Abstract
Uninvestigated dyspepsia (UD), irritable bowel syndrome (IBS), and gastroesophageal reflux disease (GERD) are common disorders universally. Many studies have assessed their epidemiological characteristics around the world. However, such information is not known for Syria. We aim to estimate the epidemiologic characteristics and possible risk factors for UD, IBS, and GERD among students at Damascus University, Damascus, Syria. A cross-sectional study was conducted in July–September 2015 at a campus of Damascus University. A total of 320 students were randomly asked to complete the survey. We used ROME III criteria to define UD and IBS, and Montreal definition for GERD. In total, 302 valid participants were included in the analysis. Prevalence for UD, IBS, and GERD was 25%, 17%, and 16%, respectively. Symptom overlap was present in 46 students (15%), with UD + IBS in 28 (9.3%), UD + GERD in 26 (8.6%), and IBS + GERD in 14 (4.6%) students. Eleven (3.6%) students had symptoms of UD + IBS + GERD. Each of these overlaps occurred more frequently than expected by chance. Significant risk factors included cigarettes smoking, waterpipe consumption, and body mass index <18.5 kg/m2 for UD; female gender and three cups of coffee/d for IBS; and two cups of tea and one to five cigarettes/d for GERD. Risk factors for these disorders remain poorly characterized and need further investigations.
Keywords: Epidemiology, Gastroesophageal reflux disease, Irritable bowel syndrome, Risk factors, Syria, Uninvestigated dyspepsia
1. Introduction
Gastrointestinal (GI) diseases are very frequent in the daily practice of medicine [1] and have a significant impact on patients’ quality of life [2]. Several studies have estimated the prevalence of those diseases, described their signs and symptoms, and studied the factors associated with them.
Dyspepsia refers to a group of GI symptoms that includes epigastric pain, fullness, and nausea. Uninvestigated dyspepsia (UD) generally refers to the dyspepsia which is not investigated by any medical test (such as upper GI endoscopy) and is not determined to be functional or organic in etiology. The global prevalence of UD varies between 7% and 45% in epidemiological studies [3]. In Asia, however, the prevalence of UD varies between 8% and 30% [4]. This variation in the prevalence estimates might be due to differences in the definition used in recognizing the disorder and differences in geographical locations [3,4].
Irritable bowel syndrome (IBS) is a common functional GI disorder. It is characterized by abdominal pain or discomfort with altered bowel habits, but without any organic damages such as tumor or inflammation [5]. The prevalence of IBS is estimated to be between 10% and 15% in Western countries [6] and between 5% and 10% in Asia [7].
The Montreal consensus defines gastroesophageal reflux disease (GERD) as a condition that develops when the reflux of gastric contents causes troublesome symptoms and/or complications [8]. Epidemiological studies classified GERD as the most common GI diagnosis in the outpatient setting [9].
Prevalence and epidemiological characteristics of UD, IBS, and GERD are unknown in Syria. As part of increasing physicians’ ability of symptoms-based approach to such diseases, we have taken the opportunity to study the prevalence and risk factors of those common GI diseases among students of Damascus University, Damascus, Syria.
2. Materials and methods
2.1. Study design
A cross-sectional study regarding the prevalence and risk factors of three GI disorders: UD, IBS, and GERD, was held between July 2015 and September 2015 at the campus of Damascus University.
2.2. Participants
Participants of the study were students, both undergraduates and graduates, at Damascus University, ranging in age from 18 years to 33 years. A total of 320 students were randomly recruited for the study. Unreturned or incomplete papers were excluded, and only 302 participants were included in the analysis.
2.3. Data collection
Students were asked to voluntarily complete a predesigned questionnaire that was structured by the researchers; trained staff answered participants’ questions on the spot. The objectives of the study were explained to the participants, and they were informed that their participation was voluntary and anonymous. All included participants gave an informed consent prior to data collection. Ethical approval of this study was obtained from the Ethics Committee of the Faculty of Medicine, Damascus University.
2.4. The questionnaire
The questionnaire was designed to collect the following data: demographics (age and gender), general health [weight and height, to calculate body mass index (BMI)], lifestyle-related factors (consumption of tea, coffee, fatty food, alcohol, cigarettes, waterpipe, and NSAIDs), and physical symptoms related to GI disorders (such as epigastric pain, burning sensation, diarrhea, etc.).
