection and drug sensitivity testing) |
300 Gel PCR 500 RT-PCR 1100 Line probe assay 1400 GeneXpert |
| Margin + non-reagent costs to the lab (Rupees)# | 85–125 | 310–510 | 700–2500 | 700–2100 |
| Price to the patient (Rupees) | 100–150 | 400–600 for a single antibody 800–1200 for TB IgG + TB IgM |
900–1500 (detection) 2000–3000 (detection and drug sensitivity) |
1000 Gel PCR 1500 RT-PCR 2500 Line probe assay 3500 GeneXpert |
| Time spent by technician | 45 min | 3–4 h* | 1 h* | 3–4 h* |
| Technician training time required | 5 days (min) | 2 days | 2–3 days | 2 days–2 weeks |
| Requirement of instrumentation (Rupees) | Microscope | ELISA reader (90,000) | Instrument (100,000–150,000)** | PCR equipment, RT-PCR, GeneXpert |
| Requirement of special working conditions | Moderate biosafety | Minimal biosafety | BSL III | Moderate biosafety, Separate room for each reaction step (except GeneXpert) |
“Margin + non-reagent costs” value is aggregated because respondents were unable to clearly state the corresponding amounts. Instead, the labs said that when calculating a price of a test to patients, they would typically assume 4–7-fold increase versus the cost of a reagent.
Several tests can be prepared at the same time (limited by centrifuge/machine capacity).
Reagent rental possible (e.g. highly discounted instrument, obligation to do 10 samples/day for sensitivity testing during 3 years).
Overall, sputum smears are the least expensive tests and also have the lowest profit margin. Therefore, private laboratories do not actively promote them. At the other extreme, liquid cultures and molecular tests (e.g. polymerase chain reaction [PCR]) ensure higher profits to both doctors and laboratories, but are rarely prescribed by doctors because patients find them too expensive. In general, laboratories are reluctant to bear very high costs of capital investment in equipment and training of technical staff without certainty that they would have a sufficient volume of samples to recoup the investment. Scarcity of laboratories which perform liquid culture and PCR tests for TB and infrequent prescription of these tests by doctors are linked by a positive feedback loop.
Serological tests have an intermediate price tag and a modest profit margin, but because of the large volume (a large number of requests from doctors) and low investment requirement (many laboratories have ELISA readers that are used for a variety of tests), private laboratories find them attractive. Serology gives higher profit margins than sputum smears and is easy to perform with minimal investment. Further, ELISA tests are prescribed frequently by doctors, many of whom consider them to be a “modern or better alternative” to the antiquated sputum microscopy. Widespread availability of ELISA tests for TB in many private laboratories and frequent orders for the test by doctors are linked by a positive feedback loop. Along the delivery chain of the serological tests for TB, private pathology laboratories and doctors draw the highest economic gains from the test. Others who profit include diagnostic manufacturers and distributors.
3.3. Why are sputum smears unpopular in the private sector?
During the interviews, additional questions were asked in order to identify clinical, practical, economic and cultural reasons (Table 2) why sputum smears are unpopular. These range from the poor sensitivity of smears, lack of utility in extrapulmonary and smear-negative TB, to low profit margins for laboratories, low referral fees for doctors, and stigma associated with sputum testing and TB. Many of these reasons provide insights into why a blood-based ELISA TB test might be more attractive to doctors, patients and laboratories in India. Blood-based tests are done for a variety of diseases, and not easily linked with TB and the stigma surrounding the disease. Unlike sputum smears where patients are required to give multiple specimens, a single blood draw is adequate for serological tests, and results are much faster than smear microscopy. More importantly, serological tests are economically more profitable for both laboratories and doctors, as compared to sputum smears.
Table 2.
