Abstract
This study examines the impact that the joint effect of household wealth quintile and urban–rural residence has on the incidence of diarrhoea among Ghanaian children. Data for this paper were drawn from the Ghana Multiple Indicator Cluster Survey (MICS) of 2006. Descriptive and logistic regression was applied to analyse data on 3466 children. Rural residents are less likely, albeit insignificant, to report diarrhoea compared with those in urban areas. Significant wealth gradients are manifested in childhood experiences of diarrhoea. However, an interaction of wealth with residence does not show significant disparities. Controlling for other important covariates of childhood, the odds of diarrhoea incidence were significantly higher among: the rural poorer (OR = 4.869; 95% CI = 0.792, 29.94), the rural middle (OR = 7.477; 95% CI = 1.300, 42.99), the rural richer (OR = 6.162; 95% CI = 0.932, 40.74) and the rural richest (OR = 6.152; 95% CI = 0.458, 82.54). Apart from residential status and wealth quintile, female children (OR = 0.441; 95% CI = 0.304, 0.640), older children (OR = 0.968; 95% CI = 0.943, 0.993), having a mother with secondary and higher education (OR = 0.313; 95% CI) had lesser odds of experiencing diarrhoea. The findings show that there is a need to apportion interventions intended to improve child health outcomes even beyond residential status and household wealth position.
Keywords: Wealth status, Residence, Diarrhoea, Children, Ghana
1. Introduction
Diarrhoea is one of the major causes of morbidity and mortality among children, particularly in developing countries, and this could be an obstacle to the achievement of the Millennium Development Goal on reducing child mortality. Globally, there are about 4 billion incidents of diarrhoea, and this accounts for about 20% of deaths in children less than five years; a child in a developing country can be predicted to have 3–5 episodes of diarrhoea, resulting in an estimated 800,000 deaths among children per annum [1,2]. Apart from its grave influence on child mortality, diarrhoea can result in long-term health effects, including depletion of immune strength and under-nutrition, as well as making children susceptible to other diseases [3]. Diarrhoea is more of a symptom than a disease. It is often a reflection of gastrointestinal infection and other diseases, such as typhoid, cholera and shigella [3].
Diarrhoea is considered a symptom of wider socioeconomic inequality within and between populations [4]. Directly or indirectly, developing countries such as Ghana continue to undertake development projects that contribute to reducing the risk of early death, and, in the last two decades, it has been reported that some improvements have been made, including improvement in water and sanitation [4]. However, the problems of diarrhoea persist, and it is reported to be the third among the top ten causes of childhood morbidity and mortality [5]. Within developing countries where diarrhoea is prevalent among children, the phenomenon is generally attributed to the standards of living as shown in levels of income, literacy level, adequacy of water supply and sanitation and access to health services, as well as behavior of households and individuals, including breastfeeding and weaning practices [6–9].
Though the enduring solution to the problem lies in improving living conditions of households, in the meantime, there is a need to examine variables that can be exploited to provide some immediate solutions to diarrhoea, since it is one of the avoidable causes of childhood mortality. Previous studies in Ghana have explored the phenomenon from different perspectives. Among these studies, only one used a nationally representative data [4], which focused on household water quality and toilet facilities. The others are limited in scope, for instance, Osumanu [10] explored household environmental factors that increased the vulnerability of children to diarrhoea. The objective of the present study is to ascertain how household wealth status, coupled with type of place of residence, correlates with the incidence of childhood diarrhoea in Ghana.
The departure of the current study from previous studies lies in its interaction of residence (urban–rural) and wealth to first explore their joint effect and, secondly, controlling for other child-level, household-level and maternal-level characteristics on the incidence of childhood diarrhoea in Ghana. The assumption is that children whose parents are within the upper wealth quintile and also resident in urban areas will have better chances of escaping the problems of childhood diarrhoea. Residence was combined with wealth in the light of the increasing spate of urbanization unfolding in Ghana with the attendant ‘slumanization’. Some studies [11,12] have suggested that urban poverty can be far worse than rural poverty owing to the high cost of living in urban areas, which increasingly subjects the urban poor to cyclic squalor and, in turn, results in poor access to a life-sustaining infrastructure, such as water and sanitation. In effect, by interacting household wealth quintile with residential status (urban–rural), the present study anticipated disentangling the dual impacts of these factors on childhood diarrhoea in Ghana. In the end, tailored interventions could be designed for populations in greater need.
