Abstract
The collection and clinical use of COVID-19 convalescent plasma (CCP) as a therapy for COVID-19 infection is under development and early use in many centers worldwide. We conducted an international survey of centers undertaking studies of CCP to provide understanding of the common themes and differences between them. Sixty-four studies in 22 countries were identified from clinical trial registries and personal contacts of the authors. Twenty of the 64 centers (31%) from 12 of 22 countries (55%) responded to the survey. Of the 20 studies, 11 were randomized controlled trials (RCTs), and 9 were case series. Only 4 of the RCTs plan to recruit 400 patients or more, and only 3 RCTs were blinded. The majority of studies will study the effect of CCP on sick patients requiring hospitalization and those requiring critical care, and none is examining the role of CCP in non-infected at-risk individuals. A wide variety of primary and secondary outcomes are being used. The donor eligibility criteria among the studies are very similar, and the use of plasmapheresis for the collection of CCP is almost universal. The planned dose of CCP ranges from as little as 200 mL to well over 1 L, but is 400 to 800 mL or 4 mL/kg or greater in all the RCTs. There is considerable variability in donor antibody testing with no consistency regarding the cut-off for antibody titer for acceptance as CCP or the use of pathogen-inactivation. Our survey provides an understanding of the similarities and differences among the studies of CCP, and that by virtue of their design some studies may be more informative than others.
Keywords: Convalescent plasma, COVID-19 infection, Survey, Clinical trials
Highlights
-
•
Twenty of the 64 centers (31%) from 12 of 22 countries (55%) responded to a survey about studies of COVID-19 convalescent plasma (CCP); 11 were randomized controlled trials (RCTs), and 9 were case series.
-
•
The majority of studies are studying the effect of CCP on sick patients requiring hospitalization and those requiring critical care.
-
•
A wide variety of primary and secondary outcomes are being used.
-
•
The donor eligibility criteria among the studies are very similar, and the use of plasmapheresis for the collection of CCP is almost universal.
-
•
Our survey provides an understanding of the similarities and differences among the studies of CCP, and that by virtue of their design some studies may be more informative than others.
There are huge efforts to find effective therapies for COVID-19 infection. Numerous trials are in progress; indeed, more than 1000 studies addressing various aspects of COVID-19 were found to be registered on ClinicalTrials.gov on 15 May 2020, including more than 600 interventional studies and randomized clinical trials (RCTs) [1].
The collection and clinical use of COVID-19 convalescent plasma (CCP) is under development and early use in many centers and countries. Those implementing CCP are likely to prepare and administer it in different ways. This variation is not surprising given the urgency of the situation, and the limited evidence base for the safety and effectiveness of convalescent plasma against the several infectious agents against which it has been used [2,3].
There are several key questions surrounding the use of CCP as a therapeutic. These include antibody testing and donor selection, methods of collection and storage, dose and duration of treatment, lot to lot variability, adverse effects, selection of the patients most likely to benefit, and measurement of efficacy. A number of publications have already addressed some of these issues and a few have provided either recommendations [[3], [4], [5], [6], [7], [8]] or preliminary results [9]. Links to some websites providing information and/or recommendations about CCP are provided in Appendix 1.
There are several key questions surrounding the use of CCP as a therapeutic. These include antibody testing and donor selection, methods of collection and storage, dose and duration of treatment, lot to lot variability, adverse effects, selection of the patients most likely to benefit, and measurement of efficacy. A number of publications have already addressed some of these issues and a few have provided either recommendations [[3], [4], [5], [6], [7], [8]] or preliminary results [9]. Links to some websites providing information and/or recommendations about CCP are provided in Appendix 1.
Before being offered for routine use, this new intervention should be rigorously tested in clinical trials designed to define both safety and efficacy. This leads to questions about the design and conduct of these trials so that valid data are provided for analysis as quickly as possible. If CCP is found to be safe and effective, the lessons learned from the trials about the optimal methods for preparing and administering CCP will need to be implemented as a matter of urgency.
We report the results of an international survey of centers undertaking early studies of CCP to provide an understanding of the common themes and differences between them in the preparation and investigation of CCP and that by virtue of their design some studies may be more informative than others.
