Abstract
There is limited information of level of drug resistance to first-line and second line anti-tuberculosis agents in treatment naïve pulmonary tuberculosis (PTB) patients from the Indian region. Therefore, the present prospective study was conducted to determine the antimicrobial susceptibility to first-line and second line anti-TB drug resistance in such patients. Sputum samples from consecutive treatment naïve PTB cases registered in Lala Ram Sarup (LRS) district, under RNTCP containing 12 Directly Observed Treatment Centre’s (DOTS), were enrolled using cluster sampling technology. A total of 453 samples were received from July 2011 to June 2012. All samples were cultured on solid medium followed by drug susceptibility to first and second line anti-tubercular drugs as per RNTCP guidelines. Primary multi-drug resistance (MDR) was found to be 18/453; (4.0%). Extensively drug resistance (XDR) was found in one strain (0.2%), which was found to be resistant to other antibiotics. Data of drug resistant tuberculosis among treatment naïve TB patients are lacking in India. The presence of XDR-TB and high MDR-TB in small population studied, calls for conducting systematic multi-centric surveillance across the country.
Keywords: Antibiotic, Primary, Pulmonary, Multi-drug resistance, Extensively drug resistance
1. Introduction
Drug resistance in patients with Mycobacterium tuberculosis (MTB) infection was witnessed soon after the introduction of anti-tuberculosis agents [1]. Disease gained international importance with outbreaks of multi drug resistant tuberculosis (MDR-TB) in patients with human immunodeficiency virus (HIV) infection in the United States and Europe [1]. The MDR-TB is defined as resistance to rifampicin (RIF) and isoniazid (INH). World-wide prevalence of MDR-TB among new cases and previously treated cases is estimated as 3.6% (95% CI: 2.1–5.1%) and 20.2% (95% CI: 13.3–27.2%) respectively [2]. This situation poses grave challenge to the TB control as prolonged, limited and expensive treatment options with 10–30% of cases resulting in treatment failure and death [2]. Last decade witnessed emergence of extensively drug resistance tuberculosis (XDR-TB) defined as resistance to any fluoroquinolone (FQ) and at least one of second-line injectable agents: amikacin (AMK), kanamycin (KAN), and capreomycin (CAP) among MDR-TB [3]. Globally, 9.6% (95% CI: 8.1–11%) of MDR cases are XDR and have already spread over 92 countries [2]. The management of these cases is even more difficult than MDR-TB as these are unresponsive to almost all anti-tubercular agents.
Though previous treatment for TB is the strongest risk factor for development of DR-TB, treatment-naïve patients may also get affected due to either transmission of resistant strains or spontaneous mutations. In M. tuberculosis, genetically encoded drug resistance mutations arise exclusively through chromosomal mutations, majority of which are single-nucleotide polymorphisms. Spontaneous mutations in Mycobacterium occur as a consequence of errors that arise during DNA replication at a rate of one mutation error at two bases in every 10,000 genome copied. The rate of mutation is possibly affected by several cellular and external factors, such as UV irradiation, smoking, anti-retroviral therapy, which are still under study [4]. With growing burden of DR-TB, such strains are transmitted from close contacts to normal population increasingly. Prevalence of primary drug resistance also serves as an epidemiological indicator to assess the success of the TB control programme. There is paucity of studies on primary drug resistance from India with no data of primary XDR-TB, based on culture and drug susceptibility testing (DST). Therefore, this prospective study was systematically designed to determine the antimicrobial susceptibility to first-line and second line anti-TB drug resistance among newly diagnosed pulmonary TB (PTB) cases, in a district under Revised National Tuberculosis Control Programme (RNTCP).
2. Methods
2.1. Study design and sampling methodology
Lala Ram Sarup (LRS) district has 12 Directly Observed Treatment Centre’s (DOTS) covering population of 0.8 million. The present study, considered new PTB cases defined as those who have taken anti-tuberculosis drugs for less than one month [5], to study the drug resistance in LRS field area under RNTCP as a prospective operational research. The required sample size for measuring the drug resistance was calculated as detailed below [6].
-
a.