2. Setting
The major environmental conditions that heighten the incidence of diarrhoea in the country are largely influenced by access to quality and quantity of water and sanitation [13]. The performance of the urban water supply by the Ghana Water Company Limited is about average (60%) [13]. About 50% of the Company’s water production is still lost through unaccounted-for water, and the total coverage of rural water is around 53%, which is largely comprised of boreholes, hand-dug wells and small-piped systems [13]. Presently, the population with improved access to sanitation is 13%. Disaggregated by residence, the proportion of rural residents with improved sanitation is 8%, while in urban areas the proportion with improved access is 19% [14]. There are also indications about individual behavioural dispositions about Ghanaians that can enhance the incidence of diarrhoea. For instance, hand-washing attitudes of many Ghanaians have not improved, in spite of a number of behavioural change messages on the practice. A comparative study of Ghana and other African and Asian countries paints a grim picture about the practice in the country. The study revealed that 3% of respondents washed their hands with soap after using the toilet, while only 1% washed their hands before feeding an index child [15].
3. Methods
3.1. Data
The study used the 2006 version of the Ghana Multiple Indicator Cluster Survey (MICS) as its data set. It was the second in the series under the auspices of the United Nations Children’s Fund (UNICEF). The data was accessed from the UNICEF information statistics department. The first series of MICS were undertaken in the mid-1990s in a number of countries and, as of 2006, about 65 countries had participated in the survey. The sampling frame was based on the 2005 Ghana Living Standard Survey (GLSS5). The frame was first stratified into the 10 administrative regions in the country, then into urban and rural enumeration areas (EAs). The 2006 MICS employed a two-stage stratified sample design. At the first stage of sampling, 300 census enumeration areas (124 urban and 176 rural EAs) were selected. These are a subsample of the 660 EAs (281 urban and 379 rural) selected for the GLSS5. Within each stratum, a specified number of census enumeration areas were selected systematically with probability proportional to size. Since the sampling frame (the 2000 Ghana Population Census) was up-to-date, a new listing of households was not conducted in all the sample EAs prior to a systematic sample selection of 15 households in each selected cluster. The sample was stratified by region, urban and rural areas and is not self-weighting since Central, Northern, Upper East and Upper West regions were over-sampled. For reporting national level results, sample weights are used. Among the sampled households, data on 3466 children under the age of five were collected [16]. Ghana has participated in the first and third rounds of MICS (1995 and 2006), and these datasets are freely available on request. At the time of the paper, the 2011 MICS had been completed, but the data were not publicly available.
3.2. Data analysis
To reduce the categories of some of the predictor variables, recoding was conducted in specific instances. These were: type of toilet and water for the household; and ethnicity and religion of the head of household. Sources of water for drinking for the household were coded as either improved or unimproved. Improved sources of water comprised piped water (inside and outside dwelling), borehole, spring water and protected well. Unimproved consisted of unprotected well, tanker-truck, river/stream, dam/lake/pond/canal/irrigation canal, sachet and bottled water. Toilet was also coded as improved1 = 1 (flush to piped sewer system, flush to septic tank, flush to pit latrine, ventilated improved pit latrine and pit latrine with slab) or unimproved = 0 (pit latrine without slab/open pit, bucket, no facility/bush). Religion (Catholic, Protestant, Pentecostal/Charismatic, Moslem and Traditional/Spiritualist and “Others”) and ethnicity (Akan, Ga-Dangme, Ewe, Mole-Dagbani and Others) were restricted to the predominant groups in Ghana.
