Abstract
Heat stress hinders growth and well-being in livestock, an effect that is perhaps exacerbated by the β1 agonist ractopamine. Heat stress deficits are mediated in part by reduced feed intake, but other mechanisms involved are less understood. Our objective was to determine the direct impact of heat stress on growth and well-being in ractopamine-supplemented feedlot lambs. Commercial wethers were fed under heat stress (40 °C) for 30 d, and controls (18 °C) were pair-fed. In a 2 × 2 factorial, lambs were also given a daily gavage of 0 or 60 mg ractopamine. Growth, metabolic, cardiovascular, and stress indicators were assessed throughout the study. At necropsy, 9th to 12th rib sections (four-rib), internal organs, and feet were assessed, and sartorius muscles were collected for ex vivo glucose metabolic studies. Heat stress increased (P < 0.05) rectal temperatures and respiration rates throughout the study and reduced (P < 0.05) weight gain and feed efficiency over the first week, ultrasonic loin-eye area and loin depth near the end of the study, and four-rib weight at necropsy. Fat content of the four-rib and loin were also reduced (P < 0.05) by heat stress. Ractopamine increased (P < 0.05) loin weight and fat content and partially moderated the impact of heat stress on rectal temperature and four-rib weight. Heat stress reduced (P < 0.05) spleen weight, increased (P < 0.05) adrenal and lung weights, and was associated with hoof wall overgrowth but not organ lesions. Ractopamine did not affect any measured indicators of well-being. Heat stress reduced (P < 0.05) blood urea nitrogen and increased (P < 0.05) circulating monocytes, granulocytes, and total white blood cells as well as epinephrine, TNFα, cholesterol, and triglycerides. Cortisol and insulin were not affected. Heat stress reduced (P < 0.05) blood pressure and heart rates in all lambs and increased (P < 0.05) left ventricular wall thickness in unsupplemented but not ractopamine-supplemented lambs. No cardiac arrhythmias were observed. Muscle glucose uptake did not differ among groups, but insulin-stimulated glucose oxidation was reduced (P < 0.05) in muscle from heat-stressed lambs. These findings demonstrate that heat stress impairs growth, metabolism, and well-being even when the impact of feed intake is eliminated by pair-feeding and that systemic inflammation and hypercatecholaminemia likely contribute to these deficits. Moreover, ractopamine improved muscle growth indicators without worsening the effects of heat stress.
Keywords: animal well-being, beta agonist, blood pressure, glucose metabolism, growth efficiency, muscle growth
Introduction
Environmental heat stress diminishes growth efficiency and jeopardizes the well-being of livestock, particularly in feedlot animals (Renaudeau et al., 2012). Engineering interventions such as construction of artificial pen shade, water misters, and pen sprinklers are effective approaches for cooling pens, but the benefits are somewhat inconsistent and the financial cost to producers can be prohibiting (Boyd et al., 2015; Hagenmaier et al., 2016). We postulate that a better understanding of the physiological mechanisms mediating health and performance deficits could lead to more effective strategies for offsetting the detrimental effects of heat stress on livestock. Much of the impact of heat stress is facilitated by reduced energy intake (Brown-Brandl et al., 2003, 2017), but our research team recently found that skeletal muscle-specific glucose metabolism was also impaired in lambs exposed to chronic heat stress (Barnes et al., 2019). Similar findings were reported in heat-stressed pigs (Zhao et al., 2018), which also had impaired skeletal muscle fatty acid metabolism. Inflammatory factors are regulators of muscle growth and metabolism (Cadaret et al., 2017, 2019a), and our earlier study revealed evidence of systemic inflammation as a component of chronic heat stress (Barnes et al., 2019). Thus, the present study sought to determine the role of systemic inflammation and other stress responses in heat stress-induced deficits when the effect of reduced feed intake was eliminated by pair-feeding. In addition, feedlot cattle are often supplemented with the β1 adrenergic agonist ractopamine HCl during the last 28 to 40 d of the feedlot phase, which stimulates muscle growth and reduces adiposity (Bittner et al., 2016, 2017). Such growth promoters help producers fulfill the U.S. and global demands for high-quality protein, which by proxy benefits the economic sustainability of the beef industry. However, studies in cattle and pigs indicate that ractopamine may (James et al., 2013; Hagenmaier et al., 2017) or may not (Baszczak et al., 2006; Mendoza et al., 2017) modify the animal’s response to external stressors. Moreover, although transient episodes of tachycardia and arrhythmias have been noted in humans when beginning or changing dosages of certain β agonists for respiratory ailments (Sears, 2002), the effects of ractopamine on cardiovascular function in livestock have not been fully elucidated. It is worth noting that ruminant cardiac tissues express moderate amounts of β1 adrenergic receptors (Odore et al., 2007) and that ractopamine has some affinity for the more common β2 adrenergic receptors (Colbert et al., 1991). Because of the social and economic importance of a supplement that has the ability to produce more lean meat from fewer animals, it is imperative to define the additional risk, if any, of supplementing ractopamine to livestock during exposure to environmental heat stress. The objective of this study was to comprehensively assess the individual and interacting effects of 30-d heat stress and daily ractopamine supplementation on growth, metabolic efficiency, and well-being and to identify mechanisms for these effects in the absence of differential feed intake in finishing lambs, which are an effective model for studying ruminant growth and metabolism in place of cattle (Sewell et al., 2009; Lundy et al., 2015).
Materials and Methods
Animals and experimental design
This study was approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln (UNL). Studies were performed at the UNL Animal Science Complex, which is accredited by AAALAC International. Twenty-six crossbred Rambouillet wethers averaging 43 ± 1 kg were utilized in this study. Lambs were purchased from a commercial feedlot and given a 21-d acclimation period during which they were housed at 25 °C and transitioned to a pelleted lamb grower/finisher diet (Complete B30; Purina Animal Nutrition, St. Louis, MO) that contained 30 g/ton lasalocid. Lambs were then individually penned and randomly assigned to thermoneutral conditions of 18 °C, 15% relative humidity (controls; n = 14) or heat stress conditions of 40 °C, 35% relative humidity for 12 h and 30 °C, 35% relative humidity for 12 h/d (heat-stressed; n = 12) conditions for a period of 30 d. High temperatures within the thermally regulated environmental chambers were transitioned to 32 °C on day −1 and then to 40 °C thereafter. Heat-stressed lambs were fed ad libitum and controls were pair-fed to the average of the heat-stressed group beginning on day −5. In a 2 × 2 factorial, lambs also received an oral capsule bolus containing no supplement (n = 14) or ractopamine HCl (60 mg/d; n = 12) delivered once daily at 0700 hours via gavage. Daily dry matter intake and water intake were determined as previously described (Barnes et al., 2019). Lambs were weighed on days −7, 0, 14, 21, and 30, and these body weights (BW) were used to calculate average daily gain and gain-to-feed ratios. Respiratory rates and rectal temperatures were determined at 0700, 1300, and 1900 hours on days −1, 2, 7, 14, 21, and 30. To estimate carcass characteristics, ultrasonic measurements were performed on days 0, 14, and 30. On day 31, lambs were euthanized by double barbiturate overdose and necropsied in random order. Loin and rib cutouts were taken from the left side of the carcass. Loins were removed between the 12th rib and the connection point of the hip bone and weighed. Four-rib cutouts were trimmed at the leading edge of the 9th rib and the distal edge of the 12th rib, weighed, and dissected into muscle, fat, and bone components. Organs were weighed and tissue samples of liver, kidney, heart, adrenal, lung, bladder, rumen, and ileum along with all feet were examined for pathologies by the UNL Veterinary Diagnostics Center.
Ultrasonic carcass measurements
Back fat (subcutaneous) thickness, loin depth, loin-eye area, and body wall thickness between the 12th and 13th ribs were estimated by ultrasonography using National Sheep Improvement Program techniques as previously described (Emenheiser et al., 2010; Tait, 2016). Ultrasonic images were captured from each lamb’s left loin area with a Classic Scanner 200 and an ASP-18 transducer (Classic Medical Supply, Tequesta, FL). A standoff guide used for measurements other than body wall thickness. Vegetable oil was used as a couplant. Measurements were interpreted from single images frozen in real time.
Cardiovascular measurements
Heart rates, cardiac rhythms, and blood pressures were determined on days −1, 2, 7, 14, 21, and 30 with a multi-parameter veterinary monitor (Cardell 9500, Midmark, Dayton, OH) as previously described (Lopes et al., 2016). Briefly, lambs remained in their pens and were manually restrained by the head, which they had been conditioned to during the acclimation period. Alligator clips were used to attach the leads to shorn areas of the skin. Lead 1 was attached over the cervical vertebrae, lead 2 was attached on the fore flank, and the ground lead was attached on the rear flank. One-minute electrocardiograms were recorded based on the manufacturer’s recommendations for waveform speed and sensitivity and were professionally evaluated for arrhythmias by a veterinarian. Heart rates were averaged across the 1-min period. An SV 4 vinyl cuff (Midmark) was placed around the metacarpal area of the forelimb equidistance between the knee and fetlock, and five consecutive blood pressure readings were recorded at 1-min intervals, per manufacturer’s recommendations. Values for systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure were averaged across these five readings. At necropsy, hearts were dissected as previously described (Antolic et al., 2015) and left ventricle thickness, right ventricle thickness, and septum thickness were measured using a digital caliper (Traceable, Webster, TX).
Blood parameters
Blood was collected via jugular venipuncture into ethylenediaminetetraacetic acid (EDTA) vacutainer tubes (~6 mL) and heparinized syringes (~0.5 mL) for the analysis of blood components as previously described (Barnes et al., 2019). Samples collected in EDTA tubes were used to quantify total and differential white blood cell (WBC) concentrations using HemaTrue Veterinary Hematology Analyzer (Heska, Loveland, CO). Samples collected into heparinized syringes were used to quantify blood glucose, lactate, pH, O2, CO2, HCO3, Ca2+, Na+, and K+ using an ABL 90 FLEX blood gas analyzer (Radiometer, Copenhagen, Denmark). Plasma was isolated from the remaining blood in the EDTA tube by centrifugation at 14,000 × g for 5 min, and commercial ELISA kits were used to determine plasma concentrations of insulin (Alpco Diagnostics, Windham, NH), epinephrine (LDN, Nordhorn, Germany), tumor necrosis factor alpha (TNFα; Wuhan Fine Biotech, Wuhan, China), and cortisol (Oxford Biomedical Research, Riviera Beach, FL) in duplicate. Intra-assay and inter-assay coefficients of variance were less than 12% for all ELISA. Plasma concentrations of urea nitrogen, cholesterol, high-density lipoprotein cholesterol, and triglycerides were determined by a Vitros-250 Chemistry Analyzer (Ortho Clinical Diagnostics, Linden, NJ) by the University of Nebraska Biomedical and Obesity Research Core.
Skeletal muscle glucose metabolism
Sartorius muscle isolation
At necropsy, sartorius muscles were collected intact (i.e., tendon to tendon) from both hindlimbs at necropsy. Longitudinal strips (~400 mg) were used to measure ex vivo skeletal muscle glucose uptake and oxidation rates as previously described (Cadaret et al., 2017; Yates et al., 2019). Briefly, muscle strips were stratified by mass and placed in 6-well tissue culture plates. Muscle strips were then preincubated at 37 °C for 1 h in gassed (95% O2, 5% CO2) Krebs–Henseleit bicarbonate buffer (KHB) containing 0 (basal) or 5 mU/mL insulin (Humulin-R, Eli Lilly, Indianapolis, IN). Preincubation media also contained 5 mM glucose, 35 mM mannitol, and 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO). Muscle strips were then washed in the respective basal or insulin-spiked KHB media with no glucose, 40 mM mannitol, and 0.1% bovine serum albumin at 37 °C for 20 min.