Firstly, total number of new PTB cases registered in all DOTS centres in the specified district, were obtained for the previous year to understand the population from which the samples would be selected.
-
b.
From information available in the institute, the expected proportion of resistance for RIF was about 15–20% of the new PTB cases.
-
c.
The absolute precision was considered as 5%.
-
d.
95% confidence interval was used for the measured proportion.
The following formula was used to calculate the sample size
where N = population size, n = required sample size, Z = Z-value (from standard normal distribution) that corresponds to the desired confidence level or level of significance is 5% for 95% confidence level, P = expected proportion of resistance in the target population. From population of about 2000 (N) cases, considering an expected proportion of 17% (p) with absolute precision as 5% with 95% confidence level, calculated sample size is 196 which is approximated to 200. Furthermore, cluster sampling was adopted for sample collection from DOTS centres, which required application of cluster design effect hence above sample size was multiplied by two. The sample size derived at 400 was further increased (to account for expected 10% loss of samples), to get size of 450.
2.2. Sampling procedure
For selection of 450 patients from all the DOTS centres under the specified area of LRS institute, thirty cluster sampling method was adopted. To determine number of patients per cluster, required total sample size was divided by the number of clusters, which was found to be 15 patients. In case of more than one cluster in a DOTS centre, the number was multiplied by the size of the cluster to calculate the total number of patients needed from that centre.
To avoid any bias in patient selection from any centre, probability proportion to size cluster technique was utilized for which, list of all the DOTS centres along with list of newly diagnosed TB patients during previous year was obtained (Table 1). The cumulative total was calculated for each row (cumulative number for second centre would be (number in first centre) + (number in second centre)), and so on. The total number of patients diagnosed in the previous year was 1942.
Table 1.
The distribution of the selected clusters from Directly Observed Treatment Centres (DOTS) in Lala Ram Sarup (LRS) district.
| No. | Centre | Number of patients | Cumulative frequency number | Cluster interval (1942/30 = 65) | Number of clusters | Patient sample size |
|---|---|---|---|---|---|---|
| 1 | Khanpur | 211 | 211 | 30, 95, 160 | 3 | 45 |
| 2 | Sangam Vihar J | 157 | 368 | 225, 290, 355 | 3 | 45 |
| 3 | Sangam Vihar I | 170 | 538 | 420, 485 | 2 | 30 |
| 4 | Sangam Vihar G | 181 | 719 | 550, 615, 680 | 3 | 45 |
| 5 | Sangam Vihar K2 | 192 | 911 | 745, 810, 875 | 3 | 45 |
| 6 | Tigri | 163 | 1074 | 940, 1005, 1070 | 3 | 45 |
| 7 | Mehrauli | 191 | 1265 | 1135, 1200, 1265 | 3 | 45 |
| 8 | Lala Ram Sarup | 184 | 1449 | 1330, 1395 | 2 | 30 |
| 9 | Fatehpur | 101 | 1550 | 1460, 1525 | 2 | 30 |
| 10 | Chattarpur | 122 | 1672 | 1590, 1655 | 2 | 30 |
| 11 | Jonapur | 62 | 1734 | 1720 | 1 | 15 |
| 12 | Ber Sarai | 208 | 1942 | 1785, 1850, 1915 | 3 | 45 |
| 1942 | 1942 | 30 | 450 |
The sampling interval was obtained by dividing 1942 by 30 (1942/30 = 65). A random number was selected between 0 and 65 from random number table (30). First cluster was selected at 30, next cluster was selected by adding 65 to 30. Similarly clusters were selected by adding 65 in the obtained number, to total of 30 clusters with each of size 15.
