Abstract
Research question
The study set out to identify corrective measures aimed at reducing the risk of aerosol-mediated viral infection within an IVF laboratory.
Design
A failure modes and effect analysis (FMEA) was conducted by a multidisciplinary IVF team. A schematic representation of new protocols and procedures adopted during COVID-19 emergency has been defined, including directives about the behaviour to adopt when entering the clinic and the laboratory, in case of face-to-face contact with patients and between staff members. In addition, the risk of cross-contamination between samples belonging to different patients during cell handling and manipulation has been evaluated. Potential failure modes for each phase of the emergency have been analysed, focusing on possible sources of error. Risk priority numbers have been calculated as products of Occurrence × Severity × Detection scores.
Results
Except for cell–cell contamination, which was considered highly unlikely, failure modes during patient–staff, staff–staff and staff–cell interactions were estimated as carrrying a moderate to high risk of infection. The main corrective measures entailed precautionary logistic measures, the implementation of additional personal protective equipment and changes in the IVF laboratory procedures and scheduling of the daily routine. Some procedures were also revised, aiming to increase staff's awareness and caution.
Conclusions
Standard laboratory protocols are insufficient to face a virus whose transmission is aerosol mediated. The measures outlined in this FMEA should thus be considered not only for facing this pandemic, but also for the future to promptly manage any aerosol-mediated virus infection, whose impact on the management of an IVF laboratory might be less severe than COVID-19 although not completely negligible.
KEYWORDS: SARS-CoV-2, FMEA, IVF laboratory, Risk assessment
Introduction
In March 2020, the World Health Organization declared the onset of a SARS-CoV-2 pandemic (COVID-19), an unprecedented disease that at the time of writing has accounted for almost 8,000,000 confirmed cases and more than 430,000 deaths worldwide (latest WHO data. Source: Health Emergency Dashboard, 16 June, 1:57 pm CEST). (https://extranet.who.int/publicemergency) In particular, Italy has reported more than 237,000 confirmed cases, with more than 34,000 deaths since the beginning of the pandemic (latest WHO data. Source: Health Emergency Dashboard, 16 June, 1:57 pm CEST). (https://extranet.who.int/publicemergency)
COVID-19 is an acute respiratory disease caused by a single-stranded RNA virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The extreme contagiousness of the infection, the high risk of developing a life-threatening infectious disease and the constant increase in cases of SARS-CoV-2 infection led to a pressing need for the widespread implementation of specific risk-reduction strategies, including the deferment of all non-essential activities (Phase I, initial stage of the pandemic in Italy of the emergency situation). National and international societies for reproductive medicine, as well as the Italian Authority for Assisted Reproductive Medicine, suggested the suspension of new IVF treatments, non-essential cryopreservation of gametes and diagnostic procedures (American Society for Reproductive Medicine, 2020a; Centro Nazionale Trapianti, 2020a; Centro Nazionale Trapianti, 2020b; European Society of Human Reproduction and Embryology, 2020a; Società Italiana Embriologia Riproduzione e Ricerca, 2020). These measures were recommended because of the imperative need to reduce the risk of infection for IVF patients and healthcare workers, and to avoid overloading an already overburdened health system (Bedford et al., 2020).
Recently, in view of the declining epidemiological spread of SARS-CoV-2 infection, and in agreement with the recommendations provided by various scientific societies (De Santis et al., 2020; European Society of Human Reproduction and Embryology, 2020b), the Italian authorities have expressed a need to restart clinical IVF activity (Phase II of emergency in italy), mainly for time-sensitive patients requiring urgent treatments (poor prognosis couples who might lose their chance to conceive) (Alviggi et al., 2020; Bosch et al., 2019; Vaiarelli et al., 2020). However, cautionary measures should be taken to ensure safe clinical practice and minimize the risks associated with COVID-19 disease during treatment. The choice to restart IVF treatments has led to a change in IVF laboratory management and a redefinition of protocols and procedures to ensure safety of personnel and prevent cross-contamination, in compliance with an effective quality management system to ensure good IVF laboratory practice in this emergency situation (Hickman et al., 2020).
Before restarting, each laboratory must build its own risk-reduction strategies based on specific risk assessment analyses. Failure mode and effects analysis (FMEA) is a step-by-step approach that can be applied to identify real or potential failures in processes and to develop measures to minimize risks according to the Joint Commission International (Cimadomo et al., 2016; Rienzi et al., 2015; Rienzi et al., 2017). All the potential process failures are ranked according the occurrence of the failure and the chance to detect it, and according to the severity of the consequences. The purpose of this FMEA is to define measures to eliminate or reduce failures associated with the different phases of IVF treatment, starting with the highest priority ones.
At the authors’ group of centres, a multidisciplinary FMEA was performed aimed at identifying corrective measures to reduce the risk of infection within the IVF laboratory. All meetings were conducted online using a web platform. This paper describes this FMEA, which can be applied during the SARS-CoV-2 pandemic as well as to minimize the risk of any other aerosol-mediated infection. This FMEA is addressed at laboratory managers, supervisors and clinical embryologists.
Materials and methods
The FMEA was conducted by a multidisciplinary team involving different members of the IVF laboratory staff operating in four different GENERA (Gynaecology, Endocrinology, Embryology, Assisted Reproduction) centres in Italy (Rome, Marostica, Naples, Umbertide) and covering different responsibilities within the organization chart: IVF clinical and laboratory directors, embryology and andrology laboratory supervisors, senior clinical embryologists, scientific coordinators and quality management managers, nurses and administrative staff. Three web meetings were necessary to conclude the evaluations. The FMEA focused specifically on the management of clinical activity in the IVF laboratory. Indeed, the administrative and counselling activities were deemed to follow the general guidelines outlined for any other work environment. Similarly, the definition of the first patient populations to be admitted to treatment had already been described elsewhere, along with the general rules to adopt for consultations, monitoring of ovarian stimulation and suggested clinical strategies (European Society of Human Reproduction and Embryology, 2020b; Vaiarelli et al., 2020).
