Abstract
The novel Coronavirus disease of 2019 (nCOV-19) is a viral outbreak noted first in Wuhan, China. This disease is caused by Severe Acute Respiratory Syndrome (SARS) Coronavirus (CoV)-2. In the past, other members of the coronavirus family, such as SARS and Middle East Respiratory Syndrome (MERS), have made an impact in China and the Arabian peninsula respectively. Both SARS and COVID-19 share similar symptoms such as fever, cough, and difficulty in breathing that can become fatal in later stages. However, SARS and MERS infections were epidemic diseases constrained to limited regions. By March 2020 the SARS-CoV-2 had spread across the globe and on March 11th, 2020 the World Health Organization (WHO) declared COVID-19 as pandemic disease. In severe SARS-CoV-2 infection, many patients succumbed to pneumonia. Higher rates of deaths were seen in older patients who had co-morbidities such as diabetes mellitus, hypertension, cardiovascular disease (CVD), and dementia. In this review paper, we discuss the effect of SARS-CoV-2 on CNS diseases, such as Alzheimer's-like dementia, and diabetes mellitus. We also focus on the virus genome, pathophysiology, theranostics, and autophagy mechanisms. We will assess the multiorgan failure reported in advanced stages of SARS-CoV-2 infection. Our paper will provide mechanistic clues and therapeutic targets for physicians and investigators to combat COVID-19.
Keywords: COVID-19, Brain, Neutralizing antibodies, Diabetes mellitus, SARS-CoV-2, Therapeutics, Multiple sclerosis
Abbreviations: ACE2, Angiotensin-converting enzyme 2; ATG, autophagy-related genes; AT2 Cells, alveolar epithelial type2 cells; BBB, blood-brain barrier; CNS, Center nervous System; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease discovered in 2019; DM, diabetes mellitus; DMV, double-membrane vesicles; EM, electron microscope; HAPE, High altitude pulmonary edema; HCQ, hydroxychloroquine; IFN, Interferons; IL, Interleukin; MERS-CoV, Middle East respiratory syndrome coronavirus; MMPs, matrix metalloproteinases; MHV, mouse hepatitis virus; MS, multiple sclerosis; NAb's, Neutralizing antibodies; NSP, non-structural protein; ORF, open reading frame; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; ssRNA, single-strand RNA; TNF, Tumor Necrosis Factor; ULK, ubiquitin-like ligase
Graphical abstract
Highlights
-
•
SARS-CoV-2 and Dementia, Multiple Sclerosis, Encephalitis, and Parkinson's disease
-
•
SARS-CoV-2 and Diabetes Mellitus Coronaviruses and Autophagy
-
•
Protease Inhibitors and Repurposed Drugs for SARS-CoV-2 USFDA approved drugs-HCQ and Remdesivir.
-
•
SARS-CoV-2 diagnostics
1. Introduction
A virus is neither a dead nor alive particle that uses a host to thrive and replicate. The host could be any healthy body (animals or plants) [[1], [2], [3], [4]]. Normally, viruses are classified by their replication and growth methods. A common virus, the influenza (flu) virus, causes cold-like symptoms such as chills, headaches, muscle aches, and fever, and survives in the human body for 20 days [5]. Viruses have a preferred host and rarely jump hosts to cause disease (e.g.; Coronavirus) [6,7]. The coronavirus family has been around for many years and is a zoonotic virus found in bats, camels, and cats. Viruses from this family do not prefer human beings as hosts possibly due to the high core body temperature (37 °C) [8]. The survival temperature of coronaviruses may be below 35 °C. However, as a result of mutations in the coronavirus, some members of this family can survive in humans. Evidence of such genetic alterations was seen in the first corona outbreak of 2003. However, SARS could not transmit as effectively across populations as the current SARS-CoV-2 [9,10]. Genetic alterations may have also led to the current SARS-CoV-2 [11,12].
The most recent outbreak from the coronavirus family was seen in December of 2019 in China and was named SARS CoV-2. The disease caused by the virus was named coronavirus disease (COVID-19) (Fig. 1 ). While the case fatality rate for the SARS-CoV outbreak was 10–12% and that of MERS was 30–34%, SARS-CoV-2 had a much lower case fatality rate of 2–3% (CDC report 2020) [13]. However, SARS-CoV-2 is more infectious than its predecessors, thus more lethal despite a lowercase fatality rate (Fig. 2 ). The SARS-CoV-2 virus spreads when the infected person sneezes and coughs into the air (droplets). The virus particles may survive in the air for 14–16 h depending on outside temperature and may travel a distance of 3–4 ft [14,15].
Fig. 1.
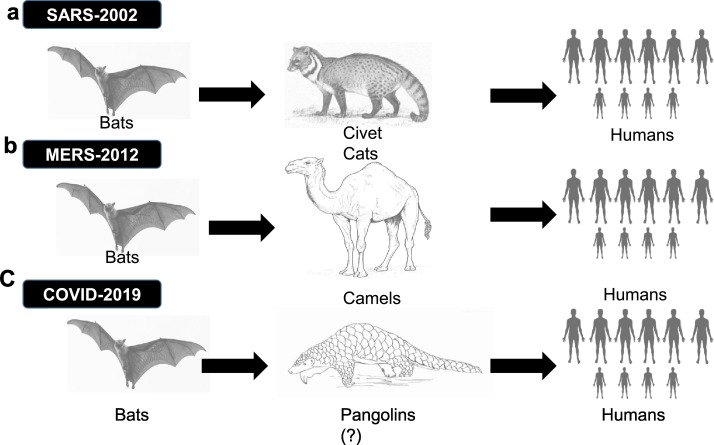
Route of Transmission of CoVs in to humans: a. SARS-CoV; Primary source is Bats and Civet Cats are intermediate reservoirs; b) MERS-CoV; Primary source is Bats and Camels are intermediate reservoirs; c) COVID-19; Primary source is Bats and Pangolins are intermediate reservoirs (putative).
Fig. 2.
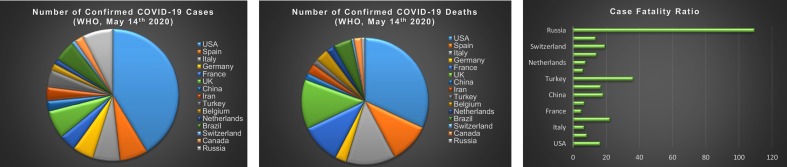
The number cases and deaths data was taken from WHO (https://who.sprinklr.com/) and CDC till May 14th, 2020. The Case Fatality was calculated as the ration of No. of Confirmed Covid-19 cases over No. of Covid-19 deaths. However, Case fatality rate is low for SARS-CoV2 (3–4%) in comparison with SARS-CoV (9.6%), MERS-CoV (34%). Case fatality rate Source from https://www.worldometers.info/coronavirus/coronavirus-death-rate/
Viral genome sequences obtained from infected patients in the United States of America are similar to those of patients in China. This similarity used to suggest a single emergence of the virus from an animal reservoir [14,16,17]. The SARS-CoV outbreak jumped from bats to civet cats, and then from civet cats to humans [16]. In 2012, the second outbreak from the coronavirus family, Middle East respiratory syndrome coronavirus (MERS-CoV), was transmitted from camels to humans in the Arabian Peninsula [16]. SARS-CoV-2 is postulated to have been transmitted from bats to humans. Pangolins may have been an intermediary host (Fig. 1). Researchers also note that SARS-CoV-2 has mutated at least once, based on the identification of two strains of the coronavirus [10,14,16,17].
In this review paper, we focus on the structure, genome, epidemiology, pathophysiology, diagnosis, and therapeutics of SARS-CoV-2. Furthermore, we emphasize the role of comorbidities such as diabetes, hypertension, coronary diseases, and obesity in SARS-CoV-2 susceptibility. We will also explore the neuroinvasive nature of SARS-CoV-2.
2. Coronavirus and COVID-19 overview
Corona in Latin means crown. Coronaviruses have a crown-like appearance under the electron microscope (EM) due to the presence of spike glycoproteins on its envelope. It belongs to the coronaviridae family and Nidovirales order. There are different groups of coronaviruses including alpha (α), beta (β), gamma (γ), and delta (δ) groups. The α-coronaviruses are Human Coronavirus-229E (HCoV229E), and Human Coronavirus NL63 (HCoV-NL63) whereas β-coronaviruses are Human Coronavirus OC43 (HCoV-OC43), SARS-CoV, HKU-1, MERS-CoV, and SARC-CoV-2. The SARS-CoV-2 is a new strain from the coronavirus family, initially named as a novel coronavirus (nCov-2019), that had not been previously identified in humans [13,16].
It is believed that COVID-2019 might have been transmitted from bats to human beings through pangolins (putative) [13,16]. The common signs of COVID-19 infection in immune-compromised individuals are fever, dry cough, shortness of breath, and muscle pain. In severe cases, this infection may cause pneumonia, renal failure, and death. Earlier studies also noted organ localization of SARS-CoV to the small intestine, kidney, stomach, liver, cerebrum, pituitary gland, parathyroid gland, and sweat glands. This localization was identified in autopsy samples by detecting N protein and viral RNA [18].
