Abstract
For certain subgroups within people living with the human immunodeficiency virus (HIV) [active tuberculosis (TB), pregnant women, children <5 years old, and serodiscordant couples], the World Health Organization recommends antiretroviral therapy (ART) irrespective of CD4 count. Another subgroup which has received increased attention is “HIV-infected presumptive TB patients without TB”. In this study, we assess the proportion of HIV-infected presumptive TB patients eligible for ART in Karnataka State (population 60 million), India. This was a cross-sectional analysis of data of HIV-infected presumptive TB patients diagnosed in May 2015 abstracted from national TB and HIV program records. Of 42,585 presumptive TB patients, 28,964 (68%) were tested for HIV and 2262 (8%) were HIV positive. Of the latter, 377 (17%) had active TB. Of 1885 “presumptive TB patients without active TB”, 1100 (58%) were already receiving ART. Of the remaining 785 who were not receiving ART, 617 (79%) were assessed for ART eligibility and of those, 548 (89%) were eligible for ART. About 90% of “HIV-infected presumptive TB patients without TB” were eligible for ART. This evidence supports a public health approach of starting all “HIV-infected presumptive TB patients without TB” on ART irrespective of CD4 count in line with global thinking about ‘test and treat’.
Keywords: ART eligibility, ART initiation criteria, HIV-infected presumptive TB patients, HIV-infected TB suspects, Operational research, WHO 2013 ART guidelines
1. Introduction
With an estimated 36.9 million people living with HIV (PLHIV), 2.0 million new HIV infections, and 1.2 million deaths in 2014, HIV continues to be the most common infectious cause of mortality in the world and has claimed >34 million lives so far [1]. Antiretroviral therapy (ART) is life-saving for PLHIV, and by the end of March 2015, about 15 million PLHIV were receiving ART. HIV is the only infectious disease where treatment is initiated only after it becomes clinically severe (assessed using CD4 counts). There have been various reasons for this strategy which include prioritizing toxic drugs for those with highest risk of progressing to AIDS, concerns about nonadherence, and risk of drug resistance if treatment is started too early. However, this situation is fast changing with the availability of newer and safer antiretroviral medicines and the evidence that early ART is beneficial even in asymptomatic PLHIV [2,3].
The World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) have both embarked on an ambitious vision of “90-90-90”: to detect 90% of all HIV-infected patients in the community, treat 90% of those detected with ART, and achieve viral suppression in 90% of those treated [4]. Given this, there is an increased demand from civil society organizations and patient groups to move toward a “test and treat” strategy. In 2013, WHO raised the threshold for ART initiation to a CD4 count ⩽500 cells/μL in adults, adolescents, and children aged 5 years and above. For certain patient groups like PLHIV having active tuberculosis (TB) disease, hepatitis B virus infection with severe chronic liver disease, pregnant and breast feeding women, children aged under 5 years, and those living in a serodiscordant relationship, ART is recommended irrespective of CD4 count (akin to a “test and treat” strategy) (Table 1) [5]. Another such subgroup which has caught global attention is “presumptive TB patients” (previously called TB suspects and defined as people with cough for 2 weeks or more with or without other symptoms suggestive of TB), but without TB.
Table 1.
Comparison of World Health Organization (WHO) guidelines for antiretroviral therapy (ART) initiation among people living with human immunodeficiency virus (HIV) in the year 2010 and 2013.