First, a schematic representation of new protocols and procedures adopted during COVID-19 emergency was outlined, including staff directives on the behaviour to adopt when entering the clinic and the embryology laboratory, in case of face-to-face contacts with patients (during patient identification or embryological consultation) and for any cases of close contact between staff members (in the changing room, during laboratory procedures, during coffee and lunch breaks, and in meetings and journal clubs). The discussion was based on the recommendations and guidelines of both national and international scientific societies (American Society for Reproductive Medicine, 2020b; British Fertility Society, 2020; De Santis et al., 2020; European Society of Human Reproduction and Embryology, 2020b). Second, the risk of cross-contamination between samples belonging to different patients during cell handling and manipulation was evaluated. Finally, potential failure modes for each phase were then analysed, focusing on possible sources of error and defined by three parameters: occurrence (O), severity (S) and detection (D). A risk priority number score (RPN) was calculated as the product of the three factors (RPN = O × S × D) according to the Joint Commission International. The scoring system was discussed by the team and is summarized in Table 1 . RPN values lower than 15 indicate a low risk of failure, those between 15 and 50 a moderate risk and those over 50 a high risk.
Table 1.
Definition of occurrence, severity and detection scores
| Score | |||
|---|---|---|---|
| Occurrence (O) | 1 | Remote | Failure unlikely |
| 2 | Low | Relatively rare failure | |
| 3 | Moderate | Occasional failure | |
| 4 | High | Recurrent failure | |
| 5 | Very high | Failure almost inevitable | |
| Severity (S) | 1 | Remote | Minimal damage to cell/patient/embryology staff |
| 2 | Low | Low damage to cell/patient/embryology staff | |
| 3 | Moderate | Moderate damage to cell/patient/embryology staff | |
| 4 | High | High damage to cell/patient/embryology staff | |
| 5 | Very high | Permanent damage to the Cell/patient/embryology staff | |
| Detection (D) | 5 | Remote | Very remote chance of detecting failure |
| 4 | Low | Low chance of detecting failure | |
| 3 | Moderate | Moderate chance of detecting failure | |
| 2 | High | High chance of detecting failure | |
| 1 | Very high | Very high chance of detecting failure |
Although preventive measures were urgently adopted to Contrast/contain the transmission of the virus during the early phase of the COVID-19 emergency, we were able to perform a risk assessment analysis during the laboratory shutdown period. This assessment considered the different nature of the viral transmission and aimed at identifying those laboratory procedures needing to be reviewed and adapted to safeguard patients, staff, gametes and embryos. It was then considered necessary to perform a second analysis to reassess the real effectiveness of the preventive measures adopted in Phase II (the restarting phase). FMEA was considered the most effective approach to estimate how the new protocols could reduce the incidence of the risk of failure and increase the chance of detecting it.
This FMEA has focused on failure modes associated with different process phases of IVF laboratory practice. Considering the limited predictive value of available assessment methods for COVID-19 infections in patients and healthcare staff (European Society of Human Reproduction and Embryology, 2020b) and given the high incidence of asymptomatic carriers, the whole population was considered to be potentially infected. Although this may lead to an overestimation of the risks, any failure mode has been considered as a potential direct or indirect source of infection.
Results
Four main areas at potential risk of bi-directional infection have been identified: patient–staff, staff–staff, staff–cell and cell–cell. All the risk modes, causes of infection and estimated occurrence, severity and detection for each phase before and after implementation of the corrective measures are described in Table 2, Table 3, Table 4, Table 5 . The estimated severity of these failures was considered ‘high’ when the person infected was the patient and/or operator, and ‘low’ when the risk was of cell cross-contamination. The occurrence of these potential situations varied between ‘low’ and ‘high’ and was calculated according to the number of times each particular step was performed during the work activity.
Table 2.
Patient–staff risk of infection
| Process phase | Risk modes | Failure modes | O | S | D | RPN | Corrective measures | O | S | D | RPN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Access to the clinic | Contact with patients (waiting room, front desk, inpatient wards) | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items (e.g. pens, patients’ form) Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Restricted access to the clinic and triage with well-trained medical staff Supply of PPE at the clinic entrance and hand sanitization Paths dedicated to patients or medical staff Interval of 1.5 h between procedures and medical appointments Interpersonal distance more than 1 m |
2 | 4 | 1 | 8 |
| Patient identification | Contact with patients in operating theatre (oocyte retrieval/embryo transfer/TESE) and at semen collection | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items (e.g. pens, patients’ form) Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Adoption of appropriate PPE (with the addition of glasses or protective visor) Patient and staff training about correct use of PPE Interval of 1.5 h between procedures Interpersonal distance more than 1 m Interview limited to the shortest duration possible |
2 | 4 | 1 | 8 |
| Embryological consultations | Contact with patient at consultation | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items (e.g. pens, patients’ form) Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Telemedicine | 1 | 4 | 1 | 4 |
D, detection; O, occurrence; PPE, personal protective equipment; RPN, risk priority number; S, severity; TESE, testicular sperm extraction.
Table 3.
