Abstract
Various neurologic syndromes have been described in patients with COVID-19 and other coronavirus infections. In this paper, we systematically reviewed the available imaging findings of patients diagnosed with neurological symptoms associated with coronavirus infections. Diverse radiologic results in the context of different neurologic presentations have been demonstrated using CT and MRI. While many patients have normal imaging evaluations, some patients present with intra-axial and extra-axial abnormalities. Stroke (both ischemic and hemorrhagic), encephalomyelitis, meningitis, demyelinating disorders such as acute disseminated encephalomyelitis (ADEM), and encephalopathy have been reported. Familiarity with these radiologic patterns will guide radiologists and referring clinicians to consider coronavirus infections in patients with worsening or progressive neurologic findings, particularly during the current COVID-19 pandemic. As data on this topic is very limited, further research and investigation are required.
Keywords: COVID-19, SARS-CoV-2, HCoV, SARS, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Brain, CNS, Neurologic sequelae, CT, MRI, Stroke, Meningitis, Encephalitis, Acute disseminated encephalomyelitis (ADEM), Encephalopathy
Introduction
The global novel Coronavirus disease (COVID-19) pandemic, first reported in Wuhan (China), has attracted intense attention around the world. As of June 11, 2020, more than 7.4 million COVID-19 cases with approximately 420 thousand deaths have been reported globally [1]. While the majority of infected patients present with fever and respiratory symptoms, several atypical manifestations have been reported recently, such as gastrointestinal complications, cardiac events, renal failure, and neurological deficits [2], [41]. In a case series of 214 hospitalized patients with COVID-19 from hospitals in Wuhan [3], 36.4% had neurological symptoms, including dizziness, headache, impaired consciousness, and acute cerebrovascular events. Moreover, in several other reports, neurologic manifestations have been reported as the initial presentation of SARS-CoV-2 infection [4], [5], [6].
Although the literature on the typical respiratory presentation of COVID-19 has been widely reported [7], thorough documentation of its neurologic manifestations, specifically the radiological findings, are lacking. Despite limited available data, radiologists and other healthcare providers should be aware of the spectrum of neurologic findings associated with COVID-19. This prompted us to conduct this systematic review on various radiological findings and concomitant neurologic symptoms in COVID-19 patients.
Lessons learned from prior coronavirus epidemics
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory syndrome (MERS-CoV), belong to the β–coronavirus family, similar to the SARS-CoV-2 strains. During the past two decades, SARS-CoV and MERS-CoV have caused epidemics affecting more than 10,000 infected patients worldwide [8], [9]. Several reports suggest that various neurologic sequelae may arise in association with respiratory coronavirus syndromes, including encephalitis, seizures, encephalopathy, Guillain-Barre syndrome (GBS), anosmia, neuromuscular disorders, and demyelinating diseases [10], [11], [12], [13]. Similarly, the neurotropism of other types of human coronaviruses (including HCoV-229E, HCoV-OC43, HCoVHKU1, and HCoV-NL63) and their possible association with neurologic diseases such as multiple sclerosis (MS) have been debated [14], [15]. The host immune response, including inflammatory cascades involving cytokine activation, has been suggested as a possible etiology for these neurologic changes. On the other hand, autopsy studies have detected viral RNA in neurons of patients who have died with SARS infection, indicating that coronaviruses might be able to infect brain cells directly [16]. Angiotensin-converting enzyme (ACE) II receptors on cerebrovascular endothelial cells may play an important role in this process.
Considering the similar viral structures and comparable post-viral neurological sequelae, we decided to analyze the available published literature on brain imaging findings associated with coronavirus strains other than COVID-19. This may provide valuable insight into coronavirus pathogenesis and guide the early detection and treatment of neurological disease associated with COVID-19 infection.
Materials and methods
Search strategy
Our research question was: “What are the reported neuroimaging findings in patients with coronavirus infections?” An extensive literature search was conducted for published articles describing relevant imaging findings by using Medline (assessed from PubMed), and Scopus online databases. The following search terms were used: “coronavirus” OR “SARS-CoV” OR “MERS” OR “COVID-19” OR “SARS-CoV-2” AND “neurologic” OR “brain” OR “Central Nervous System” OR “CNS” AND “computed tomography” OR “CT-scan” OR “MRI”. Those studies in which title, abstract, keywords or body of manuscript contained the search terms were selected for analysis. We also searched the references of selected articles to find any possible additional studies related to brain imaging findings of COVID-19. To include relevant non-indexed reports, a hand search was also performed in Google search engine and Google scholar. The search was carried out on May 6, 2020, and was updated on June 10. No language limitation was considered. Repeated studies were excluded. Several studies that generally described neurologic manifestations of COVID-19 but did not mention brain imaging findings were also excluded.
