1. Introduction
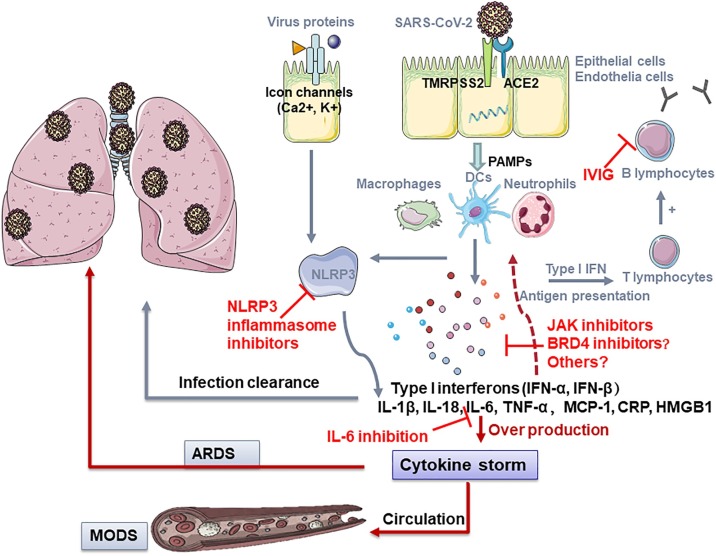
The ongoing pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused over 9.2 million confirmed cases and 478,000 deaths (as of June 24th, 2020). The high intensive care unit (ICU) admission rate and mortality of COVID-19 is continuously posing a serious strain on the global health-care system. While most patients present with mild to moderate symptoms, some patients may develop life-threatening sepsis, respiratory failure, acute respiratory distress syndrome (ARDS) and multiple organ failure. However, current antiviral and supportive therapies are not sufficient to prevent or treat the above complications especially in severely ill COVID-19 patients [1]. Thus, it is urgent to find the underlying mechanism dominating such life-threatening complications. It is well recognized that lung damage and multi-organ failure in this subgroup of patients arise due to overwhelming systemic hyper-inflammation. Therefore, promising anti-inflammatory candidate agents targeting diverse mechanisms related to cytokine storm will be therapeutically relevant (Fig. 1 ).
Fig. 1.
Potential therapies of COVID-19 by targeting inflammation and cytokine storm.
SARS-CoV-2 enters and infects cells by binding to TMPRSS2 and ACE2, subsequently releases virus RNA, one of the PAMPs, and recruits dendritic cells, macrophages and neutrophils. The released type I interferon and pro-inflammatory cytokines and chemokines induce the innate immune response while adaptive immune response is ignited by activating T and B lymphocytes to defend against the virus. Meanwhile, intact viruses or the components can directly activate the NLRP3 inflammasome, leading to IL-1β secretion. In most infections, moderate immune response and antiviral response are capable to combat the infection. Nevertheless, in individuals with immunological dysfunction, persistent hyper-inflammation triggers a cytokine storm, leading to the lung injury and ARDS or eventually swept through the whole body, causing MODS. During the pathological progression, the inhibition of NRLP3, JAK1/2, IL-6, BRD4 and the infusion of IVIG may be beneficial in suppressing the overwhelming inflammation and arrest or reverse the COVID-19 disease.
Abbreviations: ACE2, angiotensin converting enzyme 2; ARDS, acute respiratory distress syndrome; IVIG, intravenous immunoglobulin; MODS, multiple organ dysfunction syndrome; NLRP3, Nod-like receptor protein 3; PAMP, pathogen-associated molecular pattern; TMPRSS2, transmembrane serine protease 2.
2. Inflammation and cytokine storm in COVID-19
Mounting evidence has demonstrated that severely ill COVID-19 patients presented with elevated levels of cytokines and inflammatory indices, such as serum IL-1β, IL-6, IL-10 and d-dimer than those with moderate symptoms, suggesting the involvement of a cytokine storm [2]. Cytokine storm represents an exaggerated immune response characterized by overproduction of pro-inflammatory cytokines and chemokines such as IFN-γ, TNF-α, IL-6, IL-1β, IL-18, CXCL8 and CXCL10. It has also been observed in other viral diseases such as influenza, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [3]. While an adequate release of cytokines is critical for the body's defense against viral infection, uncontrolled and aberrant immune system activation can lead to organ injury. Current clinical evidence also confirmed the correlation of cytokine storm syndrome and disease severity as well as unfavorable outcomes in hospitalized COVID-19 patients [3]. The association of unfavorable prognosis with the presence of over-inflammation and cytokine storm highlights the necessity of early identification of cytokine storm and implementation of anti-inflammatory treatment. Timely control of hyper-inflammatory response is crucial to prevent the development and progression of irreversible ARDS and multi-organ dysfunction accompanying COVID-19.