Staff–staff risk of infection
| Process phase | Risk modes | Failure modes | O | S | D | RPN | Corrective measures | O | S | D | RPN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Access to the clinic | Contact with external suppliers | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Prohibition of access to external suppliers Delivery of material at reception Maintaining interpersonal distance of more than 1 m Sanitization of delivered products followed by a change of gloves |
1 | 4 | 1 | 4 |
| Contact with administrative and/or healthcare personnel | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Restricted access to staff on duty Supply of PPE and further hand sanitizing with appropriate disinfectants Triage with well-trained medical staff |
2 | 4 | 1 | 8 | |
| Changing room | Contact with other embryologists and/or healthcare personnel | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
3 | 4 | 5 | 60 | Access limited to maximum of two people at the same time Presence of disinfectants to sanitize surfaces after use Ensure proper air change (15 min) |
2 | 4 | 1 | 8 |
| Laboratory | Contact between embryologists present in the laboratory at the same time and/or engaged in the same procedure | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Organization of work schedule Proper training of staff the risks and protocols to be adopted to prevent infection Supply of PPE at laboratory entrance to be worn in place of the PPE provided at clinic entrance (with addition of glasses or protective visor and long-sleeved scrubs) Distance of at least 1 m between operators Cleaning of work stations after use between operators Facilitation of back-office work |
2 | 4 | 1 | 8 |
| Contact with healthcare staff | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Healthcare staff not allowed laboratory access Telephone communication |
2 | 4 | 1 | 8 | |
| Access to cryo-storage room | Contact between embryologists engaged in the same procedure | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Access limited to one embryologist at a time, with second supporting embryologist outside the room for emergencies | 2 | 4 | 1 | 8 |
| Coffee break | Contact with other medical staff or patients | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
3 | 4 | 5 | 60 | Minimizing frequency of coffee breaks Access to snack area limited to one person at a time |
2 | 4 | 1 | 8 |
| Lunch break | Contact with other medical staff or patients | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
3 | 4 | 5 | 60 | Reduced working hours to allow staff to have lunch safely outside the clinic | 1 | 4 | 1 | 4 |
| Staff meeting, journal club | IVF team contact | Face-to-face contact at distances of less than 1 m for more than 15 min No use of proper PPE Exchange of potentially contaminated items Sharing potentially contaminated spaces |
4 | 4 | 5 | 80 | Web communication | 1 | 4 | 1 | 4 |
O, occurrence; D, detection; PPE, personal protective equipment; RPN, risk priority number; S, severity
Table 4.
Cell–staff risk of infection
| Process phase | Risk modes | Failure modes | O | S | D | RPN | Corrective measures | O | S | D | RPN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seminal fluid management and processing | Contact with semen collection pot | Direct contact of embryologist with potentially infected semen collection pot | 3 | 3 | 5 | 45 | Use of a disposable bag for the patient to put the pot in Use of double gloves |
2 | 3 | 1 | 6 |
| Unintentional contact with seminal fluid | Direct contact of embryologist with seminal fluid (incorrect processing procedure or accidental breakage of container) Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces |
2 | 3 | 5 | 30 | Use of glasses or protective visor in addition to standard PPE Use of double gloves |
2 | 3 | 1 | 6 | |
| Follicular fluid screening | Unintentional contact with follicular fluid | Contact of embryologist with potentially infected follicular fluid (incorrect processing procedure or accidental breakage of container) Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces |
2 | 3 | 5 | 30 | Use of glasses or protective visor in addition to standard PPE Use of double gloves |
1 | 3 | 1 | 3 |
| Oocyte and embryo handling and processing | Unintentional contact with processed gametes | Contact between samples and embryologist Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces |
1 | 3 | 5 | 15 | Wearing of PPE | 1 | 3 | 1 | 3 |
| Vitrification | Unintentional contact with processed gametes Labelling of cryo-tools |
Contact between samples and/or cryo-tools and embryologist Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces |
4 | 3 | 5 | 60 | Use of glasses or protective visor in addition to standard PPE Wearing of gloves also for cryo-tool labelling |
1 | 3 | 1 | 3 |
| Embryo transfer | In the laboratory: unintentional contact with embryos | Contact between samples and embryologist | 1 | 3 | 5 | 15 | Use of glasses or protective visor in addition to standard PPE Use of double gloves |
1 | 3 | 1 | 3 |
| In the operating theatre: contact with biological fluids and/or embryo transfer catheter |
Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces | 3 | 3 | 5 | 45 | Use of glasses or protective visor in addition to standard PPE Use of double gloves |
2 | 3 | 1 | 6 | |
| Warming | Unintentional contact with embryos | Contact between samples and embryologist Contact between sample and operator by contaminated aerosol and droplets or equipment or surfaces |
4 | 3 | 5 | 60 | Use of glasses or protective visor in addition to standard PPE Use of sterilized liquid nitrogen |
1 | 3 | 1 | 3 |
| Disinfection | Contact with potentially contaminated work station | Contact between sample and operator by contaminated equipment or surfaces | 2 | 3 | 5 | 30 | Disinfection of equipment and working area at every use Extraordinary laboratory extra disinfection carried out weekly by a qualified company |
1 | 3 | 1 | 3 |
D, detection; ICSI, intracytoplasmic sperm injection; O, occurrence; S, severity; RPN, risk priority number; PPE, personal protective equipment.
Table 5.
