Abstract
Background
Corticosteroids are commonly used as adjuvant therapy for acute respiratory distress syndrome by many clinicians because of their perceived anti-inflammatory effects. However, for patients with severe viral pneumonia, the corticosteroid treatment is highly controversial.
Objectives
The purpose of this review is to systematically evaluate the effect and potential mechanism of corticosteroid administration in pandemic viral pneumonia.
Sources
We comprehensively searched all manuscripts on corticosteroid therapy for influenza, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and SARS coronavirus 2 (SARS-CoV-2) viral pneumonia from the PubMed, EMBASE, Web of Science and Cochrane Library databases.
Content
We systematically summarized the effects of corticosteroid therapy for pandemic viral pneumonia and the potential mechanism of action for corticosteroids in coronavirus disease 2019 (COVID-19).
Implications
Observational studies showed that corticosteroid treatment was associated with increased mortality and nosocomial infections for influenza and delayed virus clearance for SARS-CoV and MERS-CoV. Limited data on corticosteroid therapy for COVID-19 were reported. Corticosteroids were used in about a fifth of patients (670/2995, 22.4%). Although clinical observational studies reported the improvement in symptoms and oxygenation for individuals with severe COVID-19 who received corticosteroid therapy, case fatality rate in the corticosteroid group was significantly higher than that in the non-corticosteroid group (69/443, 15.6% versus 56/1310, 4.3%). Compared individuals with non-severe disease, those with severe disease were more likely to receive corticosteroid therapy (201/382, 52.6% versus 201/1310, 15.3%). Although there is no evidence that corticosteroid therapy reduces mortality in people with COVID-19, some improvements in clinical symptoms and oxygenation were reported in some clinical observational studies. Excessive inflammatory response and lymphopenia might be critical factors associated with severity of and mortality from COVID-19. Sufficiently powered randomized controlled trials with rigorous inclusion/exclusion criteria and standardized dose and duration of corticosteroids are needed to verify the effectiveness and safety of corticosteroid therapy.
Keywords: Corticosteroid, COVID-19, Influenza, MERS, SARS
Introduction
Since late December 2019, the outbreak of the new highly contagious coronavirus disease 2019 (COVID-19) has caused (up to 30 April 2020) 3 096 626 confirmed cases and 217 896 deaths worldwide [1]. The clinical course of this disease remains to be fully investigated, few data are available that describe the disease pathogenesis, and no specific pharmacological therapies have been proven efficacious.
Individuals with severe COVID-19 rapidly progressed to acute respiratory failure, pulmonary oedema and acute respiratory distress syndrome (ARDS) with extensive release of inflammatory cytokines at the early stage [2,3]. As a consequence, antiviral and anti-inflammatory therapies have become an increasing concern for clinicians [4,5]. Previous randomized clinical trials have showed that adjuvant corticosteroid could modulate inflammatory responses, reduce the incidence of treatment failure and shorten the time to clinical stability in community-acquired pneumonia without significant adverse events [6,7]. Recently, Villar et al. have reported that early administration of dexamethasone reduced the duration of mechanical ventilation and overall mortality for patients with moderate-to-severe ARDS through a randomized controlled clinical trial [8].
Although corticosteroid treatment for virus infection is highly controversial, they have been widely used as adjuvant therapy for epidemic viral pneumonia during outbreaks of influenza virus [[9], [10], [11], [12], [13], [14], [15]], severe acute respiratory syndrome coronavirus (SARS-CoV) [[16], [17], [18]] and Middle East respiratory syndrome coronavirus (MERS-CoV) [19,20]. However, over the past two decades, little clinical data indicated that net benefit was derived from corticosteroid administration for pandemic viral pneumonia, even when associated with poor prognosis [[9], [10], [11], [12], [13], [14], [15],[17], [18], [19], [20]]. Russell et al. thought that clinical evidence was undetermined to support corticosteroid treatment for COVID-19 lung injury based on the experience of corticosteroid therapy for influenza pneumonia, SARS and MERS from the previous published studies [21]. A Chinese expert panel have a different perspective, they recommend prudently administering short courses of corticosteroids at low-to-moderate dose for critically ill patients with COVID-19, based on the experiences of fighting the disease [22]. It prompted us to search the evidence and mechanism of action of corticosteroid in COVID-19.