Methods
A survey tool was developed to collect information from centers planning to collect and administer CCP to patients with COVID-19 infection. The centers were identified on 1st May 2020 from a search of Clinicaltrials.gov, the Chinese Clinical Trial Registry (ChiCTR) and personal contacts of the authors. The survey tool was written in English and designed to gather information on the whole process of the collection and administration of CCP from the identification of suitable donors including antibody testing, through the collection and storage of the product, the identification of patients suitable for its administration and details of the design of clinical trials. We did not ask about the planned completion dates of the studies so it is not known when the results will be available.
Results
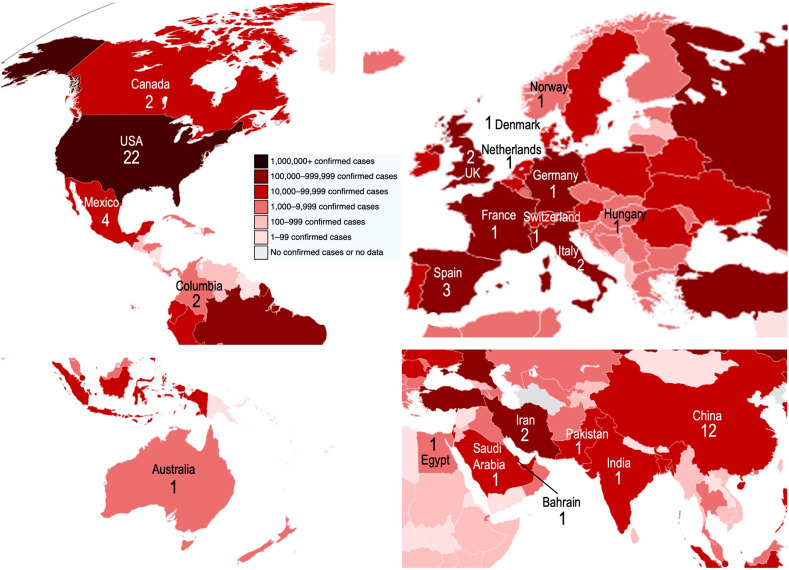
The survey was sent electronically to the study contacts for 64 studies in 22 countries shown in Fig. 1 and listed in Appendix 2 with a request to complete and return it within 7 days. We received responses from 20 of 64 (31%) studies from 12 of 22 (55%) countries, and they provide the data for this report.
Fig. 1.
World map showing the number of CCP studies and the confirmed number of cases of COVID-19 by country as of May 15, 2020.
The first survey questions were about the design of the studies. Of the 20 studies, 11 were randomized controlled trials (RCTs) and 9 were case series (Table 1A ). There was blinding of the investigators to the intervention in 3 of 11 RCTs where standard plasma was used as a comparator, and no blinding in the other 8. Among the RCTs, there was huge variation in the number of study sites (range, 1-250), and this was even more marked in the non-RCTs (range, 1-1300+). There was also considerable variation in the number of patients receiving CCP in both the RCTs (range, 40-5000) and in the case series (6-10 000).
Table 1A.
Study design.
| Study identifier | Design | Number of study sites | Number of patients receiving CCP | Age of patients (years) | Upper age limit |
|---|---|---|---|---|---|
| USA 1 | Case series | 1300+ | 10 000 | >18 | No |
| USA 2 | RCT (blinded) | 1–10 | 103 | >18 | No |
| USA 3 | RCT (blinded) | 1 | 400 | >18 | No |
| USA 4 | Case series | 1 | 30 | >18 | No |
| USA 5 | Case series | 20 | 100 | Adults | No |
| USA 6 | RCT (blinded) | 2–10 | 110 | >18 | No |
| China 1 | Case series | 1 | 6 | Not stated | No |
| Mexico 1 | Case series | 1 | 10 | >18 | No |
| Spain 1 | RCT (un-blinded) | 25 | 139 | Not stated | No |
| Spain 2 | RCT (un-blinded) | 1 | 60 | 18–69 | 69 |
| Canada 1 | RCT (un-blinded) | 53 | 800 | ≥16 | No |
| Canada 2 | RCT (un-blinded) | 16 | 100 | 0–18 | 18 |
| Iran 1 | Case series | 1 | 30 | 30–70 | 70 |
| UK 1 | RCT (un-blinded) | 120 | 1000 | >18 | No |
| UK 2 | RCT (un-blinded) | 250 | 5000 | >0 | No |
| Egypt 1 | Case series | 1 | 40 | >18 | No |
| France 1 | RCT (un-blinded) | 9 | 60 | >18 | No |
| Germany 1 | RCT (un-blinded) | 1 | 40 | <75 | 75 |
| Saudi Arabia 1 | Case series | 17 | 40 | >18 | No |
| Switzerland 1 | Case series | 1 | 10 | 18–75 | 75 |
RCT, randomized control trial.