Study selection and definitions
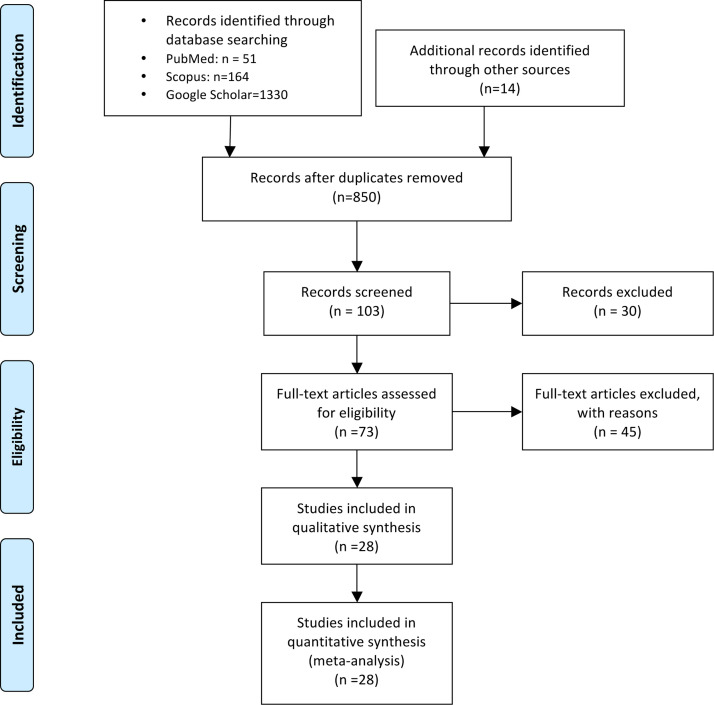
Two reviewers searched all relevant articles independently and summarized them. All studies that described brain CT or MRI findings in COVID-19 patients were included. No search filters were applied. The search strategy and article selection process are described using a flowchart in Fig. 1 , as recommended in the PRISMA statement.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of the study.
Data extraction, Quality assessments and Management
Data was extracted by the authors independently: study designs, sample size, imaging methods, main results, laboratory COVID-19 confirmatory tests (using pharyngeal or CSF samples), and important notes of different cases were included in the forms. The methodological quality of the included studies was assessed based on the 9-items featured in the National Institutes of Health Quality Assessment Tool for Case Series Studies [17].
Results
Overview of the Included Studies
A summary of articles and relevant findings included in this literature review is provided in Table 2, Table 3a, Table 3b. The electronic literature searches initially yielded 850 articles. After a manual screening of these articles based on their titles or abstracts, a total of 28 studies reporting imaging findings met the inclusion criteria for this systematic review. 21 studies were case reports, and seven were presented as case series. MR imaging was evaluated in 20 studies and CT was used in 17 reports. Sample sizes were small in most included studies, due to the rarely reported cases worldwide. The methodologic quality of the studies was generally rated as fair, indicative of scarce and low-quality data on viral neurologic findings [18].
Table 2.
Neuroimaging findings of patients with SARS-CoV2 infection (COVID-19).
| Title | Type | N. | Imaging Tool | Neurologic Symptoms | Findings |
|---|---|---|---|---|---|
| Focal status epilepticus as unique clinical feature of COVID-19: A case report [4] | Case | 1 | MRI | Focal status epilepticus as the initial presentation of SARS-CoV-2 infection in the context of a well-controlled post-encephalitic epilepsy. | Negative for acute lesions. Gliosis and atrophy involving the left temporo-parietal lobe, in the absence of new cerebral lesions. |
| COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI [33] | Case | 1 | CT, MRI | Altered mental status. | CT: Symmetric hypoattenuation within bilateral medial thalami. Normal CTA & CTV. MRI: Hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions. Dx: ANE. |
| Three unsuspected CT diagnoses of COVID-19 [5] | Case | 1 | CT, MRI | Dysarthria, Right hemiparesis, Right facial droop |
A 1.7-cm acute left basal ganglia hemorrhage |
| An Atypical Presentation of Novel Coronavirus Disease 2019 (COVID-19) [6] | Case | 1 | CT | Syncope, Altered mental status | Normal |
| Frequent Convulsive Seizures in an Adult Patient with COVID-19: A case report [20] | Case | 1 | MRI | Generalized tonic-colonic seizure | Normal |
| Encephalitis as a clinical manifestation of COVID-19 [25] | Case | 1 | CT | Myalgia, Confusion, Meningeal irritation signs, Extensor plantar response | Normal |
| COVID-19 and intracerebral haemorrhage: causative or coincidental? [27] | Case | 1 | CT | Acute loss of consciousness | A massive ICH in the right hemisphere, accompanied by intraventricular and subarachnoid haemorrhage. |
| A first case of meningitis/encephalitis associated with SARS-Coronavirus-2 [21] | Case | 1 | MRI | Headache, Generalized fatigue, Transient generalized seizure, Loss of consciousness, Neck stiffness | DWI: Hyperintensity along the wall of temporal horn of right lateral ventricle. FLAIR: Hyperintense signal changes in right mesial temporal lobe and hippocampus with slight hippocampal atrophy. No definite dural enhancement was seen. Dx: Right lateral ventriculitis and encephalitis, mainly involving the right mesial lobe and hippocampus. Additionally noted pan-sinusitis on T2-weighted images. |