Both descriptive and inferential statistics are used to present the main findings. At the first stage is the bivariate analysis of children who reported diarrhoea compared with the categories of the independent factors and the corresponding Pearson Chi-Square test of independence between the independent factors and the outcome variable (self-reported diarrhoea). Binary logistic regression was applied for the inferential analysis given its popularity in testing the statistical relationship between continuous and categorical independent factors on the one hand, and a dependent dichotomous variable on the other hand. Two separate models were estimated. The first model involved an interaction term of wealth quintile and residential status. In the final model, wealth quintile, residential status and the interaction term are retained, coupled with age of the child (measure continuously in months), sex of the child, whether the child attends early childhood education programs, age (in months) at weaning, maternal education, type of toilet and water facilities for the household, ethnicity and religious affiliation of the head of household, and the region of residence. For the logistic regression, the age of the children and age at weaning were treated as continuous rather than categorical to determine the nature (negative or positive) of the relationship between them and the incidence of diarrhoea. The logit estimation techniques assume independence of observations. However, observations in the MICS data are not strictly independent. For instance, a woman could have two children who are under five years with information on all of them collected, and such children possibly share similar household socioeconomic characteristics. In the analysis, this clustering effect is overcome by the Huber–White approach, which makes it possible to estimate robust standard errors. Also, to make the findings representative, both the descriptive and inferential analyses were weighted. All the analyses were performed with STATA (12th edition) [College Station, Texas]. The Ghana Health Service Ethics Committee provided ethical clearance for the survey from which these data emanated. UNICEF provided approval for the use of the data.
4. Findings
Table 1 shows the distribution of various explanatory variables and the proportion of children who ever experienced diarrhoea. Overall, 15.4% of children were reported to have had diarrhoea two weeks prior to the survey. The incidence of diarrhoea was slightly higher in rural (17.2%) areas than in the urban communities (14.7%), and the associations are significant at 0.05%. By wealth quintile, reported diarrhoea ranged from 11.55% among the children of the richest households to approximately 20.3% among children in the poorest households. The association between sex and diarrhoea episode was not significant. The descriptive results further point to significant differences of diarrhoea attacks by age of child. The greatest risk of diarrhoea is observed among children between 12 and 24 months (1–2 years): about one-quarter (24%) of children within this age category (12–24 months) had had diarrhoeal disease. The least was reported among children within the ages of 48–59 months (9.5%). Children residing in households with improved and unimproved water supplies had a similar risk of incidence of diarrhoea. Children resident in households using improved toilets were less prone (14.2%) to experience diarrhoea than children in houses with unimproved toilet facilities (18.48%).
Table 1.
Incidence of diarrhoea among children in Ghana socioeconomic characteristics.
| Factors | % | No. |
|---|---|---|
| Residence | ||
| Urban | 14.74 | 1011 |
| Rural | 17.19 | 2455 |
| Pearson χ2 = 3.1277; p = 0.077 | ||
| Household wealth quintiles | ||
| Poorest | 20.29 | 1035 |
| Poorer | 17.35 | 922 |
| Middle | 15.53 | 573 |
| Richer | 12.33 | 503 |
| Richest | 11.55 | 433 |
| Pearson χ2 = 25.7669; p = 0.000 | ||
| Sex | ||
| Male | 17.36 | 1780 |
| Female | 15.54 | 1686 |
| Pearson χ2 = 2.0839; p = 0.149 | ||
| Age in months | ||
| 0–11 months | 15.59 | 712 |
| 12–24 months | 24.18 | 761 |
| 25–36 months | 17.22 | 668 |
| 37–48 months | 14.4 | 722 |
| 49–59 months | 9.45 | 603 |
| Pearson χ2 = 57.3520; p = 0.000 | ||
| Age at weaning (months) | ||