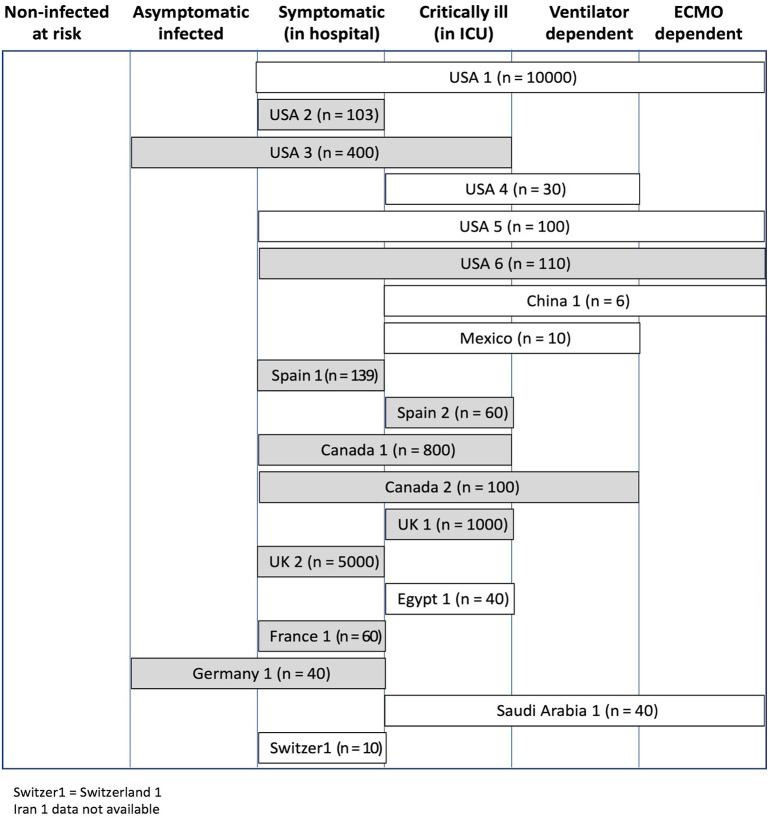
The comparison intervention to CCP was standard plasma in 3 of 11 RCTs, and no plasma in the others (although not stated in one study) (Table 1B ). The clinical stages of illness targeted by the different trials are shown in Fig. 2 . Most RCTs (9/11) included symptomatic, infected but not critically ill patients; 6 RCTs included critically ill patients; and 2 included asymptomatic infected patients. In contrast, all but one of the case series included critically ill patients. None of the studies focused on non-infected at risk individuals. Children were included as study participants in 3 of the RCTs. All studies required a positive PCR test of the recipient except for one of the studies in Iran (Iran-1) and the study in France. The collection of possible adverse effects was similar for all studies, although only 4 studies specifically included antibody dependent enhancement of infection (ADE).
Table 1B.