3. Structure of SARS-CoV-2 (COVID-19)
SARS-CoV-2 appears round and has an envelope. On its envelope, it has spike proteins (S1 and S2) and conjugated proteins (glycoproteins). The spike proteins play a crucial role in binding to Angiotensin-Converting Enzyme-2 (ACE-2) receptors of host cells to enter the cell by endocytosis. The membrane protein (M) which is present on the envelope determines the shape of the virus. The interaction of envelope (E) glycoprotein with M protein forms the viral envelope [19].
SARS-CoV-2 is a non-segmented positive sense single-strand RNA (ssRNA) 30 kb in size (Fig. 3 ). It commandeers the host's cellular machinery for its duplication. The genome contains sequences for papain-like proteases, replicases, helicases, endoribonuclease, and Spike proteins (S1 & S2). The spike proteins which are present in SARS-CoV-2 are different from those of SARS-CoV [19,20].
Fig. 3.
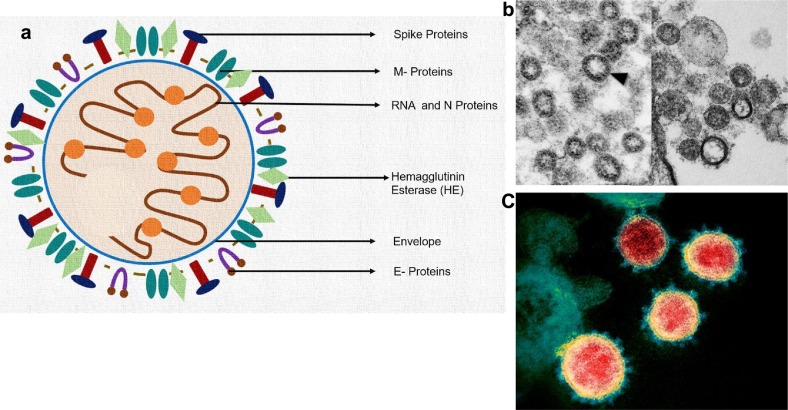
a) Structure of SARS-CoV2: Labeled with spike proteins, M-proteins, HE, E, and RNA with Nucleocapsid (N) proteins.
b) Transmission electron microscopic (TEM) images- SARS-CoV2 marked with arrow head, image credit: Centers for Disease Control and Prevention (CDC)|CS Goldsmith and TG Ksiazek (left) and NIAID (right).
c). Colored TEM with predominant spike proteins on envelope of SARS-CoV2 (COVID-19), image credit: NIAID-RML/NATIONAL INSTITUTES OF HEALTH/SCIENCE PHOTO LIBRARY.
4. Genome and proteome of SARS-CoV-2
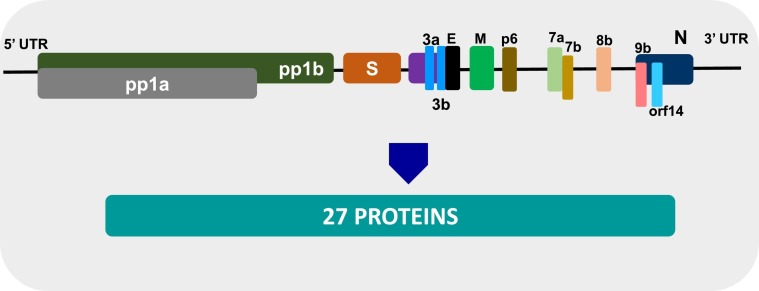
Three strains of the novel coronavirus, namely Wuhan/ IVDC-HB-01/2019 (HB01), Wuhan/IVDCHB-04/2019 (HB04), and Wuhan/IVDC-HB-05/2019 (HB05), have shown great similarity with only five nucleotide differences in their entire genome. The SARS-CoV-2 genome has 14 open reading frames (ORFs) and encodes 27 proteins. The 5′ terminus ORFs (Orf1ab and orf1a) encodes pp1 proteins and 15 non-structural protein sequences (nsps). The 3′-terminus of the SARS-CoV-2 genome contains S, M, E, and N structural proteins and accessory proteins-3a, 3b, p6, 7a. 7b, 8b, 9b, and orf14. Interestingly, at the amino acid level, SARS-CoV-2 is almost identical to SARS-CoV. It only has a few minor differences. For example, the 8a protein is present in SARS-CoV-2 but is absent in SARS- CoV. The 8b protein is longer in SARS-CoV-2 (121 amino acids) than in SARS-CoV (84 amino acids). The 3b protein is smaller in SARS-CoV-2 (22 amino acids) than in SARS-CoV (154 amino acids) (Fig. 4 ) [21]. Further functional characterization studies need to be done.
Fig. 4.
Genome of SARS-CoV2 originated in china: SARS-CoV2 (IVDC-HB-01/2019 (HB01) strain) RNA genome organization with pp1ab and pp1a proteins. The “orf1ab” is the largest gene, and it encodes for the “pp1ab” protein; contains nsp1-nsp10 and nsp12-nsp16 (15 nsps); another protein “pp1a” protein (coded by orf1a) contains nsp1-nsp10 (10 nsps). Structural proteins are encoded by the four structural genes, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) genes. NSPS; non structural protein sequence, Orf; Open reading frame Credit: Adapted from Wu et al., Cell Host Microbe. 2020 Mar 11;27(3):325–328.
5. Epidemiology of COVID-19
The R0, or reproductive ratio, is the rate of transmission for various diseases. The R0 for COVID-19 is around 2–3 while that of influenza is 1. This means that a patient who is positive for COVID-19 may spread this virus to three other people through air droplets (sneezing or coughing). Each of those individuals can spread to three more people [22].
The series interval (SI), is the time interval between the appearances of COVID-19 symptoms in the first person to the day when there is an appearance of symptoms in a second person. The SI for COVID-19 is 5–7.5 days while that of influenza is around 2.5 days [13,22]. The extended incubation period increases the virulence of the virus as it can transmit far and wide before showing itself. As of May 20th, the total number of confirmed cases exceeded 4,864,881 with 321,818 deaths globally. The United States of America (USA) had 1,528,235 confirmed cases and 91,664 deaths (Fig. 2) [23]. According to the Center for Disease Control and Prevention, as of June 22, there have been over 2,275,645 confirmed cases and 119,923 confirmed deaths in the US [17]. At the same time, the World Health Organization has recorded over 8,860,331 confirmed cases and 465,740 deaths globally due to SARS-CoV-2 [23].
6. Who is at risk for this COVID-19
The median age for SARS-CoV-2-infected patients is between the ages of 47–56 years old with males comprising more than half of the cases. People who are over 45 years old as well as those with comorbidities (hypertension patients, severe obesity (>40 kg/m2), diabetes mellitus (DM), coronary disease, and pneumonia), are at greater risk for adverse outcomes due to COVID-19 [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. In the USA, around 10.5% of the population (34.2 million) have DM and subsequently are at a higher risk for COVID-19. DM patients also had comorbidities such as severe obesity (15.5%) and hypertension (64%). As the number of comorbidities increase, so does a patient's risk of adverse outcomes if they contract COVID-19 [13].
7. Times course of SARS CoV 2/COVID-19 in the human body
The average incubation period for SARS-CoV-2 is 5.2 days, and 98% of those who develop SARS-CoV-2 symptoms do so within 11.5 days. The clinical symptoms of SARS-CoV-2 vary starting with an asymptomatic stage that can progress to acute respiratory disease (ARD) and pneumonia [24,[34], [35], [36], [37]]. However, the prevalence of asymptomatic cases is significant (20–86% of all infections). Asymptomatic individuals have a positive viral nucleic acid test without any SARS-CoV-2 symptoms [[38], [39], [40], [41], [42]]. Interestingly, respiratory viral load and transmission rates are the same in both patients (asymptomatic & symptomatic) [40,42]. Many confirmed COVID-19 patients did not have any significant abnormalities in chest imaging such as ground-glass opacities, patchy shadowing, and interstitial abnormalities [24,37]. Patients with pneumonia, on the other hand, have respiratory symptoms and positive findings in chest imaging that may progress into multi-organ failure, shock, and death [24,37,43].
8. Pathophysiology of SARS-CoV-2
The route of transmission of SARS-CoV-2 could be coughing and sneezing. The virus enters the lungs through the respiratory tract and attacks alveolar epithelial type 2 (AT2) cells. AT2 produces a surfactant to decrease the surface tension within alveoli to reduce the collapsing pressure. It has been reported that the spike proteins of SARS-CoV-2 bind to the ACE-2 receptors on AT2 cells [44,45]. ACE2 receptors are also found on the tubular epithelium of the kidney, heart, enterocytes, pancreas, and endothelial cells [[46], [47], [48], [49]]. Hoffmann et al. [20] demonstrated the role of ACE2 and TMPRSS2 (cellular serine protease) in SARS-CoV-2 entry into the host cell. Integrins may also induce conformational changes in the ACE2 receptor during its interaction with SARS-CoV-2 [20]. Once inside the host cell, the virus releases its positive sense ssRNA. The ssRNA uses the host cell ribosome to produce polyproteins. It also uses RNA dependent RNA polymerases to duplicate its RNA. The packaging structure of the cell distributes synthesized spike proteins to vesicle carriers. The proteinases in the cytoplasm cleave the synthesized polyproteins (nucleocapsid enzymes, spike proteins, M-protein, E-protein, etc.) of SARS-CoV-2 [50].