| Population | Target population | 2010 ART guidelines | 2013 ART guidelines |
|---|---|---|---|
| Adults and adolescents | HIV-infected individuals | CD4 count ⩽350 cells/mm3 or WHO clinical Stage 3 or 4 regardless of CD4 cell count | CD4 count ⩽500 cells/mm3 or WHO clinical Stage 3 or 4 regardless of CD4 cell count |
| HIV-infected pregnant and breastfeeding women | CD4 count ⩽350 cells/mm3 regardless of clinical symptoms or WHO clinical Stage 3 or 4 regardless of CD4 cell count | Regardless of CD4 cell count or WHO clinical stage | |
| HIV-infected partners in serodiscordant couple relationship(s) | No recommendation established | ||
| HIV/TB coinfection | Presence of active TB disease, regardless of CD4 cell count | No change | |
| HIV/HBV coinfection | Evidence of chronic active HBV disease, regardless of CD4 cell count | Evidence of chronic HBV disease with advanced stage liver disease (e.g., cirrhosis), regardless of CD4 cell count | |
| Children | HIV-infected children ⩾5 years old | CD4 ⩽350 cells/mm3 or WHO clinical Stage 3 or 4 regardless of CD4 cell count | CD4 count ⩽500 cells/mm3 or WHO clinical Stage 3 or 4 regardless of CD4 cell count |
| HIV-infected children 1–5 years old | 1. Between 12 and 24 months of age, regardless of CD4 count or WHO clinical stage | Regardless of CD4 cell count and clinical stage | |
| 2. Between 24 and 59 months of age with CD4 count of ⩽750 cells/mm3 or CD4% ⩽25, or whichever is lower, regardless of WHO clinical stage | |||
| HIV-infected infants <1 year old | All infants, regardless of CD4 cell count and clinical stage | No change |
ART = antiretroviral therapy; HBV = hepatitis B virus; HIV = human immunodeficiency virus; TB = tuberculosis; WHO = World Health Organization.
Several studies from sub-Saharan African countries and Asia show a high HIV prevalence among patients with presumptive TB ranging from 10% to 64%, sometimes even higher than the HIV prevalence among TB patients, prompting WHO to recommend routine HIV testing in such patients [6–14]. A prospective study from Zimbabwe showed that HIV-infected presumptive TB patients are a neglected group with only 15% getting ART, while about 85% had CD4 cell counts < 350 cells/μL and were eligible for ART at the time [4]. However, no study has systematically assessed this aspect in India.
Hence, in this study, we aim to determine the number of HIV-infected presumptive TB patients eligible for ART in a large south Indian state of Karnataka. The specific objectives were to determine among a cohort of presumptive TB patients (stratified by whether they have TB or not) attending the microscopy centers of Karnataka in May 2015: (1) number (proportion) ascertained for HIV status and found HIV positive; and (2) among HIV-infected patients, (a) number assessed for ART eligibility and found ART eligible and (b) number (proportion) initiated on ART.
2. Materials and methods
2.1. Study design
This was a cross-sectional study involving secondary analysis of data routinely recorded under the Revised National TB Control Programme (RNTCP) and National AIDS Control Programme (NACP).
2.2. Setting
India is considered a country with a concentrated HIV epidemic and contributes to about 10% of the global burden in absolute terms [1]. The HIV epidemic in India is showing a declining trend and in 2011, about 2.1 million people were living with HIV in India with an estimated 0.12 million new infections and 0.15 million deaths [15].
Karnataka State has an estimated 0.21 million PLHIV in 2011 and accounts for about 10% of the country’s HIV burden [15]. Hence, the state has been classified as “high priority” for HIV interventions by the National AIDS Control Organization (NACO) in India on the basis of consistently high HIV seroprevalence rates of >1% during sentinel surveillance at antenatal clinics [16,17]. In the state, TB control program services are available through a decentralized network of peripheral health institutions which provide general health services including diagnosis and treatment for TB.
Patients with presumptive TB are identified at the peripheral health institutions and referred for sputum smear microscopy to designated microscopy centers (DMCs), which are geographically distributed, each covering a population of 0.05–0.1 million. In situations where the patient is unable to physically visit the DMC, the sputum is collected and transported using existing systems and healthcare workers in the general health system or nongovernmental organizations. The diagnosis of TB is made in accordance with national guidelines. Presumptive TB patients are first tested using sputum smear microscopy and if found to be positive for acid-fast bacilli, patients are diagnosed as “smear-positive pulmonary TB” and initiated on treatment. Patients with negative smears are given antibiotics for 7–10 days and are tested for smear microscopy again if symptoms persist. If smears are negative in repeat sputum microscopy, patients undergo chest radiography. A diagnosis of smear-negative pulmonary TB is made if lesions consistent with TB are found on a chest radiograph. Patients with a normal chest X-ray and/or whose symptoms subsided by the trial of antibiotics were categorized as “presumptive TB without TB”. All diagnosed TB patients are treated with standardized fully intermittent thrice weekly short course regimens (6–9 months) administered under direct observation. Such patients are registered at one of the 177 subdistrict level TB program management units according to Indian program guidelines [18].