| Neurological complications of coronavirus disease (COVID-19): encephalopathy [23] | Case | 1 | CT | Headache, Altered mental status. | No acute abnormalities. Left temporal encephalomalacia, consistent with remote embolic stroke. |
| Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China [3] | Case series | 6 | CT? | Acute Cerebrovascular disease | |
| Hemisensory paresthesia as the initial symptom of a SARS-Coronavirus-2 infection. A Case report [24] | Preprint case | 1 | MRI | Refractory headache, Paresthesia of the left face, left arm and left leg. | Normal immediate MRI |
| Coronavirus Disease 2019 (COVID-19): An update on neurologic sequelae [29] | Case | 1 | CT | Right-sided vision loss, Clumsiness. Examination: Right homonymous hemianopsia with macular sparing, Dysmetria in the right extremities. | CT: Loss of gray-white matter differentiation in the left occipital and temporal lobes, with smaller areas of parenchymal hypoattenuation within the right cerebellar hemisphere. CTA: Confirmed a thrombus within the left PCA. |
| COVID-19-Associated Acute Disseminated Encephalomyelitis–A Case Report [34] | Preprint, Case | 1 | CT,MRI | Dysphagia, Dysarthria, and encephalopathy | CT: No evidence of ICH, but there were multifocal patchy hypoattenuation . MRI: Extensive patchy areas of abnormal signal involving bilateral frontoparietal white matter, anterior temporal lobes, basal ganglia, external capsules and thalami. Some showed DWI changes and corresponding ADC changes, with questionable minimal enhancement. MRA: Normal. DDX: ADEM |
| Brain MRI Findings in Patients in the Intensive Care Unit With COVID-19 Infection [22] | Case series | 27 | MRI | Neurologic symptoms in ICU patients with COVID-19 (Not described). | 15/27 (56%): Normal MRI. 12/27 (44%,): Acute MRI findings including: Cortical FLAIR signal abnormality in 10/27 (37%) patients, involving frontal lobe in 4, parietal lobe in 3, occipital lobe in 4, temporal lobe in 1, insular cortex in 3 and cingulate gyrus in 3 cases (accompanied by subcortical and deep white matter signal abnormality on FLAIR images). Acute - transverse sinus thrombosis (1 case) and acute right MCA infarction (1 case). |
| Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a Manifestation of COVID-19 Infection [29] | Case report | 2 | CT, MRI | Hemorrhagic PRES: Altered mental status, Labile blood pressure, Favorable prognosis |
1st case: CT and MRI: Focal vasogenic/cytotoxic edema in the posterior parietooccipital lobes bilaterally, with a small right-sided hemorrhage. CTV: Normal. SWI: Extensive petechial hemorrhages throughout the corpus callosum. 2nd case: MRI: Multiple areas of restricted diffusion with associated edema, mainly in the posterior parietooccipital lobes, but also in the right frontal lobe, basal ganglia, and cerebellar hemispheres. SWI: Extensive superimposed hemorrhages in the parietooccipital region along with abnormal enhancement. Dx: Hemorrhagic posterior reversible encephalopathy syndrome (PRES). |
| Cerebrovascular Disease in COVID-19 [30] | Case report | 1 | CT,CTA | stroke with left-sided hemiparesis and shortness of breath | CT: Large areas of mild hypoattenuation and loss of gray-white matter differentiation in the right MCA territory and bilateral ACAs. Extracranial CTA: High-grade stenosis at proximal ICA. Intracranial CTA: Markedly decreased flow at branches of the right MCA Follow-up CT: Worsening cerebral edema and mass effect induced by the right MCA and bilateral ACA infarcts,evolution of acute ischemia. Dx: Acute cerebral infarcts |
| Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection [31] | Case report | 1 | CT, CTA, MRI, DS | Acute aphasia, right hemiparesis. Examination: left MCA syndrome with a NIH stroke scale of 10. |
