Abstract
Documented meningioma cases in Central Texas (USA) from 1976 to 2013 were studied utilizing the Scott & White Brain Tumor Registry. All the cases examined were histologically diagnosed as meningiomas. Of the 372 cases, most were benign tumors (p < 0.05). A majority of the patients were females (p < 0.05). Elderly individuals (>45 years of age) superseded the younger patients in meningioma incidence (p < 0.05). Previous data regarding meningioma epidemiology in Texas showed a higher incidence in black patients when compared to white patients. By contrast, this study’s findings of Central Texas meningioma demographics show increased incidence of meningiomas in white patients (p < 0.05). This interesting find in meningioma prevalence warrants further investigation with a larger sample size, in order to establish validity and further parse out possible causes of meningioma development among white individuals.
Keywords: Meningioma, Central Texas, Epidemiology
1. Introduction
Meningiomas are the most common primary brain tumors in adults, constituting a third of all diagnosed primary neoplasms of the brain [1]. It has been reported that the age-adjusted incidence rate of meningioma is 7.61/100,000 individuals per year [2]. Although most meningiomas are considered histologically benign and many are clinically silent, the potential impact of morbidity these tumors have on society can be huge [3,4]. Many patients diagnosed with a meningioma live with devastating clinical symptoms that vary depending on the size and location of the growth [4,5]. Symptoms include, but are not limited to: vision loss, seizures, neurological deficits, speech dysfunction, difficulty in concentration, and motor weakness to name several [4]. Additionally, open microsurgical resection (the treatment of choice for meningiomas) has the potential to induce morbidity in and of itself, due to its complexity and time burden, especially in older patients with preexisting medical conditions. This is attributable to the benign and slow-growing nature of most meningiomas, resulting in many patients developing of large lesions over several years and thus requiring more invasive procedures. These large resections increase the risk of morbidity, particularly in older individuals with preexisting conditions [6]. Yet, despite its high prevalence rate and increased risk of morbidity, relatively little knowledge exists in the literature regarding the epidemiology of meningiomas [7]. Only recently has the Senate Appropriations Committee recognized this paucity of knowledge and subsequently made recommendations to increase attention on brain tumor research.
Recent research has led to the discovery of possible genetic and environmental risk factors for meningiomas. Genetically, it has been shown that meningiomas are the second most frequently occurring tumors in patients with neurofibromatosis type II, and to a lesser degree, can occur in the setting of multiple endocrine neoplasia type I [1].
Most nongenetic risk factors are environmental and are associated with economic development. To date, studies assessing the involvement of hair dye, cell phone use, allergens, agricultural chemicals, petrochemicals, rubber and solvent contacts, loud noise, infection, passive and active smoke exposure, and exogenous hormone use with meningioma occurrence have given rise to inconclusive or mixed results. By contrast, ionizing radiation exposure has been proven to be a definite risk factor for meningiomas, as well as for other lethal carcinomas [4,8].
The aim of this study is to undertake an analysis of meningioma occurrence in the Central Texas region (USA). Previous reports have alluded to the presence of a relatively high amount of radiation in Texas drinking water, specifically radium 226 and radium 228 [9,10]. Additionally, the Central Texas region has seen a massive increase in economic development over the past few decades. As a result, exposure to many of the risk factors previously alluded to, such as exogenous hormones, cell phones, hair dye, allergens, petrochemicals, agriculture, loud noise, and radiation, have increased and are now widespread in Central Texas at varying degrees [8–10]. According to a 2012 Centers for Disease Control and Prevention (CDC) report, 27.7% of Texas residents were obese (body mass index ⩾30), thus increasing this group’s risk of meningioma [11]. Therefore, the data accrued will also facilitate the assessment of the cumulative contribution of the aforementioned possible environmental risk factors on the occurrence of meningioma. To achieve our objectives, we evaluated cases from the Scott & White Health Care Tumor Registry ranging from 1976 to 2013. This study meets the Scott & White Health Care Central Texas institutional review board (IRB) guidelines, as well as those of the National Institutes of Health (NIH).