Ex vivo glucose uptake
Glucose uptake was measured in one subset of sartorius muscle strips by determining the incorporation rate of radiolabeled 2-deoxyglucose as previously described (Cadaret et al., 2017, 2019a). After the wash incubation, these muscle strips were incubated in their respective basal or insulin-spiked KHB media containing 1 mM [3H]2-deoxyglucose (300 μCi/mmol) and 39 mM [1-14C] mannitol (1.25 μCi/mmol) at 37 °C for 20 min. Plates containing the muscle strips were then cooled at −20 °C for 2 min and placed on ice. Muscle strips were washed three times in ice-cold phosphate-buffered saline (7.4 pH) and lysed in 2 M NaOH at 37 °C for 1 h. Ultima Gold scintillation fluid (Perkin-Elmer, Inc.; Waltham, MA) was added to the lysate, and the specific activity of 3H and 14C was measured by liquid scintillation with a BC 1900 TA LC counter (Beckman-Coulter; Brea, CA). The specific activity 3H was used to determine the amount of 2-deoxyglucose that had accumulated, and 14C was used to estimate the amount of extracellular fluid in the sample, as mannitol is not taken up by muscle. The specific activity of the media was also determined from the average of six 10-μL aliquots mixed with 500 μL distilled water and scintillation fluid. Glucose uptake rates over the 20-min period were normalized to the mass of the muscle strip.
Ex vivo glucose oxidation
Glucose oxidation was measured in the second subset of sartorius muscle strips by determining the production rate of radiolabeled CO2 from radiolabeled glucose as previously described (Cadaret et al., 2017, 2019a). After the wash incubation, muscle strips were placed in one side of sealed dual-well chambers and incubated in their respective basal or insulin-spiked KHB media containing 5 mM [14C-U] d-glucose (0.25 μCi/mmol; Perkin-Elmer) at 37 °C for 2 h. In the adjacent well, 2 M NaOH was placed to capture CO2. After 2 h, chambers were cooled at −20 °C for 2 min and 2 M HCl was added to the media through the seal. The chambers were then incubated at 4 °C for 1 h. Afterward, NaOH was collected from the chamber and mixed with UltimaGold scintillation fluid to determine the specific activity of 14CO2 via liquid scintillation. Glucose oxidation in pmol was calculated from dpm counts for 14CO2 using the specific activity of the media, which was determined as described above. Glucose oxidation rates over the 2-h period were normalized to the mass of the muscle strip.
Statistical analysis
All data except histopathology data were analyzed using the mixed procedure of SAS 9.4 (SAS Institute, Cary, NC) to determine the effects of environmental condition, supplement, and their interaction in a 2 × 2 factorial design. Lamb was considered the experimental unit, and repeated measures (day) were used for serial measurements such as BW and blood components. Ex vivo data were analyzed with environmental condition and supplement as main effects and media insulin level as a repeated measure. Glucose uptake and oxidation rates were each measured in six technical reps per media condition for each lamb, which were then averaged. Histopathological data were analyzed for differences due to environmental conditions or dietary supplements by Chi-squared test using the frequency procedure of SAS. Fisher’s exact test was used for frequency analysis in which more than 25% of cells contained expected frequencies of less than 5. These data are presented as the frequency of occurrence (%). All other data are presented as means ± standard error. Variables for which 2-way and 3-way interactions were observed are denoted in the Results section. For variables where interactions were not observed, the results for main effects are presented. The threshold for significance was P ≤ 0.05, and tendencies are noted when P ≤ 0.10.
Results
Growth and body composition
No environmental condition × supplement interactions were observed for dry matter intake, average daily gain, or gain-to-feed ratios over any of the measured timeframes. Dry matter intake did not differ between thermoneutral controls and heat-stressed lambs (1.09 ± 0.03 vs. 1.12 ± 0.08 kg/d, respectively) due to pair feeding and did not differ between supplement groups. Average daily gain between days −7 and 0 (i.e., just before starting the study) did not differ between control and heat-stressed lambs (0.129 ± 0.018 vs. 0.110 ± 0.023, respectively) or between unsupplemented and ractopamine-supplemented lambs (0.112 ± 0.021 vs. 0.120 ± 0.022, respectively), and there was no environmental condition × supplement interaction over this period. Average daily gain and gain-to-feed ratios between days 0 and 7 were reduced (P < 0.05) in heat-stressed lambs when compared with controls but did not differ subsequently (Table 1). No two-way or three-way interactions were observed among environmental conditions, supplement, or day for any ultrasonic measurements. Ultrasonic measurements for back fat thickness were initially less (P < 0.05) for ractopamine-supplemented lambs than unsupplemented lambs (2.47 ± 0.10 vs. 3.32 ± 0.28 mm, respectively) at the start of the study, but the changes over time did not differ between environmental conditions or supplements. Ultrasonic measurements for loin-eye area and loin depth indicated reduced (P < 0.05) growth (i.e., change in size) in heat-stressed lambs over the course of the 30-d study, regardless of supplementation (Table 2). Ultrasonic measurements for body wall thickness did not differ between environmental conditions or supplements.
Table 1.
Growth and efficiency metrics in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d1
| Variable | Control | Heat stress | P-value | ||||
|---|---|---|---|---|---|---|---|
| No Suppl. | Ractopamine | No Suppl. | Ractopamine | Envir. | Suppl. | E * S | |
| Dry matter intake,2 kg/d | |||||||
| Day 0 to 7 | 0.89 ± 0.04 | 0.96 ± 0.02 | 1.07 ± 0.09 | 1.05 ± 0.09 | 0.06 | NS3 | NS |
| Day 7 to 14 | 1.05 ± 0.05 | 1.06 ± 0.04 | 1.07 ± 0.17 | 1.08 ± 0.08 | NS | NS | NS |
| Day 14 to 21 | 1.19 ± 0.03 | 1.19 ± 0.03 | 1.06 ± 0.21 | 1.16 ± 0.11 | NS | NS | NS |
| Day 21 to 30 | 1.21 ± 0.02 | 1.15 ± 0.06 | 1.25 ± 0.04 | 1.19 ± 0.10 | NS | NS | NS |
| Average daily gain,2 kg/d | |||||||
| Day 0 to 7 | 0.019 ± 0.037 | 0.024 ± 0.067 | −0.122 ± 0.071 | −0.144 ± 0.097 | 0.04 | NS | NS |
| Day 7 to 14 | 0.093 ± 0.022 | 0.127 ± 0.031 | 0.155 ± 0.038 | 0.095 ± 0.063 | NS | NS | NS |
| Day 14 to 21 | 0.084 ± 0.015 | 0.100 ± 0.020 | 0.106 ± 0.039 | 0.089 ± 0.043 | NS | NS | NS |
| Day 21 to 30 | 0.053 ± 0.009 | 0.090 ± 0.026 | 0.080 ± 0.029 | 0.047 ± 0.036 | NS | NS | NS |
| Gain-to-feed ratios2 | |||||||
| Day 0 to 7 | 0.018 ± 0.036 | 0.029 ± 0.072 | −0.120 ± 0.061 | −0.165 ± 0.106 | 0.03 | NS | NS |
| Day 7 to 14 | 0.091 ± 0.024 | 0.126 ± 0.029 | 0.117 ± 0.027 | 0.078 ± 0.056 | NS | NS | NS |
| Day 14 to 21 | 0.069 ± 0.026 | 0.091 ± 0.026 | 0.073 ± 0.030 | 0.074 ± 0.028 | NS | NS | NS |
| Day 21 to 30 | 0.044 ± 0.007 | 0.030 ± 0.027 | 0.062 ± 0.023 | 0.032 ± 0.027 | NS | NS | NS |
1Ractopamine HCl (60 mg/d) was bolused daily.
2Daily averages over each period.
3NS, not significant.
Table 2.
Ultrasound-estimated loin growth in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d1
| Variable | Control | Heat stress | P-value | ||||
|---|---|---|---|---|---|---|---|
| No Suppl. | Ractopamine | No Suppl. | Ractopamine | Envir. | Suppl. | E * S | |
| Loin-eye area, cm2 | |||||||
| Day 0 | 12.9 ± 0.3 | 12.0 ± 0.4 | 13.9 ± 0.7 | 13.0 ± 0.7 | 0.07 | NS2 | NS |
| Day 14 | 13.3 ± 0.5 | 12.9 ± 0.3 | 13.6 ± 0.6 | 13.2 ± 0.8 | NS | NS | NS |
| ∆Day 0 to 142 | +0.4 ± 0.6 | +0.9 ± 0.5 | −0.3 ± 0.4 | +0.2 ± 0.4 | NS | NS | NS |
| Day 30 | 14.5 ± 0.6 | 13.1 ± 0.5 | 12.5 ± 0.6 | 13.1 ± 0.7 | 0.09 | NS | NS |
| ∆Day 0 to 30 | +1.6 ± 0.7 | +1.2 ± 0.6 | −1.4 ± 0.7 | +0.1 ± 0.4 | 0.002 | NS | NS |
| Loin depth, mm | |||||||
| Day 0 | 24.9 ± 0.5 | 24.5 ± 0.1 | 26.8 ± 1.0 | 26.7 ± 0.8 | 0.008 | NS | NS |
| Day 14 | 25.7 ± 0.7 | 25.1 ± 0.5 | 25.9 ± 1.0 | 26.2 ± 1.0 | NS | NS | NS |
| ∆Day 0 to 14 | +0.8 ± 0.7 | +0.6 ± 0.5 | −0.9 ± 0.6 | −0.5 ± 0.9 | 0.05 | NS | NS |
| Day 30 | 26.1 ± 0.5 | 25.5 ± 0.7 | 24.6 ± 0.6 | 24.9 ± 0.8 | 0.09 | NS | NS |
| ∆Day 0 to 30 | +1.2 ± 0.5 | +1.0 ± 0.7 | −2.2 ± 0.6 | −1.8 ± 1.0 | <0.001 | NS | NS |
1Ractopamine HCl (60 mg/d) was bolused daily.
2∆, change over time; NS, not significant.
Loin mass and loin mass/BW did not differ between environmental conditions but were greater (P < 0.05) in ractopamine-supplemented lambs than in unsupplemented lambs (Table 3). Four-rib mass was less (P < 0.05) in heat-stressed lambs than in controls, regardless of the supplement. An environmental condition × supplement interaction was observed (P < 0.05) for four-rib mass/BW, which was greater (P < 0.05) in unsupplemented controls than ractopamine-supplemented controls and was reduced (P < 0.05) in heat-stressed lambs regardless of supplement. Bone mass and fat mass from the dissected four-rib cutout were less (P < 0.05) for heat-stressed lambs than for controls, and fat masses were greater (P < 0.05) in ractopamine-supplemented compared with unsupplemented lambs. Dissected four-rib muscle mass did not differ among any groups, but an environmental condition × supplement interaction was observed (P < 0.05) for four-rib muscle mass/BW, which were reduced (P < 0.05) in all heat-stressed lambs compared with unsupplemented controls but not ractopamine-supplemented controls. Proximate analysis of the longissimus dorsi revealed environmental condition × supplement interactions (P < 0.05) for moisture, protein, and fat percentages. Moisture percentage was greater (P < 0.05) in muscle from unsupplemented heat-stressed lambs than any other group and did not differ among ractopamine-supplemented heat-stressed lambs, unsupplemented controls, or ractopamine-supplemented controls. Protein percentage was greater (P < 0.05) in muscle from all heat-stressed lambs than controls but was also greater (P < 0.05) in ractopamine-supplemented heat-stressed lambs compared with unsupplemented heat-stressed lambs. Fat percentage was less (P < 0.05) in all heat-stressed lambs compared with controls but was also less (P < 0.05) in unsupplemented heat-stressed lambs compared with ractopamine-supplemented heat-stressed lambs.
