Abstract
Background
A high prevalence and incidence of epilepsy has been reported in onchocerciasis-endemic regions in Central and East Africa. There is compelling epidemiological evidence suggesting that this high burden is caused by onchocerciasis-associated epilepsy (OAE). We hypothesized that OAE had also occured in West African onchocerciasis foci.
Methods
We searched PubMed, the African Journals Online platform and grey literature for population-based epilepsy studies in West African countries. Epilepsy and onchocerciasis prevalence data were extracted. The pre-control onchocerciasis endemicity in the study sites was estimated from historical data of onchocerciasis control programmes. The prevalence of epilepsy in different sites was analysed, taking into account onchocerciasis endemicity and the duration of control.
Results
The pooled prevalence of epilepsy in the West African study sites was 13.14 per 1000 (95% confidence interval 11.28–15.00). Higher pre-control endemicity and a shorter duration of onchocerciasis control were both associated with increased epilepsy prevalence (p<0.001). Two studies in Ivory Coast that provided detailed descriptions of persons with epilepsy in onchocerciasis-endemic settings revealed that most of them had features of OAE (73.7% and 83.3%, respectively).
Conclusions
Our findings suggest that before and during the early years of implementing onchocerciasis control in West Africa, high onchocerciasis endemicity resulted in a high prevalence of OAE and that subsequent control efforts significantly reduced the prevalence of OAE.
Keywords: epilepsy, prevalence, onchocerciasis, ivermectin, vector control, West Africa
Introduction
Onchocerciasis (river blindness) is known to cause skin and eye disease.1 Currently there is growing epidemiological evidence that onchocerciasis is also able to directly or indirectly cause epilepsy.2,3 A recent cohort study in the Mbam valley in Cameroon showed that skin Onchocerca volvulus microfilarial density in children <10 y of age determined the risk, in a dose-related fashion, of developing epilepsy later in life.3 Therefore the terms ‘onchocerciasis-associated epilepsy’ (OAE) and ‘river epilepsy’ have been proposed to describe this epidemiological link.4–6
A high prevalence of epilepsy in an onchocerciasis-endemic region was initially reported in 1938 in Mexico,7 and later in many meso- and hyperendemic onchocerciasis foci in Central and East Africa.2,6 However, in West Africa, reports of high epilepsy prevalence in relation to a high onchocerciasis endemicity are scarce. We hypothesized that this may be due, at least in part, to the large-scale vector control activities of the Onchocerciasis Control Programme (OCP) in West Africa, which was implemented as early as the 1970s.8 If onchocerciasis is indeed able to cause epilepsy, a high prevalence of epilepsy should also have occurred in West African foci before or during the early years of onchocerciasis control efforts. We therefore critically reviewed population-based epilepsy surveys from West Africa to investigate a possible relationship with onchocerciasis after taking the possible effect of onchocerciasis control into account.
Methods
Search strategy
We searched PubMed and the African Journals Online (AJOL) platforms for articles published in French and English until 30 June 2019. Key search terms included ‘epilepsy’, ‘prevalence’ and ‘West African countries’ (see Supplementary material, Appendix 1). We also retrieved grey literature (doctoral theses and conference abstracts) from previously published reviews on the epidemiology of epilepsy in sub-Saharan Africa.9–11 We included all population-based surveys conducted in West Africa that reported epilepsy prevalence in both the children and adult population. Two authors (SFJN, CR) independently performed the screening of articles for inclusion in this review. The risk of bias in selected studies was assessed using the ‘study participation’ and ‘outcome measurement’ domains of the Quality in Prognosis Studies (QUIPS) tool.12 The epilepsy surveys conducted in onchocerciasis foci, which provided detailed descriptions of all identified persons with epilepsy (PWE), were used to estimate the proportion of participants who fulfilled previously published criteria for OAE.2 Pre-control onchocerciasis endemicity data for the included villages were estimated from existing 1 km2 resolution maps of pre-control onchocerciasis endemicity levels in West Africa. For the OCP countries, we used a map of the predicted prevalence of microfilariae generated in a geostatistical analysis of the pre-control skin snip survey data of the OCP.13 For Liberia and Nigeria, which were not covered by the OCP but were participating countries of the African Programme for Onchocerciasis Control (APOC), we used a map of the predicted pre-control prevalence of nodules in APOC countries generated in a geostatistical analysis of Rapid Epidemiological Mapping of Onchocerciasis (REMO) data.14 We then converted the predicted nodule prevalence for the location of the study sites to the prevalence of microfilariae using the approach described by Coffeng et al.15
Data analysis
The epilepsy prevalence data that were extracted from eligible studies were entered into Review Manager version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark). The standard error (SE) of the epilepsy prevalence from each survey was calculated using the following formula: SE=√[p(1−p)/n], where p is the prevalence and n is the total study population. Pooled prevalence estimates were obtained using the inverse variance method and a random effects model.
The pre-control onchocerciasis endemicity in our study sites was expressed in terms of the prevalence of microfilariae in the total village population. We also categorized the study sites based on the duration of onchocerciasis control by the time of the epilepsy survey. Subgroup analyses were performed and forest plots generated. Multiple linear regressions, weighted by the sample size for each village (weighting coefficient=√n, where n is the sample size), were fitted to investigate the relationship between logit-transformed epilepsy prevalence, pre-control prevalence of microfilariae, duration of onchocerciasis control, type of onchocerciasis control and study year. Regression analyses were done using the software JASP version 0.10.2 (University of Amsterdam, Amsterdam, The Netherlands).
Results
PubMed and AJOL searches yielded 154 studies, while the manual search yielded 22 records. Of these, 53 were retained as epilepsy prevalence surveys after screening the abstracts. After further assessment, only 20 studies from the systematic search16–35 and 9 studies from the manual search36–44 met the inclusion criteria, and their data were extracted (Figure 1).
Figure 1.
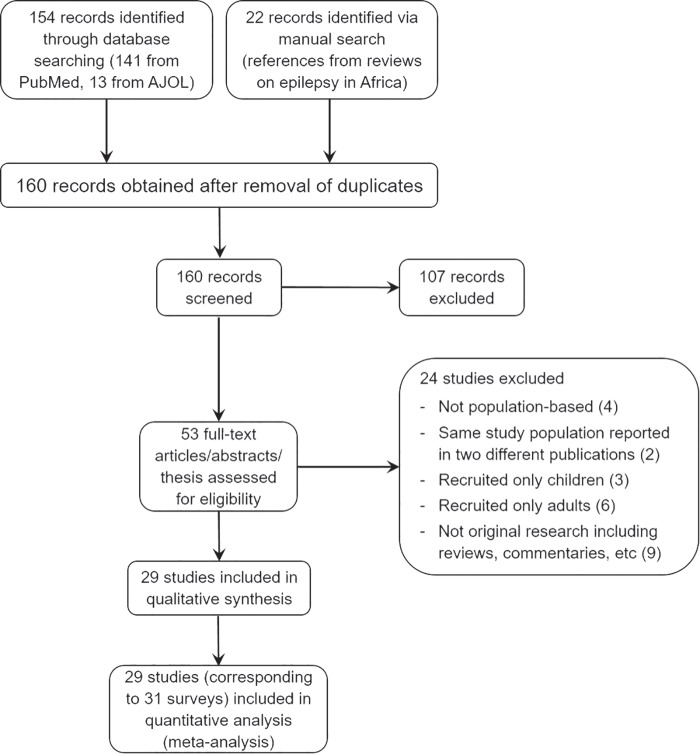
Study selection and inclusion (Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart).