2. Materials and methods
Patient details were obtained from the Scott & White Brain Tumor Registry which is a SW Temple, Texas hospital-based tumor registry. Our data are based on cerebral and spinal meningioma. For the purposes of this study, meningioma diagnoses were considered to be definitive when obtained through surgical biopsy and/or resection. As such, 372 cases in the tumor registry, ranging from 1976 to 2013, which had undergone surgical procedures where the final histological diagnoses were confirmed as meningiomas, were examined. The data were stratified according to sex, age group (0–19 years of age, 20–34 years of age, 35–44 years of age, 45–54 years of age, 55–64 years of age, 65–74 years of age, 75–84 years of age, > 85 years of age), ethnicity (white, black, Hispanic), and histology (benign or malignant). Factorial analysis of variance was utilized (general linear models; Statistica version 8.0, Statsoft – Tulsa, OK, USA). Post hoc assessments were conducted with the Duncan’s multiple range tests and a p value <0.05 qualified as statistically significant.
3. Results
3.1. Age group
Five of the eight age groups were compared with each other (0–19 years, 20–34 years, 35–44 years, 45–54 years, 55–64 years, 65–74 years, 75–84 years, >85 years). The number of patients between the ages of 45 years and 84 years (84%) exceeded those younger than 45 years and older than 85 years (F7, 21 = 7.8, p = 0.001, Fig. 1).
Fig. 1.
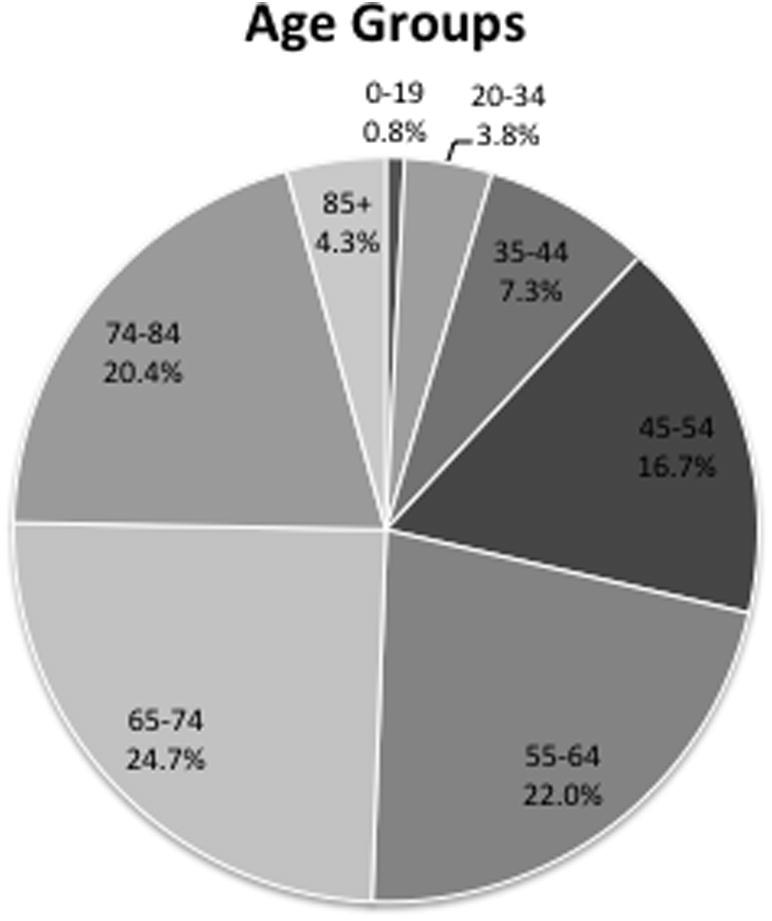
Patient demographics divided by age groups.
3.2. Sex
Of the 372 cases, 262 were female (70.4%, F1, 21 = 18.1, p = 4 × 10−4, Fig. 2).
Fig. 2.