Reasons why sputum smear microscopy is not popular in the private sector in India.
| Doctors | Patients | Labs | |
|---|---|---|---|
| Clinical | Doctors think smears are not sensitive and are antiquated | Some patients are not able to produce sputum | Labs think that smears are antiquated and are keen on replacing them with a more modern technology |
| Sputum-based tests are not suitable for diagnosis of Extrapulmonary TB, smear-negative and childhood TB | |||
| Practical | Unlike tests such as chest X-ray, doctors cannot directly see the smear result (have to rely on lab interpretation) | Patients ask for a test that requires a single visit | ELISA is a “bench” technique and is perceived as “cleaner” than smear |
| Doctors have been told that 3 sputum specimens need to be examined and this is not convenient for patients and drop-outs are likely | Technician training is necessary for microscopic examination of smear. | ||
| In case of respiratory infections or chronic fevers, patients are giving blood sample for blood counts / ESR and ELISA can be performed on the same sample | RNTCP does not reach the private labs to give guidance on the quality assurance for sputum smears | ||
| Economic | Smears are cheap and referral fees are too low (referral fees to doctors are higher for x-rays, serology, PCR, etc.) | Some patients are ready to pay more than approximately 100 Rs for the smear if they believe that they are offered a better test | Smears are cheap and give low profit margins so labs do not promote them to doctors |
| Doctors want to start TB treatment to keep patients with them for 6 months, and because smear is perceived as having low sensitivity, they do not like to use it | |||
| Cultural | Doctors want to be perceived as “modern” by the community and refrain from antiquated techniques | Patients associate sputum with TB and that increases their fear of stigmatization; so, patients may prefer a blood-based test over sputum testing | Labs want to be perceived by doctors and by patients as “modern” and refrain from antiquated techniques |
3.4. Root causes for the success of TB serological tests in India
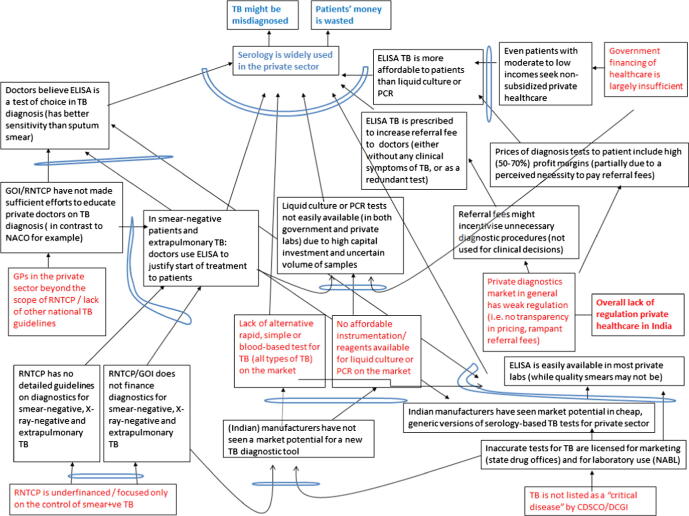
The RCA analysis suggested seven root causes (Fig. 2) that can be grouped into three categories: technical/medical, economic, and regulatory.
Figure 2.
Current Reality Tree which identifies the root causes of the widespread use of TB serodiagnostics in the private sector in India.
From a technical/medical perspective, RNTCP is currently underfunded and perceived to be focused only on sputum smear-positive cases. Indeed, most patients can only access sputum smears in the public sector. Private doctors therefore do not think the RNTCP can offer anything more than just smear microscopy for their patients, and they also believe that smears are not adequate for the types of cases they encounter (e.g. extrapulmonary TB and smear-negative disease). Private doctors also believe that smears are insensitive and inconvenient for patients. More importantly, TB culture facilities are not widely available via the RNTCP, and there is a general lack of trust in the quality of government-run services. Thus, private doctors are reluctant to refer their patients to RNTCP for diagnostic workup, even if it is free for patients. Also, because there is no accurate, WHO-endorsed, simple point-of-care test for TB, serological tests meet a perceived need among doctors for a rapid, blood-based test, especially for extrapulmonary TB, smear-negative TB, and for investigation of chronic fevers and relevant conditions (e.g. infertility in women).
From an economic perspective, several players are given incentives to use serological tests along the value chain, and this sustains their market. In contrast, there are no affordable molecular or liquid culture tests on the Indian market. This means such tests are mostly imported and are very expensive for Indian patients. Indeed, tests such as automated liquid culture (e.g. MGIT 960, BD, Sparks, USA) and molecular tests (e.g. Xpert MTB/RIF, Cepheid Inc., Sunnyvale, CA, USA) cost as much as $50–$100 in the private market, and these tests are rarely used because of the high costs. In this context, serology is perceived by doctors as a “more affordable and convenient” alternative.