Study design (continued)
| Study identifier | Comparison group for the RCTs | Exclusions | Adverse effects |
|---|---|---|---|
| USA 1 | Non-randomized patients | None | Febrile, allergic, anaphylaxis; TACO |
| USA 2 | Standard plasma | Admission to hospital for ventilation | Anaphylaxis; TACO, TRALI; TTI |
| USA 3 | Standard plasma | Pregnancy | Febrile, allergic, anaphylaxis; |
| TACO | |||
| USA 4 | Non-randomized patients | Ventilator dependent | Not stated |
| USA 5 | Non-randomized patients | None | Febrile, allergic, anaphylaxis; |
| TACO, TRALI | |||
| USA 6 | Standard plasma | Cardiac or respiratory failure; Participation in other trials | Febrile, allergic, anaphylaxis; |
| TACO | |||
| China 1 | Non-randomized patients | Pregnancy | Febrile, allergic, anaphylaxis; |
| TACO | |||
| Mexico 1 | Non-randomized patients | Renal failure; ECMO; Pregnancy | Febrile, allergic, anaphylaxis; |
| TACO | |||
| Spain 1 | Not stated | Symptoms >12 days prior; Ventilator or high flow O2; Renal failure; Participation in other trials | Febrile, allergic, anaphylaxis; |
| TACO, TRALI; ADE | |||
| Spain 2 | No plasma | Participation in other trials | Febrile, allergic, anaphylaxis; |
| TACO | |||
| Canada 1 | No plasma | Ventilator or ECMO; Symptoms >12 days prior | Febrile, allergic, anaphylaxis; |
| TACO | |||
| Canada 2 | No plasma | Not stated | Febrile, allergic, anaphylaxis; TACO |
| Iran 1 | Non-randomized patients | Pre-intubation; Ventilator dependent; | Not stated |
| Heart failure | |||
| UK 1 | No plasma | Participation in other trials | Febrile, allergic, anaphylaxis; TACO, TRALI, TAD; ADE; Thrombosis |
| UK 2 | No plasma | Participation in other trials | Febrile, allergic, anaphylaxis; TACO, TRALI; ADE |
| Egypt 1 | Non-randomized patients | Ventilator or ECMO; Cardiac, pulmonary, renal, or liver failure; | Not defined at time of survey |
| Participation in other trials | |||
| France 1 | No plasma | Ventilator or ECMO; Cardiac, pulmonary, renal, or liver failure; | Febrile, allergic, anaphylaxis; TACO; ADE |
| Pregnancy; Uncontrolled infection; | |||
| Participation in other trials | |||
| Germany 1 | No plasma | Liver failure; Pregnancy; Participation in other trials | Febrile, allergic, anaphylaxis; TACO |
| Saudi Arabia 1 | Non-randomized patients | Not defined at time of survey | Transfusion reactions per aaBB |
| Switzerland 1 | Non-randomized patients | Ventilator or ECMO; Cardiac, pulmonary failure; Pregnancy; | Febrile, allergic, anaphylaxis; TACO; Other adverse events |
| Participation in other trials |
‘No plasma’ indicates no infusion of any fluid.
TACO, transfusion associated circulatory overload; TRALI, transfusion related acute lung injury; TTI, transfusion transmitted infection; ADE, antibody dependent enhancement of infection; TAD, transfusion associated dyspnea; aaBB, American Association of Blood Banks.
All studies require a positive PCR test of the recipient except France-1 and Iran-1.
Fig. 2.
Study enrolment according to clinical stage of disease based on survey responses. The number in parentheses is the number of subjects planned to receive CCP. The shaded boxes indicate randomized controlled trials.
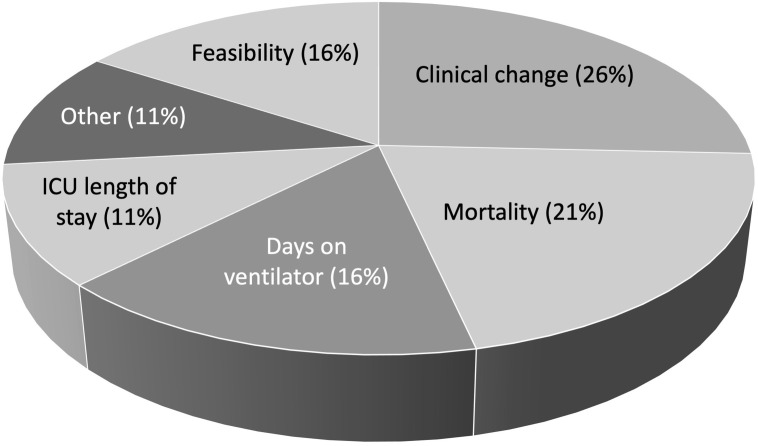
There was considerable variability in the primary and secondary outcomes for the studies (Table 2 ). Fig. 3 provides a summary of the primary outcomes with the most frequent being clinical change and mortality. The primary outcomes for the 3 largest RCTs were a composite of intubation or death at day 30 (USA-6), ventilation-free days (Canada-1) and mortality at 28 days (UK-2).
Table 2.