Included studies originated from 10 different countries of West Africa, one of which (Gambia) was non-endemic for onchocerciasis (Figure 2). Onchocerciasis prevalence at the time of the epilepsy survey was available for four endemic study sites. Table 1 summarizes the findings from each individual study; the methodology and suggested epilepsy aetiologies are provided in the supplementary material (Appendix 2). The findings from the retrieved studies will be reported country-wise and further elaborated on the Discussion.
Figure 2.
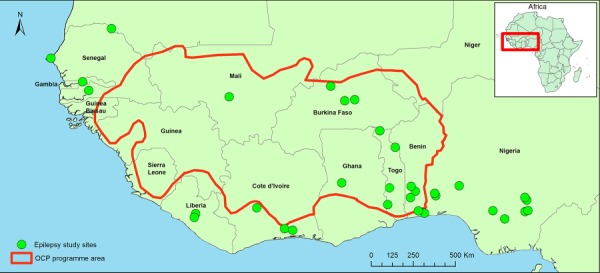
Included study sites and OCP boundaries in West Africa.
Table 1.
Epilepsy population-based surveys performed in West Africa
| Study site | Survey year | First author | Study population | Epilepsy prevalence (‰) | Pre-control onchocerciasis endemicity (%) | Onchocerciasis prevalence at time of survey (%) | Duration (y) of onchocerciasis control (VC/CDTI/overall)a |
|---|---|---|---|---|---|---|---|
| Benin (Savalou)35 | 1994 | Avode | 1443 | 15.25 | 53 | NA | 6/3/6 |
| Benin (Agbogbome)43 | 1995 | Gbenou | 503 | 25.84 | 67 | 47.4 | 7/4/7 |
| Benin (Zinvie)23 | 1997 | Debrock | 3134 | 21.06 | NE | NE | NA |
| Benin (Djidja)27 | 2005 | Houinato | 11 668 | 12.68 | 60 | NA | 14/12/17 |
| Benin (Dangbo)38 | 2007 | Houinato | 737 | 31.21 | NE | NE | NA |
| Burkina Faso (Passore, Yatenga)39 | 1989 | Debouverie | 16 627 | 10.65 | NE | NE | NA |
| Burkina Faso (Batondo, Nyonyogo, Pabré)28 | 2007 | Nitiéma | 881 | 44.27 | NE | NE | NA |
| Gambia (Farafenni)26 | 1997 | Coleman | 16200 | 4.26 | NE | NE | NA |
| Ghana (Kintampo)29 | 2010 | Ae-Ngibise | 129,812 | 1.92 | 45 | NA | 26/13/34 |
| Ivory Coast (Bonon-Frefredou)40 | 1987 | Kouassi | 1176 | 7.65 | NE | NE | NA |
| Ivory Coast (Akoungou)36 | 1989 | Kouadjo | 309 | 58.25 | 51 | NA | 0 |
| Ivory Coast (M'Brou)37 | 1993 | Kaudjhis | 920 | 41.30 | 76 | NA | 0 |
| Liberia (Grand Bassa)42 | 1981 | Goudsmit | 4436 | 27.73 | 56 | NA | 0 |
| Liberia (Grand Bassa)41 | 1983 | Gerrits | 1673 | 49.01 | 38 | NA | 0 |
| Mali (Tyenfala, Baguineda)22 | 1999 | Farnarier | 5243 | 13.35 | 71 | 23 | 12/8/12 |
| Nigeria (Igbo-Ora)19 | 1982 | Osuntokun | 18 954 | 5.33 | 29.1b | NA | 0 |
| Nigeria (Aiyete)20 | 1982 | Osuntokun | 903 | 36.54 | 39.1 | NA | 0 |
| Nigeria (Udo)21 | 1989 | Longe | 2925 | 6.15 | 34.4 | NA | 0 |
| Nigeria (Imo River basin)24 | 2004 | Dozie | 4854 | 11.95 | 49 | 26.8 | 0/5/5 |
| Nigeria (Izzi, Ebonyi state)17 | 2007 | Osakwe | 2500 | 20.80 | 49.4 | NA | 0/8/8 |
| Nigeria (Otukpo, Benue state)17 | 2007 | Osakwe | 6000 | 4.67 | 48 | NA | 0/8/8 |
| Nigeria (Ukpo)16 | 2010 | Nwani | 6800 | 4.26 | 44.5 | NA | 0/11/11 |
| Nigeria (Ilie, Osun state)31 | 2013 | Mustapha | 2212 | 4.52 | 32.5 | NA | 0/14/14 |
| Nigeria (Imo River basin)30 | 2018 | Siewe | 843 | 4.74 | 51 | 4.6 | 0/19/19 |
| Senegal (Moyenne vallée)44 | 1962 | Boutillier | 32 300 | 1.92 | NE | NE | NA |
| Senegal (Casamance, Koalack, Thiès)18 | 1982 | Ndiaye | 7682 | 8.33 | NE | NE | NA |
| Senegal (Pikine)25 | 2004 | Ndoye | 4500 | 14.22 | NE | NE | NA |
| Togo (Kloto)32 | 1989 | Balogou | 19 241 | 12.32 | 49 | NA | 0 |
| Togo (Akebou)32 | 1995 | Balogou | 4182 | 13.15 | 55 | NA | 7/0/7 |
| Togo (Tone)33 | 1995 | Balogou | 9155 | 18.57 | 71 | NA | 18/0/18 |
| Togo (Batamariba)34 | 2001 | Balogou | 6249 | 15.68 | 76 | NA | 24/10/24 |
NE: non-endemic; NA: not available
aVC/CDTI/overall: number of years of vector control/community-directed treatment with ivermectin/total duration of onchocerciasis control irrespective of the method
bReported by Wyatt (1971).45
Epilepsy prevalence in Benin
Five studies were included from Benin,23,27,35,38,43 one of which specifically investigated onchocerciasis and epilepsy in the Agbobome area; findings revealed that in 9/13 (69.2%) PWE, O. volvulus microfilariae were detected in skin snips while 8 PWE (61.5%) presented with onchocerciasis nodules.43 In that same study, one PWE also suffered from onchocercal blindness.43 Furthermore, most patients (76.9%) experienced generalized tonic–clonic seizures.43 The study by Avode et al.35 incriminated neurocysticercosis as a major cause of epilepsy in Savalou (central region of Benin), where cysticercosis prevalence was 39.5‰ and epilepsy prevalence 15.2‰. A family history of epilepsy was a predominant risk factor in Zinvie23 and Djidja,27 but not in Agbobome.43
Epilepsy prevalence in Burkina Faso
In 2007 a high prevalence of epilepsy (44.27‰) was observed in three rural villages in Burkina Faso. A case–control study in the area showed an association between epilepsy and cysticercosis seropositivity (prevalence odds ratio [OR] 3.1 [95% confidence interval {CI} 1.0–8.3]).28 All definitive and probable cases of neurocysticercosis were located in the two villages where pig breeding was common.28 Debouverie et al.39 reported an epilepsy prevalence of 10.65‰ in villages that were endemic for neither cysticercosis nor onchocerciasis; the predominant aetiology reported was neonatal asphyxia (present in 12/38 PWE with symptomatic epilepsy), and more than half of all PWE experienced their first seizure before the age of 10 y.
