Abstract
This preliminary study evaluated the transport reagent OMNIgene SPUTUM (OMS) in a real-world, resource-limited setting: a zonal hospital and national tuberculosis (TB) reference laboratory, Nepal. The objectives were to: (1) assess the performance of OMS for transporting sputum from peripheral sites without cold chain stabilization; and (2) compare with Nepal’s standard of care (SOC) for Mycobacterium tuberculosis smear and culture diagnostics. Sixty sputa were manually split into a SOC sample (airline-couriered to the laboratory, conventional processing) and an OMS sample (OMS added at collection, no cold chain transport or processing). Smear microscopy and solid culture were performed. Transport was 0–8 days. Forty-one samples (68%) were smear-positive using both methods. Of the OMS cultures, 37 (62%) were positive, 22 (36%) were negative, and one (2%) was contaminated. Corresponding SOC results were 32 (53%), 21 (35%), and seven (12%). OMS “rescued” six (i.e., missed using SOC) compared with one rescue using SOC. Of smear-positives, six SOC samples produced contaminated cultures whereas only one OMS sample was contaminated. OMS reduced culture contamination from 12% to 2%, and improved TB detection by 9%. The results suggest that OMS could perform well as a no cold chain, long-term transport solution for smear and culture testing. The findings provide a basis for larger feasibility studies.
Keywords: Culture contamination, Long-term sputum transport, OMNIgene SPUTUM
1. Introduction
The World Health Organization’s End TB Strategy [1] calls for universal access to drug susceptibility testing and systematic screening of contacts and high-risk groups, and identifies these elements as essential to eliminating Mycobacterium tuberculosis (MTB) infections. To achieve these targets, it is necessary to consider how sample transportation affects patient access to drug-susceptibility tests and how sample quality affects test results. An increasing amount of pressure is being placed on countries to test more samples using an expanding list of techniques; however, constraints differ by setting and minimal attention has been paid to practical solutions that: (1) improve sample quality; and (2) provide a flexible approach that functions seamlessly with established and novel diagnostic tests. Such solutions are critical to enable high-priority, resource-constrained countries to scale their tuberculosis (TB) testing programs.
The National TB Program in Nepal has mounted one of the most successful TB campaigns in Asia, an effort that increased the rate of successful TB treatment outcomes from 45% in 1990 to 90% by 2010 [2]. However, Nepal faces an increasing threat from multidrug-resistant TB, and faces tremendous challenges transporting samples from peripheral hospitals to a centralized testing laboratory [2]. Remote collection sites can be as far as 400 km away from the country’s two TB reference laboratories in the capital city Kathmandu, and Nepal’s mountainous geography can delay sample transport by up to 6 days. Currently, long-term transport and storage of sputum samples typically requires reliable and continuous access to refrigeration to maintain sample integrity at the level required for smear, culture, and molecular TB diagnostics [3]. Constraints on sputum sample transportation increase the costs associated with each patient diagnosis, and inadequate sample preservation during transit can result in multiple diagnostic and therapeutic issues: culture contamination; invalid test results; need for repeated patient sampling (with inherent delays to reaccess patients/collection sites and transport each sample); and, consequently, significant delays in initiating effective treatment. These constraints have led to a challenging transport situation for Nepal. Whereas the country’s standard operating guidelines state that samples should be transported within 3 days, the transport process routinely takes 4 or more days, and cold chain stabilization is not feasible due to high courier costs and the requirement for reference laboratories to return cold boxes to peripheral laboratories. National TB control programs need products that can help effectively scale their testing networks while maintaining established diagnostic algorithms and workflows. OMNIgene SPUTUM (OMS; DNA Genotek, Ottawa, ON, Canada) is a novel sample transport reagent that decontaminates and liquefies sputum, that is compatible with all gold standard TB tests (e.g., smear microscopy, solid and liquid culture, Cepheid GeneXpert, Hain Lifescience line probe assay) and other molecular assays [4], and that does not require cold chain. Versatile, reliable, diagnostically beneficial products that can be easily integrated into laboratory systems can offer a variety of solutions for TB control programs: cost reduction; increased patient access to reliable tests; improved sample quality for testing; and more rapid administration of appropriate therapy leading to better patient outcomes.