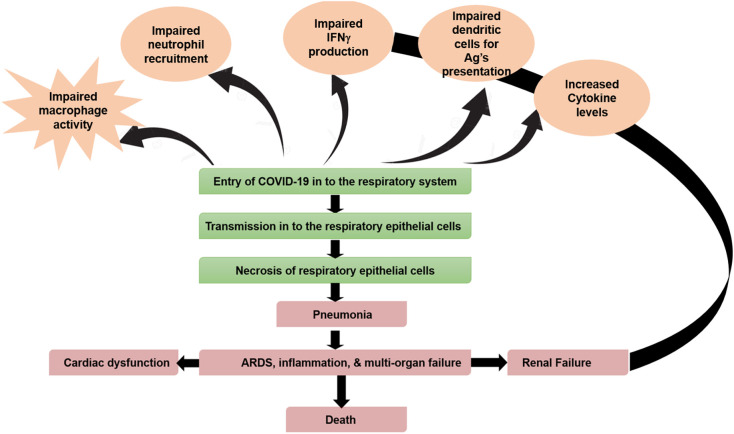
The virus also releases specific inflammatory mediators to stimulate macrophages (Fig. 5 ) [20,50,51]. Activated macrophages release cytokines (IL-1, IL-6, and TNFα) and chemokines (CXCL10 and CCL2) into the bloodstream. The release of these molecules causes vasodilation and increased capillary permeability. The leakage of plasma into the interstitial spaces of the alveoli cells will accumulate around the alveoli [24,28,32,52,53] and compress it. As a result, there is a decrease in surfactant levels in AT2 cells. The cascade events ultimately lead to alveolar collapse and impaired gaseous exchange.
Fig. 5.
Pathophysiology of SARS-CoV2.
Concurrently, there is an increase in inflammatory cytokine (cytokine storm) secretion [54]. The inflammatory mediators, through CD4+ T helper (Th1) cells, enhance the production and recruitment of neutrophils and macrophages using IL-17, IL-21, and IL-22 [53]. In the later stages of the disease, all these steps cause difficulty in breathing, hypoxemia, and cough [[55], [56], [57]].
The hypothalamus controls body temperature. The released IL-1, IL-6, and TNF-α will travel in the blood and affect the hypothalamus [24,28,32,52,53]. They will trigger the release of prostaglandin, PGE2, and causes an increase in body temperature. Considering the hypoxic condition, sympathetics can induce tachycardia. All these abnormal inflammatory responses can lead to septic shock and multi-organ failure [58,59]. In short, due to pneumonia, the vasodilation that decreases effective blood volume (BV) and peripheral resistance (PR) can lead to hypotension, reduced perfusion rate of the heart, and multi-organ failure [24,37,60].
9. Coronaviruses and dementia patients
Currently, >50 million people around the world are living with dementia. Every 3 s, a new case of dementia is being diagnosed and this number is expected to double every 20 years [61,62]. Dementia has also emerged as a pandemic disease in an aging society [63]. As a result, the hit from both pandemics, SARS-CoV-2, and dementia, is a major concern especially in China and in the United States.
In the United States, dementia patients usually stay in dedicated nursing homes or at their own homes, sometimes with a spouse. In China, however, much of the population lives in multi-generational homes where the elderly stay with their children and grandchildren. This increases the risk of exposure to the virus.
Asides from the risk of exposure, the effects of dementia, particularly that of memory- loss, make it difficult for the elderly to properly protect themselves from the virus as they may forget to follow necessary safeguards such as wearing masks [63]. Prudent care is required for these patients as a precaution.
10. The interplay of coronaviruses between brain and respiratory system
There has not been much study into how the virus affects CNS conditions or how well it infiltrates the CNS. However, researchers have noted that the infection of SARS-CoV, MERS, and SARS-CoV-2 infects the brainstem [64]. A few studies also postulate that coronaviruses might spread to the medullary cardiorespiratory center through synapses [65].
10.1. Neuroinvasive nature of HCoV-OC43
In 1980, Burks et al. found the coronavirus (CoV) infection in autopsy samples of multiple sclerosis (MS) patients [66]. In 1992, Murray et al. confirmed the presence of CoV in MS patients [67]. In 2000, Arbour et al. reported that 67% of HCoV-OC43 infections in autopsy samples had MS. This research demonstrated a significant relationship between respiratory pathogens and CNS disease [68].
In 2003, Jacomy and Talbot reported the neuroinvasive and neurotropic properties of HCoV-OC43 in mice. In brief, mice were infected intranasally (IN) with HCoV-OC43 and then viral RNA was confirmed in brain tissue by RT-PCR and later in the spleen. These findings suggested that HCoV-OC43 infected the CNS and other organs [69]. The researchers then performed intracerebral inoculation (IC) to understand brain transmission [69]. The presence of HCoV-OC43 in the cortex, hippocampus, spinal cord, brain stem, cerebellum, striatum, colliculus superior, and hypothalamus was confirmed by RT-PCR, western blotting (N-protein), immunofluorescence, and TEM. Both microgliosis and astrogliosis were seen in brain tissue. The presence of HCoV-OC43 RNA was confirmed in the lungs, spleen, heart, liver, and muscle [69]. Glass et al. found SARS-CoV in the brain of the mice [70]. In 2006, Jacomy et al. showed the effect of HCoV-OC43 on neuronal and glial cells [71].
Researchers also noted that impairments in T-cell immune responses and microglia can exacerbate the encephalitis caused by some viruses [72].
In 2004, Yeh et al. found HCoV-OC43 in the CSF and nasopharyngeal samples of children who had acute disseminated encephalomyelitis [73]. In the following year, Xu et al. detected and confirmed SARS-CoV in the brain autopsy sample of a patient using quantitative real-time PCR, ELISA, and indirect fluorescence assay (N-protein of SARS-CoV). In this study authors also investigated IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, granulocyte-macrophage–colony-stimulating factor, Mig, IP-10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and regulated on activation, normal T cell expressed and secreted (RANTES). The researchers noted that the levels of IP-10 and Mig were increased. Chemokine CXCR3 was also increased in the same patient. All these cytokines and chemokines are involved with the host defense mechanisms. Elevated IP-10 and Mig might have affected the T-cell immune system and helped the SARS-CoV to enter into the brain tissue with the aid of CD68+ monocytes/macrophages and CD3+ T lymphocytes [74].
One study reported a high incidence of coronavirus (CoV) in children with CNS diseases. In this study, cytokine analysis revealed high levels of granulocyte colony-stimulating factor (G-CSF) both in CNS illness and respiratory infection children. However, only CNS illness patients had an elevated granulocyte-macrophage colony-stimulating factor (GM-CSF). CNS illness patients also had high levels of IL-6, IL-8, and MCP-1. All these parameters indicate an alteration in the immune system in CNS infected coronavirus patients [75]. In 2004, St-Jean et al. suggested that HCoV-OC43 entered the brain through nasal infection [76]. The ablation of the olfactory bulb prevented the spread of the mouse hepatitis virus (MHV), which is genetically related to the family of HCoV-OC43, through nasal transmission [77]. These findings suggested the infection of CoV to the CNS and the spread of the virus from CNS to peripheral cells through trans-neuronal pathways.
In 2016, Morfopoulou et al. found the association of HCoV-OC43 with encephalitis using deep sequencing and quantitative real-time PCR of a brain biopsy sample from an 11- month old boy who was suffering from severe combined immunodeficiency with encephalitis symptoms. RNA sequencing of the brain biopsy was continued for two months with continuous cord blood transplantation to confirm the presence of HCoV-OC43 [78]. Studies of mice human neuronal cell lines have also found HCoV-OC43 infection of neurons in encephalitis [79,80].
10.2. Neuroinvasive nature of SARS-CoV and SARS-CoV-2
In 2003, Hung et al. showed SARS-CoV infection in the CSF sample and tracheal aspirates of a patient with neurological manifestations [81]. In the following year, Lau et al. reported SARS-CoV infection in CSF samples of a pregnant woman with a history of convulsions [82]. In 2020, Filatov et al. followed a patient with SARS-CoV-2 infection and encephalopathy. The patient was a 74-year-old male patient with a history of Parkinson's disease, COPD, atrial fibrillation, cardiac stroke (embolic), and cellulitis. The patient had ground-glass opacities (bilateral) with pleural effusion in his chest X-ray. Imaging also showed subpleural opacities and consolidations (patchy). EEG was carried out to assess the mental status of the patient. A CT scan showed encephalomalacia in the temporal region (left) that was persistent with embolic stroke. The patient was treated with lopinavir-ritonavir and hydroxychloroquine (HCQ).
Current literature on SARS-CoV-2 does not show the capability of the virus to cross the blood-brain barrier (BBB) and cause meningitis and encephalitis [83]. However, the virus is part of the coronavirus family that has been shown to affect the brain and cause CNS illness. Further study of SARS-CoV-2 neuroinvasive capabilities needs to be done.
11. SARS-CoV-2 infection and diabetes mellitus
In a 2020 meta-analysis of 76,993 patients by Emami et al., researchers found that COVID-19 patients often had many underlying comorbidities such as DM, hypertension, smoking, chronic obstructive pulmonary disease (COPD), and cardiovascular diseases [84].