Epilepsy prevalence in Gambia
A low epilepsy prevalence of 4.26‰ was reported in Gambia,26 which is non-endemic for onchocerciasis. The peak age-specific prevalence rates occurred in the 35–44 y age group.
Epilepsy prevalence in Ghana
During a wide community-based survey (n=129 812) in Kintampo, exposure to O. volvulus was identified as an independent risk factor for epilepsy (OR 2.32 [95% CI 1.12–4.78] in children <18 y and OR 2.09 [95% CI 1.29–3.40] in adults).29 Although the overall epilepsy prevalence was low (1.92‰), further analysis of the data revealed a positive trend between the onchocerciasis seropositivity rate and the prevalence of epilepsy in the different study villages: Spearman’s rho=0.54, p=0.07. Other factors associated with epilepsy in children included family history of seizures (OR 3.31 [95% CI 1.83–5.96]), abnormal delivery (OR 2.99 [95% CI 1.07–8.34]) and problems after birth (OR 3.51 [95% CI 1.02–12.06]). In addition, the adult epilepsy risk factors included pork consumption (OR 1.68 [95% CI 1.09–2.58]) and exposure to Toxoplasma gondii (OR 1.99 [95% CI 1.15–3.45]).29
Epilepsy prevalence in Ivory Coast
In the southern part of Ivory Coast, two studies reported a high epilepsy prevalence.36,37 These two studies also provided detailed descriptions of the PWE encountered during the surveys. In M'Brou, in the Agboville health district, a high burden of epilepsy had been suspected for decades. This village is located in the Agneby River basin just below the southern border of the OCP target areas and did not benefit from vector control interventions, as it was estimated that the risk for onchocercal blindness was lower in this forest area of the country.45 The OCP has undertaken detailed entomological studies in the Agneby basin, but only one epidemiological survey that was done in a village <10 km from M'Brou, which showed a prevalence of microfilariae of 76%. An epilepsy survey conducted in 1993 revealed that 38 of 920 inhabitants (41‰) had epilepsy, and 28 PWE (73.7%) fulfilled the OAE criteria.37 In addition, two PWE in M'Brou presented with nodules from which adult worms of O. volvulus were extracted.37 Studies conducted in 1989 in Akoungou village (Tiassalé health district; onchocerciasis prevalence 43.4%46) also revealed a high epilepsy prevalence; up to 18 of the 309 inhabitants (58‰) were confirmed to have epilepsy, with 15 PWE (83.3%) satisfying the OAE criteria.36 In both villages, the peak age for seizure onset was 10–15 y (Figure 3) and the frequent family history of epilepsy steered the authors to conclude genetic/consanguinity aetiologies.36,37 A low epilepsy prevalence of 7.65‰ was reported in a non-endemic study site of Ivory Coast in 1987.40 However, according to the authors, this was an underestimation because older PWEs most likely hid their condition due to stigma and thus went undetected by the research team.
Figure 3.
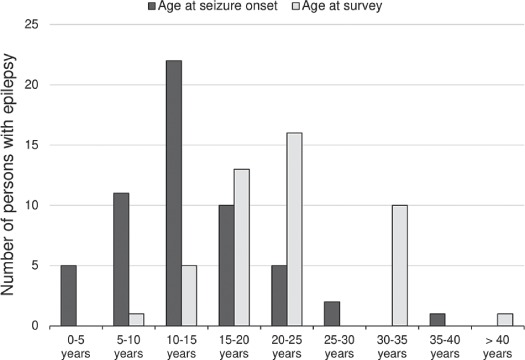
Age of persons with epilepsy and ages at seizure onset in southern Ivory Coast.
Epilepsy prevalence in Liberia
High epilepsy prevalence (27.73–49.01‰) was documented in the Grand Bassa onchocerciasis focus in Liberia.41,42 Both studies agreed that epilepsy was endemic in that region; in addition, youths were most affected.41,42 The local population distinguished two types of epilepsy: ‘to drop the head in the pan’ and ‘the big jerking’.47,48 Goudsmit et al.42,49 reported a frequent family history of epilepsy, psychological symptoms and burns among PWE in Liberia. Infection with Taenia solium was excluded as a possible cause of the high epilepsy prevalence observed.49 Other suggested aetiologies of epilepsy included genetic/environmental factors and febrile illness.49
Epilepsy prevalence in Mali
In 1999 in Mali, epilepsy was investigated in villages located in two zones with microfilariae prevalence of 9.3% for zone 1 (pre-control endemicity not known) and 23.0% for zone 2 (hyperendemic before the start of control). The epilepsy prevalence was 10.8‰ and 16.1‰ in zone 1 and zone 2, respectively (p=0.09).22 A case–control study further showed that 22.4% of PWE and 21.7% of controls had clinical and/or laboratory signs of onchocerciasis (OR 1.02 [95% CI 0.47–2.19]).22
Epilepsy prevalence in Nigeria
In Nigeria in 1982, an epilepsy prevalence of 37‰ was observed in Aiyete,20 a rural onchocerciasis-endemic village, while a 5.3‰ prevalence was observed in Igbo-Ora, a town situated 20 km away and inhabited by the same ethnic group19; both surveys found a high age-specific prevalence in the 10–19 y age group.19,20 In 2004, Dozie et al.24 reported an epilepsy prevalence of 12‰ in the then meso-endemic Imo River basin. The highest epilepsy prevalence was observed in villages with the highest onchocerciasis prevalence, with an overall positive correlation between both conditions in the study population (r=0.38, p<0.043).50 When this study site was revisited in 2018, the prevalence of microfilariae had dropped to 4.6%, with a drastic decrease of epilepsy prevalence to 5‰.30
Epilepsy prevalence in Senegal
As early as the 1960s, a low epilepsy prevalence of 1.92‰ was reported in the Moyenne Vallée of Senegal, which is non-endemic for onchocerciasis.44 However, this was most likely an underestimation of the true prevalence, as the data were not collected via a systematic approach. A more recent study conducted in non-endemic Pikine reported an epilepsy prevalence of 14.22‰.25
Epilepsy prevalence in Togo
All study sites in Togo had an epilepsy prevalence <20‰.32–34 One of the studies from Togo found an association between epilepsy and cysticercosis33; the prevalence of cysticercosis was 135‰ among PWE vs 38‰ in the general population (p<0.0001). Another study reported subcutaneous cysticercus cysts in 14/98 PWE (14.3%) and focal seizures in 38/98 (38.8%) of the PWE.34
Meta-analysis of epilepsy prevalence
Considering all included epilepsy surveys, the pooled epilepsy prevalence in the West African study sites was 13.14 per 1000 (95% CI 11.28–15.00). This prevalence ranged from 1.92‰ in study sites located in Senegal and Ghana to 58.25‰ in an onchocerciasis focus in Ivory Coast. Figure 4 shows a forest plot with the result of a meta-analysis to compare the prevalence of epilepsy between four subgroups of epilepsy studies: one group of villages from areas without onchocerciasis and three groups of sites where onchocerciasis was endemic. These latter three groups represent different durations of onchocerciasis control: no control (onchocerciasis control not yet started at the time of the epilepsy survey), 1–9 y of control (median 7 y) and ≥10 y of control (median 17.5 y). The main results are summarized in Figure 5.
