Abstract
Background
Hypertension is a leading cause of cardiovascular disease (CVD). The purpose of this study was to examine the effectiveness of community healthcare in controlling blood pressure (BP) and mitigating related risk factors after 5 y of follow-up.
Methods
Hierarchical clustering sampling was employed to choose a representative sample of 10 rural and 10 urban community populations (N=4235). The 5y prospective cohort study was completed by the medical group in the community clinical centre.
Results
The study included 4235 patients, median age 69 y (range 61–76), with hypertension in 2009; 2533 (59.81%) were female. The rate of BP control increased from 28.33% in 2009 to 64.05% in 2014. The BP control rate was higher in patients with CVD and kidney disease and lower in those with obesity than in those without. Comparing 2009 and 2014 values, the intervention resulted in median systolic BP and diastolic BP reductions of 7.0 mmHg and 6.5 mmHg, respectively. Age, medication treatment, antihypertensive agents, BP at baseline and follow-up, complications of diabetes, CVD, obesity and kidney disease, the aspartate aminotransferase:aminotransferase ratio and smoking were identified as risk factors for BP control.
Conclusions
Community management of hypertension by general practitioners achieved significant BP control over 5 y of intervention.
Keywords: hypertension, blood pressure control, risk factor, prospective cohort study
Introduction
Hypertension is one of the main contributors to the worldwide disease burden,1 especially in developing countries. Hypertension is prevalent in China, and the rate of blood pressure (BP) control is lower than recommended by the guidelines,1 even with medication. Overall, the estimated prevalence of hypertension is significantly higher in the elderly (≥65 y) than in younger individuals (<65 y), and this is particularly observed in low- and middle-income countries (LMICs).2 Indeed, the greatest increase in hypertension prevalence has occurred in developing countries, especially in China. This increase is the result of ongoing changes in lifestyle and health behaviours, including tobacco use and decreased physical activity (PA). Additionally, the elevated prevalence of obesity and hypertension has contributed to the increasing prevalence of cardiovascular disease (CVD) and CVD-induced mortality in developing countries. The prevalence of hypertension among Chinese adults rose significantly from 14.5% in 1991 to 21.4% in 2009, an increase of 6.9%.3 Moreover, this increasing hypertension prevalence is a potentially severe burden on healthcare systems, especially in developing areas of China.
Although population growth and ageing have led to an increase in the absolute burden of chronic diseases in developed countries, age-specific mortality and the incidence of CVDs and other chronic non-communicable diseases has decreased. Large-scale intervention methods4,5 and health policies,6,7 such as text messaging or internet-based reminders to improve medication adherence, and the optimization of medical insurance policies have been well implemented in some developed countries. This success is partly attributable to nationwide reductions in major health-related risk factors through improved diet, lifestyle changes and health education on topics such as smoking cessation, self-management of BP, nutrition and PA.8–10 Despite a continuing decrease in such risk factors among the populations of developed countries, the prevalence rates of hypertension and its complications have increased or remained unchanged in many LMICs.1 Dietary habits, PA and regulatory and pharmacological interventions can effectively control BP, and such measures have helped to decrease mortality from CVD in high-income settings in a cost-effective manner.11 Nonetheless, in LMICs, the ability to identify people at high risk of developing CVDs, deliver healthy management and ensure compliance with these guidelines is constrained by the number of medical staff and their professional training, as well as the cost of healthcare and the infrastructure of health facilities.
Interventions initiated in a community care setting may provide a cost-effective approach for managing cardiovascular risk factors in LMICs.11,12 Evidence from five provinces of China has revealed that community health management of patients with hypertension can reduce the annual direct medical expense per capita by 210 yuan.13 However, the primary barrier to achieving a long-term effect involves the willingness of the participants to follow the healthy lifestyle principles introduced by the intervention, which may disrupt their regular lifestyle. In general, further long-term cohort studies are needed to determine whether health management and intervention in communities can result in long-term antihypertensive effects and promote healthy habits. To date, no reported study has examined the effect of such measures on a large sample of patients with hypertension over a 5y follow-up period in southwest China. Accordingly, the purpose of this study was to assess the effectiveness of community-based healthy management of hypertension covered by the National Basic Public Health Services (NBPHS). The goal is to contribute to achieving effective long-term control of BP in individuals with hypertension. We hypothesized that such a hypertension community management programme conducted by general practitioners (GPs) would help in reducing BP, promoting healthy behaviour and, in the long term, lowering cardiovascular risk factors and reducing mortality.
Materials and methods
Intervention method
The intervention procedure was introduced in detail in a previously published paper.14 In the present study, the effect of BP control and risk factors for hypertension community management as a part of the NBPHS were evaluated after 5 y of follow-up. GPs were trained to diagnose hypertension, to measure BP and to provide medication treatment and lifestyle advice for BP control every 3 months during the follow-up period. Follow-up was conducted by a medical team that consisted of GPs, nurses, clinical pathologists and general public health practitioners. GPs also noted the dates of subsequent visits to address health issues related to hypertension. To ensure quality and adherence for the long-term follow-up, the GPs contacted patients via telephone and text messages. The clinical pathologist also performed a pathological examination and the public health practitioner offered health education and guided patients in reacting to public health events. Every year the nurses in the communities conducted physical examinations and measured fasting blood glucose and lipid levels. The enrolment and research plans were reviewed and approved by the institutional ethics committee of the Centres for Disease Control and Prevention of Jiulongpo District in Chongqing, China (reference number 066/2008). Informed written consent was obtained from the participants by the local GPs.