From a regulatory perspective, because private medical care is poorly regulated, doctors in the private sector are outside the scope of RNTCP and do not feel compelled to follow RNTCP guidelines. Apart from the RNTCP guidelines, there are no other national TB guidelines for private doctors to follow. While the International Standards for TB Care are available [15] and endorsed by the RNTCP, there is no easy mechanism to implement or mandate them in the private sector. In the past, Indian doctors were not required to get re-certified or even undergo continuing medical education (CME) to keep up-to-date with the latest guidelines. This may be changing with the Medical Council of India (MCI) proposing new rules that require mandatory CME credit hours to maintain their registration. For many private doctors, a major source of educational material is material handed out by pharmaceutical and laboratory sales representatives. Thus, some private doctors may be genuinely unaware that serological TB tests are not recommended by any agency. They may be using these tests in good faith, and not necessarily driven by economic incentives.
Regulation of in vitro diagnostics (IVDs) is weak in India. The Central Drugs Standard Control Organization (CDSCO) classifies IVDs as “critical” or “non-critical” with only a few tests (e.g. HIV, hepatitis B & C) related to blood safety (in blood banking) considered “critical”. TB tests are not classified as “critical tests” by the Drug Controller General of India (DCGI), and this allows for entry and sale of suboptimal serodiagnostics with very little independent validation [18]. In fact, a large number of imported and domestic serological kits are readily available in the market and various players have incentives to use them [4,6]. Many of the kits on the market claim to have near-perfect sensitivity and specificity, without published data to support such claims [5,6].
Lastly, the private laboratory testing market in India is also poorly regulated. A majority of Indian labs are not accredited or certified by a quality assurance body [18], and there are few requirements for establishing and running laboratories, although this may improve with the enforcement of the Clinical Establishments Act [26]. There is no transparency in pricing, and practices such as referral fees are well documented [27].
4. Discussion
Mismanagement of TB is bad for the individual patient. For example, false-positive diagnoses result in unnecessary drug treatment and side effects, personal financial losses, and public resource utilization [6]. Mismanagement is equally bad from a public health perspective because every mismanaged or undiagnosed TB patient serves as a source for new infections in the community [3]. Widespread abuse of inappropriate tests can prevent the use of good diagnostics, and this is a major challenge for implementation of new diagnostics. In a country where private out-of-pocket expenditure dominates the financing of health care costs and where such spending is one of the major reasons for people sliding into indebtedness [28], the use of inaccurate tests might have a negative impact on household budgets of many people.
For each of the root causes identified, several recommendations are suggested (Table 3). Strengthening the RNTCP, increasing its budget, and scaling-up new, validated diagnostics within RNTCP will enable more Indian TB patients to get quality-assured diagnostic work-up, and might help address the perceived need of doctors for improved diagnostic tests. Because expanding DOTS coverage and engaging all providers is critical for RNTCP, the programme should make an effort to understand why patients prefer the private sector, and why so many doctors keep referring patients to private diagnostic laboratories and not to the RNTCP. Lack of trust in quality of public services and limited availability of diagnostics other than sputum smears are issues which the RNTCP can and must address.
Table 3.
Recommendations for addressing the root causes identified for success of serological tests in India.
| Root cause | Recommendations |
|---|---|
| RNTCP is underfinanced/focused only on the control of smear-positive TB |
|
| Doctors in the private sector beyond the scope of RNTCP/lack of other national TB guidelines |
|
| TB is not listed as a “critical disease” by CDSCO & DCGI |
|
| Lack of alternative rapid, simple or blood-based test for TB (all types of TB) on the market No affordable instrumentation/reagents available for liquid culture or PCR on the market |
|
| Private diagnostics market in general has weak regulation (i.e. no transparency in pricing, widespread referral fees) |
|
| Government financing of healthcare is largely insufficient in India |
|
CDSCO, Central Drugs Standard Control Organization; DCGI, Drug Controller General of India; GOI, Government of India; RNTCP, Revised National Tuberculosis Control Programme; NABL, National Accreditation Board for Testing and Calibration Laboratories; NACO, National AIDS Control Organization.