Primary and secondary outcomes
| Study identifier | Primary outcome | Main secondary outcomes |
|---|---|---|
| USA 1 | Availability of convalescent plasma | Serious adverse events |
| USA 2 | Time to progression using outpatient ordinal scale | Not recorded |
| USA 3 | Days on ventilation | Mortality at day 90 |
| USA 4 | Feasibility of treating ICU patients | Not recorded |
| USA 5 | Not yet decided | Days on ventilation; LOS in ICU; Hospital LOS |
| USA 6 | Modified WHO score at day 14 | Days on ventilation; Hospital LOS; Change in viral load; Mortality at day 28 |
| China 1 | Change in viral load | Days on ventilation |
| Mexico 1 | Change in lung injury (Kirby index) | Mortality at day 15 & 30 |
| Spain 1 | Proportion in level 5 or higher of 7-level ordinal scale | Days on ventilation; Hospital LOS; Change in viral load; Time to clinical worsening; Mortality at 15 days |
| Spain 2 | Feasibility and safety (pilot study) | Days on ventilation; LOS in ICU |
| Canada 1 | Composite of intubation or death at day 30 | Days on ventilation; LOS in ICU; Hospital LOS; Change in viral load |
| Canada 2 | Time to recovery or discharge by day 30 | LOS in ICU; Hospital LOS; Change in viral load; Others not specified |
| Iran 1 | Mortality at days 10 & 30 | Days on ventilation; Hospital LOS; Changes to laboratory tests at day 1, 3 & 7 |
| UK 1 | Ventilator-free days at day 21 | Days on ventilation; Hospital LOS; Change in viral load; Level of respiratory support at day 15 |
| UK 2 | Mortality (date not yet specified) | Days on ventilation; LOS in ICU; Hospital LOS; Renal impairment |
| Egypt 1 | LOS in ICU | Hospital LOS |
| France 1 | Ventilation-free survival at day 14 | Days on ventilation; LOS in ICU; Hospital LOS; Disease severity (WHO scale) at day 7 & 14 |
| Germany 1 | Mortality at day 28 | Days on ventilation; LOS in ICU; Hospital LOS; Change in viral load |
| Saudi Arabia 1 | LOS in ICU | Days on ventilation; Days to clinical recovery |
| Switzerland 1 | Immune markers before vs after infusion | Clinical change (7-point ordinal scale); serious adverse events |
ICU, intensive care unit; LOS, length of stay.
Fig. 3.
Primary outcomes of CCP trials based on survey responses.
The donor eligibility criteria for the collection of CCP were very similar among the studies (Table 3 ). In 15 of 16 studies where this information was provided, the respondents indicated the requirement for a prior positive polymerase chain reaction (PCR) assay for SARS-COV2. The time required from recovery of symptoms of COVID-19 infection before collection of CCP varied from 14 to 28 days. Nearly all studies indicated that female donors would be tested for HLA or HLA and HNA antibodies to minimize the risk of transfusion-related acute lung injury (TRALI). Plasmapheresis was selected as the method of collection of CCP by nearly all investigators.
Table 3.