2.3. Clinical details
The PTB was identified based on sputum smear examination with at least 1 smear positive for AFB and/or radiographic abnormalities consistent with active PTB [5]. Clinical information form was filled at DOTS centres for each recruit to include age and sex, assess risk factors associated with PTB, such as smoking, alcohol, malnutrition, diabetes, HIV, drug abuse. In addition, clinical symptoms of cough with/without expectoration, fever, breathlessness, hemoptysis, weight loss, chest pain, diarrhea, loss of appetite, leg pain, weakness, abdominal pain, vomiting, headache and amenorrhoea were also recorded. The study was approved by institute’s ethics committee
2.4. Sample collection
One overnight and one spot sputum sample was collected from each patient and submitted to the laboratory under RNTCP. The laboratory is National Reference Laboratory (NRL), which is certified for first and second line DST for MTB by the Supra National Reference Laboratory, Institute of Tropical Medicine; Antwerp, Belgium. The laboratory caters to national level TB institute of India and is a DR-TB site for management of MDR-TB and XDR-TB patients.
2.5. Sample processing
Ziehl–Neelsen smear for detection of acid-fast bacilli (AFB) was performed for each sample and reported as per RNTCP guidelines [7]. The samples were processed by modified Petroff’s method of decontamination using 4% of sodium hydroxide (NaOH). The pellet obtained was washed twice before inoculation for culture [8].
2.6. Culture and identification
Each processed sample was cultured on two Lowenstein-Jensen (LJ) slopes. The cultures were incubated at 37 °C and observed for growth every week up to a maximum of eight weeks. All the isolates grown were identified as M. tuberculosis by their slow growth rate, colony morphology, smear microscopy from cultures and inability to grow on L-J media containing p-nitrobenzoic acid (PNB, 500 μg/ml) [8].
2.7. Drug susceptibility testing
All culture positive samples having sufficient growth for the preparation of inoculum were subjected to drug susceptibility testing (DST) for 1st line and 2nd line anti-tubercular drugs using the modified 1% proportion method on LJ medium [8]. The antibiotics (Sigma) included four 1st line anti-tubercular drugs, streptomycin; STR (4 μg/ml), INH (0.2 μg/ml), RIF (40 μg/ml), ethambutol; ETB (2 μg/ml) and seven 2nd line drugs, KAN (30 μg/ml), ethionamide; ETH (40 μg/ml), cycloserine; CYL (40 μg/ml), ofloxacin; OFL (2 μg/ml), para-amino salicylic acid; PAS (1 μg/ml), CAP (40 μg/ml) and AMK (40 μg/ml). The results were finalized on 42nd day as per the protocol. Any strain with 1% (the critical proportion) of bacilli resistant to any of the antibiotics, was classified as resistant to that drug.
2.8. Data analysis