CT and CTA: Subtle left frontal cortical hypoattenuation with surrounding hypoperfusion and distal branch occlusion. Cervical CTA: A large intraluminal floating thrombus appended to a hypoattenuated non-stenosing plaque of the left CCA. Dedicated wall imaging with 3 T MRI and DS confirmed the diagnosis of a large thrombus adherent to a thin atheromatous plaque. DWI: AIS with foci of hyperintensity scattered within left carotid territory. Dx: an AIS complicating a large floating thrombus at CCA. |
| Cerebral Venous Thrombosis associated with COVID-19 infection: causality or coincidence? [32] | Case report | 2 | CT,MRI | 1st patient: headache and altered vision, rapidly followed by sudden right hemicorporeal deficit and altered consciousness. 2nd patient: Severe headache |
CT and MRI: Large confluent ICH at left frontotemporal lobes (1st case), large hemorrhagic infarction in the left temporal lobe (2nd case). CTV and MRA: CVT of the left transverse sinus, straight vein, vein of Galen and internal cerebral veins (1st case), CVT of the left transverse sinus (2nd case). |
| Neuroradiological features in COVID-19 patients: First evidence in a complex scenario [26] | Case series | 26 | CT, MRI | Coma, Confessional state, Dizziness, Headache, Paresis, Others | 16/26 (61.6%): Non-acute events. 5/26 (19.2%): Parenchymal hemorrhage. 4/26 (15.4%): Ischemic changes. 1/26 (3.8%): Encephalitis. |
| Neurologic features in severe SARS-CoV-2 infection [35] | Case series | 13 | MRI | Unexplained encephalopathic features | 8 cases: Leptomeningeal enhancement 11/11 cases: Bilateral frontotemporal hypoperfusion in all 11 patients who underwent perfusion imaging. 2 cases: Each had a small acute ischemic stroke with focal hyperintensity on DWI, and an overlapping decreased ADC. 1 case: Subacute ischemic stroke with superimposed increased DWI and ADC signals. |
ANE: Acute Necrotizing Encephalopathy; CT: Computer Tomography; MRI: Magnetic Resonance Imaging; CTA: Computer Tomography Angiogram; CTV: Computer Tomography Venogram; MRA: Magnetic Resonance Angiogram; DS: Doppler Sonography; ADEM: Acute Disseminated Encephalomyelitis; DWI: diffusion weighted imaging; ADC: Apparent Diffusion Coefficient; ICH: Intracranial Hemorrhage; MCA: Middle Cerebral Artery; CCA: Common Carotid Artery; ACAs: Anterior Cerebral Arteries; CVT: Cerebral Venous Thrombosis; AIS: Acute Ischemic Stroke.
Table 3a.
Neuroimaging findings of patients with coronavirus infection other than COVID-19-children.
| Title | Type | N | Imaging | Symptoms | Virus | Findings |
|---|---|---|---|---|---|---|
| Coronavirus Infections in the Central Nervous System and Respiratory Tract Show Distinct Features in Hospitalized Children [15] |
Case series | 16 | CT,MRI | Acute encephalitis-like syndrome: fever, headache, neck stiffness, convulsion, altered levels of consciousness, and focal neurological signs. | HCoV | 8 cases had abnormalities. Among them, 2 (25%), including 1 with CT and 1 with MRI, had abnormity in the temporal lobe accompanied with seizures; 2 cases (25%) with MRI displayed changes in the periventricular region with headaches; and 4 patients (50%), including 1 CT and 3 with MRI, had abnormity in basal ganglia and thalamus. |
| Detection of Coronavirus in the Central Nervous System of a Child With Acute Disseminated Encephalomyelitis [36] | Case | 1 | MRI | Numbness in the lower extremities, difficulty walking. Examination: Mild distal weakness in the right hand and foot, Patchy loss of vibration and temperature sensation below T10, Mild dysmetria of the left hand. Poor heel-to-toe walking Follow-up: Resolved over several weeks without any therapeutic intervention. |
HCoV | Spine MRI: Scattered non-enhancing lesions on T2-weighted images at C4–C5 and at T7–T8 levels . Brain MRI: Patchy white matter hyperintensities particularly in the centrum semiovale, as well as the left cerebellum adjacent to the superior aspect of the left brachium pontes, with only some of the lesions demonstrating post-contrast enhancement (includingthe left centrum semiovale lesion). |
Table 3b.