Cell–cell risk of infection
| Process phase | Risk modes | Failure modes | O | S | D | RPN | Corrective measures | O | S | D | RPN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seminal fluid processing | Cross-contamination between samples belonging to different patients | Contact between samples by contaminated droplets or equipment or surfaces | 2 | 2 | 5 | 20 | Maintain safe distance between samples to be processed Disinfection of surfaces and equipment after each procedure Disinfection of incubator and incubator tray and water change at end of the day or patient cycle |
1 | 2 | 1 | 2 |
| Follicular fluid screening | Cross-contamination between samples belonging to different patients | Contact between samples by contaminated droplets or equipment or surfaces | 2 | 2 | 5 | 20 | Disinfection of surfaces and equipment after each procedure Disinfection of incubator and incubator tray and water change at end of the day or patient cycle |
1 | 2 | 1 | 2 |
| Oocyte and embryo handling and processing | Cross-contamination between samples belonging to different patients | Contact between samples by contaminated droplets or equipment or surfaces | 1 | 2 | 5 | 10 | Disinfection of surfaces and equipment after each procedure Disinfection of incubator and incubator tray and water change at end of the day or patient cycle |
1 | 2 | 1 | 2 |
| Vitrification/warming | Cross-contamination between samples belonging to different patients | Contact between samples by contaminated droplets or equipment or surfaces | 1 | 2 | 5 | 10 | Disinfection of surfaces and equipment after each procedure Sterilized liquid nitrogen Disposable liquid nitrogen trays |
1 | 2 | 1 | 2 |
| Embryo transfer | Cross-contamination between samples belonging to different patients | Contact between samples by contaminated droplets or equipment or surfaces | 2 | 2 | 5 | 20 | Disinfection of surfaces and equipment after each procedure | 1 | 2 | 1 | 2 |
O, occurrence; D, detection; RPN, risk priority number; S, severity.
Before the COVID-19 pandemic was declared, the low probability of detecting a failure and preventing the onset of risk was mainly due to the absence of triage procedures for both patients and operators and the related difficulty of making a certain and definite diagnosis of COVID-19 infection. After the first risk assessment analysis, generic health precautions were adopted as corrective measures. Access to the clinic is restricted to the patient having the IVF treatment and the IVF team on duty. Patients are screened by telephone before triage prior to attendance and instructed to postpone their treatment if they are suspected of having any possible COVID-19 symptomatology (e.g. fever, cough or dyspnoea) or they have been in close contact with a confirmed or suspected case of COVID-19.
When entering the clinic, both patients and staff undergo triage, are interviewed regarding the presence of respiratory symptoms and are screened using a temperature scanner. General personal protective equipment (PPE), such as surgical face masks, gloves and overshoes are provided at the entrance to minimize risk of transmission. Separate pathways have been defined for healthcare staff and patients, and within the clinic the different preclinical and administrative areas have been organized to guarantee a safe interpersonal distance. All non-essential visits by external providers and engineers have been postponed. For planned maintenance of critical instruments, detailed protocols and procedures have been developed together with a specific triage procedure that entails body temperature control and evaluation of technicians’ symptoms before maintenance is carried out. All the supplies for the laboratory are delivered at specific entrances.
All patients’ embryological consultations are performed via telemedicine or telephone call. Daily scheduling in the operating theatre has been revised to reduce the number of IVF operators on duty; two shift groups of operators have been defined in order to be able to guarantee laboratory activity even if a positive COVID-19 test result in one individual requires their whole group to be quarantined. The time between procedures has been increased to allow for full air exchange in the operating theatre (calculated according to the air change rate and volume of the room). All staff have been trained in these new procedures and the correct use of PPE, and their compliance has been verified through audit systems. In the laboratory area, the frequency of disinfection of all surfaces and equipment has been revised and a new regime implemented, and the planned maintenance of the air filtration system has been taken into account. The efficacy of the implemented corrective measures in reducing potential risks of infection has been confirmed in the revised FMEA: all the procedures carrying a higher risk of infection have shown a significant reduction in RPN value (Table 2, Table 3, Table 4, Table 5).
Discussion
The resumption of post-emergency COVID-19 clinical activity (Phase II of emergency) has involved a substantial reorganization of work in IVF laboratories, a revision of the existing protocols and the implementation of new operative procedures. All the basic precautions usually adopted during IVF practice have been focused on preventing a risk of cross-contamination by blood-borne viral infections such as with human immunodeficiency virus, hepatitis B virus and hepatitis C virus. Now, health professionals are having to face and manage an unprecedented emergency, not only because of the extreme contagiousness of the COVID-19 virus, but also because of its intrinsically different nature. The risk of transmission of other infectious respiratory viruses, such as influenza viruses, and the impact of their spread on the clinical activity in IVF has never previously been considered.
In the field of occupational health, the risk of infection of healthcare operators can be reduced by precautionary logistic measures to lower their exposure (Verbeek et al., 2020). Therefore, the first important precautionary measure by our clinics was a proper organization of the operational unit to avoid unnecessary close contacts. In the presence of highly contagious viruses, such as SARS-CoV-2, healthcare workers represent a category of individuals with a higher risk of infection than the general population, due to their frequent exposure of mucous membranes of the eyes, mouth and nose to biological fluids (follicular and seminal fluids) or to exhaled droplets released by potentially contaminated patients (Burke et al., 2020; Huang et al., 2020; Li et al., 2020b; Liu et al., 2020; World Health Organization, 2020b). Transmission of infection can also occur by contact between the mucous membranes and contaminated skin. Moreover, studies evaluating the persistence of SARS-CoV-2 virus on different surfaces have shown that the virus remains viable for up to 1 day on cloth and cardboard, 2 days on glass, 3–4 days on plastic and stainless steel and up to 7 days on the external surface of a medical mask (van Doremalen et al., 2020; World Health Organization, 2008).
In the authors’ group of clinics, all medical appointments and procedures have been scheduled at a time interval of at least 1.5 h in order to reduce the number of people present at the same time within the clinic and to allow appropriate and complete air exchange within the various rooms. All procedures have been therefore revised to allow an interpersonal spacing of more than 1 m, except for witnessing and embryo transfer procedures that have a shorter time duration (less than 15 min). All embryological consultations with patients, as well as team meetings, journal clubs and discussions of clinical cases are carried out via online communication. However, there is still a risk that an infected operator might spread the infection to healthy patients or act as a vector of transmission of infection between patients. Therefore, as with patients, all embryologists have been considered potentially infectious, and continuous monitoring of IVF staff by daily triage has been implemented.