3. Current anti-inflammatory and immune-modulatory agents in treating COVID-19
In COVID-19 patients, the most widely used anti-inflammatory and immune-modulatory agents include corticosteroids and intravenous immunoglobulin (IVIG). Corticosteroids, such as methylprednisolone and dexamethasone, were considered as one of the options for COVID-19 patients with cytokine storm [4] based on their inhibition on many inflammatory genes and previous clinical use in other viral diseases. On 17th June, preliminary results from a randomized, controlled clinical trial were released by the RECOVERY (Randomised Evaluation of COVid-19 thERapY) study group in the United Kingdom. This study showed that low-dose dexamethasone could reduce mortality by one-third in critically ill patients in need of mechanical ventilation [5]. However, previous studies associate the use of corticosteroid in SARS, MERS and influenza with a higher risk of a delayed viral clearance and long-term complications in survivors [6]. Yet much remains unknown regarding the impact of corticosteroids on other clinical outcomes of COVID-19, combination effect with other drugs, time window and dosage of administration, safety profile as well as possible adverse effects. Currently, there are dozens of ongoing clinical trials addressing the unsolved questions (Supplemental Table). IVIG is also used as an adjunctive drug in the treatment of severe COVID-19 patients. As a purified blood product from healthy donors, it exerts immunomodulatory effect mainly through neutralizing virus by antibodies/polyclonal immunoglobulin G (IgG). It could also block Fcγ receptors which are involved in natural and adaptive immunity. Currently, a limited number of clinical studies have shown that IVIG treatment within 48 hours of admission reduced the use of mechanical ventilation, duration of ICU stay and hospitalization, as well as 28-day mortality [7]. However, more clinical evidence for its effectiveness in managing clinical COVID-19 infection is warranted. Ongoing clinical trials of IVIG and plasma from convalescent patients are listed in Supplemental Table.
4. Therapeutic potential of NLRP3 inflammasome inhibitors in COVID-19
Nod-like receptor protein 3 (NLRP3) inflammasome protein complex, is crucial in host’s antiviral defense. During viral replication, the invading virus plus a variety of derived pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) can active NLRP3 inflammasome. Upon activation, NLRP3 inflammasome induces pyroptosis as well as the secretion of IL-1β and IL-18 which are responsible for recruitment of neutrophils to the inflammatory sites to assist in the virus eradication and induction of the adaptive immune system. However, a persistent activation of NLRP3 inflammasome, excessive production of inflammatory cells and cytokine, as well as pyroptosis-mediated cell damage jointly lead to organ injury [8]. Activation of inflammasome pathway is indicated by the elevation of IL-1β in severe COVID-19 patients. Therefore, drugs targeting at NLRP3 inflammasome and IL-1β are promising in mitigating NLRP3-inflammasome driven hyper-inflammation, pyroptosis, and cytokine storm syndrome in severely ill COVID-19 patients. Colchicine, a bioactive alkaloid compound isolated from plant Colchicum, has been commonly used for inflammatory diseases such as gout. It acts as an inhibitor of NLRP3 inflammasome and interferes with polymerization by binding to free tubulin dimers, leading to the deregulation of inflammatory cell activities and release of cytokines especially IL-6 [9]. In addition, colchicine also suppresses the production of numerous cytokines, pyrogens and superoxides mediated by white blood cells. With regard to safety profile of colchicine, 5–10 % patients administered with therapeutic dose may exhibit gastrointestinal symptoms (nausea, vomit or diarrhea) as side effects [10]. Overall, colchicine may be exploited as a potential candidate for alleviating the immune hyperactivity during SARS-CoV-2 infection. Recombinant IL-1 receptor blocker (such as anakinra) and IL-1β monoclonal antibodies (canakinumab) are also of interest. A prospective study showed that with a prospective cohort design showed that the treatment with anakinra contributes to a substantial reduction in mortality and use of invasive mechanical ventilation without bringing about serious side effects in critically ill COVID-19 patients [11]. Due to the anti-inflammatory effects of the above drugs, their possible efficacy in COVID-19 is gaining more and more attention. Currently, there are a number of registered studies on clinicaltrials. gov to test the effectiveness and safety of colchicine, anakinra and canakinumab on COVID-19 (Supplemental Table).