Donor eligibility
| Study identifier | Donor category | Prior SARS-CoV2 in donor | Other donor qualifications | Method of collection |
|---|---|---|---|---|
| USA 1 | Uncertain | Not stated | Not stated | Not stated |
| USA 2 | Males; Females negative for HLA antibodies | Positive PCR | Neg PCR if 14–28 days; | Plasmapheresis |
| ≥ 28 d after symptoms | ||||
| USA 3 | Males; Females negative for HLA & HNA antibodies | Positive PCR or antibody | ≥ 14 d after symptoms | Plasmapheresis |
| USA 4 | Males; Females negative for HLA & HNA antibodies | Positive PCR | ≥ 14 d after symptoms | Plasmapheresis |
| USA 5 | Males; Females negative for HLA & HNA antibodies | Positive PCR | ≥ 28 d after symptoms | Plasmapheresis |
| USA 6 | Males; Females negative for HLA antibodies | Positive PCR | Neg PCR if 14–28 days; | Plasmapheresis |
| ≥ 28 d after symptoms | ||||
| China 1 | Males; Females negative for HLA & HNA antibodies | Positive PCR | ≥ 14 d after symptoms | Not stated |
| Mexico 1 | Males; Females negative for HLA antibodies | Positive PCR | ≥ 14 d after symptoms | Mainly plasmapheresis |
| Spain 1 | Not stated | Not stated | Not stated | Not stated |
| Spain 2 | Males; Females negative for HLA & HNA antibodies | Positive PCR | ≥ 14 d after symptoms | Plasmapheresis |
| Canada 1 | Males; Females negative for HLA antibodies | Positive PCR | Neg PCR if 14–28 days; | Plasmapheresis |
| ≥ 28 d after symptoms | ||||
| Canada 2 | Males; Females negative for HLA & HNA antibodies | Positive PCR | ≥ 28 d after symptoms (Canadian Blood Services); | Plasmapheresis |
| ≥ 14 d after symptoms (HemaQuebec) | ||||
| Iran 1 | Not stated | Not stated | Recovery from illness | Not stated |
| UK 1 | Males; Females negative for HLA & HNA antibodies | Positive PCR plus antibody | ≥ 28 d after symptoms | Mainly plasmapheresis |
| UK 2 | Males; Females negative for HLA & HNA antibodies | Positive PCR plus antibody | ≥ 28 d after symptoms | Mainly plasmapheresis |
| Egypt 1 | Male donors only | Positive PCR | ≥ 14 d after symptoms | Plasmapheresis |
| France 1 | Males; Females negative for HLA antibodies | Clinical illness test not required | ≥ 14 d after symptoms | Plasmapheresis |
| Germany 1 | Uncertain | Uncertain at time of survey | Not stated | Plasmapheresis |
| Saudi Arabia 1 | Males; Females negative for HLA antibodies | Positive PCR | ≥ 14 d after negative PCR | Plasmapheresis |
| Switzerland 1 | Male donors only | Positive PCR | ≥ 28 d after symptoms | Plasmapheresis |
PCR, polymerase chain reaction.
The dose of plasma was 400 to 800 mL or 4 mL/kg or greater in all 10 RCTs and in 6 of 8 of the case series providing this information (Table 4 ). Protocols called for CCP to be stored in the frozen state prior to thawing before administration in all 16 studies that provided this information apart from one study (Germany-1). Six studies including only 2 of the RCTs indicated that the CCP would be pathogen-inactivated.
Table 4.
Details of plasma dosing
| Study identifier | Dose (mL) | Number of infusions | Control plasma details | Storage conditions of CCP | Pathogen inactivation |
|---|---|---|---|---|---|
| USA 1 | 200–500 | 1 | No control plasma (case series) |
Not stated | Not stated |
| USA 2 | 4–6 mL/kg⁎ | 1 | Given prior to discharge | Frozen then thawed | No |
| USA 3 | 500 | 1 | Low antibody for SARS-CoV2 | Frozen then thawed | Uncertain at time of survey |
| USA 4 | 40 mL/kg | 1 | No control plasma (case series) |
Not stated | Not stated |
| USA 5 | 200–500 | 1 | No control plasma (case series) |
Frozen then thawed | No |
| USA 6 | 500 | 2 (day 1 and 2) | 2 doses of FFP or FP24 | Frozen then thawed | No |
| China 1 | 200 | Depends on availability | No control plasma (case series) |
Frozen then thawed | Yes |
| Mexico 1 | 200 | 1 | No control plasma (case series) |
Frozen then thawed | No |
| Spain 1 | Not stated | Not stated | Not stated | Not stated | Not stated |
| Spain 2 | 600 (200 × 3) |
Every 8 h up to 3 doses | No control plasma (unblinded) | Frozen then thawed | Methylene blue or amotosalen |
| Canada 1 | 500 (250 x2) | 1 | No control plasma (unblinded) | Frozen then thawed | No |
| Canada 2 | 10 mL/kg (500 max) |
1 | No control plasma (unblinded) | Frozen then thawed | Uncertain at time of survey |
| Iran 1 | Not stated | Not stated | Not stated (case series) |
Not stated | Not stated |
| UK 1 | 400–700 (200–300 × 2) |
2 (day 1 and 2) | No control plasma (unblinded) | Frozen then thawed | No |
| UK 2 | 400–700 (200–300 × 2) |
2 (day 1 and 2) | No control plasma (unblinded) | Frozen then thawed | No |
| Egypt 1 | 400–500 | 1 | No control plasma (case series) |
Frozen then thawed | Mixture |
| France 1 | 800–880 (400–440 × 2) |
2 (day 1 and 2) | No control plasma (unblinded) | Frozen then thawed | Yes |
| Germany 1 | 400 | 1 | No control plasma (unblinded) | Stored at 4C (not frozen) | No |
| Saudi Arabia 1 | 200–400 | Daily up to 5 times | No control plasma (case series) |
Frozen then thawed | Yes |
| Switzerland 1 | 600 (200 × 3) |
3 | No control plasma (case series) |
Frozen then thawed | Yes |
Ideal body weight.