As per national policy, HIV status is routinely ascertained for all presumptive TB patients, and HIV-infected TB patients are referred to ART centers for initiation on ART and cotrimoxazole preventive therapy [19]. HIV status is ascertained at “integrated counseling and HIV testing centers” spread throughout the state, which are usually collocated with sputum microscopy services. HIV is diagnosed based on three positive rapid tests as per national guidelines [20]. Free ART is provided through a network of 64 ART centers (with at least 1 ART center in every district), where HIV-infected patients (including TB patients) are offered treatment and care. There are 194 link-ART centers in the state which offer follow up services at decentralized locations for HIV patients who are clinically stable on ART. These service delivery sites under NACP follow the national guidelines for counseling, testing, care, and treatment of HIV-infected patients [21]. In line with the higher burden of HIV, there is a higher density of HIV testing and care centers in the northern parts of the state [22].
2.3. Study population and study period
All patients with presumptive TB examined for diagnostic smear microscopy at the DMCs of Karnataka State in May 2015 constituted the study population. Patients attending for “re-examination” and follow-up examination were not included.
2.4. Data collection procedure and data variables
For objective 1, we collected aggregate data on the number of presumptive TB patients examined for smear microscopy, the number tested for HIV, and the number who were HIV positive. For the HIV-infected presumptive TB patients, individual patient data were collected using a structured, pretested data collection pro forma. The data variables included laboratory number, age, sex, TB diagnosis (smear-positive TB/smear-negative TB/extrapulmonary/no TB), TB treatment (yes/no), WHO clinical staging, latest CD4 lymphocyte count (at the time of registration for newly detected HIV patients or latest available count for patients already receiving ART), and ART status (already on ART before visiting DMC/started on ART after DMC visit/not started on ART). These variables were extracted from the TB laboratory register and TB treatment register of RNTCP and pre-ART patient register, patient ART card, and HIV-TB register of NACP. Information on whether the patient is diagnosed as smear-negative TB or extrapulmonary TB was extracted by referring to the TB register.
Data were collected by the “district PMDT/TBHIV coordinators” working for RNTCP and trained in the study protocol under the supervision of WHO consultants and state-level government program officers working for the TB and HIV programs.
2.5. Data entry and analysis
Aggregate information from each district was collected using an Excel format. For capture of patient-wise data, we used a combination of three open access sources (EpiData for data entry, Dropbox for sharing data and TeamViewer for trouble-shooting remotely). The technique has been detailed elsewhere [23]. Data entry was done by the data entry operators of the respective districts, trained for the purpose. Double data entry, validation, and analysis were done using EpiData software (version 3.1 for entry and version 2.2.2.182 for analysis, EpiData Association, Odense, Denmark). ART eligibility was assessed using WHO-2013 ART guidelines (CD4 count of <500, WHO clinical staging 3 and 4, children <5 years old, all HIV-TB patients), as the NACP in India has agreed in principle to adopt the recommendations [24,25]. In addition, if a patient was documented as “ART eligible” in the records and started on ART (even though there was no full documentation of CD4 counts and/or WHO clinical staging), we considered such patients to be eligible for ART for this analysis.
2.6. Ethics
Administrative approval to conduct the study and access the data was obtained from the State Tuberculosis Cell of Government of Karnataka. Ethics approval was obtained by the Ethics Advisory Group of International Union against Tuberculosis and Lung Disease, Paris, France. Since the study involved a secondary analysis of routine program records, the need for individual patient consent was waived by the ethics committee.