Data sources and sample size
Baseline samples were obtained from the NBPHS system according to the inclusion criteria. The inclusion criteria for the participants with hypertension was age >35 y and diagnosed with hypertension according to the guidelines from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). For patients with hypertension, those with serious diseases (e.g. cancer) were excluded from this study, as were those with secondary hypertension. Hierarchical clustering sampling was used to choose a representative sample of 10 rural and 10 urban community populations (N=4235) in southwest China (Figure 1). This study included individuals with hypertension who had completed at least 3 y of follow-up and BP measurements. Information regarding sex, age, address, body weight, height, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and other biomarkers was collected by medical groups in community clinical centres. The questionnaire we used included questions about drug use for hypertension treatment, disease history, complications (including diabetes, obesity, CVD, stroke and kidney disease) and PA. The use and dosage of drugs were confirmed through a review of medical records or by reviewing drug use at each follow-up or physical examination. A total of 4235 patients who had been diagnosed with hypertension and had undergone physical examination in 2012 were included; 3656 patients were included in the analysis in 2014, as 579 (13.67%) patients died or were lost to follow-up. Information regarding CVD, stroke, kidney disease, PA and biomarkers of hepatic and renal function was collected only at the follow-up in 2014.
Figure 1.
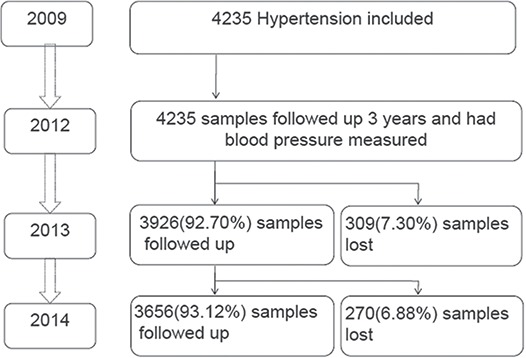
Flowchart of patients with hypertension included in this study.
Diagnosis of hypertension
Respondents were classified as having hypertension if they had at least one of the following conditions: SBP ≥140 mmHg, DBP ≥90 mmHg, diagnosed with hypertension or taking antihypertensive drugs.15 To ensure the accuracy of the diagnosis, BP was measured manually three times at baseline and at each subsequent measurement when the first BP measurement was abnormal. A validated Omron Hem-7071-CP (Omron Healthcare, Kyoto, Japan) automated upper-arm monitor was used for BP measurement.
Statistical analysis
Descriptive statistical analysis was applied to analyse the demographic characteristics of the patients. The BP control rates in 2012 and 2014 compared with 2009 were analysed using Cochran–Mantel–Haenzsel statistics. Paired Student’s t tests were employed to estimate the average effect of health management on SBP and DBP for individuals with hypertension after 5 y of follow-up. To estimate the average effect of treatment on SBP and DBP after 3 y of follow-up, we adjusted for the covariates age, sex, medication treatment in 2012, rural residence, complications, body mass index (BMI) at baseline and in 2012, and SBP and DBP at baseline. Similarly, to estimate the average effect of treatment on SBP and DBP after 5 y of follow-up, we adjusted for age, sex, medication treatment in 2014, rural residence, diabetic complications, BMI in 2014 and SBP and DBP at baseline and in 2012. A generalized linear model (GLM) was used to adjust for covariates that might affect the values of SBP and DBP. In the GLM, decreases in SBP and DBP in 2012 and 2014 compared with baseline were considered dependent variables, whereas medication treatment, sex, age, rural residence, smoking, diabetic complications and BMI were considered independent variables. We performed the above analyses for all individuals with SBP and DBP data, and all analyses were conducted separately for 2012 and 2014. The analyses were performed using the SAS software (version 9.13; SAS Institute, Cary, NC, USA). Differences were considered significant at an α level of 0.05.
Results
Patient characteristics
The demographic characteristics of the patients with hypertension are shown in Table 1. In total, 4235 patients (1702 [40.19%] males; median age 72 y [range 64–79]) in 2012 and 3656 patients (1446 [39.55%] males; median age 74 y [range 66–81]) in 2014 were included in this analysis. At baseline (in 2009), 2012 and 2014, 1398 (33.01%), 1427 (33.78%) and 2570 (70.74%) patients, respectively, received medication treatment. The rates of diabetes as a comorbidity were 23.42% (992/4235) and 23.71% (867/3656) for patients with hypertension in 2012 and 2014, respectively. Regarding lifestyle risk factors, 379 (12.44%) and 249 (9.29%) patients smoked at baseline and in 2014, respectively (χ2=13.09, p<0.01). Fewer patients drank alcohol in 2014 compared with baseline (260 [8.68%] vs 130 [4.93%]) (χ2=29.28, p<0.01). BMI (23.97 kg/m2 [standard deviation {SD} 3.40] vs 24.17 [SD 3.58]), waist circumference (82.61 cm [SD 10.14] vs 84.13 [SD 17.62]) and FBG (6.29 mmol/L (SD 1.79) vs 6.43 [SD 2.45]) showed only non-significant increases from baseline to 2014.
Table 1.
Demographic characteristics of the sample
| Characteristics | 2012 | 2014 | χ2 | p-Value |
|---|---|---|---|---|
| Sample size, n | 4235 | 3656 | ||
| Age (years), median (SD) | 71.19 (10.06) | 72.11 (9.58) | ||
| Age group (years), n (%) | ||||
| 18–39 | 19 (0.45) | 7 (0.19) | 72.83 | <0.01 |
| 40–49 | 136 (3.21) | 67 (1.83) | ||
| 50–59 | 438 (10.34) | 266 (7.28) | ||
| 60–69 | 1236 (29.19) | 928 (25.38) | ||
| ≥70 | 2406 (56.81) | 2388 (65.32) | ||
| Sex, n (%) | ||||
| Male | 1702 (40.19) | 1446 (39.55) | 0.33 | 0.56 |
| Female | 2533 (59.81) | 2210 (60.45) | ||
| Community, n (%) | ||||
| Urban | 1700 (40.14) | 1451 (39.69) | 0.17 | 0.68 |
| Rural | 2535 (59.86) | 2205 (60.31) | ||
| Medicinal treatmenta, n (%) | ||||
| Yes | 1427 (33.78) | 2570 (70.74) | 1067.97 | <0.01 |
| No | 2798 (66.22) | 1063 (29.26) | ||
| Comorbid disease, n (%) | ||||
| Diabetesb | ||||
| Yes | 992 (23.42) | 867 (23.71) | 0.09 | 0.76 |
| No | 3243 (76.58) | 2789 (76.29) | ||
| Stroke, n (%) | ||||
| Yes | 31 (1.57) | |||
| No | 1945 (98.43) | |||
| Cardiovascular disease, n (%) | ||||
| Yes | 465 (23.56%) | |||
| No | 1509 (76.44%) | |||
| Kidney disease, n (%) | ||||
| Yes | 58 (2.94) | |||
| No | 1917 (97.06) | |||
| Biomarkers, mean (SD) | ||||
| AST:ALT ratio | 1.51 (2.78) | |||
| ALT (U/L) | 20.13 (13.35) | |||
| AST (U/L) | 23.5 (13.77) | |||
| Creatinine (μmol/L) | 79.75 (28.98) | |||
| Blood urea nitrogen (mmol/L) | 6.58 (6.93) | |||
| Tbil (μmol/L) | 13.75 (11.76) |
aPrescription medication treatment for hypertension or diabetes.