Table 3.
Primal cut metrics and composition in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d1
| Variable | Control | Heat stress | P-value | ||||
|---|---|---|---|---|---|---|---|
| No Suppl. | Ractopamine | No Suppl. | Ractopamine | Envir. | Suppl. | E * S | |
| Four-rib2 | |||||||
| Mass, g | 488.8 ± 18.8 | 506.4 ± 24.1 | 422.7 ± 20.2 | 447.6 ± 17.1 | 0.006 | NS3 | NS |
| Mass/BW | 13.6 ± 0.7a | 10.2 ± 0.8b | 7.1 ± 0.9c | 8.3 ± 0.7c | — | — | 0.009 |
| Bone mass, g | 120.1 ± 5.4 | 113.2 ± 5.1 | 88.4 ± 9.1 | 99.5 ± 6.6 | 0.003 | NS | NS |
| Fat mass, g | 135.7 ± 7.3 | 155.6 ± 6.5 | 109.4 ± 5.0 | 117.4 ± 2.9 | <0.001 | 0.02 | NS |
| Lean mass, g | 219.3 ± 6.1 | 218.9 ± 11.1 | 209.8 ± 13.1 | 203.0 ± 7.5 | NS | NS | NS |
| Loin | |||||||
| Mass, g | 526.1 ± 19.5 | 583.1 ± 14.7 | 497.4 ± 19.3 | 594.4 ± 43.5 | NS | 0.009 | NS |
| Mass/BW | 11.1 ± 0.5 | 12.9 ± 0.5 | 10.2 ± 0.6 | 12.9 ± 0.5 | NS | <0.001 | NS |
| % Moisture | 71.6 ± 0.3a | 71.5 ± 0.1a | 72.7 ± 0.2b | 71.3 ± 0.1a | — | — | 0.003 |
| % Protein | 20.2 ± 0.1a | 20.0 ± 0.1a | 20.5 ± 0.1b | 21.0 ± 0.1c | — | — | 0.006 |
| % Fat | 7.0 ± 0.3a | 7.1 ± 0.1a | 5.3 ± 0.2b | 6.4 ± 0.2c | — | — | 0.03 |
1Ractopamine HCl (60 mg/d) was bolused daily.
29th to 12th rib section.
3NS, not significant.
a–cMeans with different superscripts differ (P < 0.05).
Organ size and pathology
Adrenal weights (5.1 ± 0.7 vs. 2.6 ± 0.6 g), adrenal weight/BW (0.026 ± 0.006 vs. 0.049 ± 0.007 g/kg), lung weights (893 ± 91 vs. 566 ± 80 g), and lung weight/BW (8.7 ± 1.0 vs. 5.5 ± 0.9 g/kg) were greater (P < 0.05) in heat-stressed lambs than in controls, regardless of ractopamine supplementation. Spleen weights (170 ± 21 vs. 250 ± 18 g) and spleen weight/BW (1.6 ± 0.2 vs. 2.4 ± 0.2 g/kg) were less (P < 0.05) in heat-stressed lambs than in controls, regardless of supplement. Kidney weights, kidney weight/BW, liver weights, liver weight/BW, heart weights, and heart weight/BW did not differ among groups.
Histopathological diagnoses of hepatic multifocal neutrophilic aggregates, renal nephrosis or tubule mineralization, illeal Peyer’s patch lymphoid follicles, and myocardial, adrenal, or pulmonary focal lymphoid infiltrates did not differ in frequency between heat-stressed lambs and controls or between ractopamine-supplemented and unsupplemented lambs. No pathological conditions were diagnosed in bladder or rumen tissues in any lambs. Hoof wall overgrowth was more prevalent (P < 0.05) in heat-stressed lambs than controls (91% vs. 7%) but did not differ in prevalence between ractopamine-supplemented and unsupplemented lambs. Laminitis of the hoof did not differ in prevalence among any groups.
Rectal temperature, respiration rates, and water consumption
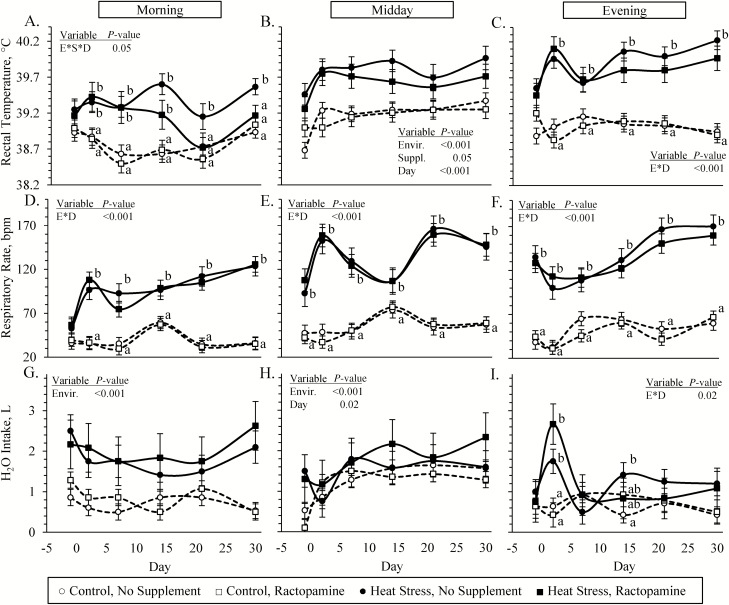
An environmental condition × supplement × day interaction was observed (P < 0.05) for morning rectal temperatures and environmental condition × day interactions were observed (P < 0.05) for evening rectal temperatures, evening water consumption, and respiratory rates at all three times of the day. Morning rectal temperatures were greater (P < 0.05) in heat-stressed lambs than controls on days 2, 7, and 14 and were greater (P < 0.05) in unsupplemented heat-stressed lambs than lambs in the other three groups on days 21 and 30 (Figure 1A). Midday rectal temperatures were greater (P < 0.05) in heat-stressed lambs than controls and were less (P = 0.05) in ractopamine-supplemented lambs than unsupplemented lambs (Figure 1B). Evening rectal temperatures were greater (P < 0.05) in heat-stressed lambs than controls for all days except day −1 (Figure 1C). Morning respiratory rates did not differ between groups on day −1 but were greater (P < 0.05) in heat-stressed lambs than controls on all other days, regardless of supplement (Figure 1D). Midday and evening respiratory rates (Figure 1E and F, respectively) were greater (P < 0.05) in heat-stressed lambs than controls for all days, regardless of the supplement. Morning and mid-day water consumption rates (Figure 1G and H, respectively) were greater (P < 0.05) in heat-stressed lambs than controls, regardless of the supplement. Evening water consumption rates were greater (P < 0.05) in heat-stressed lambs compared with controls but only on days 2 and 14 (Figure 1I).
Figure 1.
Physiological responses in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are shown for rectal temperatures (A, B, and C), respiratory rates (D, E, and F), and H2O intake (G, H, and I). Assessments were performed in the morning (0700 hours), at midday (1300 hours), and in the evening (1900 hours). Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05).
Blood metabolites and hormones
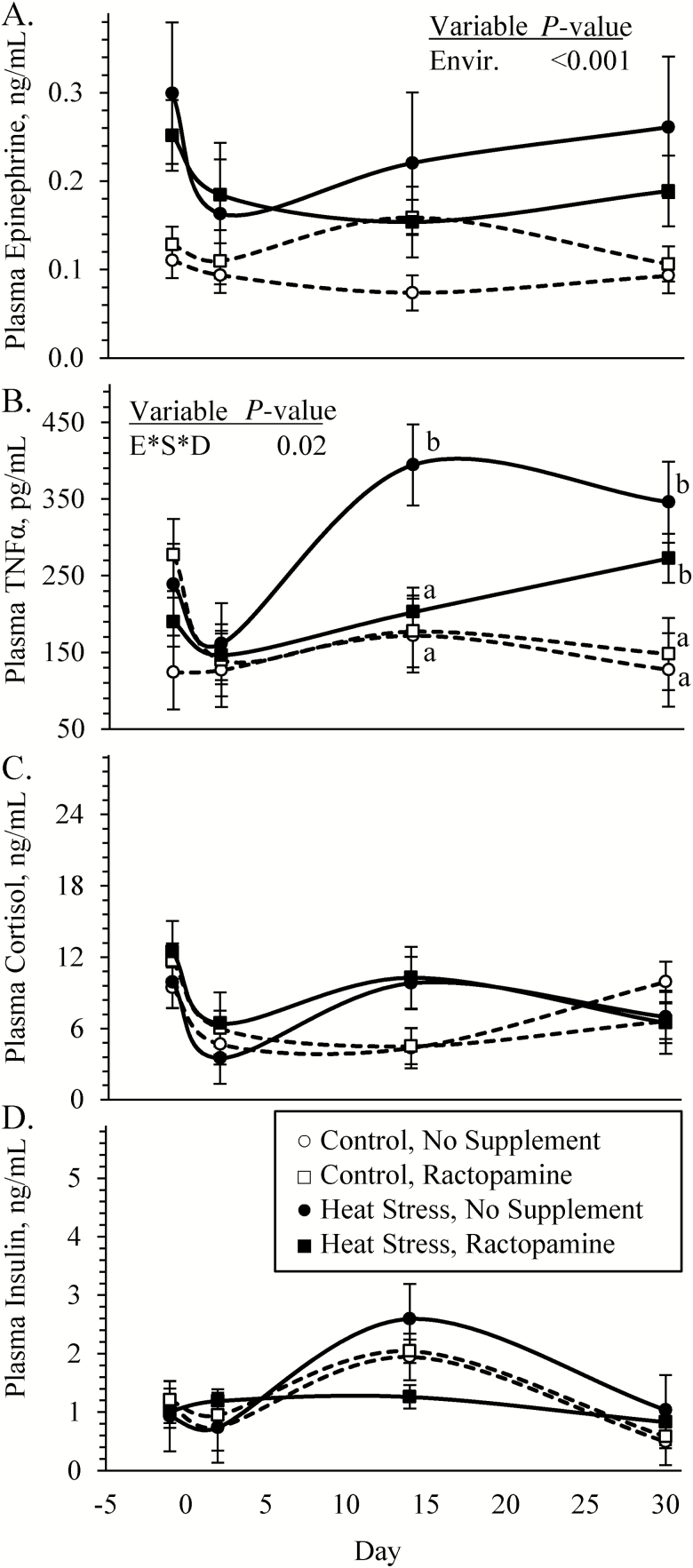
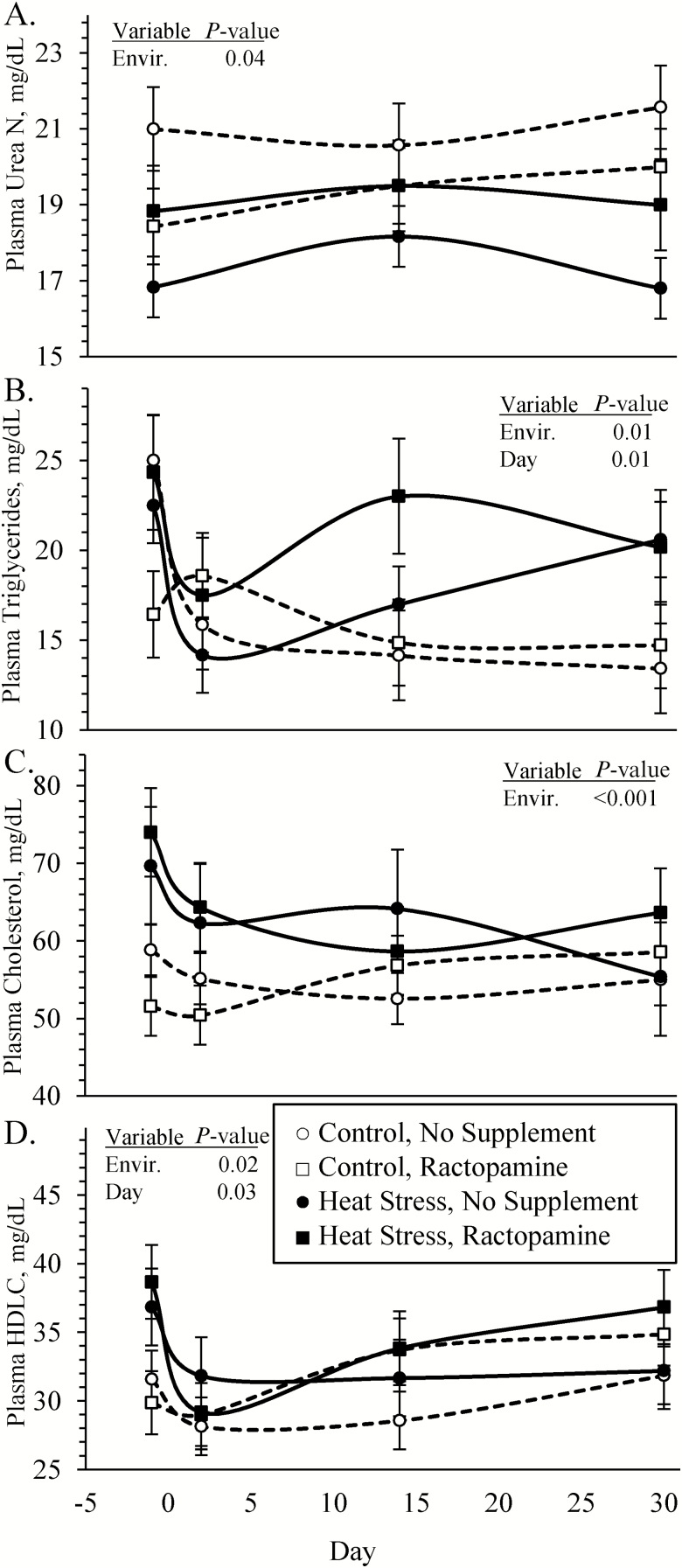