| 0–11 months | 11.11 | 117 |
| 12–24 months | 14.18 | 1509 |
| 25–36 months | 17.25 | 342 |
| Pearson χ2 = 3.2879; p = 0.193 | ||
| Main source of drinking water | ||
| Unimproved | 15.23 | 952 |
| Improved | 16.95 | 2514 |
| Pearson χ2 = 1.4743; p = 0.225 | ||
| Kind of toilet facility | ||
| Unimproved | 18.48 | 1861 |
| Improved | 14.16 | 1603 |
| Pearson χ2 = 11.6948; p = 0.001 | ||
| Mother’s education | ||
| None | 18.49 | 1677 |
| Primary | 18.18 | 671 |
| Middle/JSS | 12.43 | 901 |
| Secondary and Higher | 12.44 | 217 |
| Pearson χ2 = 19.6210; p = 0.000 | ||
| Child attends early childhood school | ||
| Yes | 11.75 | 647 |
| No | 12.62 | 705 |
| Pearson χ2 = 0.2425; p = 0.622 | ||
| Region | ||
| Western | 11.71 | 316 |
| Central | 10.00 | 260 |
| Greater Accra | 10.43 | 326 |
| Volta | 9.32 | 236 |
| Eastern | 14.24 | 337 |
| Ashanti | 16.63 | 415 |
| Brong-Ahafo | 19.01 | 242 |
| Northern | 22.84 | 578 |
| Upper East | 22.37 | 389 |
| Upper West | 19.07 | 367 |
| Pearson χ2 = 61.5398; p = 0.000 | ||
| Ethnicity of head of household | ||
| Akan | 14.32 | 1180 |
| Ga/Dangme | 10.26 | 195 |
| Ewe | 10.68 | 384 |
| Mole-Dagbani | 20.29 | 956 |
| Others | 19.68 | 742 |
| Pearson χ2 = 34.4661; p = 0.000 | ||
| Religion of head of household | ||
| Catholic | 14.71 | 476 |
| Protestant | 13.99 | 529 |
| Pentecostal/Charismatic | 13.72 | 802 |
| Moslem | 20.23 | 791 |
| Traditional/Spiritualist | 19.19 | 589 |
| No religion | 15.77 | 279 |
| Pearson χ2 = 19.2359; p = 0.002 | ||
Other important descriptive bivariate results were noted between maternal education and incidence of diarrhoea in children. Children whose mothers had attained some form of higher formal education reported the least proportion of diarrhoea: about 18.5% of children whose mothers did not have any formal education experienced diarrhoea compared with 12.4% reported in children among women who reported secondary and higher formal education and the associations are significant at 0.05%. Again, the results show significant religious and ethnic associations as depicted in Table 1.
By region of residence, diarrhoea episodes were higher in the Northern (22.8%) and Upper East (22.4%) regions and the lowest incidence was reported in the Volta region (9.3%). About one out of every five children with the head of household being a Mole-Dagbani had had a diarrhoea episode. Similarly, one-fifth of children in households with the head being affiliated with the Islamic faith had experienced a diarrheal disease.
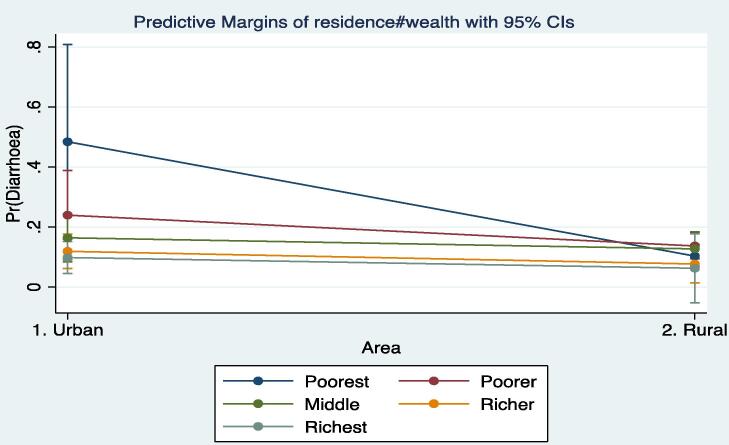
In Table 2, two different models are presented. In the first model, there is evidence to show that rural residents are less likely, albeit insignificant, to report diarrhoea compared with those in urban areas. Significant wealth gradients are manifested in childhood experiences of diarrhoea. However, an interaction of wealth with residence does not show significant disparities. Controlling for other important covariates of childhood diarrhoea in Model 2, the likelihood of a child in a rural community having an attack of diarrhoea declines substantially and significant at 0.01% (OR = 0.0971; 95% CI = 0.0204, 0.463). The interaction terms in the full model (Model 2), however, reveal some interesting findings. It becomes clear that the odds of an attack of diarrhoea were significantly higher among the rural poorer (OR = 4.869; 95% CI = 0.792, 29.94), the rural middle (OR = 7.477; 95% CI = 1.300, 42.99), the rural richer (OR = 6.162; 95% CI = 0.932, 40.74) and the rural richest (OR = 6.152; 95% CI = 0.458, 82.54). Except the odds for the rural richest, all the others are significant at 0.10%, 0.05% and 0.10%, respectively. The overall effect is that, regardless of wealth quintile, the risk of childhood diarrhoea is significantly higher in rural areas compared with the urban areas when urban–rural residence is interacted with wealth quintile (Model 2). Pictorial evidence of this is shown in Fig. 1 in the form of predictive margins. It should be noted that the incidence of diarrhoea in rural areas in relation to wealth quintile shows some form of convergence/clustering, but not the case for the urban children. In the urban areas, the differences are clearly noted, particularly between the poorest and the other categories.