In this study, we performed a comprehensive search and reviewed all previous literature on corticosteroid administration in influenza, SARS, MERS and COVID-19 pneumonia. We attempted to explore the mechanism of corticosteroids in COVID-19, so as to make more reasonable use of corticosteroids in the management of COVID-19 patients in the future.
Search strategy and data collection
Two authors independently and comprehensively searched the PubMed, EMBASE, Web of Science and Cochrane Library databases from inception to 30 April 2020. The medical subject headings terms were as follow: ‘corticosteroid’ or ‘glucocorticoid’ or ‘steroid’ or ‘dexamethasone’ or ‘methylprednisolone’ or ‘hydrocortisone’ or ‘prednisolone’ and ‘influenza’ or ‘severe acute respiratory syndrome’ or ‘SARS’ or ‘Middle East respiratory syndrome’ or ‘MERS’ or ‘novel coronavirus’ or ‘2019-nCoV’ or ‘COVID-19’. No language restrictions were set. The references of involved studies were also searched.
Two investigators independently extracted useful information and data from original studies. Disagreements were resolved by discussion and consulting a statistician. As the result of data processing and conversion analysis, some of the results may differ slightly from the published original articles.
The initial search identified 19 227 potential studies; 18 445 articles were excluded by screening of the titles and abstracts for reasons of irrelevance or redundancy. Ultimately, 782 full-text articles were reviewed, 212 of which were related to corticosteroids and influenza, 196 were related to corticosteroids and SARS, 33 were related to corticosteroids and MERS, and 341 were related to corticosteroids and COVID-19. The details of the screening process are shown in Fig. 1 .
Fig. 1.
Flow diagram.
Harm of corticosteroid therapy in influenza pneumonia
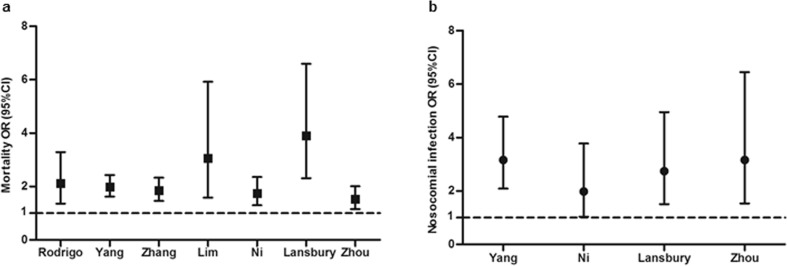
Many studies have reported associations between corticosteroid therapy and mortality, nosocomial infection, or other poor clinical outcomes of influenza [[9], [10], [11], [12], [13], [14], [15]]. Lee et al.‘s research on 2649 individuals with laboratory-confirmed influenza during 2008–2011 from three Asian cohorts (Hong Kong, Singapore and Beijing) found that corticosteroids increased superinfection (9.7% versus 2.7%) and deaths when controlled for indications (adjusted hazard ratio (HR) 1.73, 95% CI 1.14–2.62) [13]. Another study enrolled 1846 individuals with primary influenza pneumonia from 148 intensive care units (ICUs) of Spain between June 2009 and April 2014 and demonstrated that methylprednisolone was the most frequently used corticosteroid. The median daily dose was equivalent to 80 mg of methylprednisolone (interquartile range (IQR) 60–120 mg) for a median duration of 7 days (IQR 5–10 days). After propensity score matching, corticosteroid application for influenza pneumonia was associated with ICU mortality in Cox regression analysis (HR 1.32, 95% CI 1.08–1.60) and competing risks analysis (sub-hazard ratio (SHR) 1.37, 95% CI 1.12–1.68) in this study [15]. Moreover, studies from the groups of Martin-Loeches, Brun-Buisson, Han and Kim identified that early corticosteroid use for patients with severe H1N1 infection was also associated with an increased rate of superinfection, longer durations of ventilation and more prolonged ICU stays [[9], [10], [11], [12]]. Seven systematic reviews and meta-analyses have been retrieved [[23], [24], [25], [26], [27], [28], [29]], which covered almost all data from clinical studies on corticosteroid therapy in influenza pneumonia (see Supplementary material, Table S1). They showed that corticosteroid therapy was significantly associated with mortality (Fig. 2 a) and nosocomial infection (Fig. 2b).