Blood plasma epinephrine concentrations were greater (P < 0.05) in heat-stressed lambs compared with controls, regardless of ractopamine supplementation (Figure 2A). An environmental condition × supplement × day interaction was observed (P < 0.05) for plasma TNFα concentrations. Plasma TNFα did not differ among groups for day −1 or 2 but were greater (P < 0.05) in unsupplemented heat-stressed lambs than lambs from the other three groups on day 14 and were greater (P < 0.05) in all heat-stressed lambs than controls on day 30 (Figure 2B). Plasma cortisol and insulin concentrations did not differ among any groups (Figure 2C and D, respectively). Blood glucose concentrations (4.25 ± 0.09 vs. 4.46 ± 0.04 mM) tended to be less (P = 0.07) in heat-stressed lambs than controls, and an environmental condition × day interaction was observed (P < 0.05) for glucose-to-insulin ratios. These ratios (5.8 ± 1.1 vs. 9.0 ± 1.3 mmol/U) were less (P < 0.05) in heat-stressed lambs than controls on day 30 but did not differ on any other days. Blood lactate concentrations did not differ among any groups. Blood plasma urea nitrogen concentrations (Figure 3A) were reduced (P < 0.05) and plasma triglyceride, cholesterol, and high-density lipoprotein (HDL)-cholesterol concentrations (Figure 3B, C, and D, respectively) were greater (P < 0.05) in heat-stressed lambs than in controls, regardless of the supplement.
Figure 2.
Circulating hormone concentrations in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are shown for plasma epinephrine (A), TNFα (B), cortisol (C), and insulin (D) concentrations. Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05). Where interactions were observed, means with differing superscripts differ (P < 0.05) within each day.
Figure 3.
Circulating metabolites in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are presented for blood plasma urea nitrogen (A), triglyceride (B), cholesterol (C), and HDL-cholesterol (D) concentrations. Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05).
Blood gasses and electrolytes
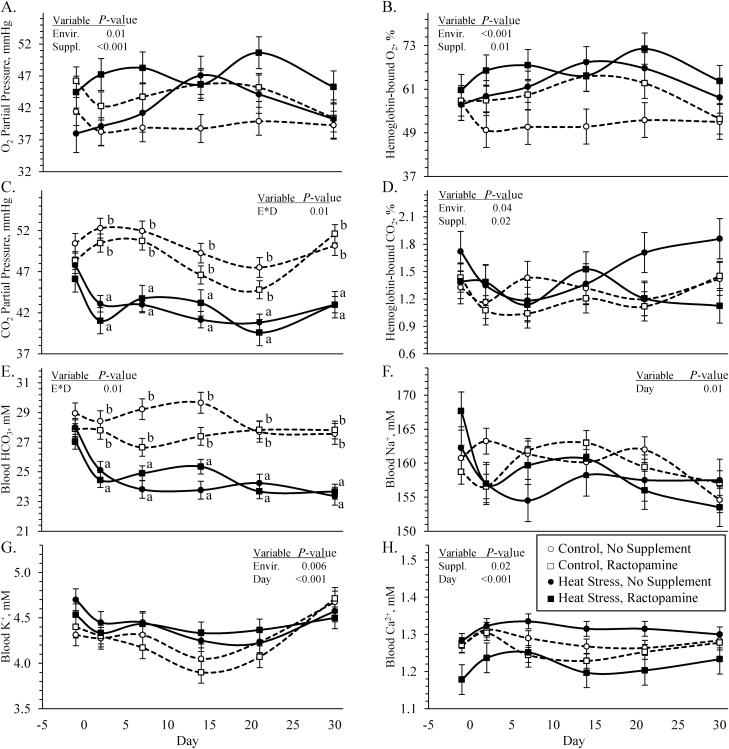
Blood O2 partial pressures and hemoglobin-bound O2 (Figure 4A and B, respectively) were greater (P < 0.05) in heat-stressed lambs compared with controls and were greater (P < 0.05) in ractopamine-supplemented compared with unsupplemented lambs. Hemoglobin-bound CO2 was greater (P < 0.05) in heat-stressed lambs compared with controls and was less (P < 0.05) in ractopamine-supplemented compared with unsupplemented lambs (Figure 4D). Environmental condition × day interactions were observed (P < 0.05) for blood CO2 partial pressures (Figure 4C), HCO3 concentrations (Figure 4E), and base excesses, which did not differ among groups on day −1 but were greater (P < 0.05) for heat-stressed lambs than controls on all other days, regardless of supplementation. Blood pH did not differ among any groups. Blood Na+ concentrations (Figure 4F) did not differ among any groups, but blood K+ concentrations (Figure 4G) were greater (P < 0.05) in heat-stressed lambs regardless of supplement, and blood Ca++ concentrations (Figure 4H) were less (P < 0.05) in ractopamine-supplemented lambs regardless of environmental condition.
Figure 4.
Blood gas and electrolyte concentrations in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are presented for O2 partial pressure (A), hemoglobin-bound O2 (B), CO2 partial pressure (C), hemoglobin-bound CO2 (D), HCO3 concentrations (E), Na+ concentrations (F), K+ concentrations (G), and Ca2+ concentrations (H). Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05). Where interactions were observed, means with differing superscripts differ (P < 0.05) within each day.
Hematology
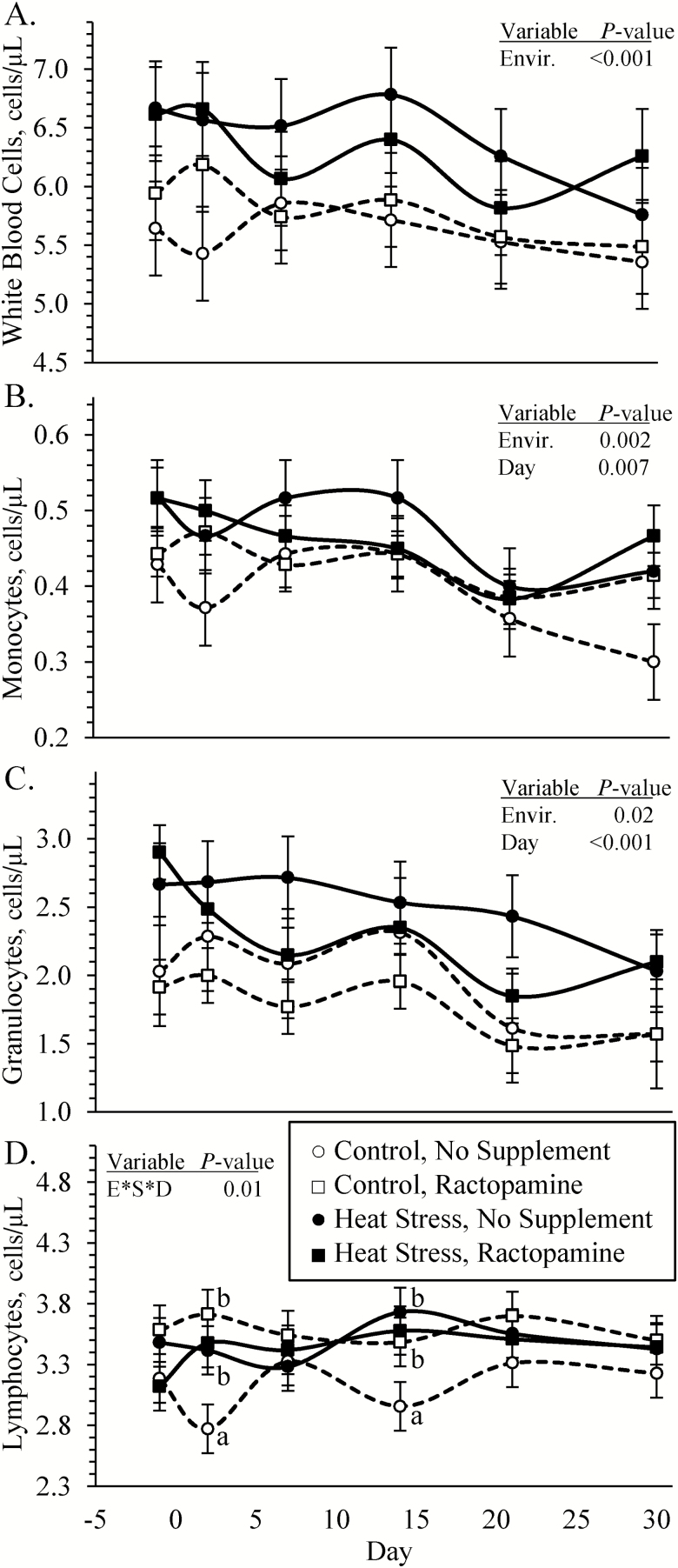
Blood WBC, monocyte, and granulocyte concentrations (Figure 5A, B, and C, respectively) were greater (P < 0.05) in blood from heat-stressed lambs than from controls. An environmental condition × supplement × day interaction was observed (P < 0.05) for blood lymphocyte concentrations (Figure 5D), which did not differ among any groups on days −1, 7, 21, or 30 but were greater (P < 0.05) in all heat-stressed lambs and ractopamine-supplemented controls compared with unsupplemented controls on days 2 and 14. Blood hematocrit, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, mean packed cell volume, red blood cell concentrations, and platelet concentrations did not differ among any groups. An environmental condition × supplement × day interaction was observed (P < 0.05) for red blood cell distribution width, which did not differ among any groups on days −1, 2, 7, and 14 but was less (P < 0.05) in ractopamine-supplemented and unsupplemented heat-stressed lambs and in ractopamine-supplemented controls compared with unsupplemented controls on day 21 (18.3 ± 0.3%, 17.8 ± 0.5%, 18.1 ± 0.4%, and 19.2 ± 0.4%, respectively) and day 30 (18.6 ± 0.3%, 17.8 ± 0.4%, 18.9 ± 0.4%, and 19.5 ± 0.6 %, respectively).