Neuroimaging findings of patients with coronavirus infection other than COVID-19-adults.
| Title | Type | N | Imaging | Symptoms | Virus | Findings |
|---|---|---|---|---|---|---|
| Possible Central Nervous System Infection by SARS Coronavirus [13] | Case | 1 | MRI | A pregnant female; generalized tonic-clonic convulsion with loss of consciousness and up-rolling eyeballs. | SARS | Normal |
| Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS–CoV) [12] | Case | 3 | MRI | Middle-aged, altered level of consciousness ranging from confusion to coma, ataxia, and focal motor deficit. | MERS | Widespread, bilateral hyperintense lesions on T2 weighted imaging involving the subcortical white matter of the frontal, temporal, and parietal lobes, the basal ganglia, and corpus callosum. None of the lesions showed enhancement. |
| Neurological Complications of Middle East Respiratory Syndrome Coronavirus: A Report of Two Cases and Review of the Literature [38] | Case | 2 | CT,MRI | Severe headache, nausea, and vomiting. consciousness level deterioration, irreversible brain stem dysfunction and she died two months later. 2nd patient: Axonal polyneuropathy, weakness in both legs and inability to walk with numbness and tingling in stocking distribution; Slowly improving in 6 months |
MERS | Brain CT: Right frontal lobe ICH, with massive brain edema, and midline shift. Normal Spine MRI. |
| Neurological Complications during Treatment of Middle East Respiratory Syndrome [10] | Case | 1 | MRI | Complete external ophthalmoplegia and mild limb ataxia. Limb Weakness, Fully recovered after 60days | MERS | Normal MRI |
| Olfactory Neuropathy in Severe Acute Respiratory Syndrome: Report of A Case [37] | Case | 1 | MRI | Complete anosmia. No changes after two years. |
SARS | No definite lesion, except for the incidental finding of an 8 mm epidermoid cyst in the left temporal lobe. The follow-up MRI examination six months later showed no changes. |
| Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome corona virus [39] | Case | 1 | CT | Suddenly became unresponsive, her GCS dropped to 3/15, and her pupils were 3 mm wide with sluggish reaction, followed by brain death | MERS | CT: Right frontal hematoma, subarachnoid hemorrhage extending to ventricles, causing midline shift and subfalcine herniation. Follow-up CT: Complete loss of gray and white matter differentiation of both cerebral hemispheres with large frontal hematoma, and complete effacement of cerebrospinal fluid spaces including lateral ventricles and basal cisterns. |
CT and MRI manifestations
COVID-19 infection
Twenty case reports/series with COVID-19 infection were included in this review. There were also two larger case series with neurological symptoms in COVID-19 patients, but they did not contain specific radiological findings [3], [19]. The first and largest study of neurologic manifestations in COVID-19 infection was an analysis of 214 patients of Wuhan hospitals [3]. They found that 36.4 percent of those patients showed some neurologists signs, with the most common being dizziness and headache. Moreover, fewer patients experienced more distinct neurological syndromes, such as acute stroke (5.7%), and consciousness impairment (15%). The authors mentioned that acute cerebrovascular disease (including ischemic and hemorrhagic stroke) had been diagnosed by clinical symptoms and head CT, albeit no CT findings have been specifically described. The study highlighted the importance of recognizing neurologic symptoms as an important presenting sign of COVID-19.
Amongst the available 20 studies, seven patients had CSF samples tested for SARS-CoV-2 infection [20], [21], [22], one of which came to be positive in the presence of meningitis/encephalitis findings [21], and the others were negative. The first case report of meningitis associated with SARS-CoV-2 [21] was a 24-year-old man with fever, and fatigue, worsening headache and sore throat. After a few days, he lost consciousness and began experiencing seizures. Diffusion-weighted imaging (DWI) revealed periventricular diffusion restriction along the temporal horn of the right lateral ventricle. On Fluid-attenuated inversion recovery (FLAIR) images, hyperintense signal abnormality was noted in the right mesial temporal lobe and hippocampus, along with slight hippocampal atrophy. Contrast-enhanced imaging showed no definite abnormal intracranial enhancement. These findings indicated right lateral ventriculitis and encephalitis. The patient's nasopharyngeal swab was negative for SARS-CoV-2 RNA, but the virus was detected in a CSF sample. The authors concluded that the COVID-19 virus might be able to invade the brain directly in rare circumstances.
In terms of radiologic findings among these 20 case reports (90 patients) with COVID-19 associated neurologic signs, 37 patients (41%) with laboratory-confirmed COVID-19 infection had no acute abnormalities on brain CT or MRI [4], [6], [20], [22], [23], [24], [25], [26]. These patients presented with the following symptoms: altered mental status, headache, frequent seizures, status epilepticus, hemisensory paresthesia, and others.