In old age, there is a higher incidence of hypertension, obesity, and DM. All these metabolic syndrome consequences may increase mortality and morbidity in SARS-CoV-2 individuals [[24], [25], [26], [27], [28], [29]]. Furthermore, patients with one comorbidity, often have others as well. It is not clear whether coronary vascular disease (CVD), severe obesity, and hypertension in DM patients contribute to the SARS-CoV-2 infection progression. However, the higher plasma glucose levels and DM are predictive factors for mortality and morbidity in SARS-CoV infection [[85], [86], [87]]. The higher mortality and morbidity in DM patients with predisposing factors like CVD, hypertension, and severe obesity could be due to the increased viral load through ACE2 receptors in the pancreas, heart, and kidney. There could be altered endosomal pH, reduced viral clearance, T-cell immune dysfunction, and hyperactivation of inflammatory signaling cascades. There is a heightened expression of ACE2 in rodent models of DM. Increased ACE2 amplifies the ability of SARS-CoV-2 to enter cells. While insulin treatment reduced ACE2 expression, hypoglycemic agents (glucagon-like peptide – 1, liraglutide, and pioglitazone), anti-hypertensive agents (like ACE inhibitors), and statins heightened the ACE2 expression [[88], [89], [90], [91], [92], [93], [94]]. In 2020, Rao et al. [95] conducted phenome-wide Mendelian randomization (MR) study and found the disease (traits) causally linked with ACE2 expression [95].
In 2018, Fernandez et al. found augmented expression of the furin protein in DM patients. Furin facilitates the SARS-CoV-2 entry by cleaving spike proteins (S1 and S2 domain). These findings show a burgeoning relationship between DM and SARS-CoV-2 that requires further investigation to elucidate molecular and cellular mechanisms. Such a study can help assess the risk of adverse outcomes in DM patients infected with SARS-CoV-2 [96]. This area of the study shows promise as such relationships have been seen before. In 2019, Kulcsar's study revealed delayed inflammation, reduced CD4+ T cells, and reduced expression Ccl2 and Cxcl10 in MERS-CoV infected humanized diabetic mice. These mice had reduced levels of TNF-α, IL-6, IL-12-b, and Arg1 and a heightened expression of IL-17a. Therefore, comorbidities, such as diabetes, can increase disease severity via a dysregulated immune response [13,[97], [98], [99]].
12. Speculations of SARS-CoV-2 on autophagy/endocytic pathway
Macro-autophagy, or autophagy, is a highly conserved process by which damaged proteins and organelles are engulfed by double-membrane vesicles called autophagosomes and degraded by lysosomes that fuse with the autophagosome to form an autolysosome [100,101]. It is involved in aging, cell survival, cell death, and immune mechanisms [102,103]. Autophagy is under the control of various autophagy-related genes (ATGs). The first step of autophagy is under the control of ubiquitin-like ligase 1 (ULK1)/ATG1 complex and it is also called the initiation step. The initiation step is a downstream target of mitochondrially targeted rapamycin (mTOR) complex 1. The second step of autophagy also called the elongation step, is under the control of the ATG-14 gene complexed with Beclin and PI3K kinases. In the last step of autophagy, all the damaged and accumulated contents are degraded by the autolysosome [104,105]. Many studies suggest that autophagy may play a role in dementia, cancer, and viral infectious diseases.
The significance of autophagy in cellular and pathological systems was highlighted in the SARS-CoV outbreak of 2002. In 2004, Prentice et al. demonstrated nsp6 of SARS-CoV might play a crucial role in mediating autophagy [106]. Autophagy in other viruses has also been investigated. Autophagy proteins ATG5 and ATG7 did not affect viral replication in MHV [107,108]. In 2011, Cottam et al. [109] found an induction of autophagy using replicase nsp6 in the infectious bronchitis virus (IBV) [109]. Overexpression of PLP2 in SARS-CoV halts autophagosome formation [110,111].
Numerous investigators have worked to inhibit coronavirus infection using the autophagy/endocytic pathway. S-protein meditated entry of the endocytic pathway by SARS can be inhibited using HCQ, Bafilomycin, A1, and NH4Cl. The clathrin-dependent endocytosis pathway, another target for possible endocytic inhibition, can be inhibited by Chlorpromazine/ MβCD and Amiodarone [[112], [113], [114], [115], [116]]. MERS and MHV infection were successfully controlled using HCQ, Bafilomycin, Chlorpromazine, Bufalin, Ouabain, A1, and NH4Cl [[117], [118], [119]]. However, there are only a few studies of such an approach being used in SARS-CoV-2 [120,121].
Currently, the role of autophagy is still debatable in SARS-CoV, MERS-CoV, and SARS-CoV-2. Regardless, targeting autophagy might have a potential role in the treatment of COVID-19. Researchers have tried drugs such as HCQ to inhibit viral replication and to elevate endosomal pH, but the efficacy and side effects are not favoring such approaches [122].
13. Identification of SARS-CoV-2 and therapeutics
13.1. COVID-19 diagnostics
-
1.
Fluid swab test: To rule out influenza flu when the individual comes with COVID-19 symptoms [123].
-
2.
Quantitative real-time PCR: The sensitivity ranges from 40 to 85% but it is the gold standard diagnostic approach in detecting COVID-19 [124].
-
3.
Nucleic acid amplification test (NAAT): Viral RNA will be amplified [125,126].
-
4.
Complete blood picture report: Estimate the counts of RBC, WBC, and platelets. There is reduced lymphopenia in 80% of individuals [13].
-
5.
Liver function tests: Estimate the AST, ALT, and acute-phase proteins to assess the prognosis of multi-organ failure [13].
-
6.
Renal function tests: Estimate the creatinine and BUN levels to assess the perfusion of kidneys [13].
-
7.
Procalcitonin levels: The viral infection may cause a superinfection with bacteria. Elevated procalcitonin levels may show a superinfection of COVID-19 with a bacterial infection [13].
-
8.
CRP/D-Dimer/Other marker levels: Elevated levels of supporting markers such as CRP, IL-6, LDH, ferritin, D-dimer, and ESR may indicate an advanced stage of SARS-CoV-2 infection [13].
-
9.
Troponin and CK-MB levels: Elevated troponins and CK-MB reveal a lack of proper perfusion to the heart that can increase mortality.
-
10.
Imaging studies: a) Chest X-Ray (CXR): COVID-19 has a ground-glass appearance
b) CT scan: COVID-19 shows ground-glass opacities, consolidation (of proteins), and crazy paving pattern (CPP) in lungs; c) UltraSound (US): COVID-19 has pleural line thickenings, increased B-lines, and consolidation with air bronchogram [[126], [127], [128], [129]].
14. Therapeutics of COVID-19
-
1.
Fluid Treatment: Providing IV fluids such as normal saline, lactate ringer's solution, and oral fluids can help control the perfusion rate by not overloading the lungs [130].
-
2.
Fever Control: It can be achieved by giving Tylenol or paracetamol.
-
3.
Remdesivir: This was a drug used for Ebolavirus treatment as it inhibited RNA dependent RNA polymerase. Earlier findings have shown an inhibitory role of Remdesivir on the replication of SARS-CoV and MERS-CoV. Researchers are postulating that it may have a therapeutic benefit on SARS-CoV-2 replication as well [131].
-
4.
Hydroxychloroquine (HCQ): HCQ is an anti-antimalarial drug that may inhibit the synthesis of new SARS-CoV-2 [122].
-
5.
Ritonavir and Lopinavir (M- Pro ) protease inhibitors: These drugs may inhibit the conversion of various polyproteins into the different components (S, M, E, HE, and enzymes) of SARS-CoV-2 [132].
- 6.
-
7.
Tocilizumab: Tocilizumab prevents the inflammatory role of IL-6 on alveolar capillaries and the accumulation of interstitial fluids [135].
-
8.
Corticosteroids: Corticosteroids can be used to reduce inflammation by acting on phospholipase 2 to prevent the formation of leukotrienes and prostaglandins (PGE2) [136].
-
9.
Tetracyclines: Tetracyclines are antibiotics that bind to the matrix metalloproteinases (MMPs) and may reduce SARS-CoV-2 infiltration. The coronaviruses utilize MMPs for its replication, infiltration, and cell-cell adhesion [137].
-
10.
Vitamin D: Vitamin D supplementation may reduce pro-inflammatory cytokines [138].
-
11.
Vaccines: Vaccines play a pivotal role to combat against SARS-CoV-2 but it may not be available for another 12–18 months. Okada et al. [139] suggests that two vaccines, SARS-Nucleocapsid (N) and SARS-M, can elicit T-cell immune responses by modulating IFN γ production and cytotoxic T-cell activity respectively to combat SARS-CoV. The application of these vaccines for SARS-CoV-2 is still questionable [139]. However, few universities and institutes have developed vaccines that are in the clinical trial stage of development. These vaccines may available for humans in 2–3 months but their efficacy may be questionable.
-
12.
Other: Walls et al. developed two antibodies to elicit a humoral response for SARS-CoV and MERS-CoV. The SARS-CoV S antibody elicited conformational changes in preventing viral entry into the host cell [51].