Table 2.
Logistic regression results on determinants of childhood diarrhoea in Ghana.
| Covariates | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Residence | ||||
| Urban | 1 | [1,1] | 1 | [1,1] |
| Rural | 0.466 | [0.175,1.242] | 0.0971** | [0.0204,0.463] |
| Household wealth quintile | ||||
| Poorest | 1 | [1,1] | 1 | [1,1] |
| Poorer | 0.641 | [0.217,1.891] | 0.290 | [0.0513,1.635] |
| Middle | 0.389+ | [0.140,1.079] | 0.172* | [0.0348,0.854] |
| Richer | 0.327* | [0.120,0.891] | 0.115** | [0.0229,0.579] |
| Richest | 0.245** | [0.0899,0.670] | 0.0919** | [0.0177,0.477] |
| Interaction terms | ||||
| Urban#Poorest | 1 | [1,1] | 1 | [1,1] |
| Urban#Poorer | 1 | [1,1] | 1 | [1,1] |
| Urban#Middle | 1 | [1,1] | 1 | [1,1] |
| Urban#Richer | 1 | [1,1] | 1 | [1,1] |
| Urban#Richest | 1 | [1,1] | 1 | [1,1] |
| Rural#Poorest | 1 | [1,1] | 1 | [1,1] |
| Rural#Poorer | 1.200 | [0.394,3.658] | 4.869+ | [0.792,29.94] |
| Rural#Middle | 1.790 | [0.614,5.214] | 7.477* | [1.300,42.99] |
| Rural#Richer | 1.289 | [0.422,3.934] | 6.162+ | [0.932,40.74] |
| Rural#Richest | 1.553 | [0.403,5.983] | 6.152 | [0.458,82.54] |
| Sex of child | ||||
| Male | 1 | [1,1] | ||
| Female | 0.441** | [0.304,0.640] | ||
| Age (in months) | 0.968* | [0.943,0.993] | ||
| Water for household use | ||||
| Unimproved | 1 | [1,1] | ||
| Improved | 1.136 | [0.727,1.776] | ||
| Type of household toilet | ||||
| Unimproved | 1 | [1,1] | ||
| Improved | 0.633+ | [0.398,1.006] | ||
| Mother’s education | ||||
| None | 1 | [1,1] | ||
| Primary | 1.611+ | [0.961,2.699] | ||
| Middle/Junior Secondary School | 1.057 | [0.610,1.833] | ||
| Secondary and Higher | 0.313+ | [0.0874,1.122] | ||
| Child attends early childhood school | ||||
| Yes | 1 | [1,1] | ||
| No | 0.754 | [0.495,1.149] | ||
| Age at weaning (in months) | 1.168 | [0.781,1.746] | ||
| Region | ||||
| Western | 1 | [1,1] | ||
| Central | 0.612 | [0.221,1.694] | ||
| Greater Accra | 1.999 | [0.738,5.414] | ||
| Volta | 0.580 | [0.160,2.101] | ||
| Eastern | 1.080 | [0.438,2.666] | ||
| Ashanti | 2.649* | [1.168,6.006] | ||
| Brong-Ahafo | 1.927 | [0.805,4.615] | ||
| Northern | 3.085* | [1.202,7.915] | ||
| Upper East | 2.529 | [0.728,8.792] | ||
| Upper West | 2.168 | [0.561,8.384] | ||
| Ethnicity of head of household | ||||
| Akan | 1 | [1,1] | ||
| Ga/Adangbe | 0.848 | [0.357,2.013] | ||
| Ewe | 1.088 | [0.501,2.362] | ||
| Mole-Dagbani | 0.639 | [0.269,1.515] | ||
| Others | 0.756 | [0.399,1.433] | ||
| Religion of head of household | ||||
| Catholic | 1 | [1,1] | ||
| Protestant | 1.029 | [0.497,2.128] | ||
| Pentecostal/Charismatic | 1.109 | [0.561,2.189] | ||
| Moslem | 1.449 | [0.700,3.002] | ||
| Traditionalist/Spiritualist | 1.560 | [0.750,3.247] | ||
| Others | 0.897 | [0.383,2.101] | ||
| Constant | 0.517 | [0.197,1.357] | 3.859 | [0.335,44.44] |
| AIC | 2964.8 | 932.8 | ||
| Log likelihood | −1472.4 | −429.4 | ||
| Chi-squared | 36.11 | 88.16 | ||
| N | 3466 | 1274 | ||
p < .10.