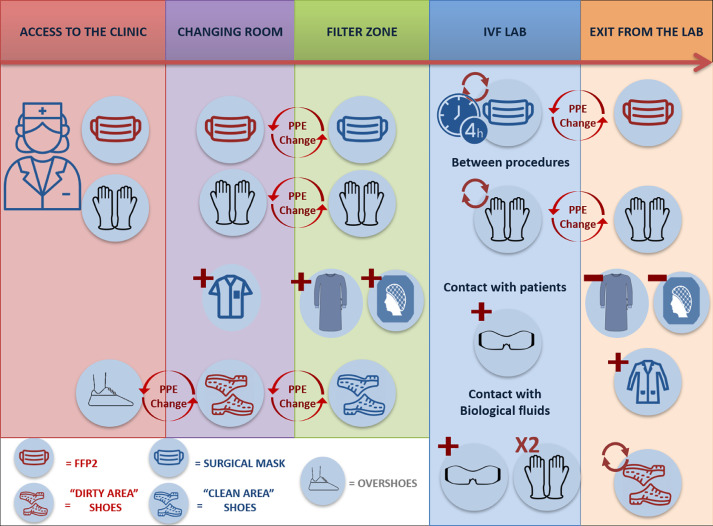
After the implementation of logistic measures, the second most important, easily implemented strategy to protect patients and embryologists is the correct use of appropriate PPE to prevent contamination of skin and mucous membranes (Adams and Walls, 2020; Chang et al., 2020). The additional PPE adopted in this group of IVF laboratories during the pandemic was chosen from the most effective ones in line with Verbeek and colleagues (Verbeek et al., 2020) and worn or removed appropriately (donning and doffing). Figure 1 summarizes the PPE implemented in the different phases.
Figure 1.
Summary of the personal protective equipment (PPE) implemented at each phase. FFP2, class 2 filtering face-piece.
The European Centre for Disease Prevention and Control (European Centre for Disease Prevention and Control, 2020) 8 april 2020 suggests the use of class 2 filtering face-piece (FFP2) masks in any case of contact with a potentially infected individual. Therefore in our clinics these have been adopted in all spaces outside the laboratory area and during contact with patients, along with goggles or face shields. However, surgical masks, which have already been shown to be efficient in reducing influenza transmission (Cheng et al., 2010; MacIntyre and Chughtai, 2015), have been considered sufficient to minimize the emission of potentially contaminated respiratory droplets within the IVF compartment. This is mainly due to the extreme control of laboratory conditions (air change and positive pressure), specific training for embryologists about the required conduct (an interpersonal distance of at least 1 m and a reduction in face-to-face contact), the limited number of operators on duty and the need for frequent replacement.
Gloves and long-sleeved water-resistant gowns to prevent body contamination are also worn throughout daily work in the IVF laboratory (de Ziegler et al., 2020). Operator compliance in wearing this PPE during the daily routine as well as the wide and easy availability of the devices are other parameters that have been considered when choosing which PPE to implement. Clearly, staff have been carefully trained in how to use them properly. Special good practice recommendations for disinfection have also been adopted (implementing standard cleaning procedures), such as sterilizing equipment and work stations, including the oculars of microscopes and the external surfaces of cryo-tanks, after each use and not only at the end of the working day. These practices have helped increase staff awareness of potential sources of transmission of infection (Hickman et al., 2020). Double gloves are used when conducting procedures with a higher risk of infection due to the presumed higher viral load (e.g. while handling biological fluids during oocyte retrieval and sperm processing), as improper sample preparation or accidental breakage of a container could expose the operator and the cells to a higher risk of infection. An additional pair of gloves decreases also the risk of contamination when doffing PPE (Casanova et al., 2012). Clearly, beyond the use of PPE, the frequency of hand washing and sanitization (for at least 20 s) has also been increased, in line with its crucial role in preventing skin contamination (Gould et al., 2010, Hickman et al., 2020).
Changes have also been made in the laboratory setting and in scheduling daily routines. In closed-controlled air systems, the airflow may increase the spread of the virus even from asymptomatic patients and/or healthcare operators (Zimmermann and Nkenke, 2020). Thus, in the authors’ clinic, the efficiency of the air filtration systems in the operating theatre and laboratory areas, as well as the air pressurization, was checked before restarting practice (Mortimer et al., 2018). Scheduled interventions every 1.5 h guarantee sufficient air renewal between one procedure and another, which, together with implementing validated disinfection procedures between interventions, means that the risk of spreading a putative infection will be minimized. In addition, an increased interval between oocyte retrievals allows procedures to operate safely and efficiently, even in presence of a reduced number of operators on duty, and always respecting the correct timings for execution of the procedures, a key process indicator for IVF laboratories (Fabozzi et al., 2020; Maggiulli et al., 2019; Maggiulli et al., 2020). Laboratory working areas have been defined so that a safety distance of at least 1 m can be guaranteed between operators. This in turn allows the number of operators on duty to be reduced without compromising the efficiency of the laboratory, mainly thanks to the automated technologies that the authors’ group of clinics had previously implemented (an electronic witnessing system, time-lapse incubation and automated equipment monitoring and alarm systems). In addition, as mentioned above, two shift working groups have been organized to limit the spread of the virus in case of infection and to guarantee laboratory activities even in the event of a positive test result requiring a complete group to be quarantined.
Before reconsidering laboratory procedures, the risk of transmission of viral contamination to embryos or gametes, from either contaminated cells or infected operators, was evaluated, with the aim of revising IVF procedures. The former possibility was assumed unlikely as recent peer-reviewed publications have suggested that SARS-CoV-2 is not present in several biological fluids (vaginal secretions, semen or testicular tissue) in patients diagnosed with COVID-19 (Paoli et al., 2020; Qiu et al., 2020; Song et al., 2020; Wang et al., 2020), and even when the presence of the SARS-CoV-2 virus was reported, potential infectivity was not proven (Li et al., 2020a).