In addition to the above therapeutics, investigators are trying to develop different drug molecules by targeting the structure of spike proteins (S1 & S2 domains), E-protein, M-protein, Nucleocapsid (N) protein, proteases, HE esterase, and helicases.
14.1. Spike proteins
Spike proteins play a pivotal role in mediating the fusion of SARS-CoV-2 to the host cell's membrane. Therefore, this mechanism can be exploited for possible therapy [19]. The spike protein is a transmembrane protein with 180–200 kDa size, the N-terminal region faces extracellularly and the C-terminal region faces intracellularly. It has three regions: transmembrane (TM) region, ectodomain (ED) region, and intracellular domain. The extracellular domain splits into S1 (three S1 heads) and S2 (trimeric stalk). The S1 has two domains: the N-terminal domain (NTD) and C-terminal domain (CTD) [19]. The spike proteins are present on the surface virus (SARS-CoV-2) in trimeric form and facilitate entry into the host cells by the interaction of the host's ACE2 (receptor binding domain, RBD) and the virus' S2 via an S2-membrane fusion subunit [10,44]. The RBD amino acid sequence of SARS-CoV-2 for spike proteins is not much different from that for Bat-RaTG13, Pangolin, Human SARS-CoV, Bat-SARS-CoV Related, and Bat-SARS-CoV. It is a true polybasic cleavage sequence and O-linked glycans sequence.
Previous studies of SARS-CoV and spike proteins may shed great light on the viral entry of SARS-CoV-2. The 18 amino acid residues of ACE2 interact with 14 amino acids of SARS-CoV spike proteins. The R453 and K341 of ACE2 are involved in the virus-host interaction. Few investigators blocked the entry of SARS-CoV by using the Anti-ACE2 antibody in E6 cells [47,140]. In 2004, an in-vitro study of 293 T cells by Kao et al. found 104 compounds with anti-SARS-CoV entry activity in host cells by targeting ACE2, H, M, and Helicases. The compounds such as VE607, HE602, and MP576 effectively inhibited viral entry (through ACE2), helicases, and M protein activity respectively [141].
14.2. S2 domain role
The S2 domain has two repeated, heptad, and hydrophobic regions known as HR1 and HR2. T20 (enfuvirtide) which is approved by USFDA against AIDS may significantly interact with the HR2 region of the SARS-CoV by inhibiting endocytosis into the host cell. However, in mutant strains of SARS-CoV, this enfuvirtide molecule fails to inhibit endocytosis [142]. Xia et al. found that the peptide OC43-HR2P, derived from the HR2 domain of HCoV-OC43, exhibits broad fusion inhibitory activity against multiple HCoVs. EK1, the optimized form of OC43-HR2P, showed substantially improved pan-CoV fusion inhibitory activity [143]. This domain needs to be further studied in SARS-CoV-2.
14.3. Other host receptor interactions
Cinanserin is a 5-HT (serotonin) receptor antagonist that comfortably binds to the substrate receptor site by interacting with H41 and E166 of MPro. Cinanserin might have an anti-SARS-CoV-2 transmission in humans. Ebselen also has shown mild antiviral activity by inhibiting MPro [144].
In 2020, Vankadri et al. hypothesized the interaction of N- and O-linked glycosylation sites of the SARS-CoV-2 spike protein with CD26 to avoid host defense. Intervening molecules might have therapeutic benefits against SARS-CoV-2 [145].
Neutralizing antibodies (NAb's) against spike proteins might have therapeutic benefits for SARS-CoV-2 as they were proven effective to combat SARS-CoV. Previous findings revealed NAb's 80R, CR3014, 3022, CR3396, B1, 201, 68, 1F8, 5E9 had a therapeutic action against SARS-CoV [146,147].
14.4. Envelope (E) protein
The envelope protein is a small structural protein in SARS-CoV that is made up of 76–109 amino acids with a molecular weight ranging from 8.4 to 12 kDa. The structure of the E protein varies among the coronavirus family. The E protein is responsible for passage and assembly in viral morphogenesis. It also acts as a virulence factor and is responsible for the formation of ion channels. The E-protein localizes around the Golgi apparatus and the endoplasmic reticulum [148,149]. In 2009, Pervushin et al. found reduced channel activity with the hexamethylene amiloride in HEK-293 cells [150].
In 2020, Gupta et al. used an in-silico approach to inhibit SARS-CoV-2 E protein with phytochemicals such as Belachinal, Macaflavanone E, and Vibsanol B. Its therapeutic applications need to be validated in humans [151].
14.5. M-protein
The M protein is responsible for maintaining the shape of the viral envelope, for stabilizing the nucleocapsid protein, and for processing virions. The structure of M-protein contains three domains (TM) and involves multiple interactions with other CoV proteins. However, in CoV assembly, M-M, M-N, and M-V are the most important domains. The M-N interaction is important for nucleocapsid-RNA complex stabilization and forms the viral core. It also activates IFN-β & NF-κB pathways through Toll-like receptor (TLR) signaling cascades (TRAF3 independent mechanism). The TLR and TLR4 antagonists through M- proteins blockers may impede the growth of SARS-CoV or CoV-2 [[152], [153], [154], [155]].
14.6. Nucleocapsid protein (N)
The N-region is highly conserved among coronaviruses and it has three major disordered regions: N-terminal domain (NTD), central linker domain, and C-terminal (tail)- domain (CTD). The CTD and NTD are the most important functional and structural domains in coronaviruses. The NTD is responsible for RNA binding. The CTD domain is responsible for the dimerization of N-proteins. The N-proteins regulate replication, transcription, and translation in all host cells. They eventually regulate host cell metabolism, cell cycle, and apoptosis. Molecules that prevent NTD binding can have therapeutic benefits against SARS-CoV-2 [[156], [157], [158], [159], [160], [161]]. In 2014, Lin et al. found reduced RNA binding and RNA replication in HCoV-OC43 with NTD inhibitors (PJ34) [158].
In 2012, Roh designed a biochip to analyze the N-protein inhibitors with the aid of nano-based ribonucleotides. In this study, the researcher examined polyphenolic compounds, (−)-catechin gallate and (−)-gallocatechin gallate reduced RNA binding with NTD [162]. NP111, NP331, and NP 351 inhibited N-protein activity in the host cell [[163], [164], [165], [166], [167]]. A few investigators targeted CTD of HCoV-229E to mitigate viral replication in the host cell with the N377–389 of C-tail sequence oligomerization inhibition.
All these findings suggest that targeting N-protein can ameliorate the COVID-19 transmission by inhibiting RNA replication.
14.7. Protease (3CL protease and papain like protease)
The SARS-CoV genome contains 16 NSPS comprised of PP1a and ab. In SARS-CoV 3C like proteases (3CLpro) and the main proteases (papain-like protease-PLpro) are involved in cleaving PP1a and ab to release NSPS [[168], [169], [170]]. In the literature, compounds like N-(benzo[1,2,3]triazol-1-yl)-N-(benzyl) acetamido) phenyl) carboxamides, ML188, and ML300 have an inhibitory activity on 3CLpro. The MPro activity gets inhibited by metal-conjugated and peptidomimetic compounds, aryl boronic acids, quinolinecarboxylate derivatives, thiophene carboxylate, phthalhydrazide-substituted ketoglutamine analogues, some flavonoids, and small molecule inhibitor N3. Commercial drugs like bepotastine, epirubicin, vapreotide, valrubicin, colistin, icatibant, epoprostenol, perphenazine, caspofungin, and aprepitant also bind similarly as HIV protease inhibitors like ritonavir. Many protease inhibitors may also act on SARS-CoV-2 and prevent its transmission [[171], [172], [173], [174], [175], [176], [177], [178], [179], [180]].
14.8. Hemagglutinin esterase (HE) and helicases
Hemagglutinin esterases are envelope glycoproteins that mediate reversible adhesion to O-acetylated sialic acids by destroying receptors and lectins. The HE inhibitors may be potential therapeutics in COVID-19 prevention [181]. Helicases are responsible for replication, transcription, and translation of the virus [182]. However, due to cell toxicity issues of the different antagonists and inhibitors, helicases may not be a therapeutic target for SARS-CoV-2 [[183], [184], [185], [186]].
14.9. Appreciation of High altitude pulmonary edema (HAPE) therapeutics for COVID-19
HAPE and COVID-19 are respiratory syndrome disorders that have similar pathophysiological pathways. The arterial blood gas analysis (ABG) in both cases presents the same pattern of disturbances associated with tachypenia and hypoxia but not limited to the ground glass opacities, CPP, consolidations, and pulmonary edema. The HAPE patients are treated with acetazolamide to reduce hypoxic vasoconstriction and to heighten the ventilation and lung vital capacity. Since COVID-19 presents similarly to HAPE, HAPE drugs, such as nifedipine, PDI, and acetazolamide, might have therapeutic benefits against SARS-CoV-2 [187].
14.10. Other potential approaches to encounter COVID-19
In the host cell, SARS-CoV-2 requires low pH for its survival. Molecules that alter this survival pH may help prevent infection progression. In 2014, Stadler et al. demonstrated inhibition of SARS-CoV infection by amiodarone in Vero cells by altering endosomal pH [188].