Figure 4.
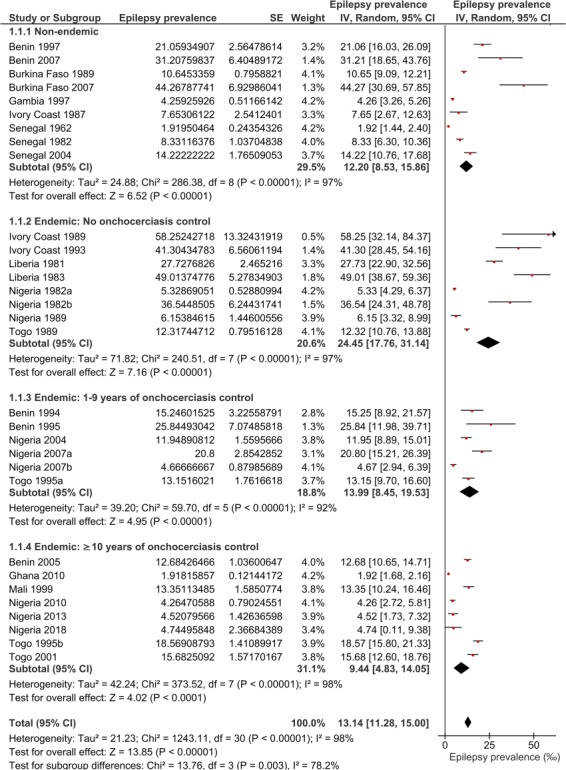
Forest plot and pooled epilepsy prevalence (per thousand) in the West African study sites.
Figure 5.
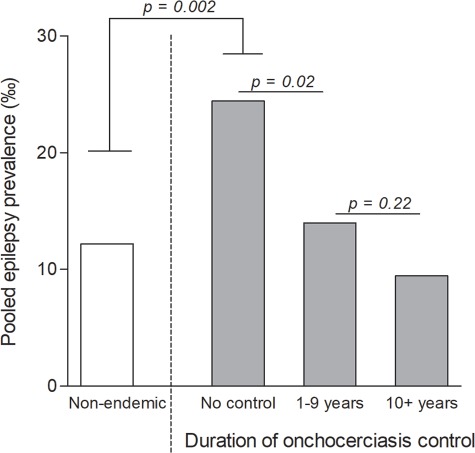
Subgroup analysis of the epilepsy prevalence stratified by duration of onchocerciasis control.
In onchocerciasis-endemic sites with no control, the prevalence of epilepsy was 24.45‰, i.e. twice as high as in the sites without onchocerciasis (12.20%). However, sites where control measures had been implemented had a significantly lower epilepsy prevalence compared with villages with no control prior to the epilepsy survey, even if control had been carried out for less than a decade (Figure 5).
Effects of pre-control onchocerciasis endemicity and duration of control on epilepsy prevalence
We used multiple linear regression to investigate possible associations between epilepsy prevalence (on the logit scale) and onchocerciasis pre-control prevalence of microfilariae, duration of onchocerciasis control, method of control (community-directed treatment with ivermectin [CDTI], vector control) and study year. The best fit was obtained with a model with two independent variables: the pre-control prevalence of microfilariae and the duration of onchocerciasis control (Table 2). Both variables showed a highly significant relationship with the prevalence of epilepsy, although with an opposite effect: the prevalence of epilepsy increases with the prevalence of microfilariae (regression coefficient 0.046, p<0.001) but decreases with the duration of control (regression coefficient −0.059, p<0.001). Thus both variables need to be taken into account at the same time when analysing the relationship between epilepsy and onchocerciasis in West Africa. The inclusion of any of the remaining variables did not result in an improvement of the model and, after controlling for pre-control prevalence of microfilariae and duration of control, there was no significant relationship with the prevalence of epilepsy for any of those variables: CDTI (p=0.68), vector control (p=0.97) and study year (p=0.22).
Table 2.
Multiple linear regression model investigating the factors associated with epilepsy prevalence in the study sites
| Modela covariates | Logit regression coefficient | 95% confidence interval | P-value | |
|---|---|---|---|---|
| Intercept | −6.343 | −7.204 | −5.483 | <0.001 |
| Pre-control onchocerciasis endemicity (prevalence of microfiladermia) | 0.046 | 0.030 | 0.063 | <0.001 |
| Duration of onchocerciasis control (years) | −0.059 | −0.076 | −0.042 | <0.001 |
aAdjusted R2=0.782.
The plots illustrating the relationship between epilepsy prevalence and pre-control onchocerciasis endemicity for the three control duration groups are shown in Figure 6. On the logit scale, each of the three groups shows a positive relationship between the prevalence of epilepsy and the prevalence of microfilariae. However, with increasing duration of control, the regression lines predict lower epilepsy prevalences for a given pre-control microfilariae prevalence, and the curve for the group of ≥10 y of control indicates a 70% reduction in the prevalence of epilepsy compared with the curve for the endemic group with no control.
Figure 6.