bHypertension and diabetes present in the same individual.
Source: Authors’ field work from 2009 to 2014.
BP control rates
The effect of hypertension management on BP control rates in 2012 and 2014 compared with 2009 was analysed; subgroup analyses conducted based on diabetes, stroke, CVD, kidney disease, age and obesity were also performed (Table 2). The BP control rate increased from 28.33% (1200/4235) to 64.45% (2730/4235) after 3 y of intervention (in 2012) and to 64.05% (2342/3656) after 5 y of intervention (in 2014). In subgroup analyses for diabetes, stroke, CVD, kidney disease, age and obesity (Table 2), the control rates increased in 2012 and 2014 compared with 2009 in each of the subgroups. BP control rates were higher in patients with than in those without CVD and kidney disease, whereas rates in patients with obesity were lower than in patients with normal weight. Moreover, the control rates in patients with diabetes or stroke or in those >65 y of age were not significantly different from those of their counterparts.
Table 2.
BP control rates in patients with different characteristics
| Characteristics | 2009 | 2012 | 2014 | p-Value |
|---|---|---|---|---|
| Patients, N | 4235 | 4235 | 3656 | |
| Diabetes, n (%) | ||||
| No | 1215 (37.7)a | 2092 (64.75)b | 1575 (62.97)b | <0.01 |
| Yes | 279 (29.94) | 623 (62.99)b | 482 (61.95)b | |
| Stroke, n (%) | ||||
| No | 719 (37.57) | 1161 (59.85) | 970 (50.1) | <0.01 |
| Yes | 7 (22.58) | 19 (61.29) | 11 (35.48) | |
| Cardiovascular disease, n (%) | ||||
| No | 554 (37.33) | 868 (57.6)a,b | 685 (45.55)a,b | <0.01 |
| Yes | 174 (37.74) | 313 (67.75)b | 299 (64.86)b | |
| Kidney disease, n (%) | ||||
| No | 700 (37.12) | 1141(59.68)b | 945 (49.53)a,b | <0.01 |
| Yes | 25 (43.1) | 40 (68.97)b | 38 (65.52)b | |
| Obesity, n (%) | ||||
| No | 984 (35.98) | 1467 (64.03)a,b | 1343 (66.16)a,b | <0.01 |
| Yes | 502 (35.99) | 796 (60.03)b | 708 (57.24)b | |
| Age (years), n (%) | ||||
| <65 | 430 (29.9)a | 715 (62.88)b | 433 (64.72)b | <0.01 |
| ≥65 | 1064 (39.16) | 2000 (64.87)b | 1624 (62.22)b | |
ap<0.05 compared with its counterparts.
bp<0.05 compared with the BP control rate in 2009.
Source: Authors’ field work, from 2009 to 2014.
BP control levels
The effect of hypertension management on decreases in SBP and DBP in 2012 and 2014 compared with 2009 was analysed and subgroup analyses were conducted based on sex, area of residence, age, medication treatment, antihypertensive agents, smoking, overweight and obesity (Table 3). Compared with the BP level in 2009 (SBP 142.19±14.91 mmHg and DBP 86.13±9.83 mmHg), patients who participated in hypertension health management exhibited a median SBP reduction of 8.00 mmHg (95% confidence interval [CI] 7.06–8.26) and a DBP reduction of 6.00 mmHg (95% CI 5.76–6.53) over 3 y of follow-up. Furthermore, these patients showed median SBP and DBP reductions of 7.00 mmHg (95% CI 5.12–6.45) and 6.50 mmHg (95% CI 5.51–6.36), respectively, over a median follow-up duration of 5 y (Table 3).
Table 3.