Responses were received to questions about donor antibody testing from 15 of 20 of survey participants (Table 5 ). Eleven of 15 of all studies and 8 of 11 of the RCTs indicated that antibody testing would be carried out before the administration of CCP, and the remainder after its administration. Eleven of 15 of all studies and 6 of 11 of the RCTs indicated that testing would include neutralizing antibodies sometimes with additional testing for non-neutralizing antibodies. Only 8 studies provided information about cut-off levels or titers of antibodies used to qualify donors.
Table 5.
Antibody testing of donor
| Study identifier | Donor antibody testing before or after infusion | Antibody test details |
|---|---|---|
| USA 1 | Not stated | Not stated |
| USA 2 | Before | Non-neutralizing titer >1:80 |
| USA 3 | Before | Non-neutralizing per FDA guidelines |
| USA 4 | Uncertain at time of survey | Uncertain at time of survey |
| USA 5 | Before | Neutralizing antibody >1:100 (Euroimmune) |
| USA 6 | Before | Neutralizing plus non-neutralizing >1:160 |
| China 1 | Before | Non-neutralizing >1:160 |
| Mexico 1 | After | Neutralizing plus non-neutralizing (no cut-off) |
| Spain 1 | Not stated | Not stated |
| Spain 2 | Before | Non-neutralizing EIA O.D. >1.0 |
| Canada 1 | Before | Neutralizing antibody >1:160 or EIA |
| Canada 2 | After | Neutralizing plus non-neutralizing (cut-off not decided) |
| Iran 1 | Not stated | Not stated |
| UK 1 | Before | Neutralizing plus non-neutralizing (cut-off not decided) |
| UK 2 | Before | Neutralizing plus non-neutralizing (cut-off not decided) |
| Egypt 1 | After | Neutralizing antibody >1:40 |
| France 1 | Before | Neutralizing >1:30 plus non-neutralizing |
| Germany 1 | Uncertain at time of survey | Uncertain at time of survey |
| Saudi Arabia 1 | Before | Neutralizing plus non-neutralizing (no cut-off) |
| Switzerland 1 | After | Neutralizing plus non-neutralizing (no cut-off) |
Discussion
The COVID-19 pandemic represents a major threat to global health and has caused enormous strain on healthcare systems worldwide. One of the major research challenges is to develop trials to determine the effectiveness of any promising therapies, and one of these treatment options is CCP. A systematic review has shown that convalescent plasma (CP) may have clinical benefit for people with acute viral diseases such as influenza and severe acute respiratory syndrome (SARS) [10], but its effectiveness in patients with COVID-19 is as yet uncertain [8]. One reason for this is that many outbreaks are regional and short-lived not providing sufficient time to collect and carefully study the safety and efficacy of CP. The current COVID-19 pandemic may not be bound by such limitations and there is likely to be sufficient time to collect CCP to treat newly infected patients. The logical first research questions are to determine the safety and effectiveness of CCP; and not surprisingly, numerous studies have been established to do this worldwide. We have undertaken an international survey of centers who have instituted studies of CCP to provide an understanding of the similarities and differences between them.
We identified 64 CCP studies in 22 countries by searching trial registries and through personal contacts. This probably represents an unprecedented upsurge in studies of any single topic in transfusion medicine. We recognize that we may not have identified all CCP studies, and that further studies will have been initiated since we began the survey. We contacted those we identified as the principal investigators by email requesting rapid completion of the survey and received 20 responses from 64 studies (31%) from 12 of 22 countries (55%).