Dyspepsia and IBS were assessed according to the ROME III criteria [5], while GERD was evaluated according to the Montreal definition [8].
UD was not directly addressed by the Rome III criteria. Rather, Rome III defines criteria for functional dyspepsia (FD), which necessitates performing upper GI endoscopy to rule out structural disease. We depended on the symptoms that were defined for FD by the Rome III criteria in order to recognize those with dyspepsia, and since no upper GI endoscopy was performed, patients who fulfill the symptomatic criteria will be diagnosed with UD. Consequently, individuals were classified as having dyspepsia if they reported experiencing at least one of the following symptoms: (1) bothersome postprandial fullness; (2) early satiation; (3) epigastric pain; and (4) epigastric burning. All the above criteria should be fulfilled for the last 3 months with symptoms onset at least 6 months prior to diagnosis.
Classification of IBS was made according to report of abdominal pain or discomfort persisting for at least 3 days, at least once a month during the previous 3 months, and accompanied by at least two of the following symptoms: (1) relief of pain or discomfort attained after defecation; (2) onset of pain or discomfort associated with a change in stool frequency; or (3) onset of pain or discomfort associated with a change in stool appearance.
Individuals were classified as having GERD if they reported experiencing frequent episodes of heartburn or acid regurgitation occurring at least twice a week for mild symptoms or once a week for moderate to severe symptoms.
For each of the GI disorders, the survey questions were translated from the original source into the Arabic language by the authors. Any individual classified with two or more of the above disorders was categorized as “GI symptom complex overlap”.
2.5. Statistical analysis
All eligible questionnaires were coded. Data were expressed as frequencies or means with standard deviations (SD). The χ2 test was used to investigate the association between possible risk factors and each of the investigated disorders. Possible risk factors were assessed again using binary logistic regression analysis, and the results were expressed as p values, along with the corresponding odds ratios (OR) and 95% confidence intervals (CI) in cases of significant associations. Exact binomial test for proportions was used to compare the observed prevalence to the calculated prevalence. Calculated p values for χ2 and binary logistic regression test were two-tailed, while calculated p values for the exact binomial test were single-tailed. A p value <0.05 was considered statistically significant. All statistical analyses were carried out with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Of the 320 students recruited in the study, 18 students refused to participate or did not complete the survey. A total of 302 valid participants were included in the analysis. Demographic data for each group of participants (normal healthy participants, those who have UD, those who have IBS, and those who have GERD) are summarized in Table 1. Overall, the study included 196 females (64.9%) and 106 males (35.1%). Participants had a mean (±SD) age of 21.6 ± 1.9 years. BMI was investigated for all participants as a possible risk factor for the disorders being investigated. The overall mean (±SD) BMI for the participants was 22.6 ± 3.9 kg/m2.
Table 1.
Characteristics of students with the investigated gastrointestinal disorders.
| Total (n = 302) | Normal (n = 186) | UD (n = 75) | IBS (n = 50) | GERD (n = 48) | ||
|---|---|---|---|---|---|---|
| Gender | Female | 196 (64.9) | 123 (66.1) | 49 (65.3) | 39 (78.0) | 27 (56.3) |
| Male | 106 (35.1) | 63 (33.9) | 26 (34.7) | 11 (22.0) | 21 (43.8) | |
| Age (y) | 21.6 ± 1.9 | 21.7 ± 1.9 | 21.4 ± 1.5 | 21.7 ± 2.2 | 21.6 ± 1.6 | |
| BMI (kg/m2) | 22.6 ± 3.9 | 22.7 ± 4.0 | 22.0 ± 3.7 | 21.8 ± 3.2 | 23.2 ± 4.0 |
Data are presented as n (%) or mean ± standard deviation.
BMI = body mass index; GERD = gastroesophageal reflux disease; IBS = irritable bowel syndrome; UD = uninvestigated dyspepsia.