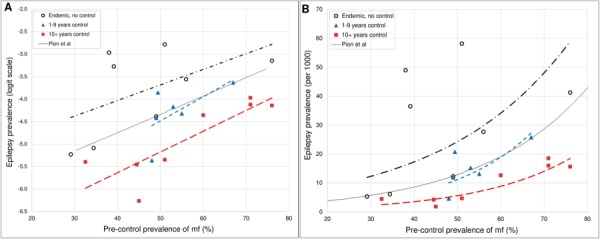
Plots and lines showing the relationship between epilepsy and pre-control onchocerciasis endemicity, taking into account the duration of onchocerciasis control in each endemic study site. (A) Logit-transformed scale for epilepsy prevalence. (B) Linear scale for epilepsy prevalence. The solid line represents the previously published curve by Pion et al.6; the black dotted line represents endemic sites with no control measures; the blue dotted line represents sites with 1–9 y of onchocerciasis control, which is almost superimposed on the previously published curve by Pion et al.; the red dotted line shows a downward shift of epilepsy prevalence in sites with ≥10 y of onchocerciasis control. (mf: detectable O. volvulus microfilariae in skin snips.)
Discussion
The pooled epilepsy prevalence in the West African study sites obtained in this study was 13.14‰, very similar to the median epilepsy prevalence in sub-Saharan Africa of 14.2‰.9 However, after taking onchocerciasis endemicity and onchocerciasis control into account, there were significant differences. First, villages in onchocerciasis-endemic areas had a double burden of epilepsy before the start of control compared with villages from areas that were onchocerciasis free. Second, in the onchocerciasis-endemic areas there was a statistically significant relationship between the prevalence of epilepsy and the pre-control onchocerciasis endemicity level. Third, after correcting for baseline endemicity, the prevalence of epilepsy declined significantly in relation to the duration of onchocerciasis control. Together, these three findings suggest that onchocerciasis has also been an important cause of epilepsy in West Africa, as was previously demonstrated in East and Central Africa. Furthermore, our results provide empirical evidence that onchocerciasis control can significantly reduce OAE. Similar findings were reported in the Kelleng village in Cameroon, where annual ivermectin distribution for more than a decade resulted in a drastic decrease in the incidence of epilepsy.51
The onchocerciasis landscape in West Africa had been particularly influenced by the activities of the OCP. In 11 West African countries, the OCP (1974–2002) was very successful in controlling onchocerciasis in the savannah areas, which were priority zones due to high blindness rates. The initial strategy was by vector control and, from the early 1990s, ivermectin was used as an additional tool.52,53 The large-scale onchocerciasis elimination control measures deployed in West Africa by the OCP came two decades earlier than those implemented later in Central and East Africa.
Given that many epilepsy prevalence studies in West Africa were performed after the introduction of OCP, only a few reported a high epilepsy prevalence in onchocerciasis-endemic regions. Our findings show that the epilepsy prevalence depended on the pre-control onchocerciasis endemicity, and longer periods of onchocerciasis control were associated with a lower epilepsy prevalence. However, the potential role of O. volvulus infection was not often discussed when researchers were confronted with an unusually high epilepsy burden as in Ivory Coast36,37 and Liberia.41,42 Most authors suggested that the high epilepsy prevalence and family clustering of PWEs were related to consanguinity,36,37,41,42 but this was never confirmed. Moreover, the fact that the household clustering of PWEs has been reported in several onchocerciasis foci and among people with very different genetic backgrounds is more suggestive of an environmental cause. Studies performed in onchocerciasis-endemic areas in Central and East Africa have repeatedly supported that the clustering of epilepsy cases in certain families and/or villages is a consequence of their proximity and common exposure to O. volvulus-infected blackflies.54,55
In Nigeria, the disparity in epilepsy prevalence observed in two study sites 20 km apart (rural Aiyete and suburban Igbo-Ora) was thought to result from more effective primary healthcare services in the suburban setting compared with the village.19 However, the rural village (Aiyete) is located closer to the Ofiki River, a known breeding site for blackflies,56 and consequently had a higher pre-control endemicity than Igbo-Ora (Table 1). A similar situation was observed in Mahenge, an onchocerciasis-endemic region in Tanzania, where an epilepsy prevalence of 35‰ was observed in rural villages close to blackfly breeding sites, compared with only 15‰ in suburban villages just 30 km away.57 Furthermore, in South Sudan, communities residing closer to the Maridi Dam (conducive environment for the onchocerciasis vector) were found to have a very high prevalence of epilepsy, up to 119‰.58
It is conceivable that other health interventions (such as malaria control or improved neonatal care) may have reduced the epilepsy burden, alongside onchocerciasis control. However, the multiple regression analysis did not show a significant association between the epilepsy prevalence and the study year, suggesting that improved healthcare in more recent studies cannot explain the disparity in epilepsy prevalence observed in the different sites. Certainly, epilepsy resulting from perinatal brain insult was more common in the past, and typically started within the early years of life as described by Collomb et al.11 in a non-endemic region in Senegal. However, in onchocerciasis-endemic areas with suboptimal control measures, epilepsy onset is typically more prevalent after the age of 5 y and before the age of 20 y2 (Figure 3). Therefore the epilepsy incidence in the 5–20 y age group would hardly be affected by improved perinatal care. Nonetheless, improved access to care would increase the life expectancy of PWEs and result in a cohort age shift of PWEs as observed in an onchocerciasis focus in central Cameroon.51
It is worth noting that in the two epilepsy studies conducted in Liberia (which was not under the umbrella of OCP, albeit with a high onchocerciasis endemicity59), a high epilepsy prevalence >25% was reported.41,42 On the other hand, the epilepsy prevalence was particularly low in non-endemic areas of Senegal44 and Gambia.26 The epilepsy prevalence was also low in Kintampo, Ghana, despite a high seroprevalence of O. volvulus Ov16 antibodies observed in these villages.29 It should be noted that by the time of the survey, Kintampo had benefited from a very efficient vector control programme by OCP followed by biannual ivermectin distribution.60 Therefore this high Ov16 IgG seropositivity rate among the adult population was more representative of a high transmission of onchocerciasis in the past that had already been controlled by the time of the survey, resulting in a drastic decline of the epilepsy burden.
The epidemiological and clinical criteria of OAE2 were observed in many endemic sites in West Africa,36,37,41,42 similar to findings from several other onchocerciasis-endemic villages.55,61,62 In Liberia, the description of early–onset focal seizures accompanied by cognitive symptoms and manifesting as rhythmic dorsoventral movements of the head49,63 most likely refer to cases of nodding syndrome,2,64 as reported in other parts of Africa. Another argument in favour of the presence of OAE in West African villages is that most PWEs encountered were ≤25 y of age, suggesting a reduced life expectancy. Analysis of a 25-year follow-up database of 295 909 persons with onchocerciasis in OCP countries revealed an increased risk of mortality with increasing skin microfilariae load, and particularly among younger age groups (<20 y old).65,66 Given that OAE typically starts between the ages of 3 and 18 y,2 we deduced that the high mortality observed among O. volvulus–infected persons <20 y of age is not a consequence of ocular manifestations of onchocerciasis, which are more frequent during adulthood. Instead, premature mortality due to epilepsy, as observed in other onchocerciasis-endemic villages,67,68 is a more plausible explanation.