The effect of hypertension management on SBP and DBP
| Variable | 2012 compared with 2009 | 2014 compared with 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP, mmHg | DBP, mmHg | SBP, mmHg | DBP, mmHg | |||||
| Median (95% CI) | p-Value | Median (95% CI) | p-Value | Median (95% CI) | p-Value | Median (95% CI) | p-Value | |
| Total | 8.00 (7.06 to 8.26) | <0.01 | 6.00 (5.76 to 6.53) | <0.01 | 7.00 (5.16 to 6.51) | <0.01 | 6.50 (5.49 to 6.39) | <0.01 |
| Sex | ||||||||
| Male | 8.00 (6.59 to 8.44) | <0.01 | 7.00 (5.7 to 6.93) | <0.01 | 7.00 (4.7 to 6.78) | <0.01 | 6.00 (5.43 to 6.77) | <0.01 |
| Female | 9.00 (6.97 to 8.54) | <0.01 | 6.00 (5.55 to 6.53) | <0.01 | 7.00 (4.95 to 6.68) | <0.01 | 6.00 (5.29 to 6.37) | <0.01 |
| Difference | −0.24 (−1.46 to 0.99) | 0.70 | 0.27 (−0.51 to 1.06) | 0.50 | −0.08 (−1.43 to 1.28) | 0.91 | 0.27 (−0.59 to 1.13) | 0.54 |
| Community | ||||||||
| Urban | 10.00 (7.55 to 9.49) | <0.01 | 6.00 (5.6 to 6.83) | <0.01 | 8.00 (5.53 to 7.73) | <0.01 | 7.00 (5.49 to 6.88) | <0.01 |
| Rural | 8.00 (6.32 to 7.84) | <0.01 | 6.00 (5.61 to 6.6) | <0.01 | 6.00 (4.4 to 6.06) | <0.01 | 6.00 (5.24 to 6.30) | <0.01 |
| Difference | 1.44 (0.22 to 2.66) | 0.02 | 0.11 (−0.68 to 0.89) | 0.78 | 1.40 (0.04 to 1.76) | 0.04 | 0.42 (−0.45 to 1.28) | 0.34 |
| Age group (years) | ||||||||
| 18–44 | 10.00 (−0.97 to 9.79) | 0.11 | 10.00 (1.48 to 10) | <0.01 | 8.00 (2.83 to 18.88) | 0.01 | 10.00 (0.28 to 12.19) | 0.04 |
| 45–54 | 8.00 (5.11 to 9.35) | <0.01 | 7.00 (5.55 to 8.62) | <0.01 | 10.00 (3.11 to 8.75) | <0.01 | 7.50 (1.92 to 6.24) | <0.01 |
| 55–64 | 10.00 (8.09 to 10.52) | <0.01 | 8.00 (6.22 to 7.87) | <0.01 | 8.00 (5.92 to 9.12) | <0.01 | 6.00 (4.77 to 7.14) | <0.01 |
| 65–74 | 10.00 (6.71 to 8.67) | <0.01 | 6.00 (5.36 to 6.59) | <0.01 | 8.00 (3.97 to 6.26) | <0.01 | 6.00 (4.86 to 6.33) | <0.01 |
| ≥75 | 8.00 (5.8 to 7.95) | <0.01 | 6.00 (5.03 to 6.37) | <0.01 | 6.00 (3.77 to 6.07) | <0.01 | 6.00 (5.28 to 6.64) | <0.01 |
| Difference | 0.04 | 0.11 | 0.10 | 0.54 | ||||
| Medicinal treatment | ||||||||
| Yes | 8.00 (7.47 to 8.91) | <0.01 | 6.00 (5.84 to 6.81) | <0.01 | 8.00 (5.4 to 6.96) | <0.01 | 7.00 (5.58 to 6.57) | <0.01 |
| No | 7.00 (5.18 to 7.29) | <0.01 | 6.00 (5.18 to 6.43) | <0.01 | 6.00 (3.5 to 6.07) | <0.01 | 6.00 (4.76 to 6.41) | <0.01 |
| Difference | 1.95 (0.7 to 3.21) | <0.01 | 0.52 (−0.29 to 1.34) | 0.21 | 1.39 (−0.07 to 2.86) | 0.06 | 0.49 (−0.44 to 1.42) | 0.30 |
| Antihypertensive agents | ||||||||
| Diuretics | 6.00 (4.13 to 8.19) | <0.01 | 6.00 (4.17 to 6.69) | <0.01 | 7.00 (3.14 to 7.06) | <0.01 | 6.00 (3.41 to 5.97) | <0.01 |
| Calcium ion antagonists | 7.00 (4.54 to 9.49) | <0.01 | 6.00 (4.28 to 6.91) | <0.01 | 6.00 (3.13 to 6.43) | <0.01 | 7.00 (4.83 to 6.82) | <0.01 |
| ACEIsa | 7.00 (4.53 to 8.48) | <0.01 | 6.00 (5.02 to 7.51) | <0.01 | 8.00 (4.82 to 9.89) | <0.01 | 7.00 (4.23 to 7.39) | <0.01 |
| Angiotensin II receptor antagonists | 2.00 (−3.19 to 5.29) | 0.62 | 4.00 (1.13 to 6.52) | <0.01 | 6.00 (1.72 to 6.79) | <0.01 | 6.00 (5.1 to 7.99) | <0.01 |
| Compound preparations | 5.00 (−2.21 to 10.57) | 0.19 | 4.50 (0.42 to 6.96) | 0.03 | 9.50 (3.31 to 11.76) | <0.01 | 7.00 (5.39 to 12.03) | <0.01 |
| Combinations of two or more drugs | 10.00 (4.65 to 9.54) | <0.01 | 8.00 (5.48 to 8.38) | <0.01 | 10.00 (5.95 to 9.05) | <0.01 | 8.00 (5.34 to 7.37) | <0.01 |
| Smoking | ||||||||
| Yes | 6.00 (4.22 to 7.97) | <0.01 | 5.00 (3.29 to 5.70) | <0.01 | 5.00 (1.57 to 6.96) | <0.01 | 5.00 (3.48 to 6.75) | <0.01 |
| No | 8.00 (6.84 to 8.36) | <0.01 | 6.00 (5.63 to 6.59) | <0.01 | 6.00 (3.93 to 5.6) | <0.01 | 6.00 (5.07 to 6.14) | <0.01 |
| Difference | −1.51 (−3.64 to 0.63) | 0.17 | −1.62 (−2.98 to −0.26) | 0.02 | −0.50 (−3.26 to 2.26) | 0.72 | −0.50 (−2.26 to 1.27) | 0.58 |
| Overweight/obesityb | ||||||||
| Yes | 7.00 (5.32 to 7.42) | <0.01 | 5.00 (4.75 to 6.12) | <0.01 | 5.00 (2.98 to 5.21) | <0.01 | 6.00 (4.81 to 6.27) | <0.01 |
| No | 8.00 (6.75 to 8.43) | <0.01 | 6.00 (5.46 to 6.49) | <0.01 | 8.00 (5.80 to 7.52) | <0.01 | 6.00 (5.50 to 6.56) | <0.01 |
| Difference | −1.22 (−2.58 to 0.14) | 0.08 | −0.54 (−1.39 to 0.31) | 0.21 | −2.56 (−3.97 to 1.16) | <0.01 | −0.49 (−1.38 to 0.40) | 0.28 |
aMeans are significantly different from those of other groups.
bUsing BMI ≥25 as a classification standard in 2012 and 2014.