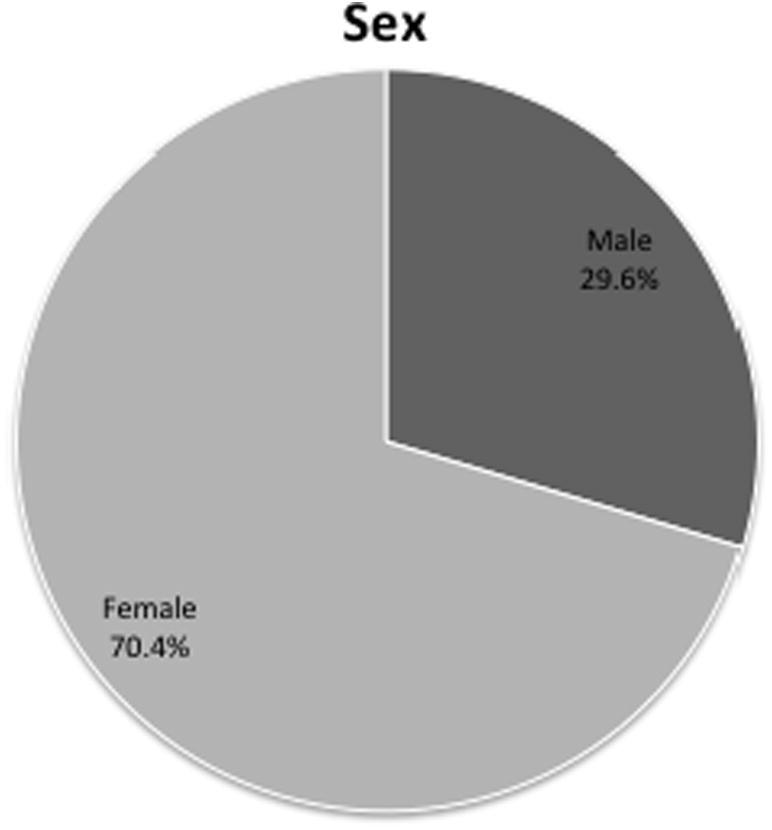
Patient demographics as determined by sex.
3.3. Ethnicity
A total of 286 (76.9%) patients were white (and non-Hispanics), 44 (11.8%) patients were black (and non-Hispanics), 23 (6.2%) patients were Hispanic, and 19 (5.1%) patients were classified as other (Fig. 3). The four groups were analyzed and white patients were more numerous than the others (F3, 21 = 56.2, p = 3.3 × 10−10).
Fig. 3.
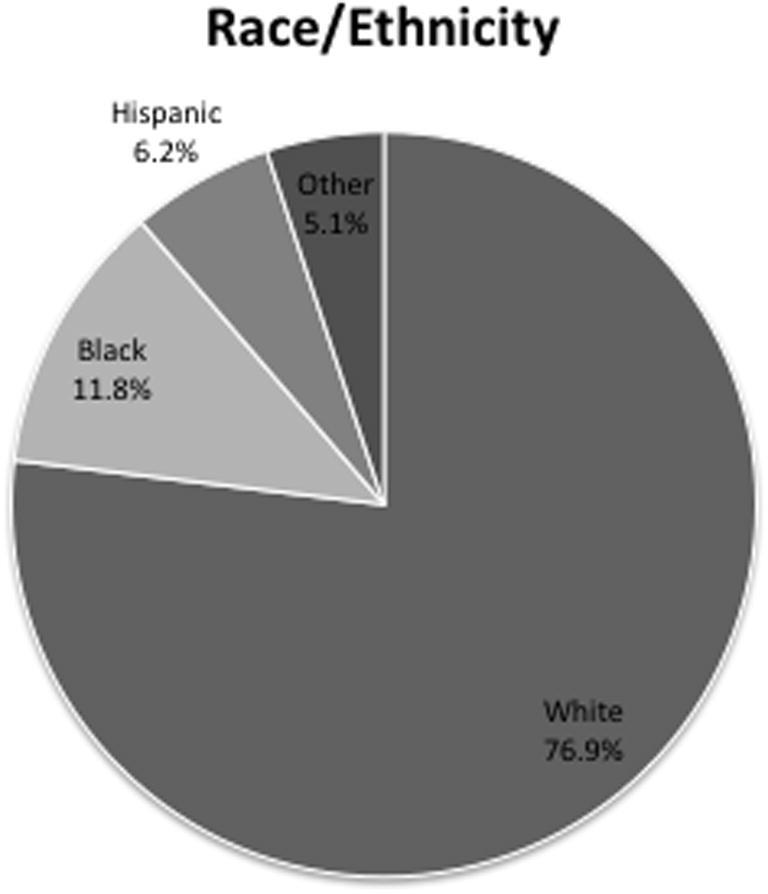
Patient demographics as determined by race.