While infection with O. volvulus may indeed be responsible for many epilepsy cases in endemic sites, neurocysticercosis was suggested as a possible aetiology in Benin,35 Togo33,34 and Nigeria.19 Generally, in communities with ongoing T. solium transmission, only about one-third of epilepsy cases can be attributed to neurocysticercosis.69 Meanwhile, the population-attributable fraction of O. volvulus infection in epilepsy was estimated at 91.7% in onchocerciasis-endemic villages in the Mbam valley in Cameroon,3 suggesting that onchocerciasis may account for a larger proportion of epilepsy cases than neurocysticercosis in co-endemic settings. A recent survey in Maridi, South Sudan (where there are no pigs and consequently no T. solium transmission to cause neurocysticercosis) showed a high prevalence of epilepsy, particularly in villages closer to blackfly breeding sites58; >85% of the PWEs in onchocerciasis-endemic villages satisfied the OAE criteria.62 Similarly, neurocysticercosis could not account for the high epilepsy prevalence observed in Liberia prior to implementing onchocerciasis control in that area.42 All these findings strongly suggest that onchocerciasis is an important, yet neglected contributor to the epilepsy burden in sub-Saharan Africa. Our results also suggest that within 10 y of implementing onchocerciasis control, the epilepsy burden could be reduced significantly in endemic communities. However, these are very imprecise estimates, as we did not take into account the method, frequency and coverage of the onchocerciasis control strategies (aerial vs ground larviciding, annual vs biannual ivermectin distribution).
The strength of our study resides in the fact that we included epilepsy data obtained during the pre-onchocerciasis control period, thus increasing the chances of finding high endemicity and consequently high epilepsy prevalence. However, we must point out that some researchers in the past had a tendency to investigate only villages with anecdotal reports of high seizure frequency, regardless of onchocerciasis endemicity. This may explain why a substantial epilepsy burden was observed in some communities with little or no onchocerciasis transmission, most likely due to a panoply of aetiologies besides infection with O. volvulus. As a major weakness of our study, the pre-control onchocerciasis prevalence for the exact study sites was generally not available and we therefore used the predicted prevalence for each location from spatial maps of onchocerciasis endemicity in West Africa. Although these maps are quite detailed, they do not always capture local fluctuations in endemicity. For a few sites, we estimated the pre-control endemicity from available survey data for nearby villages (5–10 km distance) where this was considered more reliable. For instance, the pre-control data for M'Brou village (Ivory Coast) were extrapolated from the neighbouring village of Emankono Camp (<10 km away), which had been investigated in detail by the OCP. Another limitation is the fact that obtaining precise values for the pre-control community microfilariae load (a better measure of onchocerciasis endemicity) for each study site was practically impossible. We must also point out the fact that migration into and out of the different study communities, which may have influenced the exposure of the migrants to onchocerciasis, was not taken into account during our analysis, as this information was not available. Finally, the lack of solid evidence (imaging, electroencephalography, laboratory investigations) to confirm or exclude other causes of epilepsy in most of the included studies is also a caveat worth mentioning.
Conclusions
This review adds to the existing epidemiological evidence suggesting that onchocerciasis is able to cause epilepsy in all onchocerciasis-endemic regions with high ongoing O. volvulus transmission. OAE has most likely occurred in onchocerciasis foci in West Africa prior to and during the early years of onchocerciasis control efforts. Subsequently, onchocerciasis control appears to have significantly reduced the prevalence of OAE. These findings are relevant for areas still experiencing substantial onchocerciasis transmission, as these should be prioritized for interventions in order to curb the dual burden of epilepsy and onchocerciasis.70,71
Supplementary Material
Acknowledgements:
None.
Authors’ contributions:
CR and SFJN conceived the study and performed the literature review. JHFR provided data on onchocerciasis baseline endemicity and control, while PMP provided grey data on epilepsy prevalence in West Africa. SFJN extracted the data. SFJN and JHFR performed the data analysis. SFJN wrote the first draft. All authors revised the initial draft and approved the final manuscript.
Funding:
RC receives funding from the European Research Council (ERC grant 671055).
Competing interests:
None declared.
Ethical approval:
Not applicable.
References
- 1. Burnham G. Onchocerciasis. Lancet. 1998;351(9112):1341–1346. [DOI] [PubMed] [Google Scholar]
- 2. Colebunders R, Siewe Fodjo JN, Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2018;34(3):208–216. [DOI] [PubMed] [Google Scholar]
- 3. Chesnais CB, Nana-Djeunga HC, Njamnshi AK, et al. . The temporal relationship between onchocerciasis and epilepsy: a population-based cohort study. Lancet Infect Dis. 2018;18(11):1278–1286. [DOI] [PubMed] [Google Scholar]
- 4. Kaiser C, Rubaale T, Tukesiga E, et al. . Association between onchocerciasis and epilepsy in the Itwara hyperendemic focus, West Uganda: controlling for time and intensity of exposure. Am J Trop Med Hyg. 2011;85(2):225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colebunders R, Alfred K. Njamnshi, Oijen MV, et al. . Onchocerciasis-associated epilepsy: from recent epidemiological and clinical findings to policy implications. Epilepsia Open. 2017;2(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pion SD, Kaiser C, Boutros-Toni F, et al. . Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3(6):e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casis SG. El sindrome epiléptico y sus relaciones con la oncocercosis. Bol Salubr E Hig Mex. 1938;1:11–31. [Google Scholar]
- 8. World Health Organization Onchocerciasis Control Programme (OCP). Available from: http://www.who.int/blindness/partnerships/onchocerciasis_OCP/en/ [accessed 5 July 2019].