Fig. 2.
Corticosteroid therapy for influenza increased mortality (a) and nosocomial infection (b) from published meta-analysis. Dots and whiskers represent OR and 95% CI in each study.
These findings matched the Infectious Diseases Society of America recommendations against corticosteroid adjunctive therapy in patients with influenza pneumonia unless clinically indicated for other reasons [30]. These results were largely derived from observational studies, although some statistical models were performed to adjust for confounding factors. Sufficiently powered randomized controlled trials are needed to further verify this conclusion.
Dilemma of corticosteroid therapy in SARS
During the SARS epidemic, corticosteroids were widely used in patients with progressive respiratory failure. The association of corticosteroid treatment and fatality was equivocal in different reports. A study from Chen's group revealed that proper use of corticosteroid in confirmed critical SARS patients in Guangzhou resulted in lower mortality and shorter hospitalization, and was not significantly associated with secondary lower respiratory infection and other complications [16]. In other studies, patients who received high doses or a pulse dosage of corticosteroid therapy had less requirement for oxygen and better radiographic outcome than those who did not [31,32]. Auyeung et al. found that people with SARS treated with corticosteroids were at higher risk of ICU admission or mortality [17]. Another retrospective study from Hong Kong included a total of 1287 individuals with SARS and reported that the mortality was higher in the corticosteroid group compared with that in the non-corticosteroid group (28.3% versus 17%) [18]. In addition, Lee et al. showed that early corticosteroid treatment for individuals with SARS was associated with a higher subsequent plasma viral load and corticosteroid application delayed SARS-CoV clearance [20]. Invasive fungal infection and osteonecrosis have also been reported after corticosteroid therapy in individuals with SARS [33,34].
The outcomes of corticosteroid therapy in SARS were divergent based on the published researches. We cannot reach a definite conclusion.
Harm of corticosteroid therapy in MERS
Reports on corticosteroid administration for MERS were relatively rare. Arabi et al. reported a multicentre, retrospective cohort study of 309 individuals admitted with MERS-CoV from 14 participating Saudi Arabian tertiary care hospitals between September 2012 and October 2015 [20]. In their study, 151 of 309 patients received corticosteroids. Patients who received corticosteroids were more likely to receive invasive ventilation (141/151, 93.4% versus 121/158, 76.6%, p < 0.0001) and had higher 90-day crude mortality (112/151, 74.2% versus 91/158, 57.6%, p 0.002). Using marginal structural modelling, corticosteroid therapy was not significantly associated with 90-day mortality (adjusted odds ratio (OR) 0.75, 95% CI 0.52–1.07, p 0.12) but was associated with delay in MERS RNA clearance (adjusted HR 0.35, 95% CI 0.17–0.72, p 0.005). These results revealed that corticosteroid therapy delayed clearance of MERS-CoV RNA [19].
We conclude that corticosteroid therapy in MERS may be harmful and it delays the clearance of MERS-CoV RNA.
Current status of corticosteroid therapy for COVID-19
At the time of writing, the efficacy and safety of corticosteroid therapy in COVID-19 is undetermined. Few studies have been reported.
Experts differ in their opinion. Russell et al. concluded that there is no reason to expect that individuals with COVID-19 will benefit from corticosteroid treatment, based on the increased mortality and risk of secondary infection in influenza, impaired clearance of SARS-CoV and MERS-CoV, and increased complications of survivors from available clinical data [21]. Chinese experts considered it prudent to administer short courses of corticosteroids at low-to-moderate dose for critically ill patients with COVID-19 [22].