Indeed, India’s annual healthcare spending continues to remain one of the lowest in the world. Not surprisingly, the RNTCP budget is low, even though modeling suggests that TB control has been a very cost-effective strategy for improving the health status of India’s population, with exceptional return on investment from a societal perspective [29]. It has been estimated that the scale-up of TB control in India accounted for just 1.0% of public expenditure on health over the period 1997–2006 [29]. Thus, the RNTCP’s current low budget does not allow widespread scale-up of sophisticated, WHO-endorsed technologies. For example, only a small fraction of Indian patients has access to liquid cultures and line probe assays. In 2010, India, together with DR Congo and Bangladesh, had the poorest access to culture facilities among high-burden countries (less than 0.1 per 5 million of population) [30]. A large proportion of TB patients therefore end up seeking private medical care, even if it meant higher expense for patients who mostly pay out-of-pocket [9].
The RNTCP has clearly made great progress in the past decade and has successfully scaled-up the DOTS strategy to cover 100% of the Indian population [29]. The program is currently preparing to enter a new phase, the National Strategic Plan (NSP), for the period 2012–2017. For this new phase, significantly higher budgets have been requested. Scale-up of new diagnostics is a central part of this plan, along with greater engagement of the private sector, which must be involved in creating RNTCP guidelines, and thereby have greater ownership. Creation of an “Indian Standards for TB Care,” along the lines of the “International Standards for TB Care”, for both public and private sectors, might help in overcoming the reluctance of private doctors to follow RNTCP guidelines, which they do not perceive as their own.
The central issue to appreciate is that India has the largest private health sector in the world, with 60–80% of health care sought in the private sector, and a health care market that is worth billions of rupees [31]. Despite its enormous size and importance, this sector is largely unregulated [31], although the Clinical Establishment Act 2010 attempts to address this tricky and controversial issue [26]. This Act is yet to be implemented and has been opposed by the private medical sector in India [32]. Full implementation of this Act should be a major priority for the Indian government and ministry of health.
Furthermore, the private health care market in India is heavily doctor-centric. The doctor is the sole decision maker in determining the tests that a patient will receive. While not all private doctors accept kickbacks and order unnecessary tests, the private healthcare system is influenced by monetary gains, with wide variations in practice quality [20–23]. Thus, any potential solution must address this reality, and take into account well-documented issues such as referral fees [27,33], unnecessary interventions [34], and widespread antibiotic abuse [20,35,36].
TB serological tests can bring economic benefits to doctors, although not all laboratories offer incentives and not all doctors accept incentives. Similar profit to doctors must be assured for good practice. This could be a referral fee paid to them by RNTCP for referral to their facility or to an accredited private laboratory which only performs WHO-endorsed tests. Further, free DOTS medicines could be made available to patients in the private sector so that doctors do not need to be concerned about losing their patients to the RNTCP.
The government should actively educate the public on the prevalence of referral fees in the private laboratory sector and empower patients to choose service providers. For that, patients must be enabled to judge laboratory quality on their own and make their choice based on quality and price. They must be aware of the accreditation of laboratories and must have a reasonable choice of accredited laboratories. Only then fair price competition among laboratories can be promoted.
Unfortunately, there is currently no way for patients to get data on quality of laboratories or doctors or hospitals in India. There is no objective metric or ranking system to judge quality of medical care in the country. Quality Council of India [37], an autonomous body of the Department of Industrial Policy and Promotion, Govt. of India, must address this by establishing and improving national accreditation structures, including National Accreditation Board for Testing and Calibration Laboratories (NABL), and National Accreditation Board for Hospitals and Healthcare Providers (NABH).
A majority of Indian laboratories are not accredited or certified by a quality assurance body [18]. Currently, less than 250 Indian laboratories out of an estimated 50,000 have NABL accreditation [38]. There are very few requirements as to minimal training qualifications for persons performing laboratory procedures, and skilled laboratory technicians are scarce in India. Making NABL accreditation a mandatory requirement for all laboratories is a policy worth consideration.
In the current scenario where regulation of IVDs is virtually limited to the so-called “critical devices” listed by CDSCO, putting TB tests on this list is essential to remove serological TB tests from the market. In principle, the recent WHO policy would make this regulatory action straightforward. In practice, it will require political will at the level of the DCGI, high-level support from agencies such as RNTCP and Indian Council of Medical Research, and advocacy from patient representatives, civil society and consumer groups.