- 9. Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux P-M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ngoungou EB, Quet F, Dubreuil CM, et al. . Épidémiologie de l'épilepsie en Afrique subsaharienne: une revue de la littérature. Epilepsies. 2006;16(4):225–238. [PubMed] [Google Scholar]
- 11. Collomb H, Dumas M, Ayats H, Virieu R, Simon M, Roger J. Epidemiology of epilepsy in Senegal. Afr J Med Sci. 1970;1(2):125–148. [PubMed] [Google Scholar]
- 12. Hayden JA, Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. [DOI] [PubMed] [Google Scholar]
- 13. Kim YE, Remme JHF, Steinmann P, Stolk WA, Roungou J-B, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis. 2015;9(4):e0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zouré HG, Noma M, Tekle AH, et al. . The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasit Vectors. 2014;7:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coffeng LE, Pion SDS, O’Hanlon S, et al. . Onchocerciasis: the pre-control association between prevalence of palpable nodules and skin microfilariae. PLoS Negl Trop Dis. 2013;7(4):e2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nwani P, Nwosu M, Asomugha L, Enwereji K, Arinzechi E, Ogunniyi A. Epidemiology of active epilepsy in a suburban community in southeast Nigeria: a door-to-door survey. Niger J Clin Pract. 2015;18(4):527–533. [DOI] [PubMed] [Google Scholar]
- 17. Osakwe C, Otte WM, Alo C. Epilepsy prevalence, potential causes and social beliefs in Ebonyi state and Benue state. Nigeria. Epilepsy Res. 2014;108(2):316–326. [DOI] [PubMed] [Google Scholar]
- 18. Ndiaye IP, Mauferon JB, Diagne M. Épidémiologie de l'épilepsie au Sénégal. Communication presented to the 7th Congress of the Pan- African Association of Neurological Sciences, Abidjan, Côte d’Ivoire: April 23–30, 1986. [Google Scholar]
- 19. Osuntokun BO, Adeuja AO, Nottidge VA, et al. . Prevalence of the epilepsies in Nigerian Africans: a community-based study. Epilepsia. 1987;28(3):272–279. [DOI] [PubMed] [Google Scholar]
- 20. Osuntokun BO, Schoenberg BS, Nottidge VA, et al. . Research protocol for measuring the prevalence of neurologic disorders in developing countries. Neuroepidemiology. 1982;1:143–153. [Google Scholar]
- 21. Longe AC, Osuntokun BO. Prevalence of neurological disorders in Udo, a rural community in southern Nigeria. Trop Geogr Med. 1989;41(1):36–40. [PubMed] [Google Scholar]
- 22. Farnarier G, Diop S, Coulibaly B, et al. Onchocerciasis and epilepsy. Epidemiological survey in Mali. Med Trop (Mars). 2000;60(2):151–5. [PubMed] [Google Scholar]
- 23. Debrock C, Preux P-M, Houinato D, et al. . Estimation of the prevalence of epilepsy in the Benin region of Zinvié using the capture-recapture method. Int J Epidemiol. 2000;29(2):330–335. [DOI] [PubMed] [Google Scholar]
- 24. Dozie INS, Onwuliri COE, Nwoke BEB, et al. . Onchocerciasis and epilepsy in parts of the Imo river basin, Nigeria: a preliminary report. Public Health. 2006;120(5):448–450. [DOI] [PubMed] [Google Scholar]
- 25. Ndoye NF, Sow AD, Diop AG, et al. . Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal: an ILAE/IBE/WHO study. Seizure. 2005;14(2):106–111. [DOI] [PubMed] [Google Scholar]
- 26. Coleman R, Loppy L, Walraven G. The treatment gap and primary health care for people with epilepsy in rural Gambia. Bull World Health Org. 2002;80(5):378–383. [PMC free article] [PubMed] [Google Scholar]
- 27. Houinato D, Yemadje L-P, Glitho G, et al. . Epidemiology of epilepsy in rural Benin: prevalence, incidence, mortality, and follow-up. Epilepsia. 2013;54(4):757–763. [DOI] [PubMed] [Google Scholar]
- 28. Nitiéma P, Carabin H, Hounton S, et al. . Prevalence case-control study of epilepsy in three Burkina Faso villages. Acta Neurol Scand. 2012;126(4):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ae-Ngibise K, Akpalu B, Ngugi AK, et al. . Prevalence and risk factors for active convulsive epilepsy in Kintampo. Ghana. Pan Afr Med J. 2015;21:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siewe Fodjo JN, Ukaga CN, Nwazor EO, et al. . Low prevalence of epilepsy and onchocerciasis after more than 20 years of ivermectin treatment in the Imo River basin in Nigeria. Infect Dis Poverty. 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mustapha AF, Preux P, Sanya EO, Akinleye CA. The prevalence and subjective handicap of epilepsy in Ilie—a rural riverine community in South West Nigeria: a door-to-door survey. Epilepsy Behav. 2014;37:258–264. [DOI] [PubMed] [Google Scholar]
- 32. Balogou AA, Doh A, Grunitzky KE. Neurological disorders and endemic goiter: comparative analysis of 2 provinces in Togo. Bull Soc Pathol Exot. 2001;94(5):406–410. [PubMed] [Google Scholar]
- 33. Balogou AA, Grunitzky KE, Beketi KA, Bouteille B, Dumas M. Cysticercosis and epilepsy in the city of Tone, north of Togo. Rev Neurol (Paris). 2000;156(3):270–273. [PubMed] [Google Scholar]
- 34. Balogou AA, Grunitzky EK, Belo M, et al. . Management of epilepsy patients in Batamariba district. Togo. Acta Neurol Scand. 2007;116(4):211–216. [DOI] [PubMed] [Google Scholar]
- 35. Avode DG, Capo-Chichi OB, Gandaho P, Bouteille B, Dumas M. Epilepsie provoquée par la cysticercose. A propos d'une enquête sociologique et culturelle realisée à Savalou au Bénin. Bull Soc Path Exot. 1996;89(1):45–47. [PubMed] [Google Scholar]
- 36. Kouadjo Y. Génétique et épilepsie: à propos d'un foyer d'épilepsie observe dans un village Ivoirien. Doctoral thesis, Université Nationale de Côte d'Ivoire, 1990.
- 37. Kaudjhis PJR. Les agrégats de l'épilepsie de M'brou: approche électroclinique et étiologique. Doctoral thesis, Université Nationale de Côte d'Ivoire, 1995.
- 38. Houinato DS, Adjien KC, Gnonlonfon D, Adoukonou T, Dema F, Avode DG. Etude de la prévalence de l'épilepsie á Dangbo dans le département de l'Oueme au Benin. Bénin Méd. 2007;37:14–17. [Google Scholar]
- 39. Debouverie M, Kabore J, Dumas M, Weber M, Duboz P, Vaugelade J. Epidemiology of epilepsy in Burkina Faso In: Dumas M, Giordano C, Gentilini M, Chieze F, editors. Neurologie Tropicale. Paris: John Libbey Eurotext, 1993; p. 57–61. [Google Scholar]
- 40. Kouassi B, Koffi JK, Diarra JA. Prévalence de l'épilepsie en milieu rural ivoirien. Pub Méd Afr. 1988;89:25–30. [Google Scholar]
- 41. Gerrits C. A West African epilepsy focus. Lancet. 1983;321(8320):358. [DOI] [PubMed] [Google Scholar]
- 42. Goudsmit J, Waals FW, Gajdusek C. Epilepsy in the Gbawein and Wroughbarh clan of Grand Bassa County, Liberia: the endemic occurrence of ‘See-ee’ in the native population. Neuroepidemiology. 1983;2(1–2):24–34. [Google Scholar]
- 43. Gbenou HD. [Contribution à l'étude de l'association onchocercose-épilepsie: résultats préliminaires d'une enquête neuroépidémiologique à Agbogbomé-commune de Paouignan, Sous-Préfecture de Dassa, Zoumé, au Bénin]. Doctoral thesis, Université Nationale du Bénin, 1995.