Spike proteins (S) are present in the trimeric form and are highly conserved in both SARS-CoV and SARS-CoV-2 with 77% amino acid sequence similarity and possible epitope cross-reactivity [189]. In one study CR3022, a neutralizing antibody isolated from one SARS patient's plasma binds to the RBD of SARS-CoV [190]. In 2020, Yuan et al. determined the crystal structure of CR3022 when bound to the RBD of SARS-CoV at a resolution of 3.1A0. The researchers noted 86% conservation of residues between SARS-CoV and SARS-CoV-2 in the epitope. However, there are differences in affinity for CR3022 between SARS-CoV and SARS-CoV-2 potentially as a result of differences in the non-conserved residues and N-glycosylation (N370 of SARS-CoV) [191]. Based on these findings CR3022 neutralizing antibody may confer in-vivo protection for SARS-CoV-2.
15. Conclusions and future directions
As the pandemic unfolds, further investigation into risk factors, disease progression, and drug therapy is needed. This article investigates the neuroinvasive nature of coronaviruses, the risks of comorbidities such as diabetes, and potential therapeutic targets and drugs. Future studies should strive to identify the precise molecular and cellular links between coronavirus infections and its dissemination to the pancreas, liver, kidney, heart, and brain. Such studies can, for example, help physicians treat patients with compromised immune systems as a result of liver cirrhosis or AIDS [[192], [193], [194], [195]]. Currently, drugs such as HCQ and Remdesivir are being used to prevent SARS-CoV-2 transmission. Many nations across the globe are also employing convalescent plasma therapy. The mechanism of viral entry of SARS-CoV-2, governed by spike proteins, might also play a potential role for therapeutic targets. The presentation of multiple possible targets of therapy in this review should be further investigated. The SARS-CoV-2 pandemic caught the world by surprise and the only way to keep fighting it is by learning more about it.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All the authors do not have any conflict of interest RK acknowledged DBT for awarding Ramalingaswami Re-entry fellowship. JV acknowledged for giving support in preparing this manuscript. P.H.R acknowledged NIH for funding various projects (R01AG042178, R01AG47812, R01NS105473, and AG060767).
Contributor Information
P. Hemachandra Reddy, Email: hemachandra.reddy@ttuhsc.edu.
Ramesh Kandimalla, Email: ramesh.kandimalla@iict.res.in.
References
- 1.Enquist L.W., Dermody T.S., DiMaio D. Vol. 3. 2016. Introduction, Annual Review of Virology. v. [Google Scholar]
- 2.Beachy R.N., Zaitlin M. Replication of tobacco mosiac virus, VI Replicative intermediate and TMV-RNA-related RNAs associated with polyribosomes. Virology. 1975;63:84–97. doi: 10.1016/0042-6822(75)90373-6. [DOI] [PubMed] [Google Scholar]
- 3.Reddi K.K. Degradation of tobacco mosiac virus nucleic acid with micrococcal phosphodiesterase. Biochim. Biophys. Acta. 1959;36:132–142. doi: 10.1016/0006-3002(59)90077-0. [DOI] [PubMed] [Google Scholar]
- 4.Hart R.G. Infectivity measurements of partially degraded tobacco mosiac virus. Virology. 1955;1:402–407. doi: 10.1016/0042-6822(55)90034-9. [DOI] [PubMed] [Google Scholar]
- 5.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front. Cell. Infect. Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herfst S., Ludlow M. Editorial overview: intraspecies transmission of viruses. Curr. Opin. Virol. 2018;28:v–vii. doi: 10.1016/j.coviro.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Zanin M., Wong S.S., Barman S., Kaewborisuth C., Vogel P., Rubrum A., Darnell D., Marinova-Petkova A., Krauss S., Webby R.J., Webster R.G. Molecular basis of mammalian transmissibility of avian H1N1 influenza viruses and their pandemic potential. Proc. Natl. Acad. Sci. U. S. A. 2017;114:11217–11222. doi: 10.1073/pnas.1713974114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan K.H., Peiris J.S., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.To K.K., Chan J.F., Chen H., Li L., Yuen K.Y. The emergence of influenza A H7N9 in human beings 16 years after influenza a H5N1: a tale of two cities. Lancet Infect. Dis. 2013;13:809–821. doi: 10.1016/S1473-3099(13)70167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudi S.K., Tiwari K.K. Preparedness and lessons learned from the novel coronavirus disease. Int. J. Occup. Environ. Med. 2020;11:108–112. doi: 10.34172/ijoem.2020.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html
- 18.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers as a potential target for antiviral development. Antivir. Res. 2020;104792 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS- CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syal K. COVID-19: herd immunity and convalescent plasma transfer therapy. J. Med. Virol. 2020 doi: 10.1002/jmv.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://who.sprinklr.com/
- 24.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . Vol. 2020. Centers for Disease Control and Prevention, US Department of Health and Human 278 Services; Atlanta, GA: 2020. National Diabetes Statistics Report; p. 277. [Google Scholar]
- 27.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 32.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 33.Zhong N.S., Zheng B.J., Li Y.M., Poon Z.H., Xie K.H., Chan P.H., Li S.Y., Tan Q., Chang J.P., Xie X.Q., Liu J., Xu D.X., Li K.Y. Yuen, Peiris Y. Guan. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet (London, England) 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang, Xu H., Rebaza A., Sharma L., Dela Cruz C.S. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV- 2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients, AJR. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Tomlinson A.C., Wong A.H., Zhou D., Desforges M., Talbot P.J., Benlekbir S., Rubinstein J.L., Rini J.M. The human coronavirus HCoV-229E S-protein structure and receptor binding. eLife. 2019;8 doi: 10.7554/eLife.51230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.B. Diao, C. Wang, R. Wang, Z. Feng, Y. Tan, H. Wang, C. Wang, L. Liu, Y. Liu, Y. Liu, G. Wang, Z. Yuan, L. Ren, Y. Wu, Y. Chen, Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, (medRxiv), (2020) 2020.2003.2004.20031120.
- 47.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F., Long X., Zou W., Fang M., Wu W., Li W., Zhang B., Zhang W., Chen X., Zhang Z. 2020. Highly ACE2 Expression in Pancreas May Cause Pancreas Damage After SARS- CoV-2 Infection. (medRxiv) (2020.2002.2028.20029181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system, nature reviews. Cardiology. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigrist C.J., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palm N.W., Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat. Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID- 19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. 2020. Characteristics of Lymphocyte Subsets and Cytokines in Peripheral Blood Of 123 Hospitalized Patients With 2019 Novel Coronavirus Pneumonia (NCP) (medRxiv) (2020.2002.2010.20021832) [Google Scholar]
- 58.Cowburn A.S., Macias D., Summers C., Chilvers E.R., Johnson R.S. Cardiovascular adaptation to hypoxia and the role of peripheral resistance. eLife. 2017;6 doi: 10.7554/eLife.28755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balk R.A. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5:20–26. doi: 10.4161/viru.27135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connors J.M., Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):0–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd-Sherlock P., Ebrahim S., Geffen L., McKee M. Bearing the brunt of covid-19: older people in low and middle income countries. BMJ (Clinical research ed.) 2020;368:m1052. doi: 10.1136/bmj.m1052. [DOI] [PubMed] [Google Scholar]
- 62.Alzheimer'’s Disease International World Alzheimer's report 2019: attitudes to dementia. September, 2019. https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf
- 63.Wang H., Li T., Barbarino P., Gauthier S., Brodaty H., Molinuevo J.L., Xie H., Sun Y., Yu E., Tang Y., Weidner W., Yu X. Dementia care during COVID-19. Lancet (London, England) 2020;395:1190–1191. doi: 10.1016/S0140-6736(20)30755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tacharoenmuang R., Komoto S., Guntapong R., Upachai S., Singchai P., Ide T., Fukuda S., Ruchusatsawast K., Sriwantana B., Tatsumi M., Motomura K., Takeda N., Murata T., Sangkitporn S., Taniguchi K., Yoshikawa T. High prevalence of equine-like G3P[8] rotavirus in children and adults with acute gastroenteritis in Thailand. J. Med. Virol. 2020;92:174–186. doi: 10.1002/jmv.25591. [DOI] [PubMed] [Google Scholar]
- 66.Burks J.S., DeVald B.L., Jankovsky L.D., Gerdes J.C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209:933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- 67.Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacomy H., Talbot P.J. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315:20–33. doi: 10.1016/S0042-6822(03)00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol.: 1950. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- 71.Jacomy H., Fragoso G., Almazan G., Mushynski W.E., Talbot P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349:335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler D.L., Sariol A., Meyerholz D.K., Perlman S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Invest. 2018;128:931–943. doi: 10.1172/JCI97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 74.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., Ding Y., Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y., Li H., Fan R., Wen B., Zhang J., Cao X., Wang C., Song Z., Li S., Li X., Lv X., Qu X., Huang R., Liu W. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.St-Jean J.R., Desforges M., Almazán F., Jacomy H., Enjuanes L., Talbot P.J. Recovery of a neurovirulent human coronavirus OC43 from an infectious cDNA clone. J. Virol. 2006;80:3670–3674. doi: 10.1128/JVI.80.7.3670-3674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perlman S., Evans G., Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J. Exp. Med. 1990;172:1127–1132. doi: 10.1084/jem.172.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morfopoulou S., Brown J.R., Davies E.G., Anderson G., Virasami A., Qasim W., Chong W.K., Hubank M., Plagnol V., Desforges M., Jacques T.S., Talbot P.J., Breuer J. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 79.Talbot P.J., Desforges M., Brison E., Jacomy H. Coronaviruses as encephalitis-inducing infectious agents. In: Tkachev S., editor. Nonflavivirus Encephalitis. Vol. 4. InTech; Rijeka, Croatia: 2011. pp. 185–202. [Google Scholar]