According to the World Health Organization guidelines, sputum samples must be refrigerated if they are stored or transported more than 24 h prior to testing [5]; however, it is widely known that many resource-limited countries cannot finance or logistically provide reliable cold chain transport. The aim of this preliminary study was to evaluate the effectiveness of OMS in a real-world setting and to determine the feasibility of conducting additional larger studies. Performance of OMS was compared with that of Nepal’s current standard sputum collection, shipping, and processing protocol with respect to results for smear microscopy and solid MTB culture.
2. Materials and methods
2.1. Sample collection and transport methods
The study was conducted at the GENETUP TB Reference Laboratory in Kathmandu, Nepal in February and March 2015. Sixty raw sputum samples were collected from suspected TB patients at peripheral hospitals. An individual sterile swab stick was used to manually split each sample into two equivalent portions as it was poured from one container to another. Portions were randomly assigned to one treatment method prior to being packaged for transport. As per the standard procedures for sputum collection, shipping, and processing in Nepal, one sample portion [hereafter referred to as the “standard of care (SOC) sample”] was left untreated. The second sample portion (the “OMS sample”) had an equal volume of OMS reagent added to it at the time of collection. All samples were shipped via airline courier and without refrigeration. (Note that cold chain stabilization is not required for OMS samples. Although cold chain transport is the recommended standard for sputum samples collected in Nepal, this was not feasible due to the high cost associated with this transport method.) Transport times varied from 0 days to 8 days depending on the distance from the collection site to the GENETUP laboratory. Temperatures during transport ranged from 4 °C to 24 °C, as recorded in Kathmandu during the study period.
2.2. Sample processing and testing
Upon arrival at the laboratory, each SOC sample was processed using the Nepal standard NaOH/N-acetyl L-cysteine (NALC) method: fresh preparation of a 4% NaOH, 2.9% trisodium citrate, 0.5 g NALC solution, addition of an equal volume of solution to the sample, and 15 min of incubation at room temperature, followed by neutralization using sterile phosphate buffer and centrifugation to produce a sediment. The OMS sample required no further processing and was directly centrifuged to produce a sediment.
Sediments were resuspended in sterile phosphate buffer and were assessed by smear microscopy and cultured on Lowenstein–Jensen slants in duplicate. Cultures were incubated at 37 °C for up to 56 days. Smears were categorized as negative or as one of four levels of acid-fast bacilli detection: scanty, 1+, 2+, or 3+.
2.3. Data collected and analysis
Transport times from collection site to laboratory were recorded. For each OMS sample and SOC sample, smear results were reported as negative or positive (defined as scanty, 1+, 2+ or 3+). Culture results were reported as negative, positive (i.e., growth), or contaminated. For positive cultures, the interval from date of inoculation to date of observable growth (i.e., time to culture-positive status) was recorded in days. When the duplicate culture slants from a sample yielded discrepant results, a single outcome was reported as follows: samples that yielded one contaminated and one negative culture were counted as negative; samples that yielded one contaminated and one positive culture were counted as positive; and samples that yielded two contaminated cultures were counted as contaminated.
The OMS and SOC methods were compared with respect to proportions of TB cases detected by smear and by culture, respectively, and with respect to proportions of contaminated cultures. In addition, average time to culture-positive status (in days) was compared for the two methods. Findings were compared relative to transport time, as appropriate.
3. Results
Table 1 summarizes transport times and key diagnostic information and results of each of the 60 respective pairs of OMS and SOC samples. Transport times ranged from 0 days to 8 days. Sputum volumes ranged from 0.5 mL (n = 2) to 4.5 mL, and the majority (n = 50) were ⩾2 mL. Of the 60 sputum samples collected, 41 (68% of total) were positive by smear microscopy with OMS and SOC, respectively. There were two discrepancies between the methods: Sample 1945 for which only the OMS portion was positive, and Sample 2287 for which only the SOC portion was positive. Both samples were graded as scanty by smear microscopy.
Table 1.