p < .05.
p < .01.
Fig. 1.
Predictive margins of diarrhoea by wealth status and residence.
Apart from residential status and wealth quintile, female children (OR = 0.441; 95% CI = 0.304, 0.640), older children (OR = 0.968; 95% CI = 0.943, 0.993), and having a mother with secondary and higher education (OR = 0.313; 95% CI) had lesser odds of experiencing diarrhoea. On the other hand, a child whose mother had had only a primary education (OR = 1.611; 95% CI = 0.961, 2.699), being resident in the Ashanti (OR = 2.649; 95% CI = 1.168, 6.00) and the Northern (OR = 3.085; 95%) regions had significantly higher incidences of diarrhoea.
5. Discussion
This study examined the joint effect of household wealth quintile and residential status (urban–rural) on the incidence of diarrhoea among children less than five years in Ghana. The study finds an overall incidence of diarrhoea to be around 15.4%. Decomposed by the respective background characteristics, the highest (24.2%) proportion of reported diarrhoea is among children between 12 and 24 months (1–2 years), with the lowest proportion reported in any group noted among children from the Volta region of Ghana.
The residential status and wealth quintile analysis demonstrate a mix of important findings. Prior to interacting residential status with wealth, rural children show better diarrhoea outcomes. This aspect of the study appears to support the “urban health penalty” hypothesis, which posits that the poor in urban areas are advertently or inadvertently pushed to marginal areas where environmental health conditions are unsuitable for health [17,18]. This is particularly the case in countries transitioning to development where access to water and sanitation and general socioeconomic conditions have been compromised by population movements–increased migration to urban areas, albeit unregulated and poorly managed. Associated with this is the creation of urban slums, the lack of or inadequate safe water supply, inadequate drainage and sewage networks, and absence of sanitation and solid waste removal, all of which have potentially combined to increase the risk of infectious diseases, including diarrheal diseases [12,19]. That said, however, the multivariable analyses follow the predictable pattern of inequalities in ill health in urban and rural areas. Thus, children in rural areas were at a higher risk of reporting an episode of diarrhoea. A recent discourse on child health outcomes in Kenya revealed a narrowing gap between urban and rural areas, and this was partly attributed to the deplorable living conditions in urban slums [12]. In the present study, considering all the theoretically relevant variables, urban children had a generally lower incidence of diarrhoea. Even for the urban poor, they were better off than the wealthy in rural areas [20–23]. The relationship between wealth status and health unfolds through differential access to health improving resources, which exert a greater impact on prevention and treatment of illnesses, as well as survival [20]. On the substantive point on which this study rests, it was observed that while the risk of diarrhoea remains comparatively low in rural areas, the interaction outcome shows that children in rural areas have higher odds of diarrhoea infection compared with urban children. The implication is that although urban children may experience the so-called urban penalty, the effect is attenuated when wealth and other covariates are controlled for. The implication is that residence in either urban or rural areas may not profoundly affect the incidence of diarrhoea unless other mediating variables are equally taken into consideration.