Source: Authors’ field work, from 2009 to 2014.
In the subgroup analysis for 2012 (Table 3), there was a greater SBP reduction among urban residents than among rural residents (mean difference 1.44 mmHg [95% CI 0.22–2.66], p=0.02). In contrast, no significant effect on SBP as an intervention outcome was observed in subgroup analyses based on sex, smoking and overweight/obesity. However, according to subgroup analysis, non-smoking individuals showed a greater reduction in DBP than did smoking individuals (mean difference 1.62 mmHg [95% CI 0.26–2.98], p=0.02). In addition, subgroup analyses by sex, age, residence area, medication treatment and overweight/obesity revealed no significant effects on DBP in 2012. In the subgroup analysis in 2014, the reduction in SBP was greater among urban residents than among rural residents (mean difference 1.40 mmHg [95% CI 0.04–1.76], p=0.04), among patients who received medication treatment vs those who did not (mean difference 1.39 mmHg [95% CI −0.07 to 2.86], p=0.06) and among patients with normal weight vs those with overweight/obesity (mean difference 2.56 mmHg [95% CI −3.97 to 1.16], p<0.01). Nonetheless, subgroup analyses showed no significant effects of sex, age, area of residence, medication treatment, smoking or overweight/obesity on DBP in 2014 (Table 3).
Furthermore, subgroup analysis of antihypertensive agents revealed that calcium ion antagonists (7.00 mmHg [95% CI 4.54–9.49]) and combinations of two or more drugs (10.00 mmHg [95% CI 4.65–9.54]) were more effective than were other drug regimens for SBP control in 2012. Additionally, a more significant effect on DBP was achieved using angiotensin-converting enzyme inhibitors (ACEIs) (6.00 mmHg [95% CI 5.02 to 7.51]) or combinations of two or more drugs (8.00 mmHg [95% CI 5.48–8.38]) than other drug regimens in 2012 (Table 3). Moreover, ACEIs (8.00 mmHg [95% CI 4.82–9.89]), compound preparations (9.50 mmHg [95% CI 3.31–11.76]) and combinations of two or more drugs (10.0 mmHg [95% CI 5.95–9.05]) showed greater effects on SBP than did other agents in 2014, and angiotensin II receptor antagonists (6.00 mmHg [95% CI 5.10–7.99]), compound preparations (7.00 mmHg [95% CI 5.39–12.03]) and combinations of two or more drugs (8.00 mmHg [95% CI 5.34–7.37]) had greater effects on DBP in 2014 than did other agents (Table 3).
Effects of BP control according to medication use and obesity
As shown in Figure 2, after adjusting for covariates, hypertension health management reduced SBP and DBP by a mean of 7.36 (SD 0.35) and 6.11 mmHg (SD 0.22), respectively, in the group not taking medication and by a mean of 5.52 (SD 0.45) and 4.56 mmHg (SD 0.29), respectively, in the group taking medication in 2012. Moreover, after adjusting for covariates, SBP and DBP in 2014 decreased from baseline in 2009 by a mean of 4.76 (SD 0.39) and 5.17 mmHg (SD 0.25), respectively, in the group taking medication and decreased by a mean of 3.85 (SD 0.55) and 4.38 mmHg (SD 0.36), respectively, in the group that did not take medication (Figure 2). Similarly, the data in Figure 3 show that after adjusting for covariates, SBP and DBP decreased by a mean of 5.65 (SD 0.44) and 4.77 mmHg (SD 0.28), respectively, in the overweight/obesity group in 2012 compared with baseline and decreased by a mean of 7.23 (SD 0.35) and 5.90 mmHg (SD 0.23), respectively, in the control group in 2012 compared with baseline. As depicted in Figure 3, subgroup analysis by weight showed that after adjusting for covariates, SBP and DBP decreased by a mean of 3.04 (SD 0.51) and 4.30 mmHg (SD 0.33), respectively, in the overweight/obesity group and decreased by a mean of 5.57 (SD 0.42) and 5.25 mmHg (SD 0.27), respectively, in the normal-weight group in 2014 compared with 2009.
Figure 2.
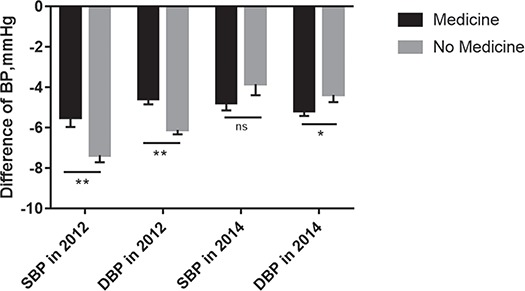
Decrease in BP in subgroup analysis of those taking medication.
Figure 3.
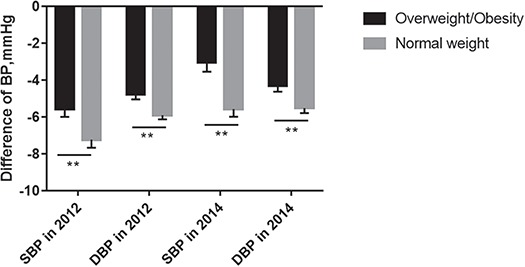
Decrease in BP in subgroup analysis of patients with overweight/obesity.
Factors affecting BP control
GLM adjusted for age and sex was employed to analyse the relationship between the net decrease in BP (in 2014 compared with 2009) and predictors including the AST:ALT ratio, ALT, AST, creatinine, blood urea nitrogen and total bilirubin (Tbil) levels, PA, kidney diseases, CVD and stroke (Supplementary Table 1). The results showed that high Tbil was a risk factor for poor SBP control and that PA, kidney disease and CVD favoured the control of SBP and DBP in 2014. The AST:ALT ratio was negatively related to the level of DBP control.