3. Results
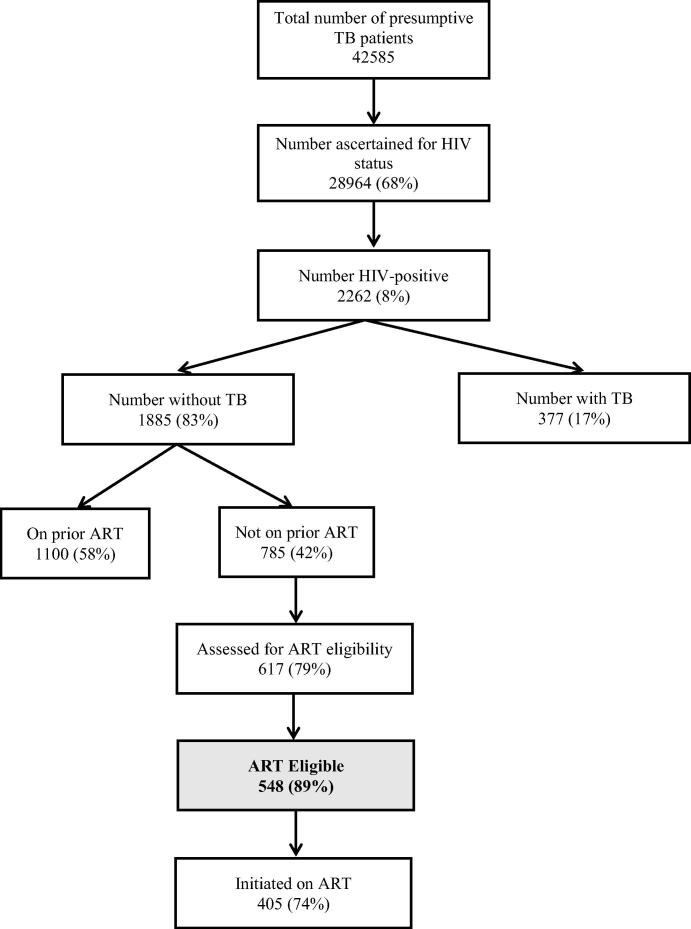
A total of 42,585 presumptive TB patients underwent sputum smear microscopy in 680 DMCs of 31 districts of Karnataka State, of whom 28,964 (68%) were ascertained for HIV status and 2262 (8%) were found to be HIV positive (Fig. 1). The median (interquartile range, IQR) age of HIV-infected patients was 38 (30–45) years and about half of them were males.
Fig. 1.
ART eligibility among human immunodeficiency virus (HIV)-infected presumptive tuberculosis (TB) patients in Karnataka, India, 2015. ART = antiretroviral therapy; HIV = human immunodeficiency virus; TB = tuberculosis. On prior ART-Person who was receiving ART prior to the current visit to sputum microscopy center (TB Clinic).
3.1. TB patients: diagnosis, TB treatment, and ART
Of 2262 HIV-infected presumptive TB patients, 377 (17%) were diagnosed to have active TB and of them, 370 (98%) were initiated on TB treatment. Of 377 TB patients, 194 (52%) had smear-positive pulmonary TB, 122 (32%) had smear-negative pulmonary TB, and 61 (16%) had extra pulmonary TB. Of the TB patients, 210 (57%) were receiving ART prior to TB diagnosis. Of the remaining 167, 122 (73%) were started on ART after TB diagnosis. Thus, a total of 332 (88%) were receiving ART during TB treatment.
3.2. Presumptive TB patients without active TB: ART eligibility and ART initiation
Among the 1885 “presumptive TB patients without active TB”, 1100 (58%) were already receiving ART prior to the current visit. Among the remaining 785 who were not receiving ART, 617 (79%) were assessed for ART eligibility and among them, 548 (89%) were found to be ART eligible as per WHO 2013 ART guidelines. Patients who were assessed for ART eligibility were similar to those not assessed by age (median age 39 years and 40 years, respectively, p = 0.95) and sex (proportion male 49% and 51%, respectively, p = 0.54). The CD4 distribution and WHO clinical staging information of 548 patients who were found to be eligible for ART is shown in Table 2. Of those found to be ART eligible, 405 (74%) were initiated on ART (Fig. 1).
Table 2.
CD4 counts and World Health Organization (WHO) clinical staging of “human immunodeficiency virus (HIV)-infected presumptive tuberculosis (TB) patients without TB” eligible for antiretroviral therapy (ART) in May 2015, Karnataka State, India.