It is again observed that the incidence of diarrhoea was not significantly associated with the quality of household water, although the risk of infection was higher among those without quality water. However, the differences were not significant. This is a major deviation from the norm [4]. Much as the sources of water for household drinking is important and in fact shows a relationship with the incidence in some previous studies, practices associated with fetching, storage and handling could contaminate even improved sources of water [10]. Thus, much as the source of water is important, storage and handling can expose households to diseases. The quality of containers for water storage has been found to be an important correlate of diarrhoea among children [10]. Unfortunately, there was no question on water storage in the data set to be used as a covariate. However, the Siamese twin of water–household sanitation–measured by the quality of toilet facility, whether improved or unimproved, significantly reduced the occurrence of diarrhoea, which is consistent with previous studies [23–25]. Households which lack improved sanitation have an elevated risk of contamination with human excreta, and there is evidence [23] to show that children in households with improved sanitation have more than a 50% probability of escaping diarrhoea.
Also, there were religious differences (albeit insignificant) in childhood diarrhoea where for children from Traditional Religion households were more likely to have diarrhoea compared with other children. While the causes of these differences are not easily discernible, the variations may be accounted for by cultural variations in childcare [26]. Further qualitative inquiries are needed to clarify these observations.
The importance of maternal education or social gradient in child health dynamics was noted in this study consistent with the extant literature. Mothers who are educated are more likely to have better skills in childcare practices, such as regular hand washing with soap prior to feeding children. Well-educated mothers are also likely to be in affluent households where water and sanitation are improved [23,26,27].
Because the information on diarrhoea was self-reported, there is the possibility of recall bias, although the recall period of illnesses in this case was limited to two weeks preceding the survey. This helps to offset some of the inherent weaknesses in self-reported data on incidence of diseases. However, findings are generally consistent with existing studies, and this gives one a higher level of certainty that the results are valid.
6. Conclusion
The study shows that household, community level characteristics, maternal characteristics and individual child’s features shape the dynamics of childhood diarrhoea in Ghana. The findings show that there is a need to apportion interventions intended to improve child health outcomes even beyond household wealth position and residential status.
Acknowledgement
The authors are grateful to UNICEF for making data set for this study freely available.
Footnotes
An improved toilet facility is considered the most efficient and hygienic method of human waste disposal, which include flush/pour flush to piped sewer, flush/pour flush to septic tank, flush/pour to pit latrine, ventilated improved pit latrine, pit latrine with slab and composting toilet.
Contributor Information
Akwasi Kumi-Kyereme, Email: akkyereme@ucc.edu.gh.
Joshua Amo-Adjei, Email: joshua.amo-adjei@ucc.edu.gh.
Competing interest
The authors declare no competing interests.
Authors’ contribution
AKK conceptualized the study. JAA participated in the conceptualization, analysed the data and drafted the manuscript. Both authors reviewed the draft manuscript and gave approval for the version to be published.
References
- [1].World Health Organisation . The world health reports 2002: reducing risks, promoting health life. Geneva: WHO; 2002. [Google Scholar]
- [2].Kosek M, Bern C, Guerrant RL. The magnitude of the global burden of diarrhoea diseases from studies published 1992–2000. Bull. World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- [3].Fuentes R, Pfütze T, Seck P. A logistic analysis of diarrhoea incidence and access to water and sanitation. Human Development Report Office; 2006. Occasional Paper Series. [Google Scholar]
- [4].Gyimah SO. Interaction effects of maternal education and household facilities on childhood diarrhoea in sub-Saharan Africa The case of Ghana. J Health Pop Dev Countries. 2003 http://www.jhpdc.unc.edu/. [Google Scholar]
- [5].Adams I, Boerma T. Ghana National burden of disease and trend analysis; Background Paper 1, Ghana Annual Health Sector Review; 2006; WHO, Geneva. [Google Scholar]