3.4. Histology
Meningiomas are meningothelial (arachnoidal) cell neoplasms that are typically attached to the inner surface of the dura mater. According to the World Health Organization (WHO), most meningiomas are benign and correspond to the WHO Grade I classification [12]. Certain histological subtypes are associated with less favorable clinical outcomes and correspond to WHO Grade II (atypical) and Grade III (anaplastic or malignant). Meningiomas display an extremely diverse microscopic appearance with numerous histologic variants. The prototypical meningioma is that of a spindle cell tumor arranged in a whorled or lobulated architecture. The neoplastic cells have a meningothelial appearance with round-to-oval nuclei, inconspicuous nucleoli, and eosinophilic cytoplasm. Psammoma bodies are often noted throughout the tumor. The vast majority of meningiomas stain for epithelial membrane antigen. In order to be characterized as an anaplastic (malignant) meningioma, there must be obviously malignant cytology resembling that of carcinoma, melanoma or high-grade sarcoma, or a markedly elevated mitotic index (20 or more mitoses per 10 high-power fields) [12]. Invasion of the brain alone is not sufficient for a diagnosis of anaplastic (malignant) meningioma.
Out of the 372 cases, 358 (96%) were diagnosed as benign meningiomas (WHO Grade I and Grade II), while 12 patients (4%) suffered frank malignancies (WHO Grade III). The number of benign tumors was more than the malignant tumors (F1, 21 = 92.6, p = 3.76 × 10−9, Fig. 6).
Fig. 6.
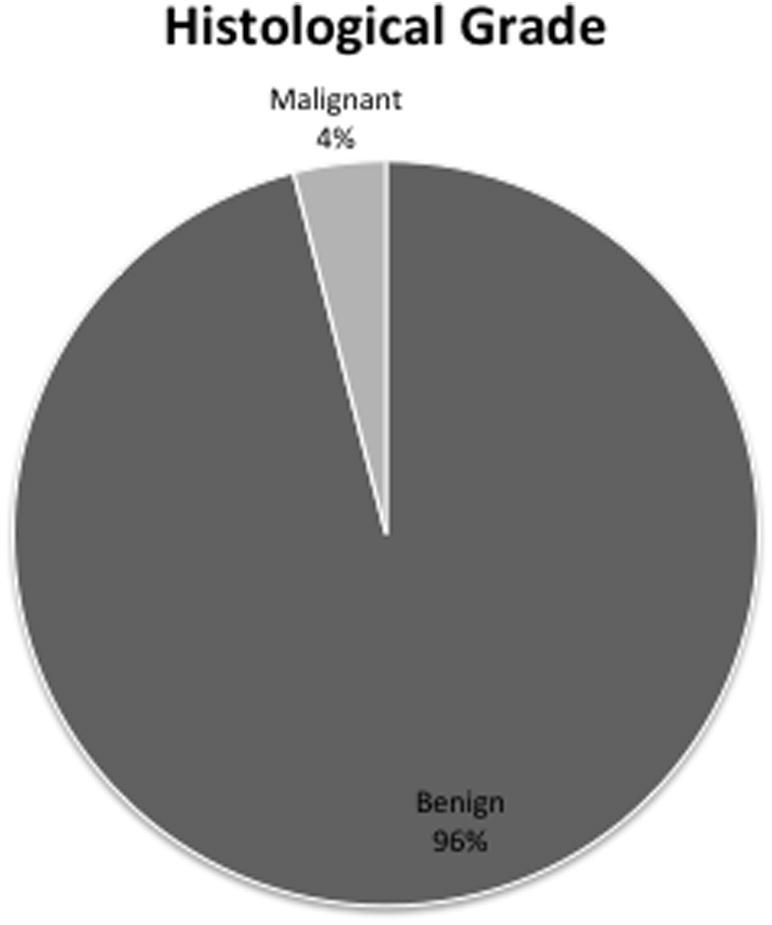
Distribution of tumors according to histological grade.