The responses raise concerns about their ability to determine the effectiveness of CCP across the clinical spectrum of COVID-19 infected patients. These concerns include the lack of randomization in 11 of 20 studies and small sample size in 10 of 20. Only 4 of the RCTs plan to recruit 400 patients or more so that the majority of studies are unlikely to have sufficient power to detect significant changes in key outcomes. A substantial proportion of survey respondents noted that mortality would be a primary outcome. Current estimates would suggest that the mortality rate of among hospitalized patients is approximately 15%, and in order to detect a 10% relative reduction in death rate (from 15% to 13.5%) with 80% power and alpha = 0.05 would require a study with over 15 000 participants. Furthermore, 8 RCTs are unblinded which may introduce bias in the assessment of outcomes other than mortality. On the other hand, the 3 blinded RCTs, where standard plasma is being used as the comparator to CCP, may have a reduced ability to detect harms from the transfusion of plasma in COVID-19 infected patients. Among those who responded to the survey, the majority of studies place emphasis on the effect of CCP on sick patients requiring hospitalization and those requiring critical care, and none is examining the role of CCP in non-infected at-risk individuals. A wide variety of primary and secondary outcomes were selected by investigators which likely reflects uncertainty regarding the most appropriate study outcome for CCP at different stages of COVID-19 infection.
The donor eligibility criteria for the collection of CCP are very similar among the studies in the almost universal requirement for a prior positive PCR assay for SARS-COV2 although there is variation in the time from recovery of symptoms of COVID-19 infection before collection of CCP. Nearly all survey respondents plan to use plasmapheresis to collect CCP and only some plan to use pathogen-inactivation. The planned dose of CCP ranges from as little as 200 mL to well over 1 L, but is 400 to 800 mL or 4 mL/kg or greater in all the RCTs. There is considerable variability in donor antibody testing with testing for neutralizing antibodies or non-neutralizing antibodies alone, or a combination of the two; and there is no consistency regarding the cut-off for antibody titer for acceptance as CCP or the use of pathogen-inactivation. Individual units of CCP would be expected to have a range of viral neutralizing capacity depending on their characteristics such as the dose, antibody titer, and antibody affinity, thereby further complicating inferences about efficacy.
As shown in Appendix 2, a large number of studies of CCP are planned worldwide. Our survey provides an informative sampling of these and indicates shared similarities and differences among them. By virtue of randomization, blinding, and sample size some studies may be more informative than others. The survey clearly shows an initial focus on sick hospitalized patients. Whether passive transfer of antibody may prove to be more effective in very recently infected individuals or non-infected persons at high risk for infection will await other studies not represented here. Results of all well-designed trials are eagerly awaited. The COVID-19 pandemic provides the first opportunity in history to rigorously define the role of convalescent plasma in a critically important viral respiratory disease.
The following are the supplementary data related to this article.
Useful links to sites providing information and/or recommendations about CCP.
CCP studies identified by May 1, 2020. Survey responses were received from those studies shown in shading.
Declaration of competing interest
The authors have no conflicts to declare.
References
- 1.Bauchner H., Fontanarosa P.B. Randomized clinical trials and COVID-19: managing expectations. JAMA. 2020 doi: 10.1001/jama.2020.8115. Published online May 04. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan H.C., Roback J.D. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev. 2020 doi: 10.1016/j.tmrv.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao H., Shi Y. Convalescent plasma: possible therapy for novel coronavirus disease 2019. Transfusion. 2020 doi: 10.1111/trf.15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein J., Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang. 2020 doi: 10.1111/vox.12939. 32319102 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020:138745. doi: 10.1172/JCI138745. Online ahead of print. PMID: 32254064 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown B.L., McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020:102790. doi: 10.1016/j.transci.2020.102790. [published online ahead of print, 2020 Apr 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan C. Convalescent serum lines up as first choice treatment for coronavirus. Nat Biotechnol. 2020 doi: 10.1038/d41587-020-00011-1. [DOI] [PubMed] [Google Scholar]
- 8.Valk S.J., Piechotta V., Chai K.L., Doree C., Monsef I., Wood E.M. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner M., Wright R.S., Fairweather D., Senefeld J., Bruno K., Klassen S. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020 Jun;11:140200. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Useful links to sites providing information and/or recommendations about CCP.
CCP studies identified by May 1, 2020. Survey responses were received from those studies shown in shading.