| WHO clinical staging | ||||||
|---|---|---|---|---|---|---|
| Baseline CD4 count (cells/μL) | Stage 1 n (%) | Stage 2 n (%) | Stage 3 n (%) | Stage 4 n (%) | Unknown n (%) | Total n (%) |
| <50 | 15 (7) | 19 (10) | 17 (22) | 9 (60) | 24 (50) | 84 (15) |
| 50–200 | 68 (32) | 86 (44) | 34 (45) | 5 (33) | 8 (17) | 201 (37) |
| 201–350 | 67 (31) | 52 (27) | 13 (17) | 1 (7) | 7 (15) | 140 (26) |
| 351–500 | 47 (22) | 24 (12) | 7 (9) | 0 (0) | 8 (17) | 86 (16) |
| ⩾501 | 14 (7)a | 13 (7)a | 3 (4) | 0 (0) | 0 (0) | 30 (5) |
| Unknown | 3 (1)a | 1 (1)a | 2 (3) | 0 (0) | 1 (2)a | 7 (1) |
| Total | 214 | 195 | 76 | 15 | 48 | 548 |
ART = antiretroviral therapy; HIV = human immunodeficiency virus; WHO = World Health Organization.
These patients (n = 32) were documented as “antiretroviral therapy (ART) eligible” in the program records and started on ART even though the CD4/World Health Organization (WHO) staging criteria were either not documented or did not match eligibility criteria. They were considered “ART eligible” for the purpose of this analysis.
3.3. Median CD4 counts
Of 2262 patients, 1998 (88%) had information on CD4 counts. The median (IQR) CD4 count among patients with active TB was 194 (100–333) as compared to 264 (123–438) among “presumptive TB patients without active TB” (p < 0.001). Among those without active TB, median (IQR) CD4 count was significantly higher (p < 0.001) among those receiving ART at 281 (147–450) cells/μL as compared to those not on ART at 226 (92–399) cells/μL.
4. Discussion
These findings confirm our hypothesis and showed that nine out of 10 HIV-infected presumptive TB patients (without TB) are eligible for ART as per current WHO guidelines. This, too, is an underestimate, as we did not assess all the criteria of ART eligibility in our study. For example, we did not have any information about whether the patients in our study had coexisting hepatitis-B infection with severe liver disease, or if they were pregnant or living in a serodiscordant relationship. Some studies have indicated that nearly 75% of PLHIV in India are serodiscordant [26,27]. If we apply this figure to our cohort, then nearly all HIV-infected presumptive TB patients would be eligible for ART. Hence, as a public health approach to ART initiation, there is a strong case to be made for recommending that all HIV-infected presumptive TB patients should be initiated on ART irrespective of CD4 count.
There are a number of advantages with adoption of this recommendation. First, this is likely to improve uptake of ART (possibly by removing barriers associated with CD4 testing) in this vulnerable group. We have observed this in the past with HIV-infected TB patients when after adopting a “test and treat” strategy for TB patients, there was a significant increase in uptake of ART. The proportion of HIV-infected TB patients receiving ART in India increased from 41% in 2008 to 91% in 2014 [22]. This is observed in our study sample too – nearly 90% of HIV-infected TB patients received ART as compared to only 74% among those without TB. Despite high uptake of ART among HIV-TB patients in India, mortality remains high at 13%, emphasizing the need for early diagnosis and ART initiation [22].
Second, this is likely to lead to early diagnosis and treatment of HIV, thus preventing avoidable mortality, morbidity, and improving the quality of life in the long term. HIV testing among presumptive TB patients implies moving the HIV testing intervention to an upstream level in the TB diagnostic pathway and hence, patients are diagnosed earlier on in the natural course of disease. This is substantiated by higher median CD4 counts in our study, among presumptive TB patients (without TB) as compared to those with active TB. If active TB can be confidently ruled out using more sensitive diagnostic tools such as Xpert MTB/RIF or mycobacterial culture, such patients could be potentially eligible for isoniazid preventive therapy, which is known to act synergistically with ART in preventing TB. A recent randomized controlled trial has shown that early ART, along with IPT, is likely to provide maximum benefit to PLHIV in terms of preventing mortality and morbidity including TB [2].
It is essential to rule out active TB confidently before starting ART, as not doing so might pose a higher risk of developing immune reconstitution inflammatory syndrome.
Third, starting these patients earlier on ART is likely to improve their immune status. As observed in our study, the median CD4 counts were higher among “HIV-infected presumptive TB patients without active TB” already receiving ART prior to the current visit as compared to those who were not on ART. A previous study from Zimbabwe showed that not starting these patients on ART led to high mortality and morbidity [4]. In addition to individual benefits, early ART is also likely to have public health benefits – ART reduces the risk of transmission of HIV to partners [28] and avoids the operational problem of poor retention in pre-ART care [29,30].