3.5. Patient distribution of ethnically white patients
Patient distribution within the white population followed the demographics of the rest of the population. Most of the tumors were histologically benign (2.4% vs. 97.6%, Fig. 6). There were more female patients than male patients (29% vs. 71%, Fig. 5). The ages of 84% of the patients were between 45 years and 84 years (Fig. 4).
Fig. 5.
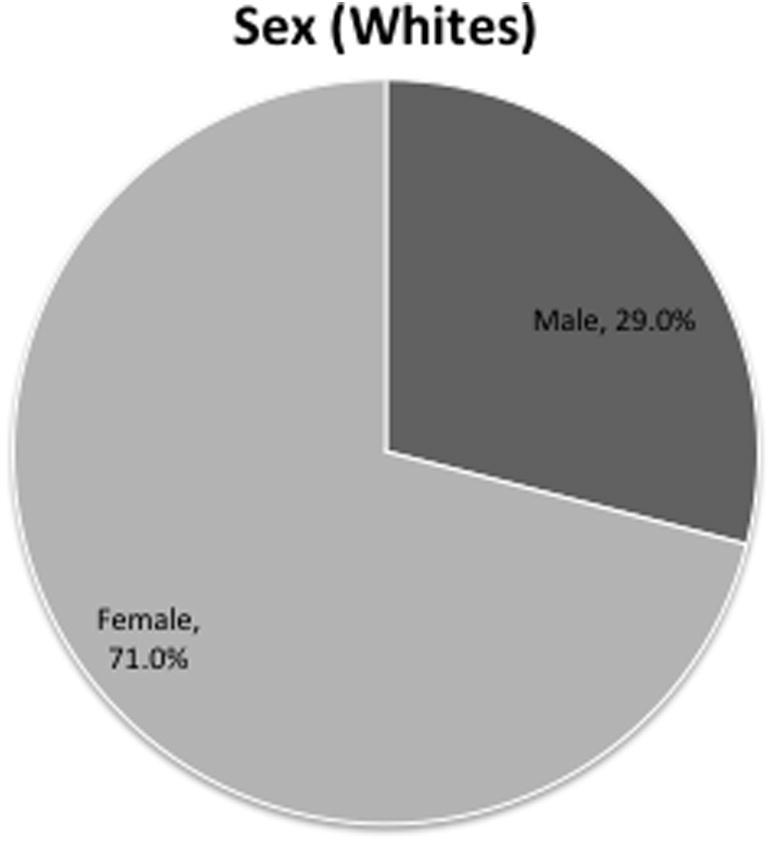
Distribution of white patients according to sex.
Fig. 4.
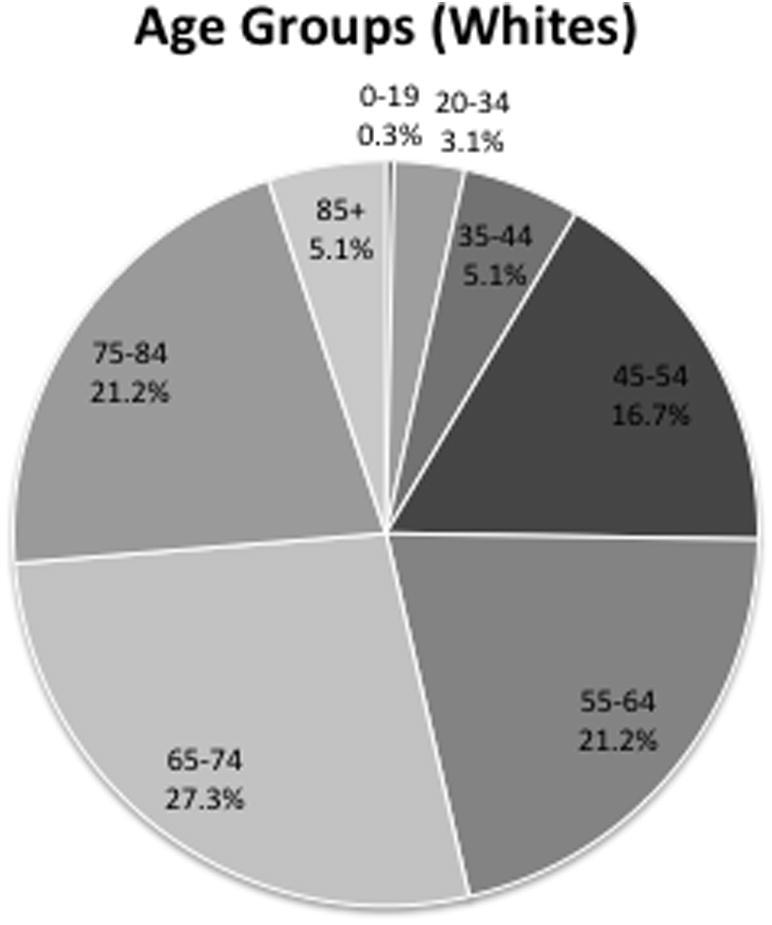
Distribution of white patients according to age.