- [6].Woldemicael G. Diarrhoea morbidity among young children in Eritrea: environmental and socioeconomic determinants. J Health Pop Nutr. 2001;19:83–90. [PubMed] [Google Scholar]
- [7].Mekasha A, Tesfahun A. Determinants of diarrhoeal diseases: a community based study in urban south-western Ethiopia. East African Med J. 2003;80:77–82. doi: 10.4314/eamj.v80i2.8650. [DOI] [PubMed] [Google Scholar]
- [8].Dasgupta R. Exploring intra-household factors for diarrhoea diseases: a study in slums of Delhi. India J Water Health. 2008;6:289–99. doi: 10.2166/wh.2008.025. [DOI] [PubMed] [Google Scholar]
- [9].Gurpreet K, Teel GE, Amal N, Paramesarvathy MR, Karuthan C. Incidence and determinants of acute diarrhoea in Malaysia: a population-based study. J Health Pop Nutr. 2011;29:103–12. doi: 10.3329/jhpn.v29i2.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Osumanu KI. Household environmental and behavioural determinants of childhood diarrhoea morbidity in the Tamale Metropolitan Area (TMA) Ghana Danish J Geog. 2007;107:59–68. doi: 10.1080/00167223.2007.10801375. [DOI] [Google Scholar]
- [11].Agtini MD, Soeharno R, Lesmana M. The burden of diarrhoea, shigellosis, and cholera in North Jakarta Indonesia: findings from 24 months surveillance. BMC Infect Dis. 2005;5 doi: 10.1186/1471-2334-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kimani-Murage EW, Fotso JC, Egondi T, Abuya B, Elungata P, Ziraba AK, et al. Trends in childhood mortality in Kenya: the urban advantage has seemingly been wiped out. Health Place. 2014;29:95–103. doi: 10.1016/j.healthplace.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Awuah E, Nyarko KB, Owusu PA. Water and sanitation in Ghana. Desalination. 2009;248:460–7. doi: 10.1016/j.desal.2008.05.088. [DOI] [Google Scholar]
- [14].World Health Organisation (WHO) & United Nations Children’s Fund (UNICEF) Progress on sanitation and drinking water – 2013 Update. France: WHO and UNICEF; 2013. [Google Scholar]
- [15].Danquah L, Curtis V, Aunger R. What do we know about hand washing practices? London: LSHTM/Hygiene Centre; 2007. [Google Scholar]
- [16].UNICEF . Monitoring the situation of children and women: findings from the Ghana Multiple Indicator Cluster Survey, 2006. New York: UNICEF; 2007. [Google Scholar]
- [17].Goebel A, Dodson B, Hill H. Urban advantage or urban penalty? A case study of female-headed households in a South African city. Health Place. 2010;2010(16):573–80. doi: 10.1016/j.healthplace.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [18].Fotso JC. Urban–rural differentials in child malnutrition in sub-Saharan Africa: trends and socioeconomic correlates. Health Place. 2007;13:205–23. doi: 10.1016/j.healthplace.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [19].Patel RB, Burke TF. Urbanization, an emerging humanitarian disaster. N Engl J Med. 2009;361(8):741–3. doi: 10.1056/NEJMp0810878. [DOI] [PubMed] [Google Scholar]
- [20].Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11(2):131–41. doi: 10.1016/s1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nundy S, Gilman RH, Xiao L, Cabrera L, Cama R, et al. Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. Am J Trop Med Hyg. 2011;84(1):38–42. doi: 10.4269/ajtmh.2011.10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diaz T, George AS, Rao SR, Bangura PS, Baimba JB, et al. Healthcare seeking for diarrhoea, malaria and pneumonia among children in four poor rural districts in Sierra Leone in the context of free health care: results of a cross-sectional survey. BMC Public Health. 2013;13(1):157. doi: 10.1186/1471-2458-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mihrete TS, Alemie GA, Teferra AS. Determinants of childhood diarrhoea among under-five children in Benishangul Gumuz Regional State, North West Ethiopia. BMC Paediatrics. 2014;14(1):102. doi: 10.1186/1471-2431-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Daniels DL, Cousens SN, Makoae LN. A case-control study of the impact of improved sanitation on diarrhea morbidity in Lesotho. Bull World Health Organ. 1990;68:455–63. [PMC free article] [PubMed] [Google Scholar]
- [25].Desalegn M, Kumie A, Tefera W. Predictors of under-five childhood diarrhoea: Mecha District, West Gojjam, Ethiopia. Ethiop J Health Dev. 2011;25(3):192–200. [Google Scholar]
- [26].Kandala NB, Magadi MA, Madise NJ. An investigation of district spatial variations of childhood diarrhoea and fever morbidity in Malawi. Soc Sci Med. 2006;62(5):1138–52. doi: 10.1016/j.socscimed.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. doi: 10.1016/s0140-6736(13)60937-x. [DOI] [PubMed] [Google Scholar]