Cluster sampling methodology was used to determine the clusters as explained above. The percentage proportion was adopted to determine the percentage of drug resistance.
3. Results
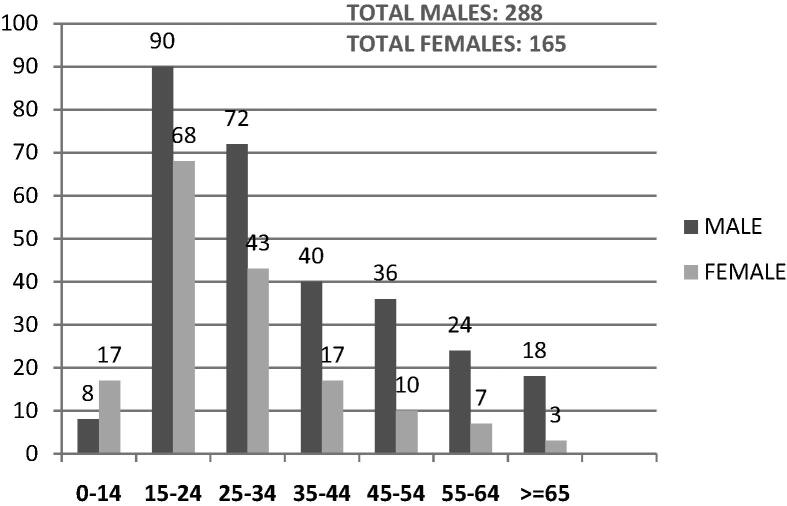
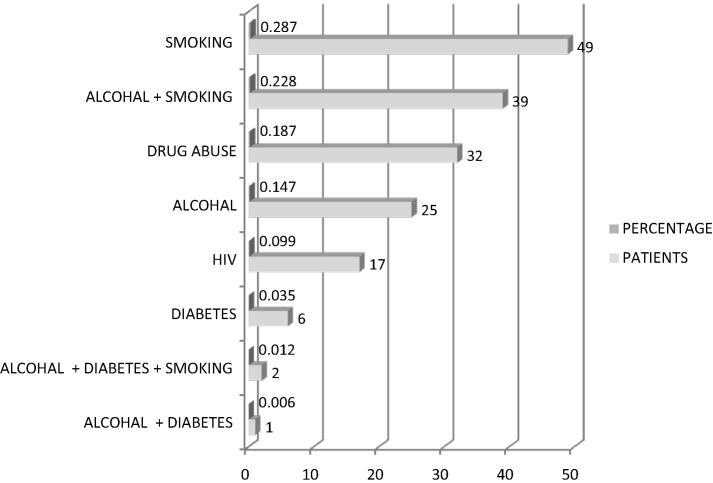
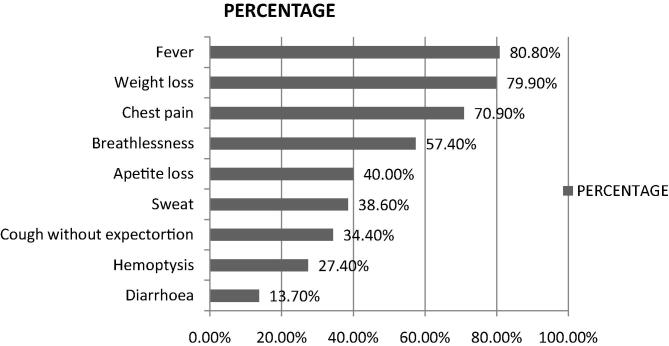
During period of July 2011 to June 2012, 453 newly diagnosed PTB cases were enrolled in this study. Age ranged from 10 year to 85 year old with median age being 27. Maximum patients were in the age group of 15–24 years (158). Overall, males predominated with ratio of males to females being 1.7:1. Females were commoner in the paediatric age group of 0–14 years of age (ratio 1.9:1) (Fig. 1). Of the 453 patients enrolled, 62.3% had no known risk factors. Smoking was found to be the commonest risk factor. The HIV positive patients formed 3.8% of the study group. Two percent of patients were found to be diabetics (Fig. 2). Commonest clinical symptoms reported by the recruits are depicted in Fig. 3. Maximum patients complained of fever (80.8%), weight loss (79.9%) and cough with expectoration (79.7%).
Fig. 1.
Age and sex distribution of the population enrolled.
Fig. 2.
Distribution of risk factors in the tuberculosis patients.
Fig. 3.
Common clinical symptoms found in newly diagnosed tuberculosis patients.
Of the sputum collected from 453 patients, 107 (23.6%) patients were smear negative, and 346 (76.4%) were smear positive. The smear positives included scanty positives 28/346 (8.1%), 1+; 126/346 (36.4%), 2+; 74/346 (21.4%) and 3+; 118/346 (34.1%). Of the total cases, 399/453 (88%) samples, at-least one LJ culture was positive for MTB. Nine (2.0%) cultures got contaminated. Total of 36 samples were both smear and culture negative, 333 samples were both smear and culture positive, 66 samples were smear negative and culture positive and 9 were smear positive and culture negative.
Out of the 399 cultures positives, DST was performed on 340 isolates with sufficient growth (more than 20 colonies) as is required for inoculum preparation to put solid culture DST. Among first line antibiotics tested, 261/340 (76.8%) cases were sensitive to all the four antibiotics whereas 5 (1.5%) cases were resistant to all four first line antibiotics. The resistance to any three first-line antibiotics was seen in 9/340 (2.6%) cases, all being resistant to STR, INH and RIF. Eighteen; (5.6%) isolates were found to be MDR. None of the patients were found to be only ethambutol resistant. None of the MDR patients were known as HIV positive. Among the MDR cases, two (11.1%) were children, both being female and one (5.5%) was male in the geriatric age group. Mono-INH resistance was found in 24 (7.1%) of isolates and mono-RIF resistance was found in 3(0.9%) of isolates. Among all cases enrolled (453), MDR prevalence was 4.0%. Antibiotic resistance pattern for first-line antibiotics is detailed in Table 2.