4. Discussion
Our study is unique as we compared only histologically confirmed meningiomas. The disadvantage of this is the inability to compare these findings with existing epidemiological findings related to meningiomas and draw final conclusions. Most of the existing studies consider meningiomas confirmed by histology and radiological diagnostic methods. However, theoretically, these comparisons should be valid. It has been shown on many occasions that radiological accuracy of meningioma diagnosis is near perfect [13]. Having said that, authors acknowledge that this can be a confounding factor in this study.
The incidence of meningiomas among females is shown to be 2.3 times that of males, nationally. The Central Texas trend that we saw mirrors that of the nation, with over two-thirds (70.4%) of patients being female. Incidence in the United States (U.S.) also increases with age, with a much steeper increase occurring after the age of 65 years. Thus, elderly individuals are more likely to be diagnosed with meningiomas when compared to younger populations, especially at the seventh and eighth decades of life. The Central Texas trend follows that of the national population, with older individuals (⩾45 years) more likely to be diagnosed with meningiomas. Additionally, this study found that Central Texas meningiomas are more likely to be benign, also following the national patterns [2].
Interestingly, current U.S. statistics indicate that the age-adjusted incidence rates of meningiomas are significantly higher among the black population, with an incidence rate ratio (white:black) of 0.8 for nonmalignant meningiomas and 0.7 for malignancies [2]. Our data reveal a stark opposing trend in the Central Texas region, with 83.1% of cases arising in white individuals (excluding Hispanics/Latinos) despite the fact that the demographic stratification of the U.S. and Central Texas (Bastrop, Bell, Burnet, Caldwell, Hays, Travis, and Williamson counties) populations according to the U.S. Census Bureau’s 2013 American Community Survey appears to be similar. Some 31.9% of the Central Texas population is ⩾45 years of age, 79.6% identified themselves as white (race alone or in combination with 1 or more other races, including Hispanic/Latino), 10.5% identified themselves as black/African-American, and 49.9% are female. The overall U.S. population aged ⩾45 years is 40.5%, with 76.2% identifying themselves as white (race alone or in combination with 1 or more other races, including Hispanic/Latino) and 13.8% as black/African American; 50.8% of the U.S. population is female [14]. Based on these data, it seems that differences in demographic distribution between Central Texas and the rest of the nation may not be a contributing factor for the increased meningioma prevalence in the Central Texas white population. However, further investigation and analysis is required to assess if there are indeed any significant differences between Central Texas and the U.S. as a whole with regard to population demographics, in order to provide a more decisive answer.
Discrepancies in healthcare access may provide insight as to the higher incidence of meningiomas in the Central Texas white population. Previous studies have found that African-Americans are more likely to be without insurance and a usual source of healthcare than white individuals [15]. In 2011, the average proportion of black individuals reporting barriers to access of care nationwide was ∼29% compared with ∼23% among white individuals [16]. Thus, it is possible that our findings regarding meningioma prevalence when stratified by race may be confounded due to an increased ability for white individuals to access various forms of healthcare. There is an abundance of data discussing healthcare access disparities among minority groups in the U.S. and further investigation into the possibility of such disparities existing in the Central Texas region are warranted in order to assess its possible contribution as a confounder to the findings of this study.