Fourth, as only about 10% of presumptive TB patients were not eligible as per current guidelines, adopting this strategy is not likely to place a large additional burden on the national ART program in India. From a public health perspective, this is a step toward “immediate and universal ART” for every person with HIV in line with the vision of NACP phase IV for the period 2012–2017 [24].
Our study had several strengths. This is the first study from India examining the issue of ART eligibility among HIV-infected presumptive TB patients. Since the data come from a large and comprehensive sample of patients in a large state in India, the results are likely to reflect ground realities and are generalizable to similar high HIV settings in India. However, we need more evidence, especially from low-HIV settings in India, before a national policy decision can be considered. We used an innovative method of quality-assured data capture and this model is likely to be useful in coordinating data collection in multicenter research studies in resource-constrained settings.
There were a few limitations mainly related to our reliance on data collected routinely by the national TB and HIV programs. However, we think this is not a major limitation as both TB and HIV programs in the state of Karnataka are well supervised and monitored, and hence the quality of data is likely to be high. Information on ART eligibility (CD4 counts and/or WHO clinical staging) was missing in about one-fifth of the patients. Since those assessed for ART eligibility were similar to those not assessed by age and sex, we think the results can be extrapolated to those not assessed for ART eligibility. We did not have any information on presumptive extrapulmonary TB patients in our study and this should be a topic of future research. Although our recommendations are justified as a public health approach, it is important to study the long-term outcomes among the subgroup of patients with CD4 count of >500/mm3 and assess if they actually benefit by early initiation of ART. We, however, think early ART will be beneficial based on the evidence of two randomized controlled trials which have confirmed the benefit of early ART in preventing death and/or AIDS related events [2,3]. These trials have also confirmed that there was no or minimal increase in adverse events due to early ART initiation, thus allaying any fears that early ART initiation could be detrimental.
Finally, as the adage goes, “what gets monitored gets done”. Hence, we need to think about ways of monitoring the ART uptake among HIV-infected presumptive TB patients. We propose the addition of an extra column in the existing TB laboratory register to capture ART initiation status. From this, two indicators can be calculated: (1) proportion of presumptive TB patients ascertained for HIV; and (2) proportion of HIV-infected presumptive TB patients receiving ART. These indicators could then be compiled and reported in the quarterly reports of the TB program from the peripheral level, to district, to state, and to national levels, and finally finding a place in global TB and HIV reports. Future operational research should test this and/or other models of recording and reporting to find out the most optimal way forward.
5. Conclusion
In conclusion, about 90% of “HIV-infected presumptive TB patients without active TB” were eligible for ART as per WHO-2013 guidelines. As a public health approach, “HIV-infected presumptive TB patients without TB” is a vulnerable subgroup qualifying for “test and treat” strategy akin to HIV-infected TB patients.
Acknowledgments
The authors are thankful to the staff of the State TB Cell and Karnataka State AIDS prevention society, District TB Officers and their staff, and the staff of the ART centers of the state of Karnataka for enthusiastically assisting in the process of data collection. We would especially like to thank by name the “district PMDT/TBHIV co-ordinators” (Deshpande JR, Surendranath M Malkapur, Manjesh Kumar R, Prabhakar Gowda, Shauraj S, Patil PR, Gududaiah, Shivkumar Swamy, Jayteerth S Ashtaputre, Shekara P, Manjunatha BK, Shivanna S, Yathisha GS, Kishore PV, Srinivasa AD, Ambiger SL, Kencharaddiyavara DM, Suresh Doddamani, Manjaraj HT, Umapathi BS, Shashikala HS, Mahesh N, Danana Gouda Patil, Madegowda KD, Mahadev KS, Praveen Kumar Hiremath, Shivananjaiah N, Kumar S, Krishna Kumar R, Pramod Kumar Thingalaya, Rajendra MS, and Mallareddy) who were primarily responsible for data collection and the district data entry operators who painstakingly performed double entry and validation and ensured highest quality of data. Finally, the authors are grateful to the HIV-infected presumptive TB patients of Karnataka State who have indirectly contributed to this learning.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Conflicts of interest
No conflicts of interest have been declared.