Figure 5.
Circulating leukocyte concentrations in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are presented for total WBC (A), monocyte (B), granulocyte (C), and lymphocyte (D) concentrations in whole blood. Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05). Where interactions were observed, means with differing superscripts differ (P < 0.05) within each day.
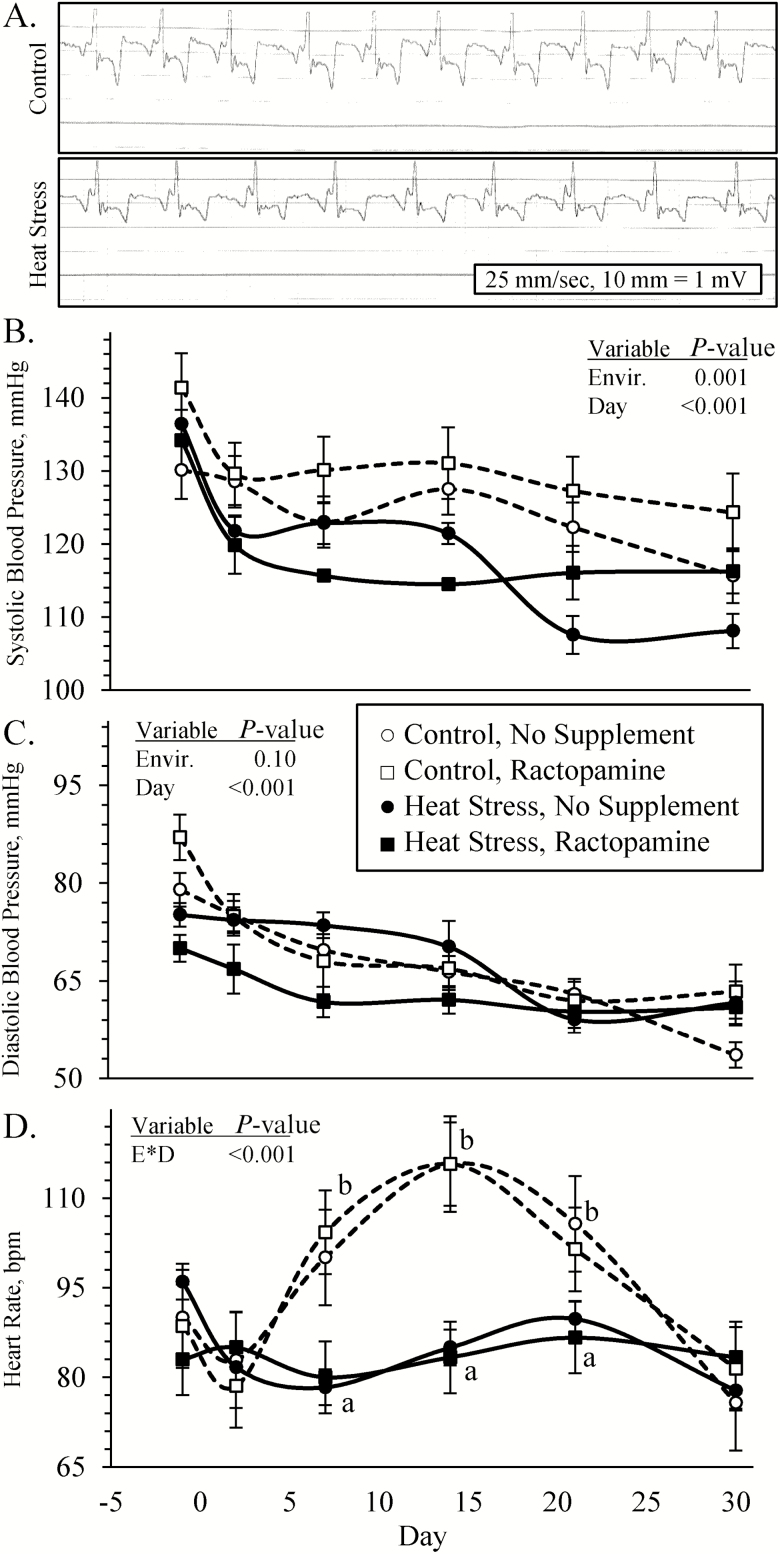
Cardiovascular parameters
Representative electrocardiograms are reported in Figure 6A. No pathological abnormalities in cardiac rhythms were detected from electrocardiograms. No interactions among environmental condition, supplement, and day were observed for blood pressures. Systolic arterial blood pressures (Figure 6B) and mean arterial blood pressures (93.0 ± 1.1 vs. 97.8 ± 1.1 mmHg)) were less (P < 0.05) in heat-stressed lambs and diastolic arterial blood pressures (Figure 6C) tended to be less (P = 0.09) in heat-stressed lambs compared with controls. An environmental condition × day interaction was observed (P < 0.05) for pulse rate (Figure 6D), which did not differ among groups on days −1, 2, or 30 but was less (P < 0.05) in heat-stressed lambs compared with controls on days 7, 14, and 21.
Figure 6.
Cardiovascular responses in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Representative electrocardiograms are shown for controls and heat-stressed lambs (A). Data are presented for systolic blood pressure (B), diastolic blood pressure (C), and heart rates (D). Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), day, and the interaction (E * S * D) are noted when significant (P < 0.05). Where interactions were observed, means with differing superscripts differ (P < 0.05) within each day.
Environmental condition × supplement interactions were observed (P < 0.05) for left ventricular thickness and left ventricle/septum, which were greater (P < 0.05) in all heat-stressed lambs than controls but were greatest (P < 0.05) in unsupplemented heat-stressed lambs (Table 4). Right ventricular thickness, septum thickness, and left ventricle/right ventricle tended to be greater (P < 0.10) in heat-stressed lambs, regardless of the supplement. Right ventricle/septum did not differ among groups.
Table 4.
Heart metrics in heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d1
| Variable | Control | Heat stress | P-value | ||||
|---|---|---|---|---|---|---|---|
| No Suppl. | Ractopamine | No Suppl. | Ractopamine | Envir. | Suppl. | E * S | |
| Heart mass, g | 282 ± 32 | 274 ± 32 | 351 ± 38 | 318 ± 34 | 0.10 | NS2 | NS |
| Heart mass/BW | 2.7 ± 0.3 | 2.7 ± 0.3 | 3.3 ± 0.4 | 3.1 ± 0.3 | NS | NS | NS |
| L. ventricular wall2, mm | 16.7 ± 0.9a | 18.1 ± 1.3ab | 22.9 ± 0.8c | 20.3 ± 0.6b | — | — | 0.03 |
| R. ventricular wall2, mm | 7.5 ± 0.6 | 7.1 ± 0.6 | 9.4 ± 0.7 | 7.6 ± 0.6 | 0.08 | NS | NS |
| Septum, mm | 17.5 ± 1.1 | 17.4 ± 0.9 | 18.9 ± 0.9 | 19.4 ± 0.9 | 0.09 | NS | NS |
| L. ventricle/septum | 0.96 ± 0.02 | 1.04 ± 0.06 | 1.24 ± 0.05 | 1.05 ± 0.04 | — | — | 0.01 |
| R. ventricle/septum | 0.43 ± 0.01 | 0.41 ± 0.02 | 0.51 ± 0.08 | 0.40 ± 0.05 | NS | NS | NS |
| L. ventricle/R. ventricle | 2.25 ± 0.15 | 2.53 ± 0.15 | 2.59 ± 0.18 | 2.75 ± 0.16 | 0.09 | NS | NS |
1Ractopamine HCl (60 mg/d) was bolused daily.
2L, left; NS, not significant; R, right.
a–cMeans with different superscripts differ (P < 0.05).
Skeletal muscle glucose metabolism
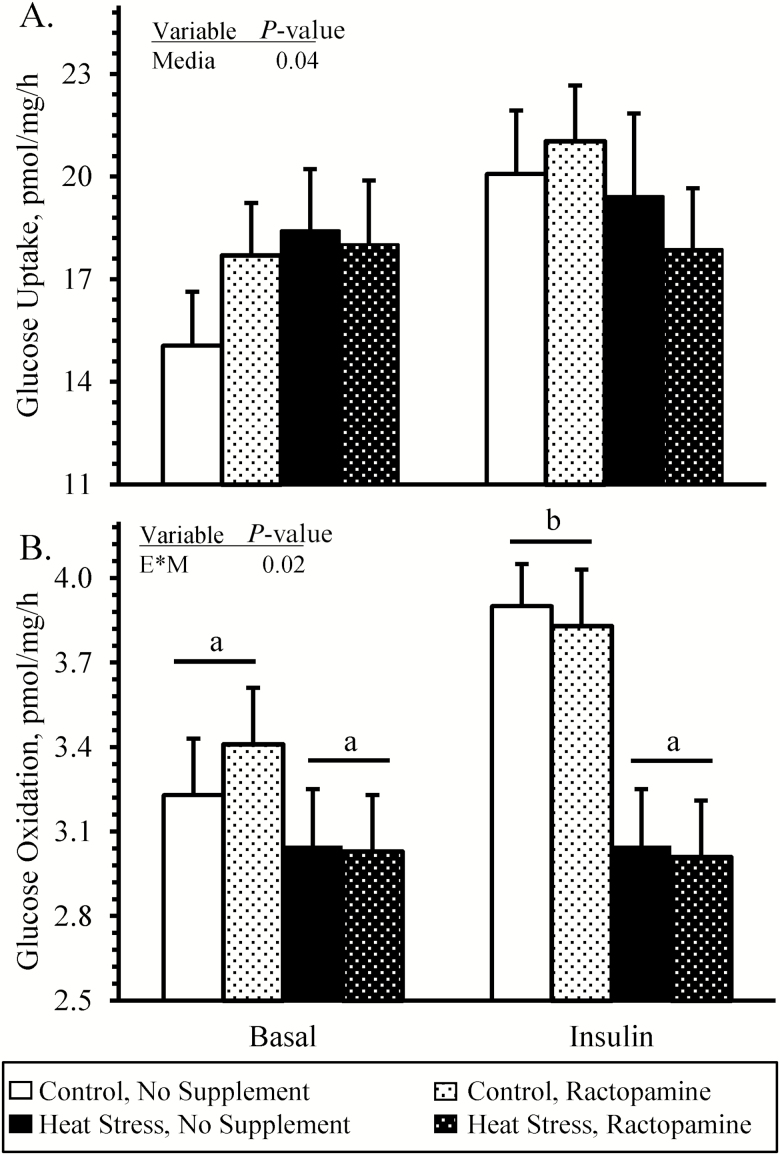
No interactions between environmental condition, supplement, and media condition were observed for ex vivo skeletal muscle glucose uptake rates (Figure 7A), which were greater (P < 0.05) in insulin-spiked media compared with basal media but did not differ among any groups. An environmental condition × media condition interaction was observed (P < 0.05) for ex vivo skeletal muscle glucose oxidation rates (Figure 7B). In basal media, glucose oxidation did not differ among groups. In insulin-spiked media, glucose oxidation was less (P < 0.05) in muscle from heat-stressed lambs than controls, regardless of the supplement.