Differences in occupational exposure may also provide insight into discrepancies of meningioma incidence between racial groups. It is possible that white individuals in Central Texas face increased occupational exposure to meningioma risk factors. Many of these factors are associated with industrial, construction, and materials transport occupations. Nationally, black men are more likely than white men to work in production, transportation, and material moving occupations, and white men are more numerous than black men in the construction industry [17]. Central Texas may yield a different workforce distribution, which may account for the prevalence divergence when compared to national meningioma statistics. Further investigation into the distribution of the Central Texas workforce is needed to ascertain discrepancies in risk factor exposures between white and black laborers.
Studies have reported that >50% of patients with meningioma experience impaired cognition and worse quality of life [3,18]. It has also been found that approximately a third of patients diagnosed with benign meningiomas had stable or worse neurological symptoms following surgery, two-thirds suffered long-term neurological sequelae, and a quarter were chronically disabled [5]. Other studies have shown that preexisting conditions such as hypertension, diabetes mellitus, and epilepsy may contribute to meningioma development and progression [6,18–21]. While the findings of this study revealed that relatively older (⩾45 years) individuals are more likely to be diagnosed with meningiomas, a younger patient demographic was diagnosed with meningiomas based on the cases isolated from the Scott & White Tumor Registry relative to the national statistics cutoff of 65 years. This is cause for concern, since it could indicate that individuals in Central Texas may suffer from the comorbidities and complications associated with meningiomas starting at an earlier age, and thus incurring a prolonged reduction in quality of life, or shortening lifespan altogether. Coupled with the increased prevalence of potential meningioma risk factors in Central Texas that were discussed earlier, it is plausible to postulate that individuals in the region are now at increased susceptibility to meningiomas and associated debilitating morbidities at a younger age than the rest of the nation. This warrants further investigation in greater detail.
In addition to the main shortcoming explained in the first chapter, we acknowledge several other drawbacks in our study design. It is common practice for physicians and patients to pursue aggressive treatment of tumors in younger patients [22,23]. Since only meningioma patients who were subjected to surgery were included in the study, this could explain why we found an increased prevalence of meningiomas in a younger demographic and thus be a confounding factor. Improved diagnostic methods can be a confounding factor at any setting when considering overdiagnosis and unnecessary aggressive treatment. Also, this study does not consider patients who sought other methods of treatment, such as radiotherapy, endovascular procedures, or conservative approaches. Many of the risk factors mentioned related to Central Texas are not well validated, and the sample from which incidence was calculated may not be representative of the region at large, due to being a hospital-based small sample. However, these findings should encourage subsequent larger scale investigations on a more extensive patient pool, in order to achieve a greater sample size. We anticipate these findings will encourage more controlled studies in this demographic area.
5. Conclusions
A relatively younger (<45 years of age) population was diagnosed with meningiomas in Central Texas compared to national statistics. Additionally, non-Hispanic white individuals appear to be at greater risk for developing meningiomas than black individuals, which is in stark contrast to the national trend. However, the mean survival rate between the elderly and younger groups failed to become statistically significant. In order to confirm whether these observations are due to confounding factors in our study design or due to environmental and occupational risk factors, as well as to assess the effects of increased possible meningioma risk factors on the Central Texas population, further detailed investigation is needed.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Conflicts of interest
There are no conflicts of interest.