References
- [1].UNAIDS . How AIDS changed everything. MDG6: 15 Years, 15 lessons of hope from the AIDS response: Joint United Nations Programme on HIV/AIDS. Geneva, Switzerland: 2015. Available at: < http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf>. Cited 09/19/2015. [Google Scholar]
- [2].Group TAS. Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/nejmoa1507198. [DOI] [PubMed] [Google Scholar]
- [3].Group ISS. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/nejmoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Joint United Nations Programme on HIV/AIDS . An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: 2014. Available at: < http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf>. Updated 09/19/2015. [Google Scholar]
- [5].World Health Organization . Consolidated guidelines for the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. June 2013. Geneva, Switzerland: World Health Organization; 2013. Available at: < http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html>. Updated 07/03/2013. [PubMed] [Google Scholar]
- [6].Srikantiah P, Lin R, Walusimbi M, Okwera A, Luzze H, Whalen CC, et al. Elevated HIV seroprevalence and risk behavior among Ugandan TB suspects: implications for HIV testing and prevention. Int J Tuberc Lung Dis. 2007;11:168–74. [PMC free article] [PubMed] [Google Scholar]
- [7].Deribew A, Negussu N, Kassahun W, Apers L, Colebunders R. Uptake of provider-initiated counselling and testing among tuberculosis suspects. Ethiop Int J Tuberc Lung Dis. 2010;14:1442–6. [PubMed] [Google Scholar]
- [8].Porskrog A, Bjerregaard-Andersen M, Oliveira I, Joaquím LC, Camara C, Andersen PL, et al. Enhanced tuberculosis identification through 1-month follow-up of smear-negative tuberculosis suspects. Int J Tuberc Lung Dis. 2011;15:459–64. doi: 10.5588/ijtld.10.0353. [DOI] [PubMed] [Google Scholar]
- [9].Odhiambo J, Kizito W, Njoroge A, Wambua N, Nganga L, Mburu M, et al. Provider-initiated HIV testing and counselling for TB patients and suspects in Nairobi, Kenya. Int J Tuberc Lung Dis. 2008;12(Suppl. 1):S63–8. [PubMed] [Google Scholar]
- [10].Munthali L, Mwaungulu JN, Munthali K, Bowie C, Crampin AC. Using tuberculosis suspects to identify patients eligible for antiretroviral treatment. Int J Tuberc Lung Dis. 2006;10:199–202. [PubMed] [Google Scholar]
- [11].Dimairo M, Macpherson P, Bandason T, Zezai A, Munyati SS, Butterworth AE, et al. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12 month cohort study in Harare, Zimbabwe. PLoS One. 2010;5:e11849. doi: 10.1371/journal.pone.0011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Adjei AA, Adiku TK, Ayeh-Kumi PF, Hesse IFA. Prevalence of human immunodeficiency virus infection among tuberculosis suspect patients in Accra, Ghana. West Afr J Med. 2006;25:38–41. doi: 10.4314/wajm.v25i1.28243. [DOI] [PubMed] [Google Scholar]
- [13].Naik B, Kumar AMV, Lal K, Doddamani S, Krishnappa M, Inamdar V, et al. HIV prevalence among persons suspected of tuberculosis: policy implications for India. J Acquir Immune Defic Syndr. 2012;59:e72–6. doi: 10.1097/qai.0b013e318245c9df. [DOI] [PubMed] [Google Scholar]
- [14].Achanta S, Kumar AMV, Burugina Nagaraja S, Jaju J, Motta Shamrao SR, Uappaluri R, et al. Feasibility and effectiveness of provider initiated HIV testing and counseling of TB suspects in Vizianagaram district, South India. PLoS One. 2012;7:e41378. doi: 10.1371/journal.pone.0041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National AIDS Control Organization, National Institute of Medical Statistics . Technical Report India HIV Estimates-2012: Directorate General of Health Services. Ministry of Health and Family Welfare, Government of India; 2013. Available at: < http://www.naco.gov.in/upload/Surveillance/Reports%20&%20Publication/Technical%20Report%20-%20India%20HIV%20Estimates%202012.pdf>. Cited 09/23/2015. [Google Scholar]
- [16].Office of the Registrar General and Census Commissioner . Provisional Population Totals paper-1: 2011 – Karnataka. New Delhi: Government of India; 2011. Available at: < http://censusindia.gov.in/2011-prov-results/prov_data_products_karnatka.html>. Cited 05/09/2011. [Google Scholar]