Figure 7.
Ex vivo glucose metabolism in skeletal muscle from heat-stressed lambs fed concentrate diets supplemented with ractopamine for 30 d. Data are presented for glucose uptake (A) and glucose oxidation (B) rates in intact sartorius muscle strips incubated with 0 (basal) or 5 mU/mL insulin. Lambs were fed in a 2 × 2 factorial: unsupplemented controls (n = 7), controls supplemented with 60 mg/d ractopamine HCl (n = 7), unsupplemented heat-stressed lambs (n = 6), and heat-stressed lambs supplemented with ractopamine HCl (n = 6). Effects of environmental condition (Envir.), dietary supplement (Suppl.), media, and the interaction (E * S * M) are noted when significant (P < 0.05). Where interactions were observed, means with differing superscripts differ (P < 0.05).
Discussion
In this study, we found that heat stress sustained over a 30-d period reduced muscle growth, impaired metabolism, and altered cardiovascular function in feedlot lambs independently of its effects on dietary intake. Heat-stressed lambs exhibited reduced weight gain and growth efficiency compared with their pair-fed thermoneutral counterparts early in the feeding period and presented smaller ultrasound-estimated ribeye areas and loin depths by the end of the feeding period. At necropsy, they possessed lighter primal cuts with less intramuscular fat content. Muscle strips isolated from heat-stressed lambs were impaired in their capacity for glucose metabolism, which coincided with hyperlipidemia throughout the study. Heat stress not only produced predictable physiological responses, including hyperthermia, hyperventilation, adrenal hypertrophy, and hypercatecholaminemia, but also induced chronic systemic inflammation that was characterized by increased circulating leukocytes and TNFα. Surprisingly, circulating cortisol concentrations were similar between heat-stressed and thermoneutral lambs throughout the study, perhaps because pair-feeding equalized nutritional status. It appeared that heat-stressed lambs developed left-ventricular hypertrophy despite having lower peripheral blood pressures and heart rates throughout the study. Although heat stress did not increase the prevalence of any internal organ lesions, it did increase adrenal and lung size, reduce spleen size, and was associated with greater prevalence of hoof wall overgrowth. Daily supplementation of the β1 adrenergic agonist ractopamine resulted in moderate improvements in muscle growth but did not improve growth efficiency or metabolic function, although the fact that feed intake was reduced may have contributed to its limited effectiveness. Importantly, ractopamine did not cause cardiovascular abnormalities, internal organ lesions, or hoof pathologies. It also did not worsen the negative effects of heat stress on well-being or performance and in some cases moderated them. Together, these findings demonstrate that heat stress impairs growth and efficiency in feedlot lambs via physiological mechanisms that are independent of and in addition to reduced feed intake. Two such mechanisms indicated by the results of this study are sustained systemic inflammation and hypercatecholaminemia. In addition, ractopamine supplementation during heat stress did not present a greater apparent risk to animal well-being than supplementation under thermoneutral conditions.
By pair-feeding thermoneutral controls to heat-stressed lambs, we were able to demonstrate that the early deficits in growth efficiency and the sustained deficits in muscle growth occurred in the absence of reduced dietary intake. The consequences of heat-stressed livestock going off of feed are well-characterized across many species (Mader, 2003; Wheelock et al., 2010; Zhao et al., 2018), and in an earlier study, we observed that heat stress caused lambs to consume about 21% less of a high-concentrate diet when offered ad libitum (Barnes et al., 2019). However, growth rates in our previous study were reduced by 36%, which provides evidence that mechanisms other than just eating less were contributing to heat stress-induced growth restriction. In the present study, growth indicators were indeed impaired when reduced feed intake was eliminated as a cause. In fact, heat-stressed lambs lost weight over the first week of this study, although their average daily gain and feed efficiency recovered and stabilized thereafter, indicating at least some acclimation to heat-stress conditions. It is important to note that this study does not discount the role of reduced feed intake in heat stress-induced growth deficits but in fact confirms its importance, as growth restriction here was less severe than in the previous study. Moreover, the rates of gain in our pair-fed thermoneutral lambs were only a fraction of those observed in ad libitum-fed thermoneutral lambs from our previous study (Barnes et al., 2019). Nevertheless, muscle growth rates estimated by ultrasonic loin measurements progressively worsened over the 30-d period, resulting in diminished size of primal cuts by the end of the study and demonstrating conclusively the existence of direct mechanisms of heat stress.
Daily supplementation of the β1 adrenergic agonist ractopamine at the recommended dosages did not impact the rate of gain or feed efficiency for lambs housed under either environmental condition, but it did increase indicators of muscle growth. When we failed to observe effects on growth or efficiency from ractopamine or zilpaterol included in the feed rations in our previous study (Barnes et al., 2019), we speculated that it was due to potential variation among lambs in the amount or timing of supplement consumed or to the short (21 d) duration of the feeding period. However, administering exact doses of ractopamine by gavage and extending the feeding period to 30 d in the present study likewise did not affect weight gain or growth efficiency. Recent studies have reported that supplementing ractopamine to bulls (Cônsolo et al., 2016; Antonelo et al., 2017) or steers (Bittner et al., 2017) for around 30 d increased average daily gain by up to 28% and feed efficiency by up to 30%, which makes it tempting to speculate that sheep are less responsive to β1 agonists than cattle. However, ractopamine did increase loin growth by about 15% in our lambs, which was comparable to the gain in loin muscle size observed in cattle. It also partially mitigated the loss of intramuscular fat due to heat stress, which was unexpected considering that it reduces marbling in cattle under normal environmental conditions (Gonzalez et al., 2010; Bittner et al., 2016).
Muscle isolated from heat-stressed lambs at the end of the feeding period exhibited intrinsically impaired capacity for insulin-stimulated glucose oxidation, although glucose oxidation rates under unstimulated conditions were normal. Impaired responsiveness of glucose oxidation to insulin confirmed our previous observations in lambs heat stressed for 21 d (Barnes et al., 2019) as well as a recent study in pigs heat stressed for 7 d (Zhao et al., 2018). Moreover, this impairment occurred in the presence of normal insulin-responsiveness glucose uptake, which demonstrates inefficient glucose metabolism that presumably contributes to poor growth efficiency. Although we did not assess skeletal muscle glucose uptake rates in our previous study, we did observe indicators of compensatory insulin sensitivity in muscle from heat-stressed lambs (Barnes et al., 2019). Moreover, skeletal muscle gene expression in pigs after 8-d heat stress indicated that oxidative metabolism capacity was reduced despite normal or even greater insulin-responsive glucose clearance (Sanz Fernandez et al., 2015; Victoria Sanz Fernandez et al., 2015). Thus, we speculate that impaired insulin-stimulated glucose oxidation by heat stress is not a product of disruption in canonical insulin signaling and, in fact, may be the impetus for enhanced insulin sensitivity. Daily supplementation of ractopamine had no effect on glucose uptake or oxidation rates, which is not surprising considering our previous observations of its effects on skeletal muscle metabolism (Cadaret et al., 2017).
Heat stress induced persistent systemic inflammation over the 30-d period, which helps to explain the observed reductions in muscle growth and metabolic function. Muscle growth requires the incorporation of stem cells called myoblasts as well as favorable rates of protein synthesis relative to protein degradation (Rhoads et al., 2016). We were not able to assess myoblast function or protein synthesis in the present study, but we previously observed that enhanced inflammatory tone impaired myoblasts isolated from fetal sheep (Posont et al., 2018). Moreover, a recent study in mice showed that chronic inflammation disrupts anabolic protein turnover patterns (Ceelen et al., 2018). In a separate study, we reported that in vitro exposure to inflammatory cytokines did not affect insulin-responsive glucose uptake of muscle from adult rats, but that insulin-responsive glucose oxidation was impaired despite a stimulatory response to cytokines in the absence of insulin (Cadaret et al., 2017). We also reported that chronic inflammatory conditions in utero reduced glucose oxidation rates in fetal muscle without affecting glucose uptake (Cadaret et al., 2019a, 2019b). Although not conclusive, the present findings in the context of our previous findings and the literature implicate a role for inflammation in the growth and metabolic deficits observed in our heat-stressed lambs.
Systemic inflammation and hypercatecholaminemia may also explain hyperlipidemia in our heat-stressed lambs. Catecholamines stimulate fat mobilization (Ferlay and Chilliard, 2018) and thus high circulating epinephrine in our heat-stressed lambs helps to explain elevated circulating triglycerides. Chronically elevated plasma epinephrine is also associated with increased circulating cholesterol (Dimsdale et al., 1983). Although we did not detect a reduction in back fat thickness via ultrasound, heat-stressed lambs had reduced fat content in primal cuts and reduced intramuscular fat percentages in the loin muscle, perhaps due to greater rates of mobilization. In addition, inflammation disrupts β oxidation of fatty acids (Remels et al., 2010, 2013), which may have resulted in lipid accumulation in the bloodstream. Blood plasma urea nitrogen concentrations were reduced by heat stress in this study, which was consistent with our previous findings in ad libitum-fed sheep near the end of a 21-d heat-stress period (Barnes et al., 2019) but not with other heat stress studies of shorter duration (Mahjoubi et al., 2015, 2016; Yazdi et al., 2016). Blood urea nitrogen concentrations are typically increased by undernutrition and high circulating cortisol concentrations (Hammon et al., 2003; Buntyn et al., 2016; Santana et al., 2020), neither of which were present in our study. Daily supplementation of ractopamine had no impact on skeletal muscle glucose metabolism, fat mobilization, or blood urea nitrogen but reduced circulating Ca2+ levels by about 5%, similar to findings in feedlot cattle (Abney et al., 2007).
Heat stress induced cardiovascular changes and was associated with organ hypertrophy/atrophy and hoof overgrowth, which demonstrates its detrimental effects on well-being. Ractopamine supplementation, however, did not diminish any well-being indicators and did not exacerbate any of the ill-effects of heat stress. Heart rates were not initially affected by heat stress but were reduced beginning 1 wk into the study, despite the presence of adrenal hypertrophy and hypercatecholaminemia. The literature indicates that heat stress typically increases heart rate, especially during acute periods (Crandall and Gonzalez-Alonso, 2010; Iguchi et al., 2012). However, chronic hyperventilation by our heat-stressed lambs caused them to be hyperoxemic, which in humans has been shown to reduce heart rates by about 10% (Siński et al., 2016). Moreover, a study in Angus bulls reported that mild heat stress (28.6 to 31.4 °C) for 10 d caused hyperventilation and reduced heart rates by about 7% (Valente et al., 2013). Heat stress also reduced peripheral blood pressures in the present study, which has been observed in humans exposed to acute heat stress (Crandall et al., 2008; Crandall and Gonzalez-Alonso, 2010; Iguchi et al., 2012) and is associated with hyperoxemia (Jones et al., 1984). Relative hypertrophy of the left ventricle in heat-stressed lambs was not consistent with the observed hyperoxemia or hypotension and was most likely the product of sustained systemic inflammation (Sani et al., 2018) and hypercatecholaminemia (Kelm et al., 1996; Kinugawa et al., 1999). Ractopamine supplementation moderated heat stress-induced left ventricular hypertrophy, perhaps by reducing adrenergic sensitivity in cardiac tissues (Odore et al., 2007; Badino et al., 2008). Heat-stressed lambs also had larger lungs, which were likely a product of sustained hyperventilation (Faridy and Yang, 1989), and smaller spleens, which were likely the product of inflammation (Ohtsu et al., 2015). The hoof overgrowth observed in heat-stressed lambs was perhaps due to greater peripheral blood flow (Wheeler et al., 1972).