References
- [1].Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. 2013;13:337. doi: 10.1007/s11910-013-0337-4. [DOI] [PubMed] [Google Scholar]
- [2].Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl. 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, Wumkes M, Waagemans M, Vandertop WP, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80:910–5. doi: 10.1136/jnnp.2007.138925. [DOI] [PubMed] [Google Scholar]
- [4].Longstreth WT, Dennis LK, McGuire VM, Drangsholt MT, Koepsell TD. Epidemiology of intracranial meningioma. Cancer. 1993;72:639–48. doi: 10.1002/1097-0142(19930801)72:3<639::aid-cncr2820720304>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [5].Van Alkemade H, de Leau M, Dieleman EMT, Kardaun JW, van Os R, Vandertop WP, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14:658–66. doi: 10.1093/neuonc/nos013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sughrue ME, Rutkowski MJ, Shangari G, Chang HQ, Parsa AT, Berger MS, et al. Risk factors for the development of serious medical complications after resection of meningiomas: clinical article. J Neurosurg. 2011;114:697–704. doi: 10.3171/2010.6.jns091974. [DOI] [PubMed] [Google Scholar]
- [7].Shao C, Bai LP, Qi ZY, Hui GZ, Wang Z. Overweight, obesity and meningioma risk: a meta-analysis. PloS One. 2014;9:e90167. doi: 10.1371/journal.pone.0090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schuz J. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany) Am J Epidemiol. 2006;163:512–20. doi: 10.1093/aje/kwj068. [DOI] [PubMed] [Google Scholar]
- [9].Landsberger SG, George G. An evaluation of 226Ra and 228Ra in drinking water in several counties in Texas, USA. J Environ Radioact. 2013;125:2–5. doi: 10.1016/j.jenvrad.2013.02.016. [DOI] [PubMed] [Google Scholar]
- [10].Lytle DA, Sorg T, Wang L, Chen A. The accumulation of radioactive contaminants in drinking water distribution systems. Water Res. 2014;50:396–407. doi: 10.1016/j.watres.2013.10.050. [DOI] [PubMed] [Google Scholar]
- [11].Carolina S. Overweight and Obesity. Available at: < http://origin.glb.cdc.gov/obesity/stateprograms/fundedstates/pdf/South-Carolina-State-Profile.pdf>; 2012 [accessed 19 January 2015].
- [12].Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].New PF, Aronow S, Hesselink JR. National Cancer Institute study: evaluation of computed tomography in the diagnosis of intracranial neoplasms. IV. Meningiomas. Radiology. 1980;136:665–75. doi: 10.1148/radiology.136.3.7403545. [DOI] [PubMed] [Google Scholar]
- [14]. American Community Survey. U.S. Census Bureau; 2014.
- [15].Waidmann TA, Rajan S. Race and ethnic disparities in health care access and utilization: an examination of state variation. Med Care Res Rev. 2000;57(4 Suppl.):55–84. doi: 10.1177/1077558700057001s04. [DOI] [PubMed] [Google Scholar]
- [16].National Healthcare Disparities Report USDHHS, Agency for Healthcare Research and Quality. Available at: < www.ahrq.gov/research/findings/nhqrdr/index.html>; 2014 [accessed 22 January 2016].
- [17].Labor Force Characteristics by Race and Ethnicity, 2013. U.S. Bureau of Labor Statistics. Available at: < www.bls.gov/cps/documentation.htm>; 2014. [accessed 22 January 2016].
- [18].Waagemans ML, van Nieuwenhuizen D, Dijkstra M, Wumkes M, Dirven CM, Leenstra S, et al. Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery. 2011;69:72–9. doi: 10.1227/neu.0b013e318212badb. [DOI] [PubMed] [Google Scholar]
- [19].Barnholtz-Sloan JS, Kruchko C. Meningiomas: causes and risk factors. doi: 10.3171/FOC-07/10/E2. Available at: < http://thejns.org/doi/full/10.3171/FOC-07/10/E2>; 2007 [accessed 19 January 2015]. [DOI] [PubMed]
- [20].Kotecha RS, Jacoby P, Cole CH, Gottardo NG. Morbidity in survivors of child and adolescent meningioma: Morbidity in pediatric meningioma. Cancer. 2013;119:4350–7. doi: 10.1002/cncr.28366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schwartzbaum J, Jonsson F, Ahlbom A, Preston-Martin S, Malmer B, Lönn S, et al. Prior hospitalization for epilepsy, diabetes, and stroke and subsequent glioma and meningioma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:643–50. doi: 10.1158/1055-9965.epi-04-0119. [DOI] [PubMed] [Google Scholar]
- [22].Reddy AT. Advances in biology and treatment of childhood brain tumors. Curr Neurol Neurosci Rep. 2001;1:137–43. doi: 10.1007/s11910-001-0009-7. [DOI] [PubMed] [Google Scholar]
- [23].Duffner PK, Horowitz ME, Krischer JP, Burger PC, Cohen ME, Sanford RA, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro Oncol. 1999;1:152. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