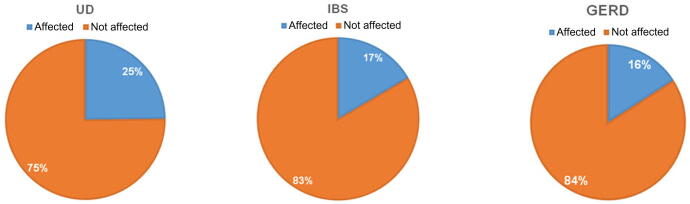
There were 186 participants (61%) who did not have any of the three investigated GI disorders; they were classified as normal participants. UD affected 75 participants, while IBS affected 50 participants, and GERD affected 48 participants. The prevalences for those three disorders in the study sample were 25%, 17%, and 16%, respectively (Fig. 1). There were no clinically significant differences in the mean age or mean BMI between the participants classified in each disorder group and those who are classified in the normal group (Table 1).
Fig. 1.
Prevalences of the investigated gastrointestinal disorders in the study sample. GERD = gastroesophageal reflux disease; IBS = irritable bowel syndrome; UD = uninvestigated dyspepsia.
GI symptom complex overlap was present in 46 students (15%), with UD + IBS in 28 students (9.3%), UD + GERD in 26 students (8.6%), and IBS + GERD in 14 students (4.6%). Eleven students (3.6%) had symptoms of all three investigated disorders (UD + IBS + GERD; Fig. 2). The expected prevalence of the overlap between each group of disorders was calculated by multiplying the prevalences of the overlap components, assuming those components were independent diseases. The true observed prevalence was compared to the calculated prevalence using the exact binomial test for proportions. All comparisons yielded statistically significant p values, indicating that each overlap had occurred more frequently than expected by chance (Table 2).
Fig. 2.
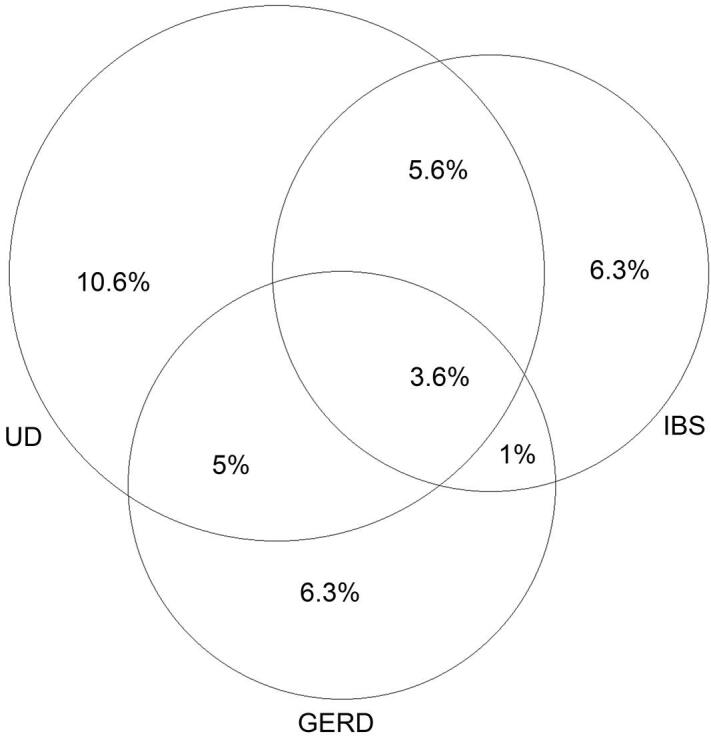
Overlaps in prevalence of the investigated gastrointestinal disorders in the study sample. GERD = gastroesophageal reflux disease; IBS = irritable bowel syndrome; UD = uninvestigated dyspepsia.
Table 2.
Relationship between the investigated gastrointestinal disorders.
| Observed prevalence | Expected prevalencea | p | |
|---|---|---|---|
| UD and IBS | 28 (9.3) | 13 (4.3) | <0.001* |
| UD and GERD | 26 (8.6) | 12 (4) | <0.001* |
| IBS and GERD | 14 (4.6) | 8 (2.7) | 0.037* |
| UD and IBS and GERD | 11 (3.6) | 2 (0.68) | <0.001* |
Data are presented as n (%).
p value is for the exact binomial test for proportions that compared the observed vs. the expected prevalence of each corresponding pair of subgroups.
GERD = gastroesophageal reflux disease; IBS = irritable bowel syndrome; UD = uninvestigated dyspepsia.
Assuming the corresponding pair of subgroups was independent.
p < 0.05.
Tables 3–5 summarize demographic and lifestyle characteristics of the participants and their statistical relationship with UD, IBS, and GERD, respectively.