- [17].National AIDS Control Organization . HIV Sentinel Surveillance and HIV Estimation, 2007, A technical brief. Ministry of Health and Family Welfare, Government of India; 2008. Available at: < http://naco.gov.in/upload/Surveillance/Reports%20&%20Publication/HIV%20Sentinel%20Surveillance%20and%20HIV%20Estimation%202007_A%20Technical%20Brief.pdf>. Cited 09/23/2015. [Google Scholar]
- [18].Central TB Division . Managing the RNTCP in your area – A Training course (module 1–4) Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2005. Available at: < http://tbcindia.gov.in/index1.php?lang=1&level=3&sublinkid=4262&lid=2906>. Updated 08/12/2012; cited 09/17/2015. [Google Scholar]
- [19].National AIDS Control Organization, Central TB Division . National framework for Joint HIV/TB collaborative activities. Ministry of Health and Family Welfare, Government of India; 2009. Available at: < http://tbcindia.gov.in/showfile.php?lid=2857>. Cited 09/23/2015. [Google Scholar]
- [20].National AIDS Control Organization . Guidelines for HIV Testing. Ministry of Health and Family Welfare, Government of India; 2007. Available at: < http://www.naco.gov.in/NACO/Quick_Links/Publication/Blood_Safety__Lab_Services/Operational__Technical_guidelines_and_policies/Guidelines_for_HIV_test/>. Cited 09/23/2015. [Google Scholar]
- [21].National AIDS Control Organization . Operational Guidelines for Integrated Counselling and Testing Centres. Ministry of Health and Family Welfare, Government of India; 2008. Available at: < http://www.naco.gov.in/NACO/Quick_Links/Publication/Basic_Services/Operational__Technical_guidelines_and_policies/Operational_Guidelines_for_Integrated_Counseling_and_Testing_Centres/>. Cited 09/23/2015. [Google Scholar]
- [22].Central TB Division . TB India 2015. Revised National TB Control Programme Annual Status Report. Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2015. Available at: < http://tbcindia.gov.in/showfile.php?lid=3166. Updated 09/17/2015>; Cited 09/17/2015. [Google Scholar]
- [23].Kumar AMV, Naik B, Guddemane DK, Bhat P, Wilson N, Sreenivas AN, et al. Efficient, quality-assured data capture in operational research through innovative use of open-access technology. Public Health Action. 2013;3:60–2. doi: 10.5588/pha.13.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].National AIDS Control Organization . Shri Ghulam Nabi Azad launches National AIDS Control Programme-Phase IV. Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2014. Available from: < http://pib.nic.in/newsite/PrintRelease.aspx?relid=103593>. Cited 09/23/2015. [Google Scholar]
- [25].Dodderi SK, Kumar AM, Naik BR, Kanchar A, Rewari BB, Harries AD. How many people living with HIV will be additionally eligible for antiretroviral treatment in Karnataka State, India as per the World Health Organization 2013 guidelines? PLoS One. 2014;9:e107136. doi: 10.1371/journal.pone.0107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saggurti N, Schensul SL, Verma RK. Migration, mobility and sexual risk behavior in Mumbai, India: mobile men with non-residential wife show increased risk. AIDS Behav. 2009;13:921–7. doi: 10.1007/s10461-009-9564-8. [DOI] [PubMed] [Google Scholar]
- [27].Arora P, Nagelkerke N, Sgaier SK, Kumar R, Dhingra N, Jha P. HIV, HSV-2 and syphilis among married couples in India: patterns of discordance and concordance. Sex Transm Infect. 2011;87:516–20. doi: 10.1136/sextrans-2011-050203. [DOI] [PubMed] [Google Scholar]
- [28].Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.3410/f.718302523.793494710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63:e64–71. doi: 10.1097/qai.0b013e31828af5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ganga Devi NP, Ajay KM, Palanivel C, Sahu S, Selvaraj M, Valan A, et al. Implementation and operational research: high loss to follow-up among children on pre-ART care under national AIDS Program in Madurai, South India. J Acquir Immune Defic Syndr. 2015;69:e109–14. doi: 10.1097/qai.0000000000000640. [DOI] [PubMed] [Google Scholar]