Summary of transport time and diagnostic results for the OMNIgene SPUTUM and standard of care methods.
| Sample ID | Days in transport | Smear | LJ culture: time to positive (d) | OMS impact on TTP (d) | Avg TTP SOC | Avg TTP OMS | Δ TTP for OMS | ||
|---|---|---|---|---|---|---|---|---|---|
| SOC | OMS | SOC | OMS | ||||||
| 4600 | 0 | 1+ | 1+ | 21 | 24 | 3 | 21 | 24 | 3 |
| 4796 | 0 | Neg | Neg | NG | NG | NG | |||
| 4820 | 0 | Neg | Neg | NG | NG | NG | |||
| 4547 | 0 | Neg | Neg | NG | NG | NG | |||
| 4582 | 0 | Neg | Neg | NG | NG | NG | |||
| 4649 | 0 | Neg | Neg | NG | NG | NG | |||
| 4671 | 0 | Neg | Neg | NG | NG | NG | |||
| 4661 | 0 | Neg | Neg | NG | NG | NG | |||
| 4817 | 0 | Neg | Neg | NG | NG | NG | |||
| 2081 | 2 | 2+ | 1+ | 17 | 23 | 6 | 17 | 21 | 4 |
| 2071 | 2 | 2+ | 1+ | 17 | 23 | 6 | |||
| 2336 | 2 | 3+ | 3+ | 16 | 16 | 0 | |||
| 2366 | 2 | 2+ | 2+ | 18 | 23 | 5 | |||
| 2374 | 2 | 1+ | Scanty | NG | NG | NG | |||
| 1966 | 3 | 2+ | 1+ | Contaminated | 28 | Rescued | 22 | 23 | 1 |
| 2064 | 3 | 1+ | Scanty | 25 | 25 | 0 | |||
| 2067 | 3 | 1+ | 3+ | 36 | 29 | −7 | |||
| 2068 | 3 | 1+ | 1+ | Contaminated/29 | 25 | −4 | |||
| 2093 | 3 | 1+ | 1+ | 6 | 19 | 13 | |||
| 2099 | 3 | 3+ | 2+ | 25 | 19 | −6 | |||
| 2114a | 3 | 2+ | 1+ | Contaminated | 23 | Rescued | |||
| 2186 | 3 | Neg | Neg | NG | NG | NG | |||
| 2200 | 3 | 3+ | 3+ | 12 | 18 | 6 | |||
| 2355 | 3 | Neg | Neg | Contaminated | NG | NG/Rescued | |||
| 2356 | 3 | Neg | Neg | NG | NG | NG | |||
| 2357 | 3 | Neg | Neg | NG | NG | NG | |||
| 2360 | 3 | Neg | Neg | NG | NG | NG | |||
| 2370 | 3 | Neg | Neg | NG | NG | NG | |||
| 2371a | 3 | Neg | Neg | NG | NG | NG | |||
| 2372 | 3 | 3+ | 3+ | 18 | 18 | 0 | |||
| 1872 | 4 | 2+ | 3+ | 16 | 23 | 7 | 22 | 22 | 0 |
| 1879 | 4 | 1+ | 1+ | Contaminated/NG | NG | NG | |||
| 1880 | 4 | 3+ | 3+ | 16 | 25 | 9 | |||
| 2054 | 4 | 3+ | 2+ | Contaminated | 34 | Rescued | |||
| 2060 | 4 | 3+ | 2+ | 18 | 18 | 0 | |||
| 2062 | 4 | 2+ | Scanty | 29 | 25 | −4 | |||
| 2075 | 4 | 2+ | 1+ | 29 | 18 | −11 | |||
| 2105 | 4 | 2+ | 2+ | Contaminated/23 | 17 | −6 | |||
| 2117 | 4 | 3+ | 1+ | Contaminated | 14 | Rescued | |||
| 2183 | 4 | Neg | Neg | NG | NG | NG | |||
| 1911 | 5 | 1+ | 1+ | 25 | 25 | 0 | 28 | 28 | 0 |
| 1929 | 5 | Scanty | Scanty | NG | NG | NG | |||
| 1941 | 5 | Neg | Neg | NG | NG | NG | |||
| 1945 | 5 | Neg | Scanty | 33 | 42 | 9 | |||
| 1946 | 5 | 1+ | 2+ | 41 | 41 | 0 | |||
| 2007 | 5 | 1+ | Scanty | 28 | 37 | 9 | |||