Table 3.
Association of demographic- and lifestyle-related factors with uninvestigated dyspepsia.
| UD | χ2, df, p | Binary logistic regression (p) | |||
|---|---|---|---|---|---|
| No. | % | ||||
| Gender | Male | 26 | 34.7 | 0.008, 1, 0.928 | — |
| Female | 49 | 65.3 | 0.931 | ||
| Age (y) | 18–21 | 41 | 54.7 | 2.729, 2, 0.255 | — |
| 22–25 | 34 | 45.3 | 0.717 | ||
| >25 | 0 | 0.0 | 0.999 | ||
| BMI (kg/m2) | 18.5–24.9 (normal) | 43 | 57.3 | 5.620, 3, 0.132 | — |
| <18.5 (underweight) | 14 | 18.7 | 0.038* | ||
| 25–30 (overweight) | 16 | 21.3 | 0.456 | ||
| >30 (obese) | 2 | 2.7 | 0.224 | ||
| Tea intake (cups/d) | 0 | 10 | 13.3 | 3.339, 4, 0.503 | — |
| 1 | 26 | 34.7 | 0.699 | ||
| 2 | 24 | 32.0 | 0.448 | ||
| 3 | 8 | 10.7 | 0.827 | ||
| >3 | 7 | 9.3 | 0.970 | ||
| Coffee intake (cups/d) | 0 | 19 | 25.3 | 2.147, 4, 0.709 | — |
| 1 | 35 | 46.7 | 0.404 | ||
| 2 | 11 | 14.7 | 0.829 | ||
| 3 | 6 | 8.0 | 0.165 | ||
| >3 | 4 | 5.3 | 0.637 | ||
| Fatty food consumption (average frequency/wk) | 0 | 0 | 0.0 | 7.312, 4, 0.120 | — |
| 1 | 12 | 16.0 | 0.999 | ||
| 2 | 28 | 37.3 | 0.999 | ||
| 3 | 20 | 26.7 | 0.999 | ||
| >3 | 15 | 20.0 | 0.999 | ||
| Alcohol consumption (cups/wk) | 0 | 72 | 96.0 | 2.291, 3, 0.514 | — |
| 1 | 2 | 2.7 | 0.919 | ||
| 2 | 0 | 0.0 | 0.999 | ||
| 3 | 0 | 0.0 | 0.999 | ||
| >3 | 1 | 1.3 | 0.999 | ||
| Cigarettes consumption (No. of cigarettes/d) | 0 | 67 | 89.3 | 13.560, 5, 0.019* | — |
| 1–5 | 3 | 4.0 | 0.334 | ||
| 6–10 | 1 | 1.3 | 0.999 | ||
| 11–15 | 0 | 0.0 | 0.999 | ||
| 16–20 | 1 | 1.3 | 0.995 | ||
| >20 | 3 | 4.0 | 0.999 | ||
| Waterpipe consumption (average frequency/wk) | 0 | 60 | 80.0 | 10.400, 4, 0.034* | — |
| 1 | 8 | 10.7 | 0.526 | ||
| 2 | 0 | 0.0 | 0.998 | ||
| 3 | 3 | 4.0 | 0.062 | ||
| >3 | 4 | 5.3 | 0.401 | ||
| Regular NSAIDs intake (pills/mo) | 0–5 | 63 | 84.0 | 3.290, 4, 0.510 | — |
| 6–10 | 9 | 12.0 | 0.889 | ||
| 11–15 | 1 | 1.3 | 0.874 | ||
| 16–20 | 2 | 2.7 | 0.196 | ||
| >20 | 0 | 0.0 | 1.000 | ||
BMI = body mass index; df = degree of freedom; NSAIDs = nonsteroidal antiinflammatory drugs; UD = uninvestigated dyspepsia.
p < 0.05; for the χ2 or binary logistic regression test investigating the association between each risk factor and uninvestigated dyspepsia.
Table 5.