Table 2.
Drug susceptibility pattern of Mycobacterium tuberculosis to first line anti-tubercular agents.
| First line anti-tubercular agents | Number | Percentage |
|---|---|---|
| All sensitive | 261 | 76.80 |
| Streptomycin | 19 | 5.60 |
| Isoniazid | 24 | 7.10 |
| Rifampicin | 3 | 0.90 |
| Streptomycin + isoniazid | 14 | 4.10 |
| Streptomycin + rifampicin | 1 | 0.30 |
| isoniazid + rifampicin | 4 | 1.20 |
| Streptomycin + isoniazid + rifampicin | 9 | 2.60 |
| Streptomycin + isoniazid + rifampicin + ethambutol | 5 | 1.50 |
| Total | 340 | 100 |
Among second line antibiotics tested, 305 (89.7%) isolates were sensitive to all the four antibiotics whereas 1 (0.3%) isolate was resistant to all six second line antibiotics tested. The XDR prevalence was found to be 0.2% among all 453 patients enrolled. The details of resistance rates (%) observed against 2nd line drugs are shown in Table 3.
Table 3.
Drug susceptibility pattern of Mycobacterium tuberculosis to second line anti-tubercular agents.
| Second line anti-tubercular agents | Number | Percentage |
|---|---|---|
| All sensitive | 305 | 89.70 |
| Kanamycin | 1 | 0.30 |
| Ethionamide | 5 | 1.50 |
| Ofloxacin | 9 | 2.60 |
| PAS | 6 | 1.80 |
| Capreomycin | 1 | 0.30 |
| Kanamycin + PAS | 1 | 0.30 |
| Ethionamide + ofloxacin | 2 | 0.60 |
| Ethionamide + PAS | 3 | 0.90 |
| Ofloxacin + PAS | 2 | 0.60 |
| Capreomycin + PAS | 1 | 0.30 |
| Kanamycin + PAS + capreomycin | 1 | 0.30 |
| Ethionamide + ofloxacin + PAS | 1 | 0.30 |
| Ofloxacin + capreomycin + PAS | 1 | 0.30 |
| Kanamycin + PAS + capreomycin + ethionamide + ofloxacin + amikacin | 1 | 0.30 |
| Total | 340 | 100 |
4. Discussion
The present prospective study depicts the drug resistance to first line and second line anti-tubercular drugs among treatment naïve PTB patients of LRS district in New Delhi. The DOTS centres within the LRS district caters mainly to urban slums comprising of low socio-economic group. Though less, in the district most patients referred from private sector and various non government organizations in the area, were included. The sampling has been done systematically as per the standard guidelines [6]. During the study period, one morning and one spot sputum sample from 453 patients were received from these patients.
We found males to be predominant in our study with 63.6%. This finding is also seen in many other studies [5–9]. As this population constitutes an economically productive section of society, the presence of disease in this segment has far reaching socio-economic implications. Pressure to continue working in-spite of poor health could lead to decreased compliance toward regular medication thereby increasing the threat of developing drug resistance.
Most PTB cases were not associated with any risk factor. Among the risk factors found, smoking was found to be the commonest. Association of TB with smoking is well established as reported in earlier studies [10]. Impaired immunity is also associated with high incidence of TB. The lifetime risk of TB in immuno-competent persons is 5–10% but in HIV-positive individuals, there is 5–15% annual risk of developing active TB disease [11]. Impaired immunity is also seen in diabetes mellitus, a metabolic disorder. India accounts for 1/5th of world’s newly diagnosed TB of which almost 50% are diabetics [11]. In the present study, 3.8% and 2.0% of PTB patients were found to be HIV positive and diabetics respectively.
Commonest symptoms reported by the patients enrolled in the study included fever (80.8%), weight loss (79.9%) and cough with expectoration (79.7%), which are known in PTB patients. Surprisingly, 40% patients only complained of loss of appetite. Appetite-regulatory hormones are altered in TB patients, which improve on treatment [12].