- 80.Arbour N., Côté G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J. Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung E.C., Chim S.S., Chan P.K., Tong Y.K., Ng E.K., Chiu R.W., Leung C.B., Sung J.J., Tam J.S., Lo Y.M. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filatov A., Sharma P., Hindi F. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. March 21, 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020;8 [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Sun G.Z., Yang G.R., Zhang X.L., Wang L., Xu X., Xu X.P., Chan J.C. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 86.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV- 2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020:e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017:18. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E., Coffman T.M., Chen S., Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 90.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 91.Romani-Perez M., Outeirino-Iglesias V., Moya C.M., Santisteban P., Gonzalez-Matias L.C., Vigo E., Mallo F. Activation of the GLP-1 receptor by liraglutide INCREASES ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of Type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 92.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Wosten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 94.Zhang W., Xu Y.Z., Liu B., Wu R., Yang Y.Y., Xiao X.Q., Zhang X. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. TheScientificWorldJournal. 2014;2014:603409. doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rao S., Lau A., So H.-C. 2020. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of 2019-nCov: A Mendelian Randomization Analysis. (medRxiv) (2020.2003.2004.20031237) [DOI] [PubMed] [Google Scholar]
- 96.Fernandez C., Rysa J., Almgren P., Nilsson J., Engstrom G., Orho-Melander M., Ruskoaho H., Melander O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J. Intern. Med. 2018;284:377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. 2019. Comorbid Diabetes Results in Immune Dysregulation and Enhanced Disease Severity Following MERS-CoV Infection, JCI Insight, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 99.Chen X., Hu W., Ling J., Mo P., Zhang Y., Jiang Q., Ma Z., Cao Q., Deng L., Song S., Zheng R., Gao S., Ke H., Gui X., Lundkvist Å., Li J., Lindahl J.F., Xiong Y. 2020. Hypertension and Diabetes Delay the Viral Clearance in COVID-19 Patients. (medRxiv) (2020.2003.2022.20040774) [Google Scholar]
- 100.Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018;20:521–527. doi: 10.1038/s41556-018-0092-5. [DOI] [PubMed] [Google Scholar]
- 101.Levine B., Kroemer G. Autophagy: machinery and regulation. Microb cell. 2016. Cell. 2019;176:11–42. [Google Scholar]
- 102.Yin Z., Pascual C., Klionsky D.J. Autophagy: machinery and regulation. Microbial cell (Graz, Austria) 2016;3:588–596. doi: 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mizushima N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2019;63:1–10. doi: 10.1016/j.ceb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Mizushima N., Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 106.Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Z., Thackray L.B., Miller B.C., Lynn T.M., Becker M.M., Ward E., Mizushima N.N., Denison M.R., Virgin H.W.T. Coronavirus replication does not require the autophagy gene ATG5. Autophagy. 2007;3:581–585. doi: 10.4161/auto.4782. [DOI] [PubMed] [Google Scholar]
- 108.Reggiori F., Monastyrska I., Verheije M.H., Cali T., Ulasli M., Bianchi S., Bernasconi R., de Haan C.A., Molinari M. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cottam E.M., Maier H.J., Manifava M., Vaux L.C., Chandra-Schoenfelder P., Gerner W., Britton P., Ktistakis N.T., Wileman T. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy. 2011;7:1335–1347. doi: 10.4161/auto.7.11.16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X., Wang K., Xing Y., Tu J., Yang X., Zhao Q., Li K., Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gassen N.C., Niemeyer D., Muth D., Corman V.M., Martinelli S., Gassen A., Hafner K., Papies J., Mosbauer K., Zellner A., Zannas A.S., Herrmann A., Holsboer F., Brack-Werner R., Boshart M., Muller-Myhsok B., Drosten C., Muller M.A., Rein T. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS- coronavirus infection. Nat. Commun. 2019;10:5770. doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eifart P., Ludwig K., Bottcher C., de Haan C.A., Rottier P.J., Korte T., Herrmann A. Role of endocytosis and low pH in murine hepatitis virus strain A59 cell entry. J. Virol. 2007;81:10758–10768. doi: 10.1128/JVI.00725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stadler K., Ha H.R., Ciminale V., Spirli C., Saletti G., Schiavon M., Bruttomesso D., Bigler L., Follath F., Pettenazzo A., Baritussio A. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am. J. Respir. Cell Mol. Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 117.Pu Y., Zhang X. Mouse hepatitis virus type 2 enters cells through a clathrin-mediated endocytic pathway independent of Eps15. J. Virol. 2008;82:8112–8123. doi: 10.1128/JVI.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo−/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burkard C., Verheije M.H., Haagmans B.L., van Kuppeveld F.J., Rottier P.J., Bosch B.J., de Haan C.A. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J. Virol. 2015;89:4434–4448. doi: 10.1128/JVI.03274-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park J.E., Li K., Barlan A., Fehr A.R., Perlman S., McCray P.B., Jr., Gallagher T. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. U. S. A. 2016;113:12262–12267. doi: 10.1073/pnas.1608147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoffman A.M., Viel L., Prescott J.F., Rosendal S., Thorsen J. Association of microbiologic flora with clinical, endoscopic, and pulmonary cytologic findings in foals with distal respiratory tract infection. Am. J. Vet. Res. 1993;54:1615–1622. [PubMed] [Google Scholar]
- 124.COVID-19 virus testing in NHS laboratories" (PDF). NHS England and NHS Improvement. 16 March 2020.
- 125.Letter from FDA". FDA. 27 March 2020. Retrieved 2 April 2020.
- 126.Patel R., Babady E., Theel E.S., Storch G.A., Pinsky B.A., George K. St, Smith T.C., Bertuzzi S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020:11. doi: 10.1128/mBio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y., Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 128.Ai T., Yang Z., Hou H. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases [published online ahead of print, 2020 Feb 26] Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee E.Y.P., Ng M.Y., Khong P.L. COVID-19 pneumonia: what has CT taught us? Lancet Infect. Dis. 2020;20:384–385. doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bein B., Bachmann M., Huggett S., Wegermann P. SARS CoV-2/COVID-19: evidence-based recommendation on diagnosis and therapy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2020;55:257–265. doi: 10.1055/a-1146-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020;64(5) doi: 10.1128/AAC.00399-20. Published 2020 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Y.W., Yiu C.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C- like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 2013;87:8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen K.D., Lee D.A. Effect of steroids and nonsteroidal antiinflammatory agents on human ocular fibroblast. Invest. Ophthalmol. Vis. Sci. 1992;33:2693–2701. [PubMed] [Google Scholar]
- 137.Sodhi M., Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy. 2020;40(5):487–488. doi: 10.1002/phar.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Okada M., Takemoto Y., Okuno Y., Hashimoto S., Yoshida S., Fukunaga Y., Tanaka T., Kita Y., Kuwayama S., Muraki Y., Kanamaru N., Takai H., Okada C., Sakaguchi Y., Furukawa I., Yamada K., Matsumoto M., Kase T., Demello D.E., Peiris J.S., Chen P.J., Yamamoto N., Yoshinaka Y., Nomura T., Ishida I., Morikawa S., Tashiro M., Sakatani M. The development of vaccines against SARS corona virus in mice and SCID-PBL/hu mice. Vaccine. 2005;23:2269–2272. doi: 10.1016/j.vaccine.2005.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prabakaran P., Xiao X., Dimitrov D.S. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem. Biophys. Res. Commun. 2004;314:235–241. doi: 10.1016/j.bbrc.2003.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kao R.Y., Tsui W.H., Lee T.S., Tanner J.A., Watt R.M., Huang J.D., Hu L., Chen G., Chen Z., Zhang L., He T., Chan K.H., Tse H., To A.P., Ng L.W., Wong B.C., Tsoi H.W., Yang D., Ho D.D., Yuen K.Y. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem. Biol. 2004;11:1293–1299. doi: 10.1016/j.chembiol.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Q., Bi W., Zhu X., Li H., Qi Q., Yu F., Lu L., Jiang S. Nonneutralizing antibodies induced by the HIV-1 gp41 NHR domain gain neutralizing activity in the presence of the HIV fusion inhibitor enfuvirtide: a potential therapeutic vaccine strategy. J. Virol. 2015;89:6960–6964. doi: 10.1128/JVI.00791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5:eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 145.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerging Microbes & Infections. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS- CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuo L., Hurst K.R., Masters P.S. Exceptional flexibility in the sequence requirements for coronavirus small envelope protein function. J. Virol. 2007;81:2249–2262. doi: 10.1128/JVI.01577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Venkatagopalan P., Daskalova S.M., Lopez L.A., Dolezal K.A., Hogue B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology. 2015;478:75–85. doi: 10.1016/j.virol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pervushin K., Tan E., Parthasarathy K., Lin X., Jiang F.L., Yu D., Vararattanavech A., Soong T.W., Liu D.X., Torres J. Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 2009;5:e1000511. doi: 10.1371/journal.ppat.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gupta M.K., Vemula S., Donde R., Gouda G., Behera L., Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1751300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hogue B.G., Machamer C.E. Nidoviruses, American Society of Microbiology. 2008. Coronavirus structural proteins and virus assembly. [Google Scholar]