Multivariable GLM analysis using the difference in SBP between 2009 and 2012 as the dependent variable revealed that SBP in 2009 was a protective factor for SBP in 2012 (p<0.01). Conversely, antihypertensive agents (diuretics, angiotensin II receptor antagonists, compound preparations and combinations of two or more drugs, all of which were compared with lifestyle intervention alone) and overweight/obesity were risk factors for poor SBP control in 2012 (p<0.01) (Table 4). GLM analysis using the difference in DBP between 2009 and 2012 as the dependent variable revealed that DBP in 2009 and age were strong protective factors for DBP control compared with baseline (p<0.01); antihypertensive agents (diuretics, ACEIs, angiotensin II receptor antagonists and combinations of two or more drugs, all of which were compared with lifestyle intervention alone), diabetic complications and overweight/obesity were risk factors for poor DBP control in 2012 (p<0.05).
Table 4.
The results of the GLM regression for SBP and DBP
| Parameter | Results in 2012 compared with 2009 (N=4235) | Results in 2014 compared with 2009 (N=3656) | ||||||
|---|---|---|---|---|---|---|---|---|
| Difference in SBP, mmHg | Difference in DBP, mmHg | Difference in SBP, mmHg | Difference in DBP, mmHg | |||||
| Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | |
| Intercept | 117.99 (3.76) | <0.01 | 73.57 (2.67) | <0.01 | 85.43 (8.81) | <0.01 | 55.48 (5.62) | <0.01 |
| Sex | ||||||||
| Male vs female | 0.19 (0.49) | 0.70 | 0.67 (0.34) | 0.04 | 0.40 (0.94) | 0.67 | 1.03 (0.60) | 0.09 |
| Age (continuous) | 0.00 (0.03) | 0.84 | −0.10 (0.02) | <0.01 | 0.09 (0.06) | 0.18 | −0.19 (0.04) | <0.01 |
| Community | ||||||||
| Urban vs rural | 0.20 (0.51) | 0.69 | 0.42 (0.35) | 0.23 | −0.99 (0.95) | 0.30 | −0.88 (0.60) | 0.15 |
| Medicinal treatment | ||||||||
| Yes vs no (in 2012) | – | – | 2.27 (1.15) | 0.04 | 2.43 (0.74) | <0.01 | ||
| Antihypertensive agents | ||||||||
| Diuretics | 2.15 (0.81) | <0.01 | 1.63 (0.57) | <0.01 | 3.73 (1.54) | 0.02 | 2.45 (0.98) | 0.01 |
| Calcium ion antagonists | −0.26 (0.97) | 0.79 | 1.04 (0.67) | 0.12 | 1.63 (1.33) | 0.22 | −0.63 (0.85) | 0.46 |
| β-blockers | −2.82 (14.34) | 0.84 | −1.38 (9.12) | 0.88 | −2.00 (5.05) | 0.69 | 0.26 (3.22) | 0.94 |
| ACEIs | 0.81 (0.92) | 0.38 | 1.78 (0.65) | <0.01 | −1.63 (2.04) | 0.43 | −0.29 (1.30) | 0.83 |
| Angiotensin II receptor antagonists | 4.91 (1.71) | <0.01 | 3.44 (1.17) | <0.01 | 2.28 (1.90) | 0.23 | −2.32 (1.21) | 0.05 |
| Compound preparations | 5.73 (2.63) | 0.03 | 3.02 (1.68) | 0.07 | −4.03 (2.67) | 0.13 | −3.17 (1.71) | 0.06 |
| Combinations of two or more drugs | 3.50 (0.97) | <0.01 | 1.47 (0.66) | 0.02 | −1.78 (1.34) | 0.19 | −2.32 (0.86) | <0.01 |
| BP in 2009, mmHg | ||||||||
| SBP | −0.94 (0.02) | <0.01 | 0.01 (0.01) | 0.48 | −0.88 (0.04) | <0.01 | 0.002 (0.02) | 0.94 |
| DBP | 0.03 (0.03) | 0.27 | −0.90 (0.02) | <0.01 | 0.004 (0.06) | 0.94 | −0.81 (0.04) | <0.01 |
| BP in 2012, mmHg | ||||||||
| SBP | – | – | 0.14 (0.04) | <0.01 | −0.02 (0.02) | 0.52 | ||
| DBP | – | – | 0.13 (0.06) | 0.04 | 0.32 (0.04) | <0. 01 | ||
| Complication | ||||||||
| Diabetes (yes vs no) | 0.23 (0.07) | <0.01 | 0.10 (0.05) | 0.03 | 1.06 (1.14) | 0.35 | 1.48 (0.72) | 0.04 |
| Overweight/obesity | ||||||||
| Yes vs no | 1.59 (0.50) | <0.01 | 1.04 (0.35) | <0.01 | 2.17 (0.94) | 0.02 | 0.72 (0.60) | 0.24 |
| Physical activity (min/d) | ||||||||
| 0, reference | ||||||||
| ~30 | – | – | −1.26 (1.47) | 0.39 | −1.32 (0.94) | 0.16 | ||
| ~60 | – | – | −1.14 (1.55) | 0.46 | −0.43 (0.99) | 0.67 | ||
| >60 | – | – | −0.70 (1.38) | 0.61 | −1.35 (0.88) | 0.13 | ||
| AST:ALT ratio | – | – | 0.26 (0.16) | 0.11 | 0.29 (0.10) | <0. 01 | ||
| Kidney disease (yes vs no) | – | – | −4.69 (2.53) | 0.06 | −2.96 (1.61) | 0.07 | ||
| Cardiovascular disease (yes vs no) | – | – | −4.83 (1.07) | <0.01 | −1.69 (0.68) | 0.01 | ||
Source: Authors’ field work, from 2009 to 2014.