Among newly diagnosed PTB patients, 76.4% were found to be smear-positive. World-wide around 56% of new cases of PTB are sputum smear positive tuberculosis [2]. Culture provided an incremental microbiological confirmation of 14.6% among the smear negatives which is less than expected. This could be because of use of harsher decontamination using modified Petroff’s method, recommended by RNTCP. Two percent of smear positives were culture negative and only 2.0% of total cultures got contaminated which is as per the acceptable standards [8].
The prevalence of MDR in our study from a RNTCP New Delhi among treatment naïve patients was found to be 4.0% which is higher than expected national average of 2.2% [2]. Treatment naïve MDR rates as reported by Indian studies from various regions, over the years ranged from 0.6% to 24% as detailed in Table 4 [5,13–18]. The high rate could be because most of the population is mainly from urban slums, i.e. low-socio economic background with over-crowded houses, lack of ventilation and poor hygiene. Much higher rates were found in studies from Lucknow and Mumbai, with treatment naïve MDR-TB to be 13.2% and 24% respectively. These studies had potential bias in the patient selection as the retreatment cases could not be fully excluded leading to high MDR rate. The high treatment naïve MDR-TB rate brings about the significance of applying newer rapid TB diagnostics such as Xpert and other nucleic acid assays in both private and public health systems in the region. Mathematical modelling studies have suggested the implementation of newer TB diagnostics and rapid DST methods, as these are cost effective alternatives in high burden settings that reduce spread of TB and DR-TB [19].
Table 4.
Indian studies on prevalence of primary multi-drug resistant tuberculosis in India.
| Authors | Year of publication & place | Study setting | Study design | Sample size | MDR rate (%) | Refs. |
|---|---|---|---|---|---|---|
| Present study | 2011–12 New Delhi, India | Lala Ram Sarup District with 12 Designated Microscopy Centres. Population of 0.8 million | Drug resistance estimation to first and second line anti-tubercular agents among new cases | 450 | 4.00 | |
| Sharma et al. | 2008–2009 New Delhi, India | Designated Microscopy Centre at Sanjay Gandhi Memorial Hospital | Drug resistance estimation to first line anti-tubercular agents among sputum positive cases | 218 | 1.10 | [5] |
| D’Souza et al. | 2004–7 Mumbai, India | Four central wards in Mumbai with high sputum-positive case load. Population 3 million | Levels of drug resistance to first line anti-tubercular agents in new and first line treatment-failure cases | 493 | 24 | [13] |
| Joseph et al. | 2006 Kerala, India | Ernakulam District | Level of drug resistance to first line anti-tubercular agents among new smear-positive pulmonary tuberculosis cases | 344 | 2 | [14] |
| Ramachandran et al. | 2005 Gujarat, India | State of Gujarat. 56 million population | Drug resistant survey to first and second line anti-tubercular agents on representative samples of new and previously treated pulmonary TB | 1571 | 2.40 | [15] |
| Paramasivan et al. | 2002 Tamil Nadu & Karnataka, India | 23 centres in North Arcot district and 20 centres in Raichur district | Levels of drug resistance to first line anti-tuberculars and ofloxacin in new and previously treated cases of pulmonary TB | 320, 314 | 3 | [16] |
| Jain et al. | 2000–2, Lucknow, India | One Primary Level Centre and one District Tuberculosis Centre and One Tertiary Centre Hospital | Prevalence of multi-drug resistance in three different levels of health care | 1014 | 13.20 | [17] |
| Jain et al. | 1990–91, New Delhi, India | Intermediate Reference Laboratory | Retrospective study of drug resistance levels to first line anti-tubercular agents in new and previously treated | NA | 0.60 | [18] |
NA: not available.