- 154.Arndt A.L., Larson B.J., Hogue B.G. A conserved domain in the coronavirus membrane protein tail is important for virus assembly. J. Virol. 2010;84:11418–11428. doi: 10.1128/JVI.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y., Liu L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a Toll-Like-Receptor-Related TRAF3-independent mechanism. mBio. 2016;7 doi: 10.1128/mBio.01872-15. (e01872–01815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chang C.K., Lo S.C., Wang Y.S., Hou M.H. Recent insights into the development of therapeutics against coronavirus diseases by targeting N protein. Drug Discov. Today. 2016;21:562–572. doi: 10.1016/j.drudis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin S.Y., Liu C.L., Chang Y.M., Zhao J., Perlman S., Hou M.H. Structural basis for the identification of the N-terminal domain of coronavirus nucleocapsid protein as an antiviral target. J. Med. Chem. 2014;57:2247–2257. doi: 10.1021/jm500089r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chang C.K., Hou M.H., Chang C.F., Hsiao C.D., Huang T.H. The SARS coronavirus nucleocapsid protein–forms and functions. Antivir. Res. 2014;103:39–50. doi: 10.1016/j.antiviral.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang C.K., Jeyachandran S., Hu N.J., Liu C.L., Lin S.Y., Wang Y.S., Chang Y.M., Hou M.H. Structure-based virtual screening and experimental validation of the discovery of inhibitors targeted towards the human coronavirus nucleocapsid protein. Mol. BioSyst. 2016;12:59–66. doi: 10.1039/c5mb00582e. [DOI] [PubMed] [Google Scholar]
- 161.Lo Y.S., Lin S.Y., Wang S.M., Wang C.T., Chiu Y.L., Huang T.H., Hou M.H. Oligomerization of the carboxyl terminal domain of the human coronavirus 229E nucleocapsid protein. FEBS Lett. 2013;587:120–127. doi: 10.1016/j.febslet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roh C. A facile inhibitor screening of SARS coronavirus N protein using nanoparticle- based RNA oligonucleotide. Int. J. Nanomedicine. 2012;7:2173–2179. doi: 10.2147/IJN.S31379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao J., Huang Q., Wang W., Zhang Y., Lv P., Gao X.M. Identification and characterization of dominant helper T-cell epitopes in the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:6079–6088. doi: 10.1128/JVI.02568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheung Y.K., Cheng S.C., Sin F.W., Chan K.T., Xie Y. Induction of T-cell response by a DNA vaccine encoding a novel HLA-A*0201 severe acute respiratory syndrome coronavirus epitope. Vaccine. 2007;25:6070–6077. doi: 10.1016/j.vaccine.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lo Y.S., Lin S.Y., Wang S.M., Wang C.T., Chiu Y.L., Huang T.H., Hou M.H. Oligomerization of the carboxyl terminal domain of the human coronavirus 229E nucleocapsid protein. FEBS Lett. 2013;587(2):120–127. doi: 10.1016/j.febslet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kariwa H., Noda H., Nakauchi M. Characterization and epitope mapping of monoclonal antibodies to the nucleocapsid protein of severe acute respiratory syndrome coronavirus. Jpn J Vet Res. 2008;55(4):115–127. [PubMed] [Google Scholar]
- 167.Zhao J., Huang Q., Wang W., Zhang Y., Lv P., Gao X.M. Identification and characterization of dominant helper T-cell epitopes in the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:6079–6088. doi: 10.1128/JVI.02568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: a systematic review. Indian J. Pharm. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lindner H.A., Fotouhi-Ardakani N., Lytvyn V., Lachance P., Sulea T., Menard R. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J. Virol. 2005;79:15199–15208. doi: 10.1128/JVI.79.24.15199-15208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu T., Zhang Y., Li L., Wang K., Chen S., Chen J., Ding J., Jiang H., Shen X. Two adjacent mutations on the dimer interface of SARS coronavirus 3C-like protease cause different conformational changes in crystal structure. Virology. 2009;388:324–334. doi: 10.1016/j.virol.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsu M.F., Kuo C.J., Chang K.T., Chang H.C., Chou C.C., Ko T.P., Shr H.L., Chang G.G., Wang A.H., Liang P.H. Mechanism of the maturation process of SARS-CoV 3CL protease. J. Biol. Chem. 2005;280:31257–31266. doi: 10.1074/jbc.M502577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barretto N., Jukneliene D., Ratia K., Chen Z., Mesecar A.D., Baker S.C. The papain- like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005;79:15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Turlington M., Chun A., Tomar S., Eggler A., Grum-Tokars V., Jacobs J., Daniels J.S., Dawson E., Saldanha A., Chase P., Baez-Santos Y.M., Lindsley C.W., Hodder P., Mesecar A.D., Stauffer S.R. Discovery of N-(benzo[1,2,3]triazol-1-yl)-N- (benzyl)acetamido)phenyl) carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: identification of ML300 and noncovalent nanomolar inhibitors with an induced-fit binding. Bioorg. Med. Chem. Lett. 2013;23:6172–6177. doi: 10.1016/j.bmcl.2013.08.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jacobs J., Grum-Tokars V., Zhou Y., Turlington M., Saldanha S.A., Chase P., Eggler A., Dawson E.S., Baez-Santos Y.M., Tomar S., Mielech A.M., Baker S.C., Lindsley C.W., Hodder P., Mesecar A., Stauffer S.R. Discovery, synthesis, and structure-based optimization of a series of N-(tert-butyl)-2-(N-arylamido)-2-(pyridin-3-yl) acetamides (ML188) as potent noncovalent small molecule inhibitors of the severe acute respiratory syndrome coronavirus (SARS-CoV) 3CL protease. J. Med. Chem. 2013;56:534–546. doi: 10.1021/jm301580n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perera K.D., Rathnayake A.D., Liu H., Pedersen N.C., Groutas W.C., Chang K.O., Kim Y. Characterization of amino acid substitutions in feline coronavirus 3C-like protease from a cat with feline infectious peritonitis treated with a protease inhibitor. Vet. Microbiol. 2019;237:108398. doi: 10.1016/j.vetmic.2019.108398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shahab S., Sheikhi M. Triazavirin - Potential inhibitor for 2019-nCoV Coronavirus M protease: A DFT study [published online ahead of print, 2020 May 20] Curr Mol Med. 2020 doi: 10.2174/1566524020666200521075848. [DOI] [PubMed] [Google Scholar]
- 179.Han Y.S., Chang G.G., Juo C.G., Lee H.J., Yeh S.H., Hsu J.T., Chen X. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44:10349–10359. doi: 10.1021/bi0504761. [DOI] [PubMed] [Google Scholar]
- 180.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zeng Q., Langereis M.A., van Vliet A.L., Huizinga E.G., de Groot R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Frick D.N., Lam A.M. Understanding helicases as a means of virus control. Curr. Pharm. Des. 2006;12:1315–1338. doi: 10.2174/138161206776361147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karpe Y.A., Lole K.S. NTPase and 5′ to 3' RNA duplex-unwinding activities of the hepatitis E virus helicase domain. J. Virol. 2010;84:3595–3602. doi: 10.1128/JVI.02130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Banerjee T., Aggarwal M., Sommers J.A., Brosh R.M., Jr. Biochemical and cell biological assays to identify and characterize DNA helicase inhibitors. Methods (San Diego, Calif.) 2016;108:130–141. doi: 10.1016/j.ymeth.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bertram S., Glowacka I., Muller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C., Soilleux E.J., Jahn O., Steffen I., Pohlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85:13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Solaimanzadeh I. Acetazolamide, nifedipine and phosphodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ghosh A.K., Brindisi M., Shahabi D., Chapman M.E., Mesecar A.D. Drug Development and Medicinal Chemistry Efforts toward SARS-Coronavirus and Covid-19 Therapeutics. ChemMedChem. 2020;15(11):907–932. doi: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019- nCoV and naming it SARS-CoV-2Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yuan M., Wu N.C., Zhu X., Lee C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lleo A., Invernizzi P., Lohse A.W., Aghemo A., Carbone M. Highlights for management of patients with autoimmune liver disease during COVID-19 pandemia. J. Hepatol. 2020;S0168-8278(20):30212–30219. doi: 10.1016/j.jhep.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.E.B. Tapper, S.K. Asrani, COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care, J. Hepatol. [DOI] [PMC free article] [PubMed]
- 194.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xiao Y., Pan H., She Q., Wang F., Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5(6):528–529. doi: 10.1016/S2468-1253(20)30080-7. (pii: S2468- 1253(20)30080-7) [DOI] [PMC free article] [PubMed] [Google Scholar]