From these results, we conclude that heat stress reduces growth and metabolic efficiency in finishing lambs at least in part through chronic inflammatory and adrenergic responses, as we previously postulated (Barnes et al., 2019). Even when the effect of reduced feed intake was eliminated by pair-feeding, heat stress reduced skeletal muscle growth, impaired glucose metabolism, and compromised well-being in feedlot lambs. Daily supplementation of the β1 agonist ractopamine did not compromise well-being and, in fact, moderated the impact of heat stress on several growth and health indicators. Ractopamine also increased some indicators of muscle growth independent of heat stress but did not affect any metabolic indicators. Achieving optimal growth without compromising well-being remains a priority for the livestock industry, even as it faces the challenges of climate change, greater production demands, and expanding scrutiny from consumers. Heat stress-induced reductions in feed intake are difficult for producers to address, but the findings of this study demonstrate that additional mechanisms independent of nutritional intake contribute to deficits in growth and efficiency associated with heat stress. Identifying physiological targets such as systemic inflammation and hypercatecholaminemia is a fundamental step for new strategies to improve outcomes in heat-stressed livestock.
Acknowledgments
This manuscript is based on research that was supported in part by the USDA National Institute of Food and Agriculture Foundational Grant 2019-67015-29448, the National Institute of General Medical Sciences Grant 1P20GM104320 (J. Zempleni, Director), and the Nebraska Agricultural Experiment Station with funding from the Hatch Act (accession number 1009410) and Hatch Multistate Research capacity funding program (accession numbers 1011055, 1009410) through the USDA National Institute of Food and Agriculture. The Biomedical and Obesity Research Core (BORC) in the Nebraska Center for Prevention of Obesity Diseases (NPOD) receives partial support from NIH (NIGMS) COBRE IDeA award NIH 1P20GM104320.
Glossary
Abbreviations
- BW
body weight
- EDTA
ethylenediaminetetraacetic acid
- HDL
high-density lipoprotein
- KHB
Krebs–Henseleit buffer
- TNFα
tumor necrosis factor alpha
- WBC
white blood cells
Conflict of interest statement
The contents of this publication are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or NIGMS. The authors have no conflicts of interest to declare.
Literature Cited
- Abney, C. S., Vasconcelos J. T., McMeniman J. P., Keyser S. A., Wilson K. R., Vogel G. J., and Galyean M. L.. . 2007. Effects of ractopamine hydrochloride on performance, rate and variation in feed intake, and acid-base balance in feedlot cattle. J. Anim. Sci. 85:3090–3098. doi: 10.2527/jas.2007-0263 [DOI] [PubMed] [Google Scholar]
- Antolic, A., Feng X., Wood C. E., Richards E. M., and Keller-Wood M.. . 2015. Increased maternal nighttime cortisol concentrations in late gestation alter glucose and insulin in the neonatal lamb. Physiol. Rep. 3(9):e12548. doi: 10.14814/phy2.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelo, D. S., Mazon M. R., Nubiato K. E. Z., Gomez J. F. M., Brigida D. J., Gomes R. C., Netto A. S., Leme P. R., and Silva S. L.. . 2017. Effects of immunocastration and beta-adrenergic agonists on the performance and carcass traits of feedlot finished Nellore cattle. Animal 11:2103–2110. doi: 10.1017/S1751731117000842 [DOI] [PubMed] [Google Scholar]
- Badino, P., Odore R., Pagliasso S., Schiavone A., Girardi C., and Re G.. . 2008. Steroid and beta-adrenergic receptor modifications in target organs of broiler chickens fed with a diet containing beta2-adrenergic agents. Food Chem. Toxicol. 46:2239–2243. doi: 10.1016/j.fct.2008.02.025 [DOI] [PubMed] [Google Scholar]
- Barnes, T. L., Cadaret C. N., Beede K. A., Schmidt T. B., Petersen J. L., and Yates D. T.. . 2019. Hypertrophic muscle growth and metabolic efficiency were impaired by chronic heat stress, improved by zilpaterol supplementation, and not affected by ractopamine supplementation in feedlot lambs1. J. Anim. Sci. 97:4101–4113. doi: 10.1093/jas/skz271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baszczak, J. A., Grandin T., Gruber S. L., Engle T. E., Platter W. J., Laudert S. B., Schroeder A. L., and Tatum J. D.. . 2006. Effects of ractopamine supplementation on behavior of British, Continental, and Brahman crossbred steers during routine handling. J. Anim. Sci. 84:3410–3414. doi: 10.2527/jas.2006-167 [DOI] [PubMed] [Google Scholar]
- Bittner, C. J., Crawford G. I., Berger L. L., Holt S., Pritchard R. R., Platter W. J., Van Koevering M. T., Pyatt N. A., and Erickson G. E.. . 2016. Effect of ractopamine hydrochloride (Optaflexx) dose and duration on growth performance and carcass characteristics of finishing steers. J. Anim. Sci. 94:5382–5392. doi: 10.2527/jas.2016-0807 [DOI] [PubMed] [Google Scholar]
- Bittner, C. J., Greenquist M. A., Burken D. B., Shreck A. L., MacDonald J. C., Klopfenstein T. J., Platter W. J., Van Koevering M. T., Pyatt N. A., and Erickson G. E.. . 2017. Evaluation of ractopamine hydrochloride (Optaflexx) on growth performance and carcass characteristics of finishing steers across different feeding durations. J. Anim. Sci. 95:485–498. doi: 10.2527/jas.2016.0806 [DOI] [PubMed] [Google Scholar]
- Boyd, B. M., Shackelford S. D., Hales K. E., Brown-Brandl T. M., Bremer M. L., Spangler M. L., Wheeler T. L., King D. A., and Erickson G. E.. . 2015. Effects of shade and feeding zilpaterol hydrochloride to finishing steers on performance, carcass quality, heat stress, mobility, and body temperature. J. Anim. Sci. 93:5801–5811. doi: 10.2527/jas.2015-9613 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl, T. M., Chitko-McKown C. G., Eigenberg R. A., Mayer J. J., Welsh T. H., Davis J. D., and Purswell J. L.. . 2017. Physiological responses of feedlot heifers provided access to different levels of shade. Animal 11:1344–1353. doi: 10.1017/S1751731116002664 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl, T. M., Nienaber J. A., Eigenberg R. A., Hahn G. L., and Freetly H.. . 2003. Thermoregulatory responses of feeder cattle. J. Therm. Biol. 28:149–157. doi: 10.1016/s0306-4565(02)00052-9 [DOI] [Google Scholar]
- Buntyn, J. O., Burdick Sanchez N. C., Schmidt T. B., Erickson G. E., Sieren S. E., Jones S. J., and Carroll J. A.. . 2016. The metabolic, stress axis, and hematology response of zilpaterol hydrochloride supplemented beef heifers when exposed to a dual corticotropin-releasing hormone and vasopressin challenge. J. Anim. Sci. 94:2798–2810. doi: 10.2527/jas.2015-0192 [DOI] [PubMed] [Google Scholar]
- Cadaret, C. N., Beede K. A., Riley H. E., and Yates D. T.. . 2017. Acute exposure of primary rat soleus muscle to zilpaterol HCl (β2 adrenergic agonist), TNFα, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine 96:107–113. doi: 10.1016/j.cyto.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret, C. N., Merrick E. M., Barnes T. L., Beede K. A., Posont R. J., Petersen J. L., and Yates D. T.. . 2019a. Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished β-cell function in fetal sheep1,2. J. Anim. Sci. 97:4822–4833. doi: 10.1093/jas/skz321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret, C. N., Posont R. J., Beede K. A., Riley H. E., Loy J. D., and Yates D. T.. . 2019b. Maternal inflammation at midgestation impairs subsequent fetal myoblast function and skeletal muscle growth in rats, resulting in intrauterine growth restriction at term. Transl. Anim. Sci. 3:867–876. doi: 10.1093/tas/txz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen, J. J. M., Schols A. M. W. J., Kneppers A. E. M., Rosenbrand R. P. H. A., Drożdż M. M., van Hoof S. J., de Theije C. C., Kelders M. C. J. M., Verhaegen F., and Langen R. C. J.. . 2018. Altered protein turnover signaling and myogenesis during impaired recovery of inflammation-induced muscle atrophy in emphysematous mice. Sci. Rep. 8:10761. doi: 10.1038/s41598-018-28579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert, W. E., Williams P. D., and Williams G. D.. . 1991. β-adrenoceptor profile of ractopamine HCl in isolated smooth and cardiac muscle tissues of rat and guinea-pig. J. Pharm. Pharmacol. 43:844–847. doi: 10.1111/j.2042-7158.1991.tb03192.x [DOI] [PubMed] [Google Scholar]
- Cônsolo, N. R., Mesquita B. S., Rodriguez F. D., Rizzi V. G., and Silva L. F.. . 2016. Effects of ractopamine hydrochloride and dietary protein content on performance, carcass traits and meat quality of Nellore bulls. Animal 10:539–546. doi: 10.1017/S1751731115001895 [DOI] [PubMed] [Google Scholar]
- Crandall, C. G., and González-Alonso J.. . 2010. Cardiovascular function in the heat-stressed human. Acta Physiol. (Oxf). 199:407–423. doi: 10.1111/j.1748-1716.2010.02119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall, C., Wilson T., Marving J., Vogelsang T., Kjaer A., Hesse B., and Secher N.. . 2008. Effects of passive heating on central blood volume and ventricular dimensions in humans. J. Physiol. 586:293–301. doi: 10.1113/jphysiol.2007.143057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale, J. E., Herd J. A., and Hartley L. H.. . 1983. Epinephrine mediated increases in plasma cholesterol. Psychosom. Med. 45:227–232. doi: 10.1097/00006842-198306000-00005 [DOI] [PubMed] [Google Scholar]
- Emenheiser, J. C., Greiner S. P., Lewis R. M., and Notter D. R.. . 2010. Validation of live animal ultrasonic measurements of body composition in market lambs. J. Anim. Sci. 88:2932–2939. doi: 10.2527/jas.2009-2661 [DOI] [PubMed] [Google Scholar]
- Faridy, E. E., and Yang W. Z.. . 1989. Role of hyperventilation in hypoxia on lung growth in rats. Respir. Physiol. 76:179–190. doi: 10.1016/0034-5687(89)90096-0 [DOI] [PubMed] [Google Scholar]