The XDR prevalence among all enrolled cases was found to be 0.2% with one isolate being XDR. This strain was resistant to other first line and second line antibiotics as well. The isolate belonged to a 64 year old adult male, HIV negative with history of diabetes. Presently, there are no data available on the prevalence of XDR among the newly diagnosed TB patients from any region of India. However few studies have reported XDR rates in previously treated TB patients ranging from 1.5% to 22.2% [20–24]. Worldwide, 6.7–11.2% of MDR cases are found as XDR [2]. Such patients are usually treatment failures to the available regimens. The transmission of primary XDR has been reported in South Africa, among HIV positive patients [25]. In India, facility for performing second line anti-tubercular DST is limited to few advanced laboratories, which contemplate detection of resistance to second line usually when patient is XDR-TB suspect. Besides, most such laboratories are not certified or accredited leading to paucity of authentic and validated drug susceptibility data [24].
This study has some limitations as, in spite of generating useful data of primary MDR-TB and XDR-TB prevalence in a RNTCP district, may not be representative of the whole country. A drug surveillance survey comprising RNTCP centres from different regions of the country, inclusive of private practitioners, is possibly warranted. The study also could not capture rural population as the area covered caters mainly to urban slums which are epidemiologically different.
5. Conclusion
To the best of our knowledge, this is the first systematic study from a district under RNTCP in India of determination of both MDR-TB and XDR-TB among treatment naïve TB cases. The high MDR-TB and XDR-TB in the small population studied, warrants accurate nation-wide multi-centric drug resistance surveillance in the country. Given the high level of MDR-TB, its rapid detection using WHO approved newer modalities for all diagnosed cases in the country, may be considered. Furthermore, setting up of more certified or accredited laboratories for diagnosis of any DR-TB is significant in ensuring accurate diagnosis of the condition. This in turn would determine accurate treatment of the patient eventually leading to decreased spread of DR-TB in the community and therefore control of the disease.
Acknowledgements
Authors acknowledge the technical help of Mr Kamaaluddin, Mr Neeraj Sharma and Mr Ved Prakash. No external funding was taken for the study, the study was supported by the parent institute.
Contributor Information
Vithal Prasad Myneedu, Email: tbmicro@gmail.com.
Ritu Singhal, Email: ritugo@hotmail.com.
Khalid Umer Khayyam, Email: dr.khaliduk@yahoo.com.
Prem Prakash Sharma, Email: ppsharma@hotmail.com.
Manpreet Bhalla, Email: mnprtbhalla@yahoo.com.
Digamber Behera, Email: dirlrsi@gmail.com.
Rohit Sarin, Email: tbmicro@gmail.com.
Conflict of interest
None declared.
References
- [1].Dooley SW, Jarvis WR, Martone WJ, Snider D., Jr Multidrug-resistant tuberculosis. Ann Intern Med. 1992;117:257–9. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization . Global Tuberculosis Report, Geneva, Switzerland, WHO Report 2013. Geneva, Switzerland: WHO/HTM/TB; 2013. [Google Scholar]
- [3].Shah NS, Wright A, Bai GH, Barerra L, Boulahbal F. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2014;13:30–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McGrath M, Pittius Gey van NC, Van Helden PD, Warren RM, Warner DF. Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2014;69:292–302. doi: 10.1093/jac/dkt364. [DOI] [PubMed] [Google Scholar]
- [5].Sharma SK, Kaushik G, Jha B. Prevalence of multi-drug resistant tuberculosis among newly diagnosed cases of sputum positive pulmonary tuberculosis. Indian J Med Res. 2011;133:308–11. [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization . Guidelines for surveillance of drug resistance in tuberculosis: WHO Report 2009. Geneva, Switzerland: WHO/HTM/TB; 2009. [Google Scholar]
- [7].Central Tuberculosis Division, Directorate General of Health Services, Ministry of Health and Family Welfare . Revised National Tuberculosis Control Programme, Module for laboratory technicians: RNTCP. New Delhi, India: 2005. [Google Scholar]