- 44. Boutillier J-L, Cantrelle P, Caussé J, Laurent C, N’Doye T. La moyenne vallée du Sénégal (étude socio-économique). Paris: Presses Universitaires de France, 1962. [Google Scholar]
- 45. Dadzie KY, Remme J, Rolland A, Thylefors B. Ocular onchocerciasis and intensity of infection in the community. II. West African rainforest foci of the vector Simulium yahense. Trop Med Parasitol. 1989;40(3):348–354. [PubMed] [Google Scholar]
- 46. Koudou BG, Kouakou M-M, Ouattara AF, et al. . Update on the current status of onchocerciasis in Côte d'Ivoire following 40 years of intervention: progress and challenges. PLoS Negl Trop Dis. 2018;12(1):e0006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gerrits C. Epilepsy care in a non-clinical setting. A medical-anthropological study among the Bassa and Kpelle in the rainforest of Liberia, West Africa. Trop Geogr Med. 1994;46(3 Suppl):S13–S17. [PubMed] [Google Scholar]
- 48. Gerrits C. Conceptions and explanations of sii epilepsy: a medical-anthropological study among the Bassa and Kpelle in Liberia. Curare. 1983;6:33–40. [Google Scholar]
- 49. Goudsmit J, Van Der Waals FW. Endemic epilepsy in an isolated region of Liberia. Lancet. 1983;321(8323):528–529. [DOI] [PubMed] [Google Scholar]
- 50. Dozie I, Onwuliri C, Nwoke B. Onchocerciasis in Imo state, Nigeria (2): the prevalence, intensity and distribution in the upper Imo river basin. Int J Environ Health Res. 2004;14(5):359–369. [DOI] [PubMed] [Google Scholar]
- 51. Siewe Fodjo JN, Tatah G, Tabah EN, et al. . Epidemiology of onchocerciasis-associated epilepsy in the Mbam and Sanaga river valleys of Cameroon: impact of more than 13 years of ivermectin. Infect Dis Poverty. 2018;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boatin BA. The current state of the Onchocerciasis Control Programme in West Africa. Trop Doct. 2003;33(4):209–214. [DOI] [PubMed] [Google Scholar]
- 53. Leveque C. The use of insecticides in the Onchocerciasis Control Programme and aquatic monitoring in West Africa In: Bourdeau P, Haines JA, Klein W, Krishna Murti CR, editors. Ecotoxicology and Climate. Chichester: John Wiley & Sons, 1989; p. 317–335. [Google Scholar]
- 54. Boussinesq M, Pion SD, Ngangue D, Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans R Soc Trop Med Hyg. 2002;96(5):537–541. [DOI] [PubMed] [Google Scholar]
- 55. Levick B, Laudisoit A, Tepage F, et al. . High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2017;11(7):e0005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brieger WR, Oshiname FO, Ososanya OO. Stigma associated with onchocercal skin disease among those affected near the Ofiki and Oyan rivers in Western Nigeria. Soc Sci Med. 1998;47(7):841–852. [DOI] [PubMed] [Google Scholar]
- 57. Mmbando BP, Suykerbuyk P, Mnacho M, et al. . High prevalence of epilepsy in two rural onchocerciasis endemic villages in the Mahenge area, Tanzania, after 20 years of community directed treatment with ivermectin. Infect Dis Poverty. 2018;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Colebunders R, Carter JY, Olore PC, et al. . High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: a community-based survey. Seizure. 2018;63:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. White AT, Newland HS, Taylor HR, et al. . Controlled trial and dose-finding study of ivermectin for treatment of onchocerciasis. J Infect Dis. 1987;156(3):463–470. [DOI] [PubMed] [Google Scholar]
- 60. Turner HC, Osei-Atweneboana MY, Walker M, et al. . The cost of annual versus biannual community-directed treatment of onchocerciasis with ivermectin: Ghana as a case study. PLoS Negl Trop Dis. 2013;7(9):e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siewe Fodjo JN, Ngarka L, Tatah G, et al. . Clinical presentations of onchocerciasis-associated epilepsy (OAE) in Cameroon. Epilepsy Behav. 2019;90:70–78. [DOI] [PubMed] [Google Scholar]
- 62. Colebunders R, Abd-Elfarag G, Carter JY, et al. . Clinical characteristics of onchocerciasis-associated epilepsy in villages in Maridi County. Republic of South Sudan. Seizure. 2018;62:108–115. [DOI] [PubMed] [Google Scholar]
- 63. Waals FW, Goudsmit J, Gajdusek DC. See-ee: clinical characteristics of highly prevalent seizure disorders in the Gbawein and Wroughbarh clan region of Grand Bassa County, Liberia. Neuroepidemiology. 1983;2:35–44. [Google Scholar]
- 64. Dowell SF, Sejvar JJ, Riek L, et al. . Nodding syndrome. Emerg Infect Dis. 2013;19(9):1374–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Little M, Breitling L, Basáñez M-G, Alley E, Boatin B. Association between microfilarial load and excess mortality in onchocerciasis: an epidemiological study. Lancet. 2004;363(9420):1514–1521. [DOI] [PubMed] [Google Scholar]
- 66. Walker M, Little MP, Wagner KS, Soumbey-Alley EW, Boatin BA, Basáñez M-G. Density-dependent mortality of the human host in onchocerciasis: relationships between microfilarial load and excess mortality. PLoS Negl Trop Dis. 2012;6(3):e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kamgno J, Pion SDS, Boussinesq M. Demographic impact of epilepsy in Africa: results of a 10-year cohort study in a rural area of Cameroon. Epilepsia. 2003;44(7):956–963. [DOI] [PubMed] [Google Scholar]
- 68. Kaiser C, Asaba G, Kasoro S, Rubaale T, Kabagambe G, Mbabazi M. Mortality from epilepsy in an onchocerciasis-endemic area in West Uganda. Trans R Soc Trop Med Hyg. 2007;101(1):48–55. [DOI] [PubMed] [Google Scholar]
- 69. Gripper LB, Welburn SC. The causal relationship between neurocysticercosis infection and the development of epilepsy – a systematic review. Infect Dis Poverty. 2017;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Colebunders R, Siewe Fodjo JN, Hopkins A, et al. . From river blindness to river epilepsy: implications for onchocerciasis elimination programmes. PLoS Negl Trop Dis. 2019;13(7):e0007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gumisiriza N, Mubiru F, Siewe Fodjo JN, Mbonye Kayitale M, Hotterbeekx A, Idro R, et al. . Prevalence and incidence of nodding syndrome and other forms of epilepsy in onchocerciasis-endemic areas in northern Uganda after the implementation of onchocerciasis control measures. Infect Dis Poverty. 2020;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