| 2014 | 5 | 3+ | 3+ | 25 | 21 | −4 | |||
| 2027 | 5 | 1+ | 1+ | 37 | 28 | −9 | |||
| 2034 | 5 | 1+ | Scanty | 21 | 25 | 4 | |||
| 2167 | 5 | 3+ | 3+ | 19 | 19 | 0 | |||
| 2165 | 5 | 3+ | 3+ | Contaminated | 19 | Rescued | |||
| 2326 | 5 | 1+ | 2+ | 25 | 20 | −5 | |||
| 1932 | 6 | Neg | Neg | NG | NG | NG | 19 | 18 | −1 |
| 2038 | 6 | 3+ | 3+ | 18 | 22 | 4 | |||
| 2287 | 6 | Scanty | Neg | NG | NG | NG | |||
| 2289 | 6 | 1+ | 2+ | 26 | Contaminated | Missed | |||
| 2292 | 6 | 3+ | 3+ | 14 | 17 | 3 | |||
| 2307 | 6 | 2+ | 1+ | 16 | 16 | 0 | |||
| 2181 | 7 | 1+ | 1+ | 21 | 21 | 0 | 21 | 21 | 0 |
| 2244 | 8 | 3+ | 3+ | Contaminated | 21 | Rescued | n/a | 21 | n/a |
Avg = average; d = days; LJ = Lowenstein–Jensen; Neg = negative; NG = no growth after 56 days; OMS = OMNIgene SPUTUM; SOC = standard of care; TTP = time to culture-positive; Δ TTP = difference in TTP between the methods.
Collected sample volume was 0.5 mL.
The impact of OMS on smear microscopy was negligible, as smear categorization was similar for the two methods. Note that low-positive sputum samples (i.e., those categorized as scanty or 1+) were not negatively affected by transport in OMS, even after 7 days in transit (Table 1).
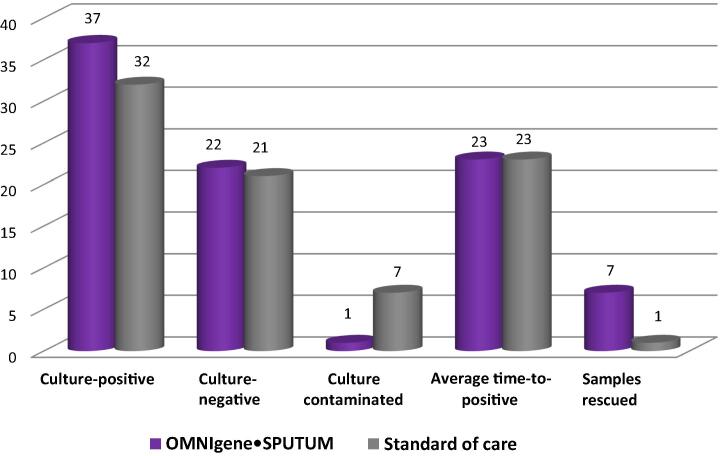
Regarding culture results, of the 60 OMS samples, 37 (62%) were culture-positive, 22 (36%) were culture-negative, and one culture (2% of total) was contaminated (Fig. 1). In contrast, 32 (53%) of the 60 SOC samples were culture-positive, 21 (35%) were culture-negative, and seven (12%) of the SOC cultures were contaminated (Fig. 1).
Fig. 1.
Comparison of OMNIgene SPUTUM and standard of care: solid culture results overall.
Overall average time to culture-positive was not significantly affected by treatment method (23 days for both treatment methods; Table 1, Fig. 1). The largest variation in time to culture-positive was observed in samples that were 2 days in transport. In this group, the OMS-treated samples took an average of 4 days longer to become culture-positive (range, 16–23 days) compared with the SOC-treated samples (range, 16–18 days; Table 1). However, only five samples were transported for 2 days and each treatment method had 80% detection. When numbers of culture positives per group were compared relative to transport time, the OMS group had two more positives at 3 days and 4 days of transport, and one more positive at 5 days of transport; the other transport category comparisons were identical.