We reviewed the studies about COVID-19 from inception to 30 April 2020. Limited data on corticosteroid therapy were reported [2,[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] (Table 1 ). Corticosteroids were used in 22.4% (670/2995). Case fatality rate in these patients was 4.2% (127/2995), which was higher than the national fatality rate (3.2%) [47]. It may be due to the relatively high proportion of early critically ill patients of Wuhan in this population. The case fatality rate in the corticosteroid group was higher than in the non-corticosteroid group (69/443, 15.6% versus 56/1310, 4.3%). As expected, corticosteroid use was significantly higher in the individuals with severe disease than in those with non-severe disease (201/382, 52.6% versus 301/1310, 15.3%), which seems to be responsible for the higher case fatality rate in the corticosteroid group.
Table 1.
Studies on corticosteroid therapy for COVID-19
| Reference | Patients | Location | Types of study | Period | CS used for all | CFR | Corticosteroid |
CFR |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe | Non-severe | CS | Non-CS | |||||||
| Huang et al. [2] | 41 | Wuhan, Jinyintan Hospital | SCR | inception to 2/1/2020 | 9/41 | 6/41 | 6/13 | 3/28 | 4/9 | 2/32 |
| Chen et al. [35] | 99 | Wuhan, Jinyintan Hospital | SCR | 1/1/2020 to 20/1/2020 | 19/99 | 11/99 | 11/23 | 8/76 | 6/19 | 5/80 |
| Zhou et al. [42] | 15 | Wuhan | SCR | 1/1/2020 to 29/1/2020 | 15/15 | 7/15 | 15/15 | NA | 7/15 | NA |
| Wang et al. [36] | 138 | Wuhan, Zhongnan Hospital | SCR | 1/1/2020 to 3/2/2020 | 72/138 | 6/138 | 26/36 | 36/102 | 4/62 | 2/76 |
| Xu et al. [37] | 62 | Zhejiang | MCR | 10/1/2020 to 26/1/2020 | 16/62 | 1/62 | 1/1 | 15/61 | 1/16 | 0/46 |
| Guan et al. [38] | 1099 | China | MCR | inception to 29/1/2020 | 204/1099 | 15/1099 | 77/173 | 127/926 | 6/204 | 9/895 |
| Yang et al. [41] | 52 | Wuhan, Jinyintan Hospital | SCR | late Dec 2019 to 9/2/2020 | 30/52 | 32/52 | 30/52 | NA | 16/30 | 16/22 |
| Yang et al. [39] | 149 | Wenzhou | MCR | 17/1/2020 to 15/2/2020 | 5/149 | 0/149 | NA | NA | NA | NA |
| Wu et al. [40] | 80 | Jiangsu | MCR | 22/1/2020 to 14/2/2020 | 12/80 | 0/80 | NA | NA | NA | NA |
| Li et al. [44] | 225 | Hanchuan City People's Hospital | SCR | 20/1/2020 to 14/2/2020 | 100/225 | 2/225 | NA | NA | NA | NA |
| Wang et al. [43] | 46 | Union Hospital of Huazhong University of Science and Technology | SCR | 20/1/2020 to 25/2/2020 | 26/46 | 3/46 | 3/46 | NA | 2/26 | 1/20 |
| Wu et al. [45] | 201 | Wuhan, Jinyintan Hospital | SCR | 25/12/2019 to 26/1/2020 | 62/201 | 44/201 | 50/84 | 12/117 | 23/62 | 21/139 |
| Lian et al. [46] | 788 | Zhejiang | MCR | 17/1/2020 to 12/2/2020 | 100/788 | 0/788 | NA | NA | NA | NA |
Abbreviations: CFR, case fatality rate; CS, corticosteroid; MCR, multicentre retrospective study; NA, not available; SCR, single-centre retrospective study.
Corticosteroid therapy was associated with better clinical outcomes in severe COVID-19 in clinical practice. Zhou et al. and Wang et al. reported that corticosteroid therapy improved the clinical symptoms and oxygenation of patients with COVID-19 [42,43]. Wang et al. also found that, for severe COVID-19, corticosteroid therapy reduced the lengths of hospitalization and ICU stay [43]. Wu et al. reported that treatment with methylprednisolone decreased the risk of death for individuals with COVID-19 with ARDS (HR 0.38; 95% CI 0.20–0.72) [45]. These results should be interpreted cautiously owing to potential bias and confounding factors from the single-centre observational studies with limited sample size.