- [8].Central Tuberculosis Division, Directorate General of Health Services, Ministry of Health and Family Welfare . Manual of standard operating procedures of culture of Mycobacterium tuberculosis and drug susceptibility testing on solid medium: RNTCP. New Delhi, India: 2009. Revised National Tuberculosis Control Programme. [Google Scholar]
- [9].Shao Y, Yang D, Xu W, Lu W, Song H. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health. 2011;11:110. doi: 10.1186/1471-2458-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alcaide J, Altet MN, Plans P, Parrón I, Folguera L. Cigarette smoking as a risk factor for tuberculosis in young adults: a case-control study. Tuber Lung Dis. 1996;77:112–6. doi: 10.1016/s0962-8479(96)90024-6. [DOI] [PubMed] [Google Scholar]
- [11].Anamika G, Jitendra PM, Surya KS, Anil KG, Shampa A. Antitubercular drug resistance in four healthcare facilities in North India. J Health Popul Nutr. 2011;29:583–92. [PMC free article] [PubMed] [Google Scholar]
- [12].Chang SW, Pan WS, Beltran DL, Baldelomar LO, Solano MA, Friedland JS, et al. Gut harmones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One. 2013;8:545–64. doi: 10.1371/journal.pone.0054564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mistry NF, Vira TS, Dholakia Y, Hoffner S, Pasvo G. High levels of multidrug resistant tuberculosis in new and treatment-failure patients from the Revised National Tuberculosis Control Programme in an urban metropolis (Mumbai) in Western India. BMC Public Health. 2009;211:1–9. doi: 10.1186/1471-2458-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Joseph MR, Shoby CT, Amma GR, Chauhan LS, Paramasivan CN. Surveillance of anti-tuberculosis drug resistance in Ernakulam District, Kerala State, South India. Int J Tuberc Lung Dis. 2007;11:439–43. [PubMed] [Google Scholar]
- [15].Ramachandran R, Nalini S, Chandrasekar V, Dave PV, Sanghvi AS, Wares F. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154–60. [PubMed] [Google Scholar]
- [16].Paramasivan CN, Venkataraman P, Chandrasekaran V, Bhat S, Narayanan PR. Surveillance of drug resistance in tuberculosis in two districts of south India. Int J Tuberc Lung Dis. 2002;6:479–84. doi: 10.5588/09640569512977. [DOI] [PubMed] [Google Scholar]
- [17].Jain A, Mondal R, Prasad R, Singh K, Ahuja RC. Prevalence of multidrug resistant Mycobacterium tuberculosis in Lucknow, Uttar Pradesh. Indian J Med Res. 2008;128:300–6. [PubMed] [Google Scholar]
- [18].Jain NK, Chopra KK, Prasad G. Initial and acquired isoniazid and rifampicin resistance to Mycobacterium tuberculosis and its implication for treatment. Indian J Tuberc. 1992;39:12–4. [Google Scholar]
- [19].Zwerling A, White RG, Vassall A, Cohen T, Dowdy DW, Houben RM. Modeling of novel diagnostic strategies for active tuberculosis – a systematic review: current practices and recommendations. PLoS One. 2014;9(10):e110558. doi: 10.1371/journal.pone.0110558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases – a retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011;58:54–9. [PubMed] [Google Scholar]
- [21].Chakraborty N, De C, Bhattacharyya S, Mukherjee A, Santra S, Banerjee D, et al. Drug susceptibility profile of Mycobacterium tuberculosis isolated from HIV infected and uninfected pulmonary tuberculosis patients in eastern India. Trans R Soc Trop Med Hyg. 2010;104:101–95. doi: 10.1016/j.trstmh.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [22].Thomas A, Ramachandaran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, et al. Management of multi-drug resistance tuberculosis in the field: tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- [23].Mondal R, Jain A. Extensively drug resistant Mycobacterium tuberculosis, India. Emerg Infect Dis. 2007;13:1429–31. doi: 10.3201/eid1309.070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Michael JS, John TJ. Extensively drug-resistant tuberculosis in India: a review. Indian J Med Res. 2012;1(36):504–99. [PMC free article] [PubMed] [Google Scholar]
- [25].Horsburg J. Primary transmission of multidrug-resistant and extensively drug-resistant tuberculosis among HIV-infected persons. J Infect Dis. 2008;198:1577–8. doi: 10.1086/592992. [DOI] [PubMed] [Google Scholar]