For analysis, “rescued” was used to indicate instances where one portion of a sample (i.e., OMS or SOC) was identified MTB-positive or MTB-negative by culture, whereas the corresponding portion yielded no usable diagnostic results (i.e., a contaminated culture, which provides neither a negative nor a positive result). Use of the OMS method resulted in seven samples being rescued (i.e., seven additional actionable results that would have been missed using SOC alone) and one sample being “missed” (i.e., for 1 of the 60 total samples, the culture for the SOC portion was positive whereas the culture for the OMS portion was contaminated). In contrast, the SOC method missed seven positives and rescued one sample (Table 1, Fig. 1).
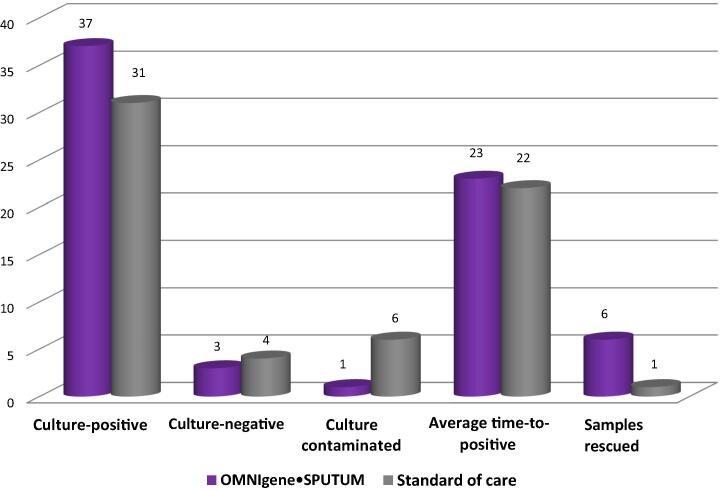
Of the smear-positive samples (i.e., 41 total for each method), the proportions identified as culture-positive for MTB were 90% (n = 37) for the OMS method and 76% (n = 31) for the SOC method. Within the smear-positive subgroup, the numbers of culture-negative results with the two methods were comparable; however, there were more contaminated cultures with the SOC method (i.e., 6 for SOC vs. 1 for OMS; Fig. 2).
Fig. 2.
Comparison of OMNIgene SPUTUM and standard of care: solid culture results for smear-positive samples.
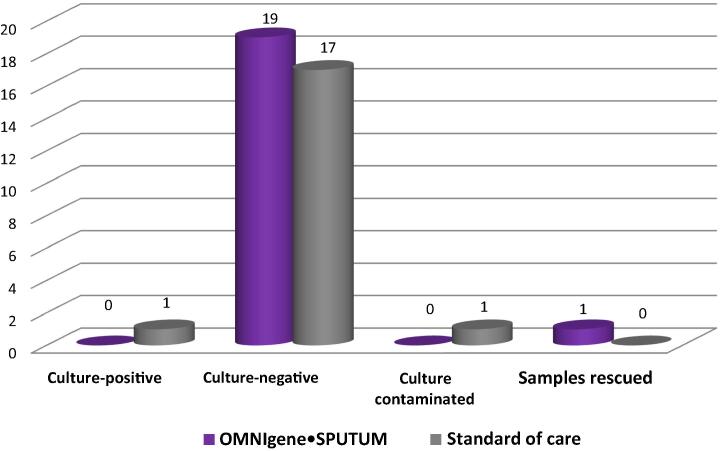
Within the smear-negative subgroup, the OMS treatment method identified 19 samples as culture-negative, while the SOC treatment method identified 17 culture-negative, one culture-positive, and one culture-contaminated. Two discrepant smear microscopy results were identified: Sample 1945 was smear-positive for OMS only and Sample 2287 was smear-positive for SOC only. Sample 1945 was culture-positive for both methods (average times to culture-positive: 33 days and 42 days for SOC and OMS, respectively), while Sample 2287 was culture-negative for both methods. One smear-negative sample was rescued by OMS treatment. Sample 2355 was culture-negative (i.e., an actionable diagnostic result) following OMS treatment, but was culture-contaminated (i.e., an unusable diagnostic result) after SOC treatment (Table 1 and Fig. 3).
Fig. 3.
Comparison of OMNIgene SPUTUM and standard of care: solid culture results for smear-negative samples.