In addition, medication treatment in 2012, diuretic treatment, SBP and DBP in 2012 and overweight/obesity were risk factors for poor SBP control in 2014. In contrast, SBP in 2009 and CVD were protective factors for SBP control in 2014 (p<0.05) and kidney disease was a borderline-significant protective factor for SBP control (p=0.06) (Table 4). Moreover, medication treatment in 2012, diuretic treatment, DBP in 2012, diabetes and AST:ALT ratio were risk factors for poor DBP control in 2014, whereas age, antihypertensive agents (angiotensin II receptor antagonists and combinations of two or more drugs compared with lifestyle intervention alone), DBP in 2009 and CVD were protective factors for DBP control in 2014 (p<0.05). Compound medication treatment and kidney disease were borderline-significant protective factors for DBP control (both p=0.06). Residence in urban vs rural areas was not associated with SBP or DBP in 2012 or 2014 after adjusting for covariates.
Discussion
The purpose of this study was to examine the effectiveness of community healthcare in controlling BP and mitigating related risk factors over 5 y of follow-up. After 3 y of intervention, rates of BP control were improved and BP level itself decreased; the effect continued to 5 y of follow-up, indicating that the intervention may be effective in the long term. However, the effect decreased after adjusting for covariates.
Our results confirm the importance of timely follow-up and medical compliance management in the treatment of hypertension, as reported in other studies,16 and further confirm the effect of self-monitoring of BP with the guidance of GPs as part of primary care.8 Our outcomes were consistent with the results of studies in other geographic regions,17 indicating that health management is associated with improved outcomes, including better BP control, improved lifestyle and (PA) and decreased risk factors for CVD. The results were also in agreement with the outcomes of cohort and randomized controlled studies reporting the effectiveness of home BP monitoring, Web communication and pharmacist care on hypertension control and of comparisons of the effectiveness of immediate and delayed interventions.18
An innovation of this study was that the medication treatment group achieved optimal BP control compared with its counterpart, although the effects decreased after adjusting for covariates. This result may be due to reduced total mortality and cardiovascular comorbidity in patients who take numerous antihypertensive and antidiabetic agents,19,20 along with the decreased rate of BP control in the older population. Our results demonstrate the effects of different antihypertensive agents on BP control, revealing that calcium ion antagonists, ACEIs and combinations of two or more antihypertensive drugs are more effective than are other drugs and that combined antihypertensive medication therapy may be the most effective approach for patients with high cardiovascular risk.21 The underlying mechanism is probably related to antioxidant activity or a reduction in the activity of the renin–angiotensin–aldosterone system (RAAS) by ACEIs or calcium antagonists.22 In addition, we found an increased medication treatment rate in 2014, and the rate of medication compliance in hypertensive patients has been associated with BP control.23
Moreover, our study found that the effectiveness of BP control decreased with increasing age; the mechanism of this change is that age correlates with left ventricular concentric/functional changes.24 In addition, SBP improved to a greater extent in urban areas than in rural areas, but the effect disappeared after adjusting for covariates, suggesting that the effect of urban residence on BP control was elicited by differences in lifestyle, education, occupation and other risk factors between urban and rural residents.25 Additionally, the effectiveness of BP control was lower in smokers than in non-smokers, as smoking is an independent risk factor of hypertension,17 and the intensity of smoking was associated with increased SBP and DBP. Furthermore, PA was positively associated with BP control after adjusting for age and sex, which corroborates other studies;26 however, the effect did weaken after adjusting for other variables.
Our study found that BP control was less effective among patients with overweight/obesity than among those with normal weight, which is consistent with another study;27 therefore, maintaining appropriate body weight favours BP control in hypertension. This study also demonstrated that the effect was more pronounced for patients whose SBP or DBP was higher at baseline, who are also more difficult to treat and who have greater cardiovascular risk factors, as reported in another trial.20 This result indicates that GPs might enhance the benefit of BP control measures by identifying new and undiagnosed cases of hypertension in the community and starting intervention as early as possible.16 Our study found the AST:ALT ratio to be negatively associated with DBP control, corroborating the results of previous studies showing that an elevated AST:ALT ratio is an independent risk factor for arterial stiffness28 and CVD.29 This correlation might be attributable to the metabolic effects of AST and ALT in reducing blood glutamate levels. Moreover, hypertensive patients with kidney disease and CVD exhibited better BP control, which may be contrary to stringent guidelines that set a low target level of BP in kidney disease and CVD.30
Our study has several strengths. First, to our knowledge, this study is the first large, long-term, high-quality prospective representative health management cohort survey measuring individual-level risk factors, sociodemographic variables, medication treatment, disease complications and physical examination results associated with hypertension in southwest China. Second, we considered the effects of community health management of hypertension and different antihypertensive agents on BP control. Third, our three-wave longitudinal data allowed examination of not only the cross-sectional association, but also changes in SBP and DBP; therefore this study adds knowledge to prior retrospective cohort and cross-sectional studies. Fourth, the impact of health management on BP was effectively captured and residual confounding by other common risk factors was unlikely, as we adjusted for the main risk factors of age, sex, medication treatment, rural residence, complications, BMI and baseline SBP and DBP. Finally, we applied GLM statistical approaches to analyse the main risk factors that impact changes in BP, control for potential confounding effects and assess the average treatment effect after adjusting for the main potential confounders. Our conclusions regarding the effectiveness of the intervention were consistent between univariate and multivariate analyses.
Nonetheless, this study has several limitations. First, this study was limited to a before-and-after design and did not include a comparative control group in the analysis, as hypertension was registered and followed up after diagnosis in 2009. Second, we collected biomarkers of hepatic and renal function, PA and cardiovascular and renal complications only in the 2014 follow-up. We were also unable to analyse quality of life after health management because we did not investigate quality of life as an outcome. Finally, we did not examine the cost-effectiveness of health management in this study; we will explore this consideration in future studies.
Conclusions
This study shows that long-term follow-up on community management for hypertension may improve the rate and degree of BP control, alter smoking and drinking habits, increase the rate of medication compliance and reduce cardiovascular complications. Despite the relatively large sample size, this cohort study of a community-based intervention had limitations in its study design. Further well-designed randomized controlled trials with large sample sizes are needed to demonstrate which community intervention methods can achieve optimal BP control and how the interventions exert their effects; the cost-effectiveness of such programmes should also be explored.