There are several limitations to this analysis. First, all studies are observational studies and the interpretations of the results from these studies are limited. Second, there may be overlap of enrolled patients in some studies affecting the results. Finally, and importantly, propensity score adjustment and regression analysis to resolve confounding factors including the presence of competing risks were not performed in these studies.
So far, although there is no evidence to support corticosteroid therapy reducing the mortality of people with COVID-19 based on the published data, some benefits of improvements in clinical symptoms and oxygenation were observed in some clinical observational studies [42,43,45]. Especially individuals with COVID-19 and ARDS may benefit from corticosteroid therapy [45]. Currently, methylprednisolone is the most reported treatment for COVID-19. Whether other types of corticosteroids work in COVID-19 is uncertain. For the dosages and duration, short courses of about 1 week and low-to-moderate dose (methylprednisolone ≤0.5–1 mg/kg/day) may be reasonable based on our experience fighting against the COVID-19 and the Chinese Expert Consensus [22]. Hence, for critically ill patients with COVID-19 who have overwhelming inflammation and cytokine-related lung injury, timely and appropriate use of corticosteroids may be considered to prevent the development of ARDS [3,22]. Unfortunately, there are no confirmed biomarkers reported to guide the use of corticosteroids for COVID-19.
In conclusion, randomized controlled clinical trials to evaluate the effectiveness and safety of early administration of a low-to-moderate dose of methylprednisolone for individuals with COVID-19 and ARDS are urgently needed.
Exploring the potential mechanism of corticosteroids in COVID-19
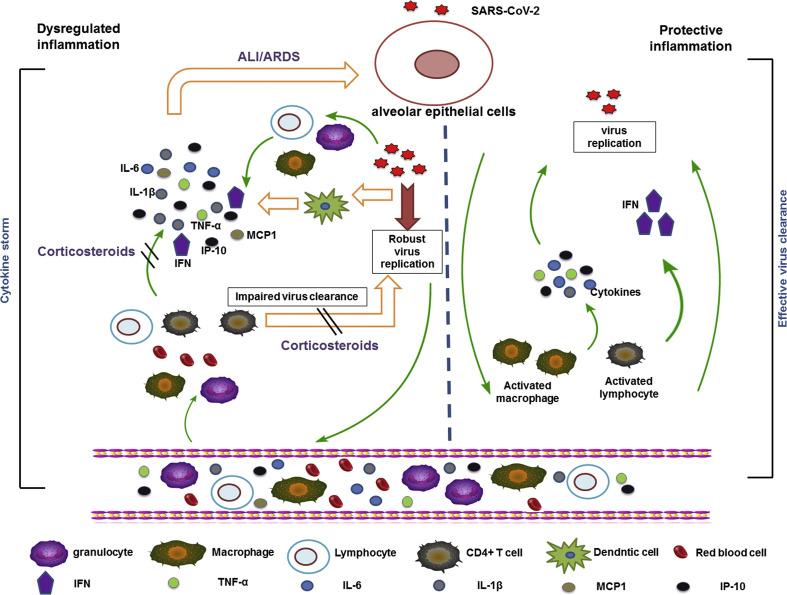
According to current research, some individuals with COVID-19 developed severe pneumonia at the 8th to 10th day after onset, with severe dyspnoea, ARDS, multiple organ dysfunction, even death [2,35,36]. Higher concentration of cytokines and chemokines were detected in patients with severe disease [2]. When the virus invades airway epithelial cells and alveolar epithelial cells, which stimulate specific immune cells (including monocytes, T cells, B cells, natural killer cells) are stimulated to produce massive amounts of cytokines and chemokines rapidly, such as tumour necrosis factor-α, interleukin-1 (IL-1), IL-6, IL-12, interferon-α (IFN-α), IFN-β, IFN-γ, monocyte chemoattractant protein 1 (MCP1), IFN-γ-induced protein 10 (IP10) and IL-8. They are in turn promoting infiltration of inflammatory cells to reproduce more cytokines [[48], [49], [50]]. The process was called ‘cytokine storm’ and it causes damage to the lung as well as acting against the virus.