4. Discussion
This evaluation showed that using the OMS reagent at point of collection might improve diagnostic results and reduce the complexity associated with SOC methods. The study demonstrated positive impacts on several key endpoints: (1) costs, OMS decreased culture contamination rates, thus potentially reducing the expense of repeat testing; (2) improved workflow, OMS ensured the highest quality sputum samples even after 8 days of transport at ambient temperature, eliminated the need for daily preparation of NaOH/NALC, and simplified laboratory processing procedures; and (3) improved TB case detection, OMS yielded a greater proportion of MTB-positive test results. Most importantly, in seven cases, the samples treated with the OMS reagent yielded usable diagnostic results, whereas the corresponding samples treated with the SOC method resulted in the need for a second sputum collection to enable repeat testing by culture. This difference has significant implications for patient care, as patients may be lost during follow up, and repeat collection and retesting will delay the initiation of appropriate antibiotic therapy [6].
OMS offers several key advantages over the sputum collection and processing method currently used in Nepal. The OMS reagent is a highly stable product (1 year shelf life) that requires no additional preparation in the laboratory. This means that capturing efficiencies through task shifting of technician time would be easily achievable. Further, the ability to add the reagent at the point of collection helps ensure that the highest quality sample is received by the laboratory, because the product reduces putrefaction and downstream culture contamination rates. Maintaining sample integrity facilitates accurate and timely TB diagnosis, which is critical for countries that are implementing large-scale testing networks.
This study identified another advantage of the OMS reagent; it allows samples to be transported for extended periods of time without the need for cold chain. This could significantly reduce costs associated with sample transport [7] and could simplify TB testing algorithms for countries with remote populations that, prior to this advancement, have been difficult to access for testing due to sample transportation challenges [8]. In addition to markedly facilitating transport and maintaining sample integrity, samples prepared using OMS are easily integrated into existing diagnostic workflows without the need for costly infrastructure investment or retooling of established laboratory methods. As the present study indicates, sediments from OMS-treated sputa are amenable to smear microscopy and solid culture methods. Other sputum decontamination solutions have been evaluated as either laboratory-added reagents [9] or transport alternatives [10], but these products have limitations related to shelf-life stability or compatibility with liquid culture systems. We have previously demonstrated that OMS is stable for 1 year prior to use and is compatible with a broad range of additional diagnostic methods, including liquid culture (Mycobacteria Growth Indicator Tube, Becton Dickinson and Company, Franklin Lakes, NJ, USA) [4], Cepheid GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) [11], Hain Lifescience line probe assays (Hain Lifescience, Nehren, Germany) [12], and other molecular applications [11]. Further, the versatility and fundamental sample preservation features of the OMS method lend it not only to the latest current diagnostic advancements for TB, but also to test platforms that will be developed and used in the foreseeable future.
Minor differences were observed between the OMS and SOC methods with respect to smear categorization and time to culture-positive, and these likely reflect the imprecision of manually splitting a complex biological sample in half prior to liquefaction. The study had limitations: (1) manually splitting sputum samples can lead to uneven distribution of bacilli, which is of particular importance with low-positive samples; (2) a considerable number of samples that were identified as MTB-negative (by smear and culture) had required no transport prior to testing (i.e., 9/10 samples with 0 days in transport were negative on both diagnostic tests; Table 1); and (3) most samples underwent 3–5 days of transport, whereas only a small number of samples underwent prolonged transit (7 days or 8 days). The higher-than-expected proportion of culture-negative results for smear-negative samples was most likely an artifact of sample splitting, which would have the greatest impact on samples with negative or scanty smear grades. Additional studies will be required to further evaluate this reagent in different regional settings; however, extended transport in OMS (i.e., longer than 4 days) demonstrated that this reagent performed better than Nepal’s SOC method in ensuring the integrity of sputum samples for culture, as two of the six OMS-rescued samples were in this transport-time category.
5. Conclusion
Challenges with long-term transport of sputum samples from peripheral sites to a centralized laboratory include high cost and logistics of providing cold chain stabilization, loss of samples through putrefaction, reduced case detection due to loss of viable MTB, and high rates of culture contamination. These issues exacerbate delays in reporting clinically relevant results to the clinician, and they can impact a patient’s health when repeat testing is required prior to initiating antibiotic therapy.