Although limited data are available, it was assumed unlikely that spermatozoa, oocytes and embryos might be infected as all IVF procedures require systematic washing and processing of the samples, which involves a significant dilution of any putative virus (https://www.eshre.eu/Press-Room/ESHRE-News). Indeed, the international guidelines for reducing the risk of cross-contamination in cases of sexually transmitted diseases recommend repeated sample washing as the primary strategy to reduce the viral load in biological samples (especially seminal fluid) and manage the risk of transmission during IVF treatment (Practice Committee of the American Society for Reproductive, 2006). Moreover, the putative role of the zona pellucida in protecting against viruses and reducing the susceptibility of the ovum to viral infections should be considered (Van Soom et al., 2010). Therefore, universal good standard laboratory practices have been applied while handling gametes and embryos within the embryology laboratory, and no significant technical changes have been made to the existing protocols for micromanipulation procedures.
Nevertheless, the authors considered that an infected operator might spread Sars-CoV-2 during the various laboratory manipulations, as well as during all preliminary phases of the effective handling and micromanipulation procedures (e.g. cryo-device labelling before cryopreservation), which previously did not require the mandatory use of PPE. In particular, the risk assessment analysis reported a higher possibility of failure associated with the management of biological material in the cryo-storage room, which can be considered as a high-risk area. The classification of this environment did not involve specific PPE (except for cryogenic PPE) to reduce the risk of viral infection per se, but all cryo-device storage procedures require a second witness, who could equally be a source of contamination. Therefore, cryostorage was ranked as the most sensitive step of the whole IVF procedure.
As SARS-CoV-2 is an enveloped RNA virus, it may maintain its viability even at the low temperatures of liquid nitrogen, thus leading to possible contamination and/or cross-contamination between samples (Bielanski, 2012). However, as mentioned before, the multiple washings to which gametes and embryos are subject before being cryopreserved, associated with the small volumes involved in the vitrification procedure (about 0.l µl), suggest that if a minimal viral load is present, it will not be sufficient to represent a source of infection. Therefore, so as not to affect the effectiveness of the procedure, the protocols for cryopreservation of gametes and embryos, even if they involved the use of open systems and direct contact with liquid nitrogen, were not revised. However, disinfection procedures and the use of proper PPE (gloves, surgical masks and face shields) have been adopted when handling biological material in non-classified environments. Furthermore, as it was not possible to estimate the risk of nitrogen infection when treating samples processed and cryopreserved in geographical areas with a higher prevalence of the infection during phase I of the emergency situation, sterile liquid nitrogen has been used to wash cryodevices at warming time (Parmegiani et al., 2012), and will be used when importing gametes from other laboratories when the pandemic is over (Yakass and Woodward, 2020). In fact, due to the fact that it has been impossible for patients to travel around Italy or abroad, the demand for imported samples has increased and will need to be carefully managed from now on.
In all other laboratory procedures, the risk of contamination has been reduced by simply suggesting the use of PPE at all stage and increasing the frequency of disinfection of equipment and surfaces (with quaternary ammonium salts), not only between different patients, but also between operators. During culture, in order to reduce the number of handlings of embryos, single-step culture has been adopted as preferential culture system.
This is the perspective of a group of four Italian IVF laboratories and represents an example that could be adopted with all due adjustments by other IVF laboratories worldwide. Every IVF laboratory is in fact invited to perform its own risk assessment analyses. This FMEA is addressed to clinical embryologists who manage and/or work in an IVF laboratory, as the authors considered this to be a peculiar work environment requiring specific procedures and corrective measures to prevent or minimize the risks of infection.
The efficacy of these corrective measures cannot be supported by specific data regarding actual infections. Nevertheless, according to a survey of the Italian Society of Embryology, Reproduction and Research (SIERR), updated on 15 May 2020, 11% of the responding Italian IVF laboratories (n = 5/46) reported at least one infected operator (European Society of Human Reproduction and Embryology, 2020d). Luckily, and possibly also due to the corrective measures implemented and described here, there have so far been no reported cases of COVID-19 among treated patients and operators working at the authors’ group of IVF centres in four Italian regions, one of which is located in the Veneto region (i.e. one of the country's worst affected areas).
This FMEA is based on current knowledge of SARS-CoV-2 infectivity and its effect on human reproduction, and on a clinical situation that is still fluid at present. Thus, it is subject to possible future modifications. However, as previously stated, the authors preferred to overestimate the risks and adopt a conservative approach with the aim of preventing any infection among patients, personnel and gametes.
Conclusions
During the COVID-19 pandemic, the risk of infection of IVF laboratory workers and patients from accidental exposure to biological fluids or contaminated material has become a greater concern. The authors considered their standard laboratory protocols to be insufficient to face a virus whose transmission is aerosol mediated. The revision of all procedures and the implementation of further preventive measures has provided guidelines for the precautionary approach required now, as well as in future, to aim to reduce the consequences of any aerosol-mediated infection. Indeed, these measures could be adopted to deal with even less severe viruses such as influenza, whose impact on the management of an IVF laboratory is less severe than SARS-CoV-2 but is not completely negligible (i.e. in terms of staff reduction and patients’ health).
Biography

Roberta Maggiulli is an ESHRE-certified Senior Clinical Embryologist. She obtained her MSc in Biotechnology and Postgraduate School of Specialization in Clinical Biochemistry at the University of Parma, and is the Laboratory Supervisor of the GENERA centre in Rome, Italy. Her interests encompass the validation and standardization of laboratory practices in IVF.
Key message.
This failure modes and effects analysis describes the precautionary administrative measures, the additional personal protective equipment required, and the modifications of IVF laboratory settings and procedures required to reduce the risk of aerosol-mediated viral infections.