If a new diagnostic is to succeed in the Indian private market, what characteristics should it have? Based on the lessons learned in this analysis, we think the new diagnostic should provide rapid results (e.g. same-day diagnosis), be more sensitive than sputum microscopy, work well with specimens other than sputum (e.g. blood or urine), and be able to detect extrapulmonary and smear-negative disease (Table 4). The test should not be too cheap or too expensive, but rather be in the middle range of about Rupees 500 [approximately US $10] (price to the patient) in the private sector, and the new test should not force labs to make big investments in equipment, maintenance or training.
Table 4.
If a new diagnostic is to succeed in the Indian private market, then the popularity of commercial serology tests in India suggests that it should have the following characteristics.
| Test characteristic | Rationale |
|---|---|
| Should be perceived by doctors as a more sensitive and sophisticated test than sputum smears | Doctors often fear under-diagnosis of TB. They do not want to miss a TB case for ethical as well as monetary reasons (the patient will be under their treatment for months). They fear that their reputation will suffer if they offer to patients sputum smears or refer them to an RNTCP centre |
| Should be a rapid test – either a point-of-care* test which can be done in the clinic or a laboratory test that can produce results within the same day | Given the doctor-centric nature of the private healthcare, doctors need to draw monetary benefit from the procedure. A rapid test result ensures that patients will stay with the doctors and will not drop-out. Tests such as cultures are very unpopular among doctors because of the lengthy time delays and because they rarely influence doctor’s clinical decisions |
| Should be done on blood or urine sample and a single test should be sufficient for diagnosis | Stigma related to TB makes sputum a less desired sample. Also, patients with suspected TB or chronic fevers often give blood samples for other lab tests (ESR, CBC) and this will make a test based on sputum disadvantaged as compared to a test which can be done on the same blood sample. Also, doctors might be afraid that patients will not show up for a second visit if more than one test is needed to make diagnosis |
| Should be suitable for the detection of extrapulmonary TB | Neither sputum smear nor X-ray is suitable for detection of extrapulmonary TB. There is a highly unmet need for a test for this type of TB (genito-urinary TB in particular because it is considered a major cause of infertility in India). |
| Labs should not need to make big investments in infrastructure/equipment | Labs might be reluctant to invest in equipment/facility if they are not certain of a good volume of samples. This applies also to reagent rental schemes which oblige labs to buy a certain amount of reagents in a given time |
| It should not be too cheap or too expensive, but be in the middle range of about rupees 500 (price to the patient) in the private sector | The current private health care system is to a large extent driven by referral fees which are about 20–50% of the price which patients pay for the test. Any diagnostic test to be successful in the current scenario must assure a referral fee to doctors in a range of 150–300 Rupees per patient. Patients’ affordability dictates that the test should not significantly exceed rupees 500 (approx. 10 US$) or so |
Most Indian doctors do not perform any testing themselves in the clinic and they prefer to send patients to the labs, either because they are too busy to be doing testing, or they are nervous about interpreting rapid tests themselves. Also, sending patients to labs is much easier because kickbacks are assured. If they do the POC test themselves, then they have to charge the patient their consultation fees PLUS the rapid test fees and that might be seen as a problem for patients (who will not mind paying the lab). So, contrary to what is often thought, a POC test in India might not actually get used at the point of care.
5. Conclusion
Although the WHO recommendation is a major progress in advocating against the use of inaccurate TB tests, the WHO policy, by itself, is unlikely to change the reality in the Indian private sector. A clear understanding of the ground realities and the root causes should facilitate market-based strategies that can help replace serological tests with accurate, validated tools. Our analysis suggests that increased funding for TB control, scale-up new diagnostics via the RNTCP, stronger regulation of the private healthcare sector (including improved regulation of TB diagnostics), rapid development of in-country R&D to bring new diagnostic options to the market, and greater engagement of the private health sector with the RNTCP are critical ingredients for success.