Association of demographic- and lifestyle-related factors with gastroesophageal reflux disease.
| GERD | χ2, df, p | Binary logistic regression (p) | |||
|---|---|---|---|---|---|
| Number | % | ||||
| Gender | Male | 21 | 43.8 | 1.875, 1, 0.171 | — |
| Female | 27 | 56.3 | 0.259 | ||
| Age (y) | 18–21 | 25 | 52.1 | 0.089, 2, 0.957 | — |
| 22–25 | 22 | 45.8 | 0.752 | ||
| >25 | 1 | 2.1 | 0.616 | ||
| BMI (kg/m2) | 18.5–24.9 (normal) | 33 | 68.8 | 2.047, 3, 0.563 | — |
| <18.5 (underweight) | 3 | 6.3 | 0.230 | ||
| 25–30 (overweight) | 9 | 18.8 | 0.428 | ||
| >30 (obese) | 3 | 6.3 | 0.903 | ||
| Tea intake (cups/d) | 0 | 3 | 6.3 | 5.768, 4, 0.217 | — |
| 1 | 17 | 35.4 | 0.055 | ||
| 2 | 17 | 35.4 | 0.010* | ||
| 3 | 6 | 12.5 | 0.253 | ||
| >3 | 5 | 10.4 | 0.154 | ||
| Coffee intake (cups/d) | 0 | 17 | 35.4 | 2.958, 4, 0.565 | — |
| 1 | 18 | 37.5 | 0.224 | ||
| 2 | 5 | 10.4 | 0.087 | ||
| 3 | 4 | 8.3 | 0.760 | ||
| >3 | 4 | 8.3 | 0.865 | ||
| Fatty food consumption (average frequency/wk) | 0 | 1 | 2.1 | 3.290, 4, 0.511 | — |
| 1 | 11 | 22.9 | 0.546 | ||
| 2 | 15 | 31.3 | 0.678 | ||
| 3 | 10 | 20.8 | 0.796 | ||
| >3 | 11 | 22.9 | 0.239 | ||
| Alcohol consumption (cups/wk) | 0 | 46 | 95.8 | 1.128, 3, 0.770 | — |
| 1 | 1 | 2.1 | 0.558 | ||
| 2 | 1 | 2.1 | 0.220 | ||
| 3 | 0 | 0.0 | 0.999 | ||
| >3 | 0 | 0.0 | 0.999 | ||
| Cigarettes consumption (No. of cigarettes/d) | 0 | 42 | 87.5 | 8.447, 5, 0.133 | — |
| 1–5 | 3 | 6.3 | 0.044* | ||
| 6–10 | 1 | 2.1 | 0.193 | ||
| 11–15 | 1 | 2.1 | 0.287 | ||
| 16–20 | 1 | 2.1 | 0.776 | ||
| >20 | 0 | 0.0 | 0.999 | ||
| Waterpipe consumption (average frequency/wk) | 0 | 39 | 81.3 | 3.740, 4, 0.442 | — |
| 1 | 4 | 8.3 | 0.755 | ||
| 2 | 1 | 2.1 | 0.344 | ||
| 3 | 2 | 4.2 | 0.149 | ||
| >3 | 2 | 4.2 | 0.703 | ||
| Regular NSAIDS intake (pills/mo) | 0–5 | 41 | 85.4 | 2.966, 4, 0.564 | — |
| 6–10 | 5 | 10.4 | 0.946 | ||
| 11–15 | 2 | 4.2 | 0.270 | ||
| 16–20 | 0 | 0.0 | 0.999 | ||
| >20 | 0 | 0.0 | 1.000 | ||
BMI = body mass index; df = degree of freedom; GERD = gastroesophageal reflux disease; NSAIDs = nonsteroidal antiinflammatory drugs.
p < 0.05; for the χ2 or binary logistic regression test investigating the association between each risk factor and gastroesophageal reflux disease.
Table 4.
Association of demographic- and lifestyle-related factors with irritable bowel syndrome.