Alt-text: Unlabelled box
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Adams J.G., Walls R.M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- Alviggi C., Esteves S.C., Orvieto R., Conforti A., La Marca A., Fischer R., Andersen C.Y., Buhler K., Sunkara S.K., Polyzos N.P., Strina I., Carbone L., Bento F.C., Galliano D., Yarali H., Vuong L.N., Grynberg M., Drakopoulos P., Xavier P., Llacer J., Neuspiller F., Horton M., Roque M., Papanikolaou E., Banker M., Dahan M.H., Foong S., Tournaye H., Blockeel C., Vaiarelli A., Humaidan P., Ubaldi F.M., Group P. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod. Biol. Endocrinol. 2020;18:45. doi: 10.1186/s12958-020-00605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. 2020a.https://www.asrm.org/news-and-publications/covid19/statements/patient-management-and-clinical-recommendations-during-the-coronavirus-covid19-pandemic <![if !supportAnnotations]>.
- American Society for Reproductive Medicine American Society for Reproductive Medicine. Patient management and clinical recommendationsduring the coronavirus (COVID-19) pandemic. UPDATE #4. 2020 https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforceupdate4.pdf (May 11, 2020 through June 8, 2020) [Google Scholar]
- Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M.D., Sall A.A., Schuchat A., Ungchusak K., Wieler L.H., Strategic W.H.O., TECHNICAL ADVISORY GROUP FOR INFECTIOUS, H. COVID-19: towards controlling of a pandemic. Lancet. 2020 doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielanski A. A review of the risk of contamination of semen and embryos during cryopreservation and measures to limit cross-contamination during banking to prevent disease transmission in ET practices. Theriogenology. 2012;77:467–482. doi: 10.1016/j.theriogenology.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Bosch E., Bulletti C., Copperman A.B., Fanchin R., Yarali H., Petta C.A., Polyzos N.P., Shapiro D., Ubaldi F.M., Garcia Velasco J.A., Longobardi S., D'hooghe T., Humaidan P., Delphi T.T.P.C.G. How time to healthy singleton delivery could affect decision-making during infertility treatment: a Delphi consensus. Reprod. Biomed. Online. 2019;38:118–130. doi: 10.1016/j.rbmo.2018.09.019. [DOI] [PubMed] [Google Scholar]
- British Fertility Society. 2020. The Association of Reproductive and Clinical Scientists (ARCS) and British Fertility Society (BFS) U.K. best practice guidelines for reintroduction of routine fertility treatments during the COVID-19 pandemic.https://www.britishfertilitysociety.org.uk/wp-content/uploads/2020/05/ARCS-BFS-COVID-19-guideline-v1.1-1.pdf.
- Burke R.M., Midgley C.M., Dratch A., Fenstersheib M., Haupt T., Holshue M., Ghinai I., Jarashow M.C., Lo J., Mcpherson T.D., Rudman S., Scott S., Hall A.J., Fry A.M., Rolfes M.A. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 - United States, January-February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Effect of single- versus double-gloving on virus transfer to health care workers' skin and clothing during removal of personal protective equipment. Am. J. Infect. Control. 2012;40:369–374. doi: 10.1016/j.ajic.2011.04.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centro Nazionale Trapianti. 2020a. Prot. 504/CNT 2020.http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_232_0_file.pdf
- Centro Nazionale Trapianti. 2020b. Prot. 605/CNT2020.http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_233_0_file.pdf.CHANG
- Cheng V.C., Tai J.W., Wong L.M., Chan J.F., Li I.W., To K.K., Hung I.F., Chan K.H., Ho P.L., Yuen K.Y. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J. Hosp. Infect. 2010;74:271–277. doi: 10.1016/j.jhin.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang XuH, Rebaza A., Sharma L., Dela Cruz C.S. Protecting healthcare workers from subclinical coronavirus infection. Lancet Respiratory Medicine. 2020;8:e13. doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadomo D., Ubaldi F.M., Capalbo A., Maggiulli R., Scarica C., Romano S., Poggiana C., Zuccarello D., Giancani A., Vaiarelli A., Rienzi L. Failure mode and effects analysis of witnessing protocols for ensuring traceability during PGD/PGS cycles. Reprod. Biomed. Online. 2016;33:360–369. doi: 10.1016/j.rbmo.2016.06.002. [DOI] [PubMed] [Google Scholar]
- De Santis L., Anastasi A., Cimadomo D., Klinger F.G., Licata E., Pisaturo V., Sosa Fernandez L., Scarica C. COVID-19: the perspective of Italian embryologists managing the IVF laboratory in pandemic emergency. Hum. Reprod. 2020;35:1004–1005. doi: 10.1093/humrep/deaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ziegler D, Pirtea, P, Scott RT Jr., Rienzi L., Poulain M., Ayoubi J.M. 2020. ART during the SARS-CoV-2 pandemic: a new context, new challenges, and new recommendations.https://www.fertstertdialog.com/rooms/871-covid-19/posts/art-during-the-sars-cov-2-pandemic-a-new-context-new-challenges-and-new-recommendations.
- European Centre for Disease Prevention and Control. 2020. Technical reports, February 2020:https://www.ecdc.europa.eu/en/coronavirus/guidance-and-technical-reports.
- European Society of Human Reproduction and Embryology. 2020a.
- European Society of Human Reproduction and Embryology. 2020b. A statement from ESHRE for phase 2 – ESHRE Guidance on recommencing ART treatments.https://www.eshre.eu/Press-Room/ESHRE-News#COVID19P2.