Acknowledgements
We are grateful to all the key informants who generously contributed their time and expertise. We are also grateful to Karen Steingart, David Dowdy, and Andrew Ramsay for providing constructive feedback on an earlier version of this manuscript. Szymon Jaroslawski is supported by an allowance from France Volontaires, Ivry sur Seine, France. Madhukar Pai is supported by research grants from the Canadian Institutes of Health Research (CIHR), European and Developing Countries Clinical Trials Partnership (EDCTP) (TB-NEAT grant) and European Commission (EU-FP7; TBSusgent grant), and Grand Challenges Canada. These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author contributions
Szymon Jaroslawski and Madhukar Pai conceived and designed the study. Jaroslawski conducted the key informant interviews, analyzed the data and wrote the first draft of the manuscript. Pai provided input in the data analysis and interpretation, and edited and revised the manuscript for important intellectual content. Both authors have approved the final article and agree with its contents.
Conflicts of interest
None of the authors have any financial/industry conflicts to declare. Szymon Jaroslawski works on research projects co-financed by the Institut Merieux, France. Madhukar Pai was engaged in the WHO policy recommendation against serological tests and has published meta-analyses and cost-effectiveness studies on TB serological tests. He serves as a consultant to the Bill & Melinda Gates Foundation (BMGF).
References
- [1].Steingart KR, Henry M, Laal S, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization . Diagnostics Evaluation Series No.2. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. Geneva: World Health, Organization; 2008. [Google Scholar]
- [3].Pai M. Improving TB diagnosis: difference between knowing the path and walking the path. Expert Rev Mol Diagn. 2011;11:241–4. doi: 10.1586/erm.11.6. [DOI] [PubMed] [Google Scholar]
- [4].Grenier J, Pinto LM, Nair D, et al. Widespread use of serological tests for tuberculosis: data from 22 high-burden countries. Eur Resp J. 2012;39:502–5. doi: 10.1183/09031936.00070611. [DOI] [PubMed] [Google Scholar]
- [5].Steingart KR, Flores LL, Dendukuri N, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8:e1001062. doi: 10.1371/journal.pmed.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dowdy DW, Steingart KR, Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001074. doi: 10.1371/journal.pmed.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].World Health Organization . Policy statement: Commercial serodiagnostic tests for diagnosis of tuberculosis. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- [8].DDG Advisory on TB serodiagnostics Directorate General of Health Services, Ministry of Health and Family Welfare. 2011 (Accessed 4 October, 2011, at < http://www.tbcindia.org/pdfs/Letter_Serodiagnosis.pdf>).
- [9].Satyanarayana S, Nair SA, Chadha SS, et al. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community-based survey of 30 districts. PLoS One. 2011;6:e24160. doi: 10.1371/journal.pone.0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Udwadia ZF, Pinto LM, Uplekar MW. Tuberculosis management by private practitioners in Mumbai, India: has anything changed in two decades? PLoS ONE. 2010;5:e12023. doi: 10.1371/journal.pone.0012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uplekar M, Juvekar S, Morankar S, Rangan S, Nunn P. Tuberculosis patients and practitioners in private clinics in India. Int J Tuberc Lung Dis. 1998;2:324–9. [PubMed] [Google Scholar]
- [12].Uplekar MW, Rangan S. Private doctors and tuberculosis control in India. Tuber Lung Dis. 1993;74:332–7. doi: 10.1016/0962-8479(93)90108-a. [DOI] [PubMed] [Google Scholar]
- [13].Uplekar MW, Shepard DS. Treatment of tuberculosis by private general practitioners in India. Tubercle. 1991;72:284–90. doi: 10.1016/0041-3879(91)90055-w. [DOI] [PubMed] [Google Scholar]
- [14].Bhargava A, Pinto LM, Pai M. Mismanagement of tuberculosis in India: causes, consequences, and the way forward. Hypothesis. 2011;9:1–13. doi: 10.5779/hypothesis.v9i1.214. [DOI] [Google Scholar]