| IBS | χ2, df, p | Binary logistic regression (p) | |||
|---|---|---|---|---|---|
| No. | % | ||||
| Gender | Male | 11 | 22.0 | 4.514, 1, 0.034* | — |
| Female | 39 | 78.0 | 0.096 | ||
| Age (y) | 18–21 | 26 | 52.0 | 0.123, 2, 0.941 | — |
| 22–25 | 23 | 46.0 | 0.594 | ||
| >25 | 1 | 2.0 | 0.452 | ||
| BMI (kg/m2) | 18.5–24.9 (normal) | 34 | 68.0 | 1.076, 3, 0.783 | — |
| <18.5 (underweight) | 6 | 12.0 | 0.551 | ||
| 25–30 (overweight) | 9 | 18.0 | 0.985 | ||
| >30 (obese) | 1 | 2.0 | 0.341 | ||
| Tea intake (cups/d) | 0 | 8 | 16.0 | 0.559, 4, 0.967 | — |
| 1 | 16 | 32.0 | 0.774 | ||
| 2 | 14 | 28.0 | 0.767 | ||
| 3 | 8 | 16.0 | 0.621 | ||
| >3 | 4 | 8.0 | 0.807 | ||
| Coffee intake (cups/d) | 0 | 15 | 30.0 | 5.744, 4, 0.219 | — |
| 1 | 20 | 40.0 | 0.426 | ||
| 2 | 6 | 12.0 | 0.131 | ||
| 3 | 7 | 14.0 | 0.039* | ||
| >3 | 2 | 4.0 | 0.293 | ||
| Fatty food consumption (average frequency/wk) | 0 | 0 | 0.0 | 4.046, 4, 0.400 | — |
| 1 | 8 | 16.0 | 0.999 | ||
| 2 | 19 | 38.0 | 0.999 | ||
| 3 | 14 | 28.0 | 0.999 | ||
| >3 | 9 | 18.0 | 0.999 | ||
| Alcohol consumption (cups/wk) | 0 | 50 | 100 | 2.052, 3, 0.562 | — |
| 1 | 0 | 0.0 | 0.999 | ||
| 2 | 0 | 0.0 | 0.999 | ||
| 3 | 0 | 0.0 | 0.999 | ||
| >3 | 0 | 0.0 | 1.000 | ||
| Cigarettes consumption (No. of cigarettes/d) | 0 | 50 | 100 | 4.708, 5, 0.453 | — |
| 1–5 | 0 | 0.0 | 0.999 | ||
| 6–10 | 0 | 0.0 | 0.999 | ||
| 11–15 | 0 | 0.0 | 0.999 | ||
| 16–20 | 0 | 0.0 | 0.999 | ||
| >20 | 0 | 0.0 | 0.999 | ||
| Waterpipe consumption (average frequency/wk) | 0 | 41 | 82.0 | 3.199, 4, 0.525 | — |
| 1 | 5 | 10.0 | 0.065 | ||
| 2 | 0 | 0.0 | 0.999 | ||
| 3 | 1 | 2.0 | 0.593 | ||
| >3 | 3 | 6.0 | 0.187 | ||
| Regular NSAIDS intake (pills/mo) | 0–5 | 41 | 82.0 | 5.822, 4, 0.213 | — |
| 6–10 | 6 | 12.0 | 0.684 | ||
| 11–15 | 1 | 2.0 | 0.688 | ||
| 16–20 | 2 | 4.0 | 0.060 | ||
| >20 | 0 | 0.0 | 1.000 | ||
BMI = body mass index; df = degree of freedom; IBS = irritable bowel syndrome; NSAIDs = nonsteroidal antiinflammatory drugs.
p < 0.05; for the χ2 or binary logistic regression test investigating the association between each risk factor and irritable bowel syndrome.
Among the investigated factors, cigarette smoking and waterpipe consumption were significantly related to UD (p = 0.019 and p = 0.034, respectively). Having a BMI < 18.5 kg/m2 was significantly associated with UD after controlling for other possible factors (p = 0.038, OR = 2.365, 95% CI: 1.048–5.336).
Female gender was significantly associated with IBS in the study sample (p = 0.034). However, there was no significant difference in IBS prevalence among females compared to males after controlling for other possible factors (p = 0.096). Drinking three cups of coffee/d was significantly associated with IBS after controlling for other possible factors (p = 0.039, OR = 3.953, 95% CI: 1.071–14.583).
GERD was significantly associated with drinking two cups of tea (p = 0.010, OR = 6.872, 95% CI: 1.579–29.901) and smoking one to five cigarettes/d (p = 0.044, OR = 7.191, 95% CI: 1.056–48.993) after controlling for other possible factors.
4. Discussion
In this study, we investigated the prevalence, associated risk factors, and overlap of three common GI disorders (UD, IBS, and GERD) among Syrian students at Damascus University. To our knowledge, this is the first study to investigate those variables in Syria.