Supplementary Material
Acknowledgements
None.
Authors’ contributions
XL analysed the data and drafted the manuscript. LX and HZ conducted the investigation. All authors read and approved the final manuscript. XL and HZ made equal contributions to this research.
Funding
This work was supported by the Natural Science Foundation of Youth Project (81502826), the National Key Research and Development Project (2017YFC0211705), the China Postdoctoral Science Foundation (2014 M562289) and Chongqing Postdoctoral Research Funded Projects (Xm2014129). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interests
None declared.
Ethical approval
The enrolment and research plans were reviewed and approved by the institutional ethics committee of the Centers for Disease Control and Prevention of Jiulongpo District in Chongqing, China (reference number 066/2008). Informed written consent was obtained from the participants by local GPs.
References
- 1. James PA, Oparil S, Carter BL, et al.. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 2. Sarki AM, Nduka CU, Stranges S, et al.. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(50):e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xi B, Liang Y, Reilly KH, et al.. Trends in prevalence, awareness, treatment, and control of hypertension among Chinese adults 1991–2009. Int J Cardiol. 2012;158(2):326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaugg V, Korb-Savoldelli V, Durieux P, et al.. Providing physicians with feedback on medication adherence for people with chronic diseases taking long-term medication. Cochrane Database Syst Rev. 2018;1:CD012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adler AJ, Martin N, Mariani J, et al.. Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;4:CD011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okoro CA, Zhao G, Fox JB, et al.. Surveillance for health care access and health services use, adults aged 18–64 years – Behavioral Risk Factor Surveillance System, United States, 2014. MMWR Surveill Summ. 2017;66(7):1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang J, Zhao G, Wang G, et al.. Insurance status among adults with hypertension—the impact of underinsurance. J Am Heart Assoc. 2016;5(12):e004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McManus RJ, Mant J, Franssen M, et al.. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalesi S, Irwin C, Sun J. Dietary patterns, nutrition knowledge, lifestyle, and health-related quality of life: associations with anti-hypertension medication adherence in a sample of Australian adults. High Blood Press Cardiovasc Prev. 2017;24(4):453–462. [DOI] [PubMed] [Google Scholar]
- 10. Lin YY, Lee SD. Cardiovascular benefits of exercise training in postmenopausal hypertension. Int J Mol Sci. 2018;19(9):2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell NR, Lackland DT, Lisheng L, et al.. Using the Global Burden of Disease study to assist development of nation-specific fact sheets to promote prevention and control of hypertension and reduction in dietary salt: a resource from the World Hypertension League. J Clin Hypertens (Greenwich). 2015;17(3):165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim SS, Gaziano TA, Gakidou E, et al.. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370(9604):2054–2062. [DOI] [PubMed] [Google Scholar]
- 13. Liang XH, Gu DF, Zhang H, et al.. The analysis of drug cost and direct medical expense in community health management of hypertensive patients. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(8):732–736. [PubMed] [Google Scholar]
- 14. Liang X, Chen J, Liu Y, et al.. The effect of hypertension and diabetes management in southwest China: a before- and after-intervention study. PLoS One. 2014;9(3):e91801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuddy ML. Treatment of hypertension: guidelines from JNC 7 (the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 1). J Pract Nurs. 2005;55(4):17–21quiz 22–3. [PubMed] [Google Scholar]
- 16. Xu W, Goldberg SI, Shubina M, et al.. Optimal systolic blood pressure target, time to intensification, and time to follow-up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levesque V, Poirier P, Despres JP, et al.. Assessing and targeting key lifestyle cardiovascular risk factors at the workplace: effect on hemoglobin A1c levels. Ann Med. 2015;47(7):605–614. [DOI] [PubMed] [Google Scholar]
- 18. Falaschetti E, Mindell J, Knott C, et al.. Hypertension management in England: a serial cross-sectional study from 1994 to 2011. Lancet. 2014;383(9932):1912–1919. [DOI] [PubMed] [Google Scholar]
- 19. Benetos A, Rossignol P, Cherubini A, et al.. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314(2):170–180. [DOI] [PubMed] [Google Scholar]
- 20. Green BB, Cook AJ, Ralston JD, et al.. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299(24):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weir S, Juhasz A, Puelles J, et al.. Relationship between initial therapy and blood pressure control for high-risk hypertension patients in the UK: a retrospective cohort study from the THIN general practice database. BMJ Open. 2017;7(7):e015527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwakernaak AJ, Roksnoer LC, Lambers Heerspink HJ, et al.. Effects of direct renin blockade on renal & systemic hemodynamics and on RAAS activity, in weight excess and hypertension: a randomized clinical trial. PLoS One. 2017;12(1):e0169258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butler MJ, Tanner RM, Muntner P, et al.. Adherence to antihypertensive medications and associations with blood pressure among African Americans with hypertension in the Jackson Heart Study. J Am Soc Hypertens. 2017;11(9):581–588e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toba A, Kariya T, Aoyama R, et al.. Impact of age on left ventricular geometry and diastolic function in elderly patients with treated hypertension. Blood Press. 2017;26(5):264–271. [DOI] [PubMed] [Google Scholar]
- 25. Dastan I, Erem A, Cetinkaya V. Urban and rural differences in hypertension risk factors in Turkey. Anatol J Cardiol. 2017;18(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Igarashi Y, Nogami Y. The effect of regular aquatic exercise on blood pressure: a meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2018;25(2):190–199. [DOI] [PubMed] [Google Scholar]
- 27. Swedlund M, Norton D, Birstler J, et al.. Effectiveness of a Best Practice Alerts at improving hypertension control. Am J Hypertens. 2019;32(1):70–76. [DOI] [PubMed] [Google Scholar]
- 28. Liu YM, Zhao PL, Cheng ML, et al.. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018;17:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao F, Chen C, Lu J, et al.. De Ritis ratio (AST/ALT) as an independent predictor of poor outcome in patients with acute ischemic stroke. Neuropsychiatric Dis Treat. 2017;13:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whelton PK, Carey RM, Aronow WS, et al.. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017, 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