- [15].Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–25. doi: 10.1016/s1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- [16].Singla N, Sharma PP, Singla R, Jain RC. Survey of knowledge, attitudes and practices for tuberculosis among general practitioners in Delhi, India. Int J Tuberc Lung Dis. 1998;2:384–9. [PubMed] [Google Scholar]
- [17].Prasad R, Nautiyal RG, Mukherji PK, Jain A, Singh K, Ahuja RC. Diagnostic evaluation of pulmonary tuberculosis: what do doctors of modern medicine do in India? Int J Tuberc Lung Dis. 2003;7:52–7. [PubMed] [Google Scholar]
- [18].Pai M. Tuberculosis control in India: time to get dangerously ambitious? Natl Med J India. 2011;24:65–8. [PubMed] [Google Scholar]
- [19].Wells WA, Ge CF, Patel N, Oh T, Gardiner E, Kimerling ME. Size and usage patterns of private TB drug markets in the high burden countries. PLoS One. 2011;6:e18964. doi: 10.1371/journal.pone.0018964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Greenlagh T. Drug prescription and self-medication in india: an exploratory survey. Social Sci Med. 1987;25:307–18. doi: 10.1016/0277-9536(87)90233-4. [DOI] [PubMed] [Google Scholar]
- [21].Das J, Hammer J. Money for nothing: the dire straits of medical practice in Delhi, India. J Dev Econo. 2007;83:1–36. doi: 10.1016/j.jdeveco.2006.05.004. [DOI] [Google Scholar]
- [22].Das J, Hammer J, Leonard K. The quality of medical advice in low-income countries. J Econo Persp. 2008;22:93–114. doi: 10.1257/jep.22.2.93. [DOI] [PubMed] [Google Scholar]
- [23].Nandraj S. Beyond the law and the LORD: quality of private health care. Econo Political Weekly. 1994;29:1680–5. [Google Scholar]
- [24].Rooney JJ, Vanden Heuvel LN. Root cause analysis for beginners. Quality progress. 2004 [Google Scholar]
- [25].Doggett M. Root cause analysis: a framework for tool selection. Quality Manage J. 2005;12:34–45. [Google Scholar]
- [26].Government of India; Report on the Working Group on Clinical Establishments, Professional Services Regulation and Accreditation of Health Care Infrastructure For the 11th Five-Year Plan. (Accessed 24 November, 2011, at < http://planningcommission.nic.in/aboutus/committee/wrkgrp11/wg11_hclinic.pdf>). [Google Scholar]
- [27].Rajagopalan A. Misuse of diagnostic tests. Indian J Med Ethics. 2008;5:121–2. doi: 10.20529/IJME.2008.044. [DOI] [PubMed] [Google Scholar]
- [28].Shahrawat R, Rao KD. Insured yet vulnerable: out-of-pocket payments and India’s poor. Health Policy Plan. 2011 doi: 10.1093/heapol/czr029. [DOI] [PubMed] [Google Scholar]
- [29].Goodchild M, Sahu S, Wares F, et al. A cost-benefit analysis of scaling up tuberculosis control in India. Int J Tuberc Lung Dis. 2011;15:358–62. [PubMed] [Google Scholar]
- [30].Global tuberculosis control . WHO report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- [31].Bhat R. Regulation of the private health sector in India. Int J Health Plann Manage. 1996;11:253–74. doi: 10.1002/(SICI)1099-1751(199607)11:3<253::AID-HPM435>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [32].Phadke A. The Indian Medical Association and the Clinical Establishment Act, 2010: irrational opposition to regulation. Indian J Medi Ethics. 2010;7:229–32. doi: 10.20529/ijme.2010.084. [DOI] [PubMed] [Google Scholar]
- [33].Bajaj R. It is time to wash the linen. Natl Med J India. 2007;20 [PubMed] [Google Scholar]
- [34].Pai M, Sundaram P, Radhakrishnan KK, Thomas K, Muliyil JP. A high rate of caesarean sections in an affluent section of Chennai: is it cause for concern? Natl Med J India. 1999;12:156–8. [PubMed] [Google Scholar]
- [35].Ganguly NK. Global Antibiotic Resistance Partnership India National Working Group, ed. Delhi: Center for Disease Dynamics, Economics & Policy; 2011. Situation Analysis: Antibiotic Use and Resistance in India. [Google Scholar]
- [36].Phadke A. New Delhi: Sage Publications; 1998. Drug supply and use: toward a rational policy in India. [Google Scholar]
- [37].Quality Council of India 2011 (Accessed 23 November 2011, at < http://www.qcin.org>).
- [38].National Accreditation Board for Testing & Calibration Laboratories Government of India. 2011 doi: 10.4103/IJPM.IJPM_630_17. (Accessed 23 November 2011, at < www.nabl-india.org>). [DOI] [PubMed]