In the remaining 12 articles (53 patients) with COVID-19 associated neurologic signs, abnormal brain radiologic findings have been reported, such as: hemorrhage [5], [26], [27] (seven cases), hemorrhagic posterior reversible encephalopathy syndrome [28] (PRES; two cases), vascular thrombosis (14 cases) [22], [26], [29], [30], [31], [32], [35], Acute hemorrhagic Necrotizing Encephalopathy [33] (ANE; one case), Acute Disseminated Encephalomyelitis [34] (ADEM; one case), meningitis/encephalitis [21], [26] (two cases), cortical FLAIR signal abnormality (10 cases) [22]. In a case-series by Helms et al. [35], bilateral frontotemporal hypoperfusion, leptomeningeal enhancement, and stroke-related findings were observed in 13 cases who had undergone brain MRI. All of the aforementioned findings have been fully described in Table 1, Table 2 .
Table 1.
Neuroimaging findings among patients with COVID-19 associated neurologic signs.
| Neuroimaging findings | Number of cases and Description (Total cases = 90) | |
|---|---|---|
| Normal Imaging (no acute lesion) |
37 (41%) | |
| Hemorrhage | 7 | 1: Intra-parenchymal/intra-axial basal ganglia hemorrhage. 1: Massive intra-parenchymal hemorrhage involving the right hemisphere, with intraventricular extension and subarachnoid component. 5: Intra-parenchymal hemorrhage as: 2 frontal, 1 hemispheric, 1 cerebellar, and 1 parietal. |
| Hemorrhagic PRES | 2 | |
| Vascular thrombosis | 14 | 1: Thrombosis in the posterior cerebral artery (PCA). 1: Acute transverse sinus thrombosis. 1: Acute right Middle Cerebral Artery (MCA) infarction. 1: Infarcts of the right middle cerebral artery (MCA) and bilateral anterior cerebral artery (ACA) territories. 4: Ischemic changes (no additional details). 2: Cerebral Venous Thrombosis (CVT), superimposed by hemorrhage (hemorrhagic infarction). 1: Acute Ischemic Stroke complicating a large floating thrombus at common carotid artery. 3: Two cases had a small acute ischemic stroke, manifesting as focal diffusion restriction on DWI and associated decreased ADC value, and the other one displayed a subacute ischemic stroke and superimposed increased DWI and ADC signals. |
| Non-specific terms | 26 | 10: Cortical FLAIR signal abnormality. 10: Bilateral frontotemporal hypoperfusion, and leptomeningeal enhancement. 6: Acute cerebrovascular strokes. |
| Others | 4 | ANE (1), ADEM (1), meningitis/encephalitis (2). |
Other Coronavirus infections
Pediatrics
In 2004, a study was performed on a total of 183 hospitalized children with acute encephalitis-like syndrome. Twenty-two children were found to have a CoV infection involving the central nervous system [15]. Among these 22 patients, 16 individuals underwent MRI or CT, with 8 patients (50%) showing abnormal findings, as non-specific changes. Out of these eight patients, two cases with seizure (25%) displayed signal abnormity in the temporal lobe; two patients with headaches (25%) had periventricular signal abnormity; and four patients with fever and vomiting (50%) had signal abnormity in the basal ganglia and thalami. This study illustrates the possible association between CoV infection and CNS manifestations in these children, manifested as non-specific radiologic changes in different brain areas.
In another case report of a child with acute disseminated encephalomyelitis (ADEM) [36], MRI demonstrated patchy lesions in the white matter tracts, particularly in the centrum semiovale, as well as non-enhancing lesions in the spine. Subsequently, HCoV infection was detected both in pharyngeal and CSF sample of the child. These findings indicated ADEM, possibly due to the CoV infection (Table 3a ).
Adults
Similar studies on adults with SARS and MERS are also present. Two cases with SARS [13], [37] and one with MERS [10] who presented with neurological symptoms were reported to have a normal brain MRI. In a case series by Arabi et al. [12], three MERS patients with severe neurological symptoms were evaluated with MRI, which displayed striking changes characterized by widespread, bilateral T2 hyperintense lesions within the white matter and subcortical areas of the frontal, temporal, and parietal lobes, the basal ganglia, and corpus callosum. None of the lesions showed enhancement. In another case series by Algahtani et al. [38], two patients with MERS had intracerebral hemorrhage with significant edema on CT images. Similarly, another case report described intracerebral hemorrhagic changes on CT in a patient with MERS infection [39]. These findings have been fully described in Table 3b .
Discussion
Various neurologic clinical manifestations have been described in patients with COVID-19 and other coronavirus infections. However, the data is sparse on this topic. When it comes to associated neuroimaging findings, available data is even more limited, as most of these studies do not include the corresponding brain imaging findings.