Previous studies have shown that the release of excessive pro-inflammatory cytokines, for example IL-1β, IL-6, IFN-γ, promoted patients with SARS-CoV and MERS-CoV to ARDS, multiple organ dysfunction and death [[48], [49], [50]]. This phenomenon was also investigated in individuals with COVID-19. Huang et al. showed that the cytokines IL-1β, IFN-γ and the chemokines IP10, MCP1 were significantly increased in individuals with severe COVID-19 [2], indicating that the immune response of T helper type 1 (Th1) cells was activated. In addition, serum levels of granulocyte colony-stimulating factor, IP10, MCP1, macrophage inflammatory protein 1A and tumour necrosis factor were significantly elevated in patients with severe disease, suggesting that ‘cytokine storms’ were associated with the severity of COVID-19.
Recent studies have shown that the receptor gene of SARS-CoV-2 is angiotensin-converting enzyme 2. Most of the angiotensin-converting enzyme 2 of the lung is located on the surface of type II alveolar epithelial cells [[51], [52], [53]]. We supposed that when SARS-CoV-2 invades alveolar epithelial cells, it robustly replicates at the early stage, which activate the lymphocytes, macrophages, natural killer cells, etc., to produce extensive cytokines and chemokines. They in turn promote massive migration of inflammatory cells into the lungs. Excessive immune responses cause damage to the lung. The process is consistent with the processes on autopsy [3]. Corticosteroid treatment is a double-edged sword, which may exacerbate an excessive immune response [22,54]. Based on this mechanism the clinical use of corticosteroids should be very cautious, if at all.
Lymphopenia might be a critical factor associated with disease severity and mortality [2,3,35,36,38,41]. Guo and his colleagues showed that in viral pneumonia the absolute counts of CD3+ T cells, CD3+ CD4+ T cells and CD3+ CD8+ T cells in the patients who died were significantly lower than in survivors [54], suggesting that the cellular immune function of individuals with severe viral pneumonia was significantly inhibited. T-cell immunity is probably an important antiviral mechanism, especially regarding the role of CD4+ T cells [[55], [56], [57]]. For severe COVID-19, autopsy has found that counts of CD4 and CD8 T cells were substantially reduced, and hyperactivated. Further research showed that the concentration of highly pro-inflammatory CCR6+ Th17 in CD4 T cells increased and high concentrations of cytotoxic granules were found in CD8 T cells. These results imply that over-activation of T cells with elevation of Th17 and high cytotoxicity of CD8 T cells may account for the severe immune injury [3]. For these patients, corticosteroid use may inhibit T-lymphocyte immunity and lead to persistent viral replication and ensuing delays in clearance.
A summary of the potential mechanism of COVID-19 and corticosteroid action is shown in Fig. 3 .
Fig. 3.
Potential mechanism of COVID-19 and corticosteroid action.
Conclusions and future perspectives
In conclusion, corticosteroid use in viral pneumonia remains a challenging clinical puzzle. Although corticosteroid therapy was reported to improve the clinical features of viral pneumonia in some cases, there is no confirmed evidence of corticosteroid therapy reducing the mortality of COVID-19 patients. The clinical use of corticosteroid for COVID-19 should be cautious, if at all. Cytokine storm and the suppression and deficiency of T-cell immunity may be the main potential mechanisms of severe COVID-19. Randomized controlled clinical trials of early administration of a low-to-moderate dose of methylprednisolone for COVID-19 patients with ARDS are required to confirm the effectiveness and safety of corticosteroid therapy and further study the long-term outcomes after discharge.
Author contributions
JFX, JWY, LY and RGL contributed to the study design, data analysis and interpretation, and to drafting and revision of the manuscript.
Support statement
This work was supported by the National Natural Science Foundation of China for Distinguished Young Scholars to JFX (81925001), Shanghai Leading Talent Programme to JFX (2016036) and the Project of the Shanghai Hospital Development Center to JFX (16CR3036A), but it did not play any role in this systematic review.