- Ferlay, A., and Chilliard Y.. . 2018. Responses of body fat mobilization to isoproterenol or epinephrine challenge in adult cows: influence of energy level, breed, and body fatness. J. Anim. Sci. 96:331–342. doi: 10.1093/jas/skx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, J. M., Johnson S. E., Stelzleni A. M., Thrift T. A., Savell J. D., Warnock T. M., and Johnson D. D.. . 2010. Effect of ractopamine-HCl supplementation for 28 days on carcass characteristics, muscle fiber morphometrics, and whole muscle yields of six distinct muscles of the loin and round. Meat Sci. 85:379–384. doi: 10.1016/j.meatsci.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Hagenmaier, J. A., Reinhardt C. D., Bartle S. J., Henningson J. N., Ritter M. J., Calvo-Lorenzo M. S., Vogel G. J., Guthrie C. A., Siemens M. G., and Thomson D. U.. . 2017. Effect of handling intensity at the time of transport for slaughter on physiological response and carcass characteristics in beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 95:1963–1976. doi: 10.2527/jas.2016.0821 [DOI] [PubMed] [Google Scholar]
- Hagenmaier, J. A., Reinhardt C. D., Bartle S. J., and Thomson D. U.. . 2016. Effect of shade on animal welfare, growth performance, and carcass characteristics in large pens of beef cattle fed a beta agonist in a commercial feedlot. J. Anim. Sci. 94:5064–5076. doi: 10.2527/jas.2016-0935 [DOI] [PubMed] [Google Scholar]
- Hammon, H. M., Sauter S. N., Reist M., Zbinden Y., Philipona C., Morel C., and Blum J. W.. . 2003. Dexamethasone and colostrum feeding affect hepatic gluconeogenic enzymes differently in neonatal calves. J. Anim. Sci. 81:3095–3106. doi: 10.2527/2003.81123095x [DOI] [PubMed] [Google Scholar]
- Iguchi, M., Littmann A. E., Chang S. H., Wester L. A., Knipper J. S., and Shields R. K.. . 2012. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J. Athl. Train. 47:184–190. doi: 10.4085/1062-6050-47.2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. W., Tokach M. D., Goodband R. D., Nelssen J. L., Dritz S. S., Owen K. Q., Woodworth J. C., and Sulabo R. C.. . 2013. Effects of dietary l-carnitine and ractopamine HCl on the metabolic response to handling in finishing pigs. J. Anim. Sci. 91:4426–4439. doi: 10.2527/jas.2011-4411 [DOI] [PubMed] [Google Scholar]
- Jones, R., Zapol W. M., and Reid L.. . 1984. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am. J. Pathol. 117:273–285 [PMC free article] [PubMed] [Google Scholar]
- Kelm, M., Schäfer S., Mingers S., Heydthausen M., Vogt M., Motz W., and Strauer B. E.. . 1996. Left ventricular mass is linked to cardiac noradrenaline in normotensive and hypertensive patients. J. Hypertens. 14:1357–1364. doi: 10.1097/00004872-199611000-00015 [DOI] [PubMed] [Google Scholar]
- Kinugawa, T., Mori M., Ogino K., Endo A., Kato M., Kato T., Osaki S., Ohtahara A., Igawa O., Hisatome I., . et al. 1999. Hypertensive left ventricular hypertrophy is linked to an enhanced catecholamine response to submaximal exercise. Eur. J. Clin. Invest. 29:594–602. doi: 10.1046/j.1365-2362.1999.00501.x [DOI] [PubMed] [Google Scholar]
- Lopes, D. I., Sousa M. G., Ramos A. T., and Maruo V. M.. . 2016. Cardiotoxicity of Senna occidentalis in sheep (Ovis aries). Open Vet. J. 6:30–35. doi: 10.4314/ovj.v6i1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy, E. L., Loy D. D., and Hansen S. L.. . 2015. Influence of distillers grains resulting from a cellulosic ethanol process utilizing corn kernel fiber on nutrient digestibility of lambs and steer feedlot performance. J. Anim. Sci. 93:2265–2274. doi: 10.2527/jas.2014-8572 [DOI] [PubMed] [Google Scholar]
- Mader, T. L. 2003. Environmental stress in confined beef cattle1. J. Anim. Sci. 81:E110–E119. doi: 10.2527/2003.8114_suppl_2E110x [DOI] [Google Scholar]
- Mahjoubi, E., Amanlou H., Hossein Yazdi M., Aghaziarati N., Noori G. R., Vahl C. I., Bradford B. J., and Baumgard L. H.. . 2016. A supplement containing multiple types of gluconeogenic substrates alters intake but not productivity of heat-stressed Afshari lambs. J. Anim. Sci. 94:2497–2505. doi: 10.2527/jas.2015-9697 [DOI] [PubMed] [Google Scholar]
- Mahjoubi, E., Yazdi M. H., Aghaziarati N., Noori G. R., Afsarian O., and Baumgard L. H.. . 2015. The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs. J. Anim. Sci. 93:1632–1640. doi: 10.2527/jas.2014-8641 [DOI] [PubMed] [Google Scholar]
- Mendoza, S. M., Boyd R. D., Ferket P. R., and van Heugten E.. . 2017. Effects of dietary supplementation of the osmolyte betaine on growing pig performance and serological and hematological indices during thermoneutral and heat-stressed conditions. J. Anim. Sci. 95:5040–5053. doi: 10.2527/jas2017.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odore, R., Badino P., Barbero R., Cuniberti B., Pagliasso S., Girardi C., and Re G.. . 2007. Regulation of tissue beta-adrenergic, glucocorticoid and androgen receptors induced by repeated exposure to growth promoters in male veal calves. Res. Vet. Sci. 83:227–233. doi: 10.1016/j.rvsc.2006.12.011 [DOI] [PubMed] [Google Scholar]
- Ohtsu, H., Yamazaki M., Abe H., Murakami H., and Toyomizu M.. . 2015. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 52:282–287. doi: 10.2141/jpsa.0150062 [DOI] [Google Scholar]
- Posont, R. J., Beede K. A., Limesand S. W., and Yates D. T.. . 2018. Changes in myoblast responsiveness to TNFα and IL-6 contribute to decreased skeletal muscle mass in intrauterine growth restricted fetal sheep. Transl. Anim. Sci. 2(Suppl 1):S44–S47. doi: 10.1093/tas/txy038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remels, A. H., Gosker H. R., Bakker J., Guttridge D. C., Schols A. M., and Langen R. C.. . 2013. Regulation of skeletal muscle oxidative phenotype by classical NF-κB signalling. Biochim. Biophys. Acta 1832:1313–1325. doi: 10.1016/j.bbadis.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Remels, A. H., Gosker H. R., Schrauwen P., Hommelberg P. P., Sliwinski P., Polkey M., Galdiz J., Wouters E. F., Langen R. C., and Schols A. M.. . 2010. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 24:5052–5062. doi: 10.1096/fj.09-150714 [DOI] [PubMed] [Google Scholar]
- Renaudeau, D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. . 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Rhoads, R. P., Baumgard L. H., El-Kadi S. W., and Zhao L. D.. . 2016. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Roles for insulin-supported skeletal muscle growth. J. Anim. Sci. 94:1791–1802. doi: 10.2527/jas.2015-0110 [DOI] [PubMed] [Google Scholar]
- Sani, C. M., Pogue E. P. L., Hrabia J. B., Zayachkowski A. G., Zawadka M. M., Poniatowski A. G., Długosz D., Leśniak W., Kruszelnicka O., Chyrchel B., . et al. 2018. Association between low-grade chronic inflammation and depressed left atrial compliance in heart failure with preserved ejection fraction: a retrospective analysis. Folia Med. Cracov. 58:45–55. doi: 10.24425/fmc.2018.124657 [DOI] [PubMed] [Google Scholar]
- Santana, P. F., Rocha V. R. Jr, Ruas J. R. M., Moncao F. P., Borges L. A., Sousa T. E. S., Silva F. V. E., Rabelo W. O., Carvalho C., and Sales E. C. J.. . 2020. Nutritional efficiency of feed-restricted F1 Holstein/Zebu cows during the middle third of lactation. Asian-Australas. J. Anim. Sci. 33(2):236–244. doi: 10.5713/ajas.18.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Fernandez, M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. . 2015. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3(8):e12478. doi: 10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears, M. R. 2002. Adverse effects of β-agonists. J. Allergy Clin. Immunol. 110:S322–S328. doi: 10.1067/mai.2002.129966 [DOI] [PubMed] [Google Scholar]
- Sewell, J. R., Berger L. L., Nash T. G., Cecava M. J., Doane P. H., Dunn J. L., Dyer M. K., and Pyatt N. A.. . 2009. Nutrient digestion and performance by lambs and steers fed thermochemically treated crop residues. J. Anim. Sci. 87:1024–1033. doi: 10.2527/jas.2008-0974 [DOI] [PubMed] [Google Scholar]
- Siński, M., Lewandowski J., Dobosiewicz A., Przybylski J., Abramczyk P., and Gaciong Z.. . 2016. The effect of hyperoxia on central blood pressure in healthy subjects. Arch. Med. Sci. 12:992–999. doi: 10.5114/aoms.2015.49038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait, R. G.Jr. 2016. Ultrasound use for body composition and carcass quality assessment in cattle and lambs. Vet. Clin. North Am. Food Anim. Pract. 32:207–218. doi: 10.1016/j.cvfa.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Valente, E. E. L., Chizzotti M. L., Oliveira C. V. R., Galvão M. C., Domingues S. S., Rodrigues A. C., Ladeira M. M., Ferreira R. A., and Chizzotti F. H. M.. . 2013. Effect of heat stress on intake and metabolism of Bos taurus (Angus) and Bos indicus (Nellore). In: Oltjen, J. W., Kebreab E., and Lapierre H., editors. Energy and protein metabolism and nutrition in sustainable animal production: 4th International Symposium on Energy and Protein Metabolism and Nutrition Sacramento; September 9 to 12, 2013; California. Wageningen:Wageningen Academic Publishers; p. 291–292. [Google Scholar]
- Victoria Sanz Fernandez, M., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C., . et al. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3(2):e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, J., Bennett J., and Hutchinson J.. . 1972. Effect of ambient temperature and daylength on hoof growth in sheep. J. Agric. Sci. 79:91–97. doi: 10.1017/S0021859600025405 [DOI] [Google Scholar]
- Wheelock, J. B., Rhoads R. P., Vanbaale M. J., Sanders S. R., and Baumgard L. H.. . 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Yates, D. T., Camacho L. E., Kelly A.C., Steyn L. V., Davis M. A., Antolic A. T., Anderson M. J., Goyal R., Allen R. E., Papas K. K., . et al. 2019. Postnatal beta2 adrenergic treatment improves insulin sensitivity in lambs with IUGR but not persistent defects in pancreatic islets or skeletal muscle. J. Physiol. 597:5835–5858. doi: 10.1113/JP278726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi, M. H., Mirzaei-Alamouti H. R., Amanlou H., Mahjoubi E., Nabipour A., Aghaziarati N., and Baumgard L. H.. . 2016. Effects of heat stress on metabolism, digestibility, and rumen epithelial characteristics in growing Holstein calves. J. Anim. Sci. 94:77–89. doi: 10.2527/jas.2015-9364 [DOI] [PubMed] [Google Scholar]
- Zhao, L., McMillan R. P., Xie G., Giridhar S. G. L. W., Baumgard L. H., El-Kadi S., Selsby J., Ross J., Gabler N., Hulver M. W., . et al. 2018. Heat stress decreases metabolic flexibility in skeletal muscle of growing pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315:R1096–R1106. doi: 10.1152/ajpregu.00404.2017 [DOI] [PubMed] [Google Scholar]