Previous studies have suggested that coronaviruses have neurotropic and neuroinvasive properties, even in the absence of pulmonary symptoms. Similarly, during the current COVID-19 outbreak, several new case reports again suggested a possible association between neurologic symptoms and COVID-19. A very recent review has found that myalgia, anosmia, headache, cerebrovascular disease, and encephalopathy are amongst the most common neurological manifestations associated with SARS-CoV-2 infection [42].
Different hypotheses have been offered to explain these abnormalities. Many authors believe that a hyperimmune response secondary to cytokine storms may account for these neurologic presentations, while the others have proposed direct viral invasion of human brain cells through hematogenous, transcribrial, and neuronal retrograde dissemination pathways. In addition, the neurotropism of CoV (especially SARS-CoV-2) might be mediated by angiotensin-converting enzyme 2 (ACE2) receptors, that are expressed by brain capillary endothelial cells. Cerebral endothelial rupture then leads to irreversible brain damage, which contributes to pathophysiology of SARS-CoV-2 neurologic manifestations [40]. Furthermore, elevated levels of CRP and D-dimer due to a high inflammatory state and hypercoagulation cascade activation, may lead to cerebrovascular events in coronavirus patients. Hence, although it is still too early to know for sure, the possible mechanisms might be a combination of immune, vascular, and neuronal factors.
CNS involvement in coronavirus infection is reported to occur in more than one third of the hospitalized patients [42], with various degrees from mild to life-threatening conditions. Therefore, it should be considered in the differential diagnosis of any patients with unexplained or worsening neurologic presentations, specifically during the current ongoing pandemic. Neurologic imaging may be helpful in these cases, as an early diagnosis is of utmost importance to minimize further neurological damage.
In this paper, we have reviewed the radiological manifestations of CNS infection by different strains of coronaviruses (SARS-CoV, MERS-CoV, COVID-19, and other CoV strains). To the best of our knowledge, this is the first systematic review describing neuroimaging results in patients with coronavirus infections, specifically COVID-19. As mentioned earlier, neuroimaging modalities such as CT and MRI have revealed various findings in the context of different clinical scenarios. Some COVID-19 patients have displayed normal results (40%), while the others have demonstrated imaging abnormalities in different areas of the brain, including acute cerebrovascular events (ischemic and hemorrhagic types), myelitis, demyelinating disorders (ADEM), meningitis, encephalopathy, and so on.
Cerebrovascular events (both ischemic and hemorrhagic) are found to be the most common neuroradiologic abnormality seen among COVID-19 patients (27%, Table 1). It is believed that patients with coronavirus infection, especially ill elderly patients with various vascular risk factors, are more vulnerable to cerebrovascular disease. As mentioned above, coagulation dysfunction and a hyperinflammtory response may play an important role in cerebrovascular pathogenesis in these patients. Munhoz RP et al. [42] found that 2.8–5.7% of infected patients present with acute cerebrovascular disease (mostly ischemic, rarely hemorrhagic or with venous thrombosis). Mao L [3] and Asadi-Pooya AA et al. [18] both reported that ischemic or hemorrhagic CVD account for 5-5.7% of neurologic manifestations associated with COVID-19. Thus extra care should be taken with patients who have severe coronavirus infection as well as vascular risk factors as they have a higher risk of acute cerebrovascular events.
Other neuroimaging abnormalities such as myelitis and encephalopathy-related changes were reported in a few cases, manifesting as parenchymal signal abnormality in different parts of the brain (Table 1, Table 2, Table 3a, Table 3b).
Overall, the neurologic events associated with coronavirus infection (especially SARS-CoV-2) suggest a possible causal or synergetic relationship between cerebral ischemic/hemorrhagic/inflammatory events and CoV infection. However, it should be noted that coincidental events, rather than casual association might explain some of these results. Moreover, long-term neurological sequelae have not yet been explored. Thus further investigation is warranted to answer these unsolved questions.