Our preliminary findings from this in-country study suggest that OMS could negate or substantially mitigate key challenges associated with traditional sputum sample transport. Compared with the SOC method in Nepal, transporting samples in OMS reduced culture contamination rates from 12% to 2%, and improved detection of MTB-positive patients by 9%. The results suggest that OMS performs well at maintaining sample integrity for smear and solid culture, and has potential as an easy-to-implement solution that could reduce costs of testing (at the laboratory and national program levels) and improve patient access to timely results and clinical decision-making. Future investigations with larger sample sizes will be valuable, and will ideally include testing via liquid culture, testing smear-negative sputa with extended transport, and analysis of cost savings.
Acknowledgments
The authors wish to thank GENETUP staff members Sajana Tandukar, Meera Shrestha, Sujt Maharjan, and Chandish Shrestha, who carried out all the work for this study. We also wish to thank Rajendra Dhami (Seti Zonal Hospital, Dhangadhi, Nepal), who collected sputum samples. BM and BS participated in the study design, acquisition of data, data analysis and manuscript review. AW, AS and CKC participated in the study design, data analysis and interpretation, and manuscript preparation. All authors have reviewed and approved the final manuscript prior to submission.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Conflicts of interest
BM and BS have no competing interest to declare. AW, AS and CKC are employees of DNA Genotek, Inc. DNA Genotek provided financial support to GENETUP in the form of reimbursement for reagents and consumables used during the study.
References
- [1].World Health Organization Post-2015 Global TB Strategy Global strategy and targets for tuberculosis prevention, care and control after 2015. Available at: http://www.who.int/tb/post2015_strategy/en/. [Accessed 22 Mar 2016].
- [2].The Guardian Nepal has lessons to teach on TB. Available at: < http://www.theguardian.com/global-development/2010/sep/27/who-fighting-tuberculosis-medical-nepal-health>. [Accessed 22 Mar 2016]; 2010.
- [3].Pang Y, Du J, Zhang ZY, Ou XC, Li Q, Xia H, et al. The feasibility of sputum transportation system in China: effect of sputum storage on the mycobacterial detection. Biomed Environ Sci. 2014;27:982–6. doi: 10.3967/bes2014.137. [DOI] [PubMed] [Google Scholar]
- [4]. DNA Genotek Product Data Sheet PD-BR-00195. Available at: www.dnagenotek.com. [Accessed 22 Mar 2016].
- [5].World Health Organization . Mycobacteriology Laboratory Manual. 1st ed. Global Laboratory Initiative; 2014. Available at: www.who.int/tb/laboratory/mycobacteriology-laboratory-manual.pdf. [Accessed 22 Mar 2016]. [Google Scholar]
- [6].Parsons LM, Somoskovi A, Gutierrez C, Lee E, Parmasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–50. doi: 10.1128/cmr.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dowdy DW, Lourenco MC, Cavalcante SC, Saraceni V, King B, Golub JE, et al. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLoS ONE. 2008;3:e4057. doi: 10.1371/journal.pone.0004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paramasivan CN, Narayana A, Prabhakar R, Rajagopal MS, Somasundaram PR, Tripathy SP. Effect of storage of sputum specimens at room temperature on smear and culture results. Tuber. 1983;64:119–24. doi: 10.1016/0041-3879(83)90036-3. [DOI] [PubMed] [Google Scholar]
- [9].Asmar S, Drancourt M. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol. 2015;15:6. doi: 10.1186/s12866-015-0479-4. pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bobadilla-del-Valle M, Ponce-de-Leon A, Kato-Maeda M, Hernandez-Cruz A, Calva-Mercado JJ, Chavez-Mazari BA, et al. Comparison of sodium carbonate, cetyl-pyridinium chloride and sodium borate for preservation of sputa for culture of Mycobacterium tuberculosis. J Clin Microbiol. 2003;41:4487–8. doi: 10.1128/jcm.41.9.4487-4488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kelly-Cirino C, Niles J, Ray B, Stewart A. Maximizing centralized testing models and GeneXpert hubs with OMNIgene SPUTUM. DNA Genotek Whitepaper PD-WP-00044. Available at: www.dnagenotek.com. [Accessed 22 Mar 2016].
- [12]. DNA Genotek Product Data Sheet PD-BR-00196. Available at: www.dnagenotek.com. [Accessed 22 Mar 2016].