Transparency declaration
All authors have nothing to disclose.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.06.020.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO . 2020. Coronavirus disease (COVID-19) outbreak situation.http://covid19.who.int 30 April. Available from: [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan E.D., Chan M.M., Chan M.M., Marik P.E. Use of glucocorticoids in the critical care setting: science and clinical evidence. Pharmacol Ther. 2020;206:107428. doi: 10.1016/j.pharmthera.2019.107428. [DOI] [PubMed] [Google Scholar]
- 6.Torres A., Sibila O., Ferrer M., Polverino E., Menendez R., Mensa J. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 7.Blum C.A., Nigro N., Briel M., Schuetz P., Ullmer E., Suter-Widmer I. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 8.Villar J., Ferrando C., Martinez D., Ambros A., Munoz T., Soler J.A. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 9.Brun-Buisson C., Richard J.C., Mercat A., Thiebayt A.C., Brochard L., REVA-SRLF A/H1N1v 2009 Registry Group Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;183:1200–1206. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Loeches I., Lisboa T., Rhodes A., Moreno R.P., Silva E., Sprung C. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1) influenza A infection. Intensive Care Med. 2011;37:272–283. doi: 10.1007/s00134-010-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.H., Hong S.B., Yun S.C., Choi W.I., Ahh J.J., Lee Y.J. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183:1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 12.Han K., Ma H., An X., Su Y., Chen J., Lian Z. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis. 2011;53:326–333. doi: 10.1093/cid/cir398. [DOI] [PubMed] [Google Scholar]
- 13.Lee N., Leo Y.S., Cao B., Chan P.K., Kyaw W.M., Uyeki T.M. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J. 2015;45:1642–1652. doi: 10.1183/09031936.00169714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B., Gao H., Zhou B., Deng X., Hu C., Deng C. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44:e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 15.Moreno G., Rodríguez A., Reyes L.F., Gomez J., Sole-Violan J., Díaz E. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44:1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q. Treatment of severe acute respiratory syndrome with glucosteroids. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auyeung T.W., Lee J.S., Lai W.K., Choi C.H., Lee J.S., Li P.C. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yam L.Y., Lau A.C., Lai F.Y., Shung E., Chan J., Wong V. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M. Corticosteroid therapy for critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 20.Lee N., Allen Chan K.C., Hui D.S., Ng E.K.O., Wu A., Chiu R.W.K. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;39:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J.S., Lim W.S. Effect of corticosteroid therapy on influenza-related mortality: a systematic review and meta-analysis. J Infect Dis. 2015;212:183–194. doi: 10.1093/infdis/jiu645. [DOI] [PubMed] [Google Scholar]
- 24.Yang J.W., Fan L.C., Miao X.Y., Mao B., Li M.H., Lu H.W. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:956–963. doi: 10.1016/j.cmi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Sun W., Svendsen E.R., Tang S., MacIntyre R.C., Yang P. Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care. 2015;19:46. doi: 10.1186/s13054-015-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J.S., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406. doi: 10.1002/14651858.CD010406.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med. 2020;48:e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y., Fu X., Liu X., Huang C., Tian G., Ding C. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-59732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68:895–902. doi: 10.1093/cid/ciy874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho J.C., Ooi G.C., Mok T.Y., Chan J.W., Hung I., Lam B. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 32.Sung J.J., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Ding Y., Li X., Yang L., Zhang W., Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med. 2003;349:507–508. doi: 10.1056/NEJM200307313490519. [DOI] [PubMed] [Google Scholar]
- 34.Zhao R., Wang H., Wang X., Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos Int. 2017;28:1027–1034. doi: 10.1007/s00198-016-3824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5:57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R., Tian J., Yang F., Lv L., Yu J., Sun G. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. doi: 10.1016/j.jcv.2020.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 48.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:1–4. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson T.M., Li C.K., Chui C.S., Huang A.K., Perkins M., Liebner J.C. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 56.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N.B. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J., Zhao J., Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.