Conclusion
During the current global COVID-19 pandemic, several case reports have suggested a possible association between SARS-CoV-2 virus infection and neurologic symptoms, similar to CNS findings reported during and after the previous SARS and MERS epidemics. Being aware of these patterns will ensure clinicians consider COVID-19 infection when encountering unexplained neurologic findings, particularly during the current COVID-19 pandemic. Due to limited data on this topic, further investigation is needed, including research on long-term neurologic consequences.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.https://www.worldometers.info/coronavirus
- 2.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. International Journal of Infectious Diseases. 2020 doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L., Jin H., Wang M., Hu Y. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vollono C., Rollo E., Romozzi M. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020 doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vu D., Ruggiero M., Choi W.S. Three unsuspected CT diagnoses of COVID-19. Emergency Radiology. 2020:1–4. doi: 10.1007/s10140-020-01775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhania N., Bansal S., Singhania G. An Atypical Presentation of Novel Coronavirus Disease 2019 (COVID-19) The American Journal of Medicine. 2020 doi: 10.1016/j.amjmed.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. American Journal of Roentgenology. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2004. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003 [EB/OL] [Google Scholar]
- 9.World Health Organization . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV). November 2019[EB/OL] [Google Scholar]
- 10.Kim J.E., Heo J.H., Kim H.O. Neurological complications during treatment of Middle East respiratory syndrome. Journal of Clinical Neurology. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai L.K., Hsieh S.T., Chao C.C., Chen Y.C., Lin Y.H., Chang S.C. Neuromuscular disorders in severe acute respiratory syndrome. Archives of neurology. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 12.Arabi Y.M., Harthi A., Hussein J. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerging infectious diseases. 2004;10(2):342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmi A., Ziola B., Hovi T., Reunanen M. Antibodies to coronaviruses OC43 and 229E in multiple sclerosis patients. Neurology. 1982;32(3):292. doi: 10.1212/wnl.32.3.292. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Li H., Fan R., Wen B. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q.L., Ding Y.Q., Hou J.L. Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization. Di 1 jun yi da xue xue bao Academic journal of the first medical college of PLA. 2003;23(11):1125–1127. [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute website. Study quality assessment tools. www.nhlbi.nih. gov/health-topics/study-quality-assessment-tools.
- 18.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. Journal of the Neurological Sciences. 2020:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020 doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimi N., Sharifi Razavi A., Rouhani N. Frequent Convulsive Seizures in an Adult Patient with COVID-19: A Case Report. Iranian Red Crescent Medical Journal. 2020 [In Press] [Google Scholar]
- 21.Moriguchi T., Harii N., Goto J. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases. 2020 doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandemirli S.G., Dogan L., Sarikaya Z.T. Findings in Patients in the Intensive Care Unit with COVID-19 Infection [published online ahead of print, 2020 May 8] Radiology. 2020:201697. [Google Scholar]
- 23.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (covid-19): encephalopathy. Cureus. 2020;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrnberger M, Durmazel N, Birklein F. Hemisensory paresthesia as the initial symptom of a SARS-Coronavirus-2 infection. A Case report. Preprint. Doi: 10.21203/rs.3.rs-26305/v1.
- 25.Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain, behavior, and immunity. 2020 doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrea G., Vinacci G., Edoardo A., Anna M., Fabio B. Neuroradiological features in COVID-19 patients: first evidence in a complex scenario. Journal of Neuroradiology. 2020 doi: 10.1016/j.neurad.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes and New Infections. 2020:35. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a Manifestation of COVID-19 Infection. American Journal of Neuroradiology. 2020 doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields B., Demirjian N., Balakrishnan S., Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): An update on neurologic sequelae. Neurodiem. 2020 [Google Scholar]
- 30.Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular Disease in COVID-19. American Journal of Neuroradiology. 2020 doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viguier A., Delamarre L., Duplantier J., Olivot J.M., Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. Journal of neuroradiology. 2020 doi: 10.1016/j.neurad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poillon G., Obadia M., Perrin M., Savatovsky J., Lecler A. Cerebral Venous Thrombosis associated with COVID-19 infection: causality or coincidence? Journal of neuroradiology. 2020 doi: 10.1016/j.neurad.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poyiadji N., Shahin G., Noujaim D. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Rodricks M.B., Hirsh E. COVID-19-Associated Acute Disseminated Encephalomyelitis: A Case Report. medRxiv. 2020 [Google Scholar]
- 35.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. New England Journal of Medicine. 2020 doi: 10.1056/NEJMc2015132. [DOI] [PubMed] [Google Scholar]
- 36.Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1):e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 37.Hwang C. Olfactory Neuropathy in Severe Acute Respiratory Syndrome: Report of A Case. Acta Neurologica Taiwanica. 2006;15(1):26. [PubMed] [Google Scholar]
- 38.Algahtani H., Subahi A., Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case reports in neurological medicine. 2016:2016. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Hameed F.M. Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome corona virus. Saudi medical journal. 2017;38(2):196. doi: 10.15537/smj.2017.2.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS chemical neuroscience. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 41.Behzad S., Aghaghazvini l, Radmard A., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiological and clinical overview. Clinical imaging. 2020 doi: 10.1016/j.clinimag.2020.05.013. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munhoz R.P., Pedroso J.L., Nascimento F.A. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq Neuropsiquiatr. 2020;78(5):290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]