- European Society of Human Reproduction and Embryology COVID-19 does not stop fertility preservation: the Italian situation during the pandemic emergency. Oral presentation. 2020 [Google Scholar]
- Fabozzi G., Cimadomo D., Maggiulli R., Vaiarelli A., Ubaldi F.M., Rienzi L. Which key performance indicators are most effective in evaluating and managing an in vitro fertilization laboratory? Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.04.054. [DOI] [PubMed] [Google Scholar]
- Gould D.J., Moralejo D., Drey N., Chudleigh J.H. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.CD005186.pub3. CD005186. [DOI] [PubMed] [Google Scholar]
- Hickman C., Rogers S., Huang G., Macarthur S., Meseguer M., Nogueira D., Portela R., Rienzi L., Sharp T., Ye H. MANAGING AN IVF LABORATORY DURING A PANDEMIC: Perspectives from laboratory managers. Reprod. Biomed. Online. 2020 doi: 10.1016/j.rbmo.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liao X., Qian S., Yuan J., Wang F., Liu Y., Wang Z., Wang F.S., Liu L., Zhang Z. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020;26:1320–1323. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre C.R., Chughtai A.A. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350 doi: 10.1136/bmj.h694. h694. [DOI] [PubMed] [Google Scholar]
- Maggiulli R., Cimadomo D., Fabozzi G., Papini L., Dovere L., Ubaldi F.M., Rienzi L. The effect of ICSI-related procedural timings and operators on the outcome. Hum. Reprod. 2020;35:32–43. doi: 10.1093/humrep/dez234. [DOI] [PubMed] [Google Scholar]
- Maggiulli R., Giancani A., Cimadomo D., Ubaldi F.M., Rienzi L. Human Blastocyst Biopsy and Vitrification. J. Vis. Exp. 2019 doi: 10.3791/59625. [DOI] [PubMed] [Google Scholar]
- Mortimer D., Cohen J., Mortimer S.T., Fawzy M., Mcculloh D.H., Morbeck D.E., Pollet-Villard X., Mansour R.T., Brison D.R., Doshi A., Harper J.C., Swain J.E., Gilligan A.V. Cairo consensus on the IVF laboratory environment and air quality: report of an expert meeting. Reprod. Biomed. Online. 2018;36:658–674. doi: 10.1016/j.rbmo.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O., Antonelli G., Lenzi A., Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Invest. 2020 doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmegiani L., Accorsi A., Bernardi S., Arnone A., Cognigni G.E., Filicori M. A reliable procedure for decontamination before thawing of human specimens cryostored in liquid nitrogen: three washes with sterile liquid nitrogen (SLN2) Fertil. Steril. 2012;98:870–875. doi: 10.1016/j.fertnstert.2012.06.028. [DOI] [PubMed] [Google Scholar]
- PRACTICE COMMITTEE OF THE AMERICAN SOCIETY FOR REPRODUCTIVE, M. Guidelines for reducing the risk of viral transmission during fertility treatment. Fertil. Steril. 2006;86:S11–S17. doi: 10.1016/j.fertnstert.2006.07.1485. [DOI] [PubMed] [Google Scholar]
- Qiu L., Liu X., Xiao M., Xie J., Cao W., Liu Z., Morse A., Xie Y., Li T., Zhu L. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L., Bariani F., Dalla Zorza M., Albani E., Benini F., Chamayou S., Minasi M.G., Parmegiani L., Restelli L., Vizziello G., Costa A.N., ITALIAN SOCIETY OF EMBRYOLOGY, R. & RESEARCH, I. Comprehensive protocol of traceability during IVF: the result of a multicentre failure mode and effect analysis. Hum. Reprod. 2017;32:1612–1620. doi: 10.1093/humrep/dex144. [DOI] [PubMed] [Google Scholar]
- Rienzi L., Bariani F., Dalla Zorza M., Romano S., Scarica C., Maggiulli R., Nanni Costa A., Ubaldi F.M. Failure mode and effects analysis of witnessing protocols for ensuring traceability during IVF. Reprod. Biomed. Online. 2015;31:516–522. doi: 10.1016/j.rbmo.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Società Italiana Embriologia Riproduzione e Ricerca. 2020.;https://www.sierr.it/images/Documenti/Raccomandazioni_SIERR_per_COVID-19.pdf.
- Song C., Wang Y., Li W., Hu B., Chen G., Xia P., Wang W., Li C., Diao F., Hu Z., Yang X., Yao B., Liu Y. Absence of 2019 Novel Coronavirus in Semen and Testes of COVID-19 Patients. Biol. Reprod. 2020 doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiarelli A., Bulletti C., Cimadomo D., Borini A., Alviggi C., Ajossa S., Anserini P., Gennarelli G., Guido M., Levi-Setti P.E., Palagiano A., Palermo R., Savasi V., Pellicer A., Rienzi L., Ubaldi F.M. COVID-19 and ART: the view of the Italian Society of Fertility and Sterility and Reproductive Medicine. Reprod. Biomed. Online. 2020 doi: 10.1016/j.rbmo.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., De Wit E., Munster V.J. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soom A., Wrathall A.E., Herrler A., Nauwynck H.J. Is the zona pellucida an efficient barrier to viral infection. Reprod. Fertil. Dev. 2010;22:21–31. doi: 10.1071/RD09230. [DOI] [PubMed] [Google Scholar]
- Verbeek J.H., Rajamaki B., Ijaz S., Sauni R., Toomey E., Blackwood B., Tikka C., Ruotsalainen J.H., Kilinc Balci F.S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020;5 doi: 10.1002/14651858.CD011621.pub5. CD011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2008. Essential environmental health standards in health care. [Google Scholar]
- World Health Organization. 2020b. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19).https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- Yakass M.B., Woodward B. COVID-19: should we continue to cryopreserve sperm during the pandemic. Reprod. Biomed. Online. 2020 doi: 10.1016/j.rbmo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M., Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J. Craniomaxillofac. Surg. 2020;48:521–526. doi: 10.1016/j.jcms.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]