Many studies around the world have yielded different results about the prevalence of UD, IBS, and GERD; these differences are probably due to various study populations, lifestyles, and different diagnostic criteria. Older Asian studies used Rome I or Rome II criteria to evaluate individuals for UD [10–12]. Studies that are more recent used ROME III criteria and estimated the prevalence of UD to be 1.6% in Beijing, China [13], 5.67% in Zhejiang Province, China [14], and 18% in India [15]. Our study showed that the prevalence of UD among students in Damascus reached 25%, which is relatively high compared to other regional studies. Similarly, prevalence of IBS was estimated to be 16.5% in India [15], 8.34% in China [16], while prevalence of GERD was estimated to be 15.6% in Japan [17]. Results from our study were similar, with a prevalence of 17% for IBS and 16% for GERD. Since IBS prevalence is more frequent in young adults [18], the younger age of our study participants may have contributed to the elevated prevalence of IBS. Investigation for FD would need performing endoscopy for all participants in order to exclude any possible structural defects, according to Rome III criteria [5]. This kind of study is not feasible in countries with limited resources like Syria. However, since most people with UD will turn out to have FD [4], a rough estimate for the prevalence of FD can be concluded.
Multiple lifestyle-related factors were shown to be related to UD or IBS, including poor socioeconomic status, smoking, alcohol, fatty food, increased caffeine intake, and ingestion of nonsteroidal antiinflammatory drugs [3,15,19]. Our results revealed relationships between UD and both cigarette and waterpipe smoking, as well as BMI. While female gender and coffee intake were related to IBS, cigarette smoking and tea intake were related to GERD. The significant increase in prevalence of IBS in females was expected because functional GI disorders have a higher prevalence in women [20]. Although not investigated, additional risk factors, like diet, physical activity, psychological factors, and type of faculty, may have important effects on these GI disorders and can be further investigated in future studies.
The data show a significant overlap between IBS, UD, and GERD among the studied population. This overlap was documented in several previous studies [21,22]. However, no precise mechanism for this overlap was ascertained, and many theories were suggested to explain it, including visceral hypersensitivity and delayed gastric emptying [23,24]. Jung et al. [21] concluded a higher than expected overlap between IBS and GERD by comparing the observed prevalence to a calculated prevalence that assumed no direct relationship between those diseases. We have used a similar approach to investigate the significance of the overlaps between the studied disorders, and we concluded similar observations.
Symptomatic diagnosis of GI disorders was found to be confounded by the overlap among GI symptoms [17]. It is important to consider this issue when interpreting results of our study, particularly regarding UD–GERD overlap. In such an overlap, questionnaire-based differential diagnosis cannot recognize those who have UD with underlying GERD and who will be falsely diagnosed with UD–GERD overlap.
The limitations of the study were as follows. Firstly, the diagnosis of each of the three investigated disorders lacks accuracy because it was based on questionnaires without any further investigations. These disorders were investigated only among students at Damascus University; results may not be generalizable to the general population of Damascus.
Secondly, the small sample size in this study may have affected the generalizability of the results and caused wide CI for risk estimates. Although it has a small number of participants, the study can be used as a pilot study to direct further research with larger samples.
5. Conclusion
Prevalences of UD, IBS, and GERD among students of Damascus University were 25%, 17%, and 16%, respectively. Significant overlaps were observed between these three GI diseases. Multiple risk factors were found to be associated with each disease. However, further research is needed in order to provide a clearer understanding of the epidemiology and risk factors for these diseases.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Conflict of interest
The authors have no conflict of interest.
References
- [1].Everhart J, Ruhl C. Burden of digestive diseases in the United States part I: Overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- [2].Agarwal N, Spiegel B. The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am. 2011;40:11–9. doi: 10.1016/j.gtc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [3].Mahadeva S, Goh K. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–6. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ghoshal U, Singh R, Chang F, Hou X, Wong B, Kachintorn U. Epidemiology of uninvestigated and functional dyspepsia in Asia: Facts and fiction. J Neurogastroenterol Motil. 2011;17:235–44. doi: 10.5056/jnm.2011.17.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley N, Thompson WG, et al. Rome III: The functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates, Inc.; 2006. pp. 1–29. [Google Scholar]
- [6].Hungin A, Whorwell P, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- [7].Chang F, Lu C. Irritable bowel syndrome in the 21st century: perspectives from Asia or South-east Asia. J Gastroenterol Hepatol. 2007;22:4–12. doi: 10.1111/j.1440-1746.2006.04672.x. [DOI] [PubMed] [Google Scholar]
- [8].Vakil N, van Zanten S, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- [9].Shaheen N, Hansen R, Morgan D, Gangarosa L, Ringel Y, Thiny M, et al. The burden of gastrointestinal and liver diseases. Am J Gastroenterol. 2006;101:2128–38. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- [10].Hu W, Wong W, Lam C, Lam K, Hui W, Lai K, et al. Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther. 2002;16:2081–8. doi: 10.1046/j.1365-2036.2002.01377.x. [DOI] [PubMed] [Google Scholar]
- [11].Jeong J. Chronic gastrointestinal symptoms and quality of life in the Korean population. World J Gastroenterol. 2008;14:6388. doi: 10.3748/wjg.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mahadeva S, Yadav H, Rampal S, Goh K. Risk factors associated with dyspepsia in a rural Asian population and its impact on quality of life. Am J Gastroenterol. 2010;105:904–12. doi: 10.1038/ajg.2010.26. [DOI] [PubMed] [Google Scholar]
- [13].Hu J, Yang YS, Peng LH, Sun G, Guo X, Wang WF. Investigation of the risk factors of FD in Beijing university students. Disan Junyi Daxue Xuebao. 2009;31:1498–501. [Google Scholar]
- [14].Li M. Prevalence and characteristics of dyspepsia among college students in Zhejiang Province. World J Gastroenterol. 2014;20:3649. doi: 10.3748/wjg.v20.i13.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Basandra S, Bajaj D. Epidemiology of dyspepsia and irritable bowel syndrome (IBS) in medical Students of Northern India. J Clin Diagn Res. 2014;8:JC13–6. doi: 10.7860/jcdr/2014/10710.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong Y, Chen F, Yu Y, Du C, Qi Q, Liu H, et al. A school-based study with Rome III criteria on the prevalence of functional gastrointestinal disorders in Chinese college and university students. PLoS One. 2013;8:e54183. doi: 10.1371/journal.pone.0054183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ohara S, Kawano T, Kusano M, Kouzu T. Survey on the prevalence of GERD and FD based on the Montreal definition and the Rome III criteria among patients presenting with epigastric symptoms in Japan. J Gastroenterol. 2011;46:603–11. doi: 10.1007/s00535-011-0382-1. [DOI] [PubMed] [Google Scholar]
- [18].Shinozaki M, Fukudo S, Hongo M, Shimosegawa T, Sasaki D, Matsueda K, et al. High prevalence of irritable bowel syndrome in medical outpatients in Japan. J Clin Gastroenterol. 2008;42:1010–6. doi: 10.1097/mcg.0b013e318150d006. [DOI] [PubMed] [Google Scholar]
- [19].Shah SS, Bhatia SJ, Mistry FP. Epidemiology of dyspepsia in the general population in Mumbai. Indian J Gastroenterol. 2001;20:103–6. [PubMed] [Google Scholar]
- [20].Lydiard RB. Increased prevalence of functional gastrointestinal disorders in panic disorder: clinical and theoretical implications. CNS Spectr. 2005;10:899–908. doi: 10.1017/s1092852900019878. [DOI] [PubMed] [Google Scholar]
- [21].Jung H, Halder S, Mcnally M, Locke G, III, Schleck C, Zinsmeister A, et al. Overlap of gastro-oesophageal reflux disease and irritable bowel syndrome: prevalence and risk factors in the general population. Aliment Pharmacol Ther. 2007;26:453–61. doi: 10.1111/j.1365-2036.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- [22].Kaji M, Fujiwara Y, Shiba M, Kohata Y, Yamagami H, Tanigawa T, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25:1151–6. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- [23].Cremonini F, Talley N. Review article: the overlap between functional dyspepsia and irritable bowel syndrome – a tale of one or two disorders? Aliment Pharmacol Ther. 2004;20:40–9. doi: 10.1111/j.1365-2036.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- [24].Gwee K, Seng Boon Chua A. Functional dyspepsia and irritable bowel syndrome, are they different entities and does it matter? World J Gastroenterol. 2006;12:2708–12. doi: 10.3748/wjg.v12.i17.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]