5. Therapeutic potential of IL-6 inhibition in COVID-19
The level of IL-6 was significantly increased in COVID-19 patients and was closely related to ARDS severity and outcome. SARS-CoV-2 infection and subsequent release of virus particles recruits monocytes and macrophages, leading to the release of cytokine and initiation of adaptive immune responses. In this process, IL-6 played an essential role. In most cases, the immune response eliminates lung infection and the patients recovered. However, in others, abnormal immune response triggered a cytokine storm, which was characterized by persistent hypercytokinemia (elevated IL-6, TNF-α, IL-1β), eventually aggravated the lung injury and even had ripple effects through the entire body. The immune dysregulation in severe COVID-19 individuals was driven by IL-6 [12] and the IL-6 deficiency was shown to reduce the severity in acute lung injury in mouse model [13]. Therefore, IL-6 may be a potential target cytokine to treat COVID-19 related ARDS. IL-6 inhibitors, such as tocilizumab, a recombinant humanized monoclonal IL-6 blocking antibody, could curb IL-6 mediated pro-inflammatory pathway through binding to the soluble and membrane fraction IL-6 receptor (IL-6R) (probably via the nuclear factor kappa-B and STAT3 signaling pathway). Tocilizumab seemed to be effective in COVID-19 patients, which decreased the use of mechanical ventilation, improved hypoxemia and CT opacity changes in some severe cases and clinical trials [14]. Tocilizumab has been prescribed for treatment in severe patients with elevated IL-6 level in China since March 2020 according to the Diagnosis and Treatment Program for COVID-19 (National Health Commission of China, 7th Version). Most notably, IL-6 could either inhibit or accelerate viral replication and function in the lung suffering from infection or chemical insults, thus the optimal timing for anti-IL-6 administration will be essential. Therefore, IL-6 blockers could be an efficient therapeutic strategy for the treatment of COVID-19. At present, there are several registered studies under way to assess the safety and efficacy of IL-6 blockers (Supplemental Table). Further researches are required to evaluate the short-term and long-term outcomes of anti-IL6 administration and determine the optimal time for drug administration in patients from different ethnic groups.
6. Therapeutic potential of BRD4 inhibitors in COVID-19
Bromodomain containing protein 4 (BRD4), an epigenetic reader of acetylated lysines, is a member of the bromodomain and extra terminal domain (BET) family. BRD4 regulates cell growth and cell cycle-related gene transcription through combining with acetylated histones. When suffering from virus infection, BRD4 plays a role in epithelial-driven and NF-κB dependent innate inflammation. NF-κB recruits BRD4 and facilitates the interaction between NF-κB and BRD4, activates NF-κB mediated pro-inflammatory signaling pathway. Moreover, BRD4 inhibitors was shown to d decrease the recruitment of macrophages and the infiltration of T cells [15]. Previous researches have indicated the potential role of BRD4 inhibition in both antiviral response through DNA damage manner to block the attachment of DNA and RNA [16], as well as in alleviating the lung fibrosis, one of the common sequelae of COVID-19 in animal models and ex vivo experiments [17]. Furthermore, recent reports demonstrated that the transmembrane E protein of SARS-CoV-2, resident on ERGIC and Golgi membranes, could bound to BRD4 [18]. BET inhibitors like Apabetalone (RVX-208) and JQ-1 inhibit vascular inflammation-related gene expression through suppressing BET-mediated transcription triggered by multiple pro-inflammatory stimuli. By doing so, BET inhibitors have many reported effects in inflammation, airway remodeling, cardiovascular disease, and cancers [19]. Though there is no reported cases of COVID-19 who have been treated with BRD4 inhibitors to date, BRD4 inhibitors could be considered as a potential candidate to ameliorate hyperinflammation and cytokine storm accompanying COVID-19.
7. Conclusion and outlook
The pathophysiology of severe COVID-19 [20] is related to aggressive inflammatory responses and damage to the lung and even ripple effects across the body. Thus, the severity depends on the balance between the viral virulence and the host immune response. When the infection occurs, the release and recognition of PAMPs trigger the production of pro-inflammatory cytokines and chemokines involving IL-1β, IL-6, IL-18, TNF-α and macrophage inflammatory proteins. Normally, a healthy immune response will clear the infection, while an inadequate immune response will result in further accumulation of activated immune cells in the lungs, leading to a cytokine storm and eventually damaging the lung, or causing multiple organ failure through circulation. Therefore, one strategy for the treatment against COVID-19 is to block the overwhelming inflammation. Emerging evidence has suggested the potential effects of inflammation inhibitors on the treatment for COVID-19, especially in severe cases. Further immunomodulatory and immunosuppressive therapies aimed at curbing immune mediated damage in COVID-19 are ongoing (Supplemental Table). It is necessary to confirm the therapeutic effects, investigate the administration timing and clarify the mechanism, all of which may render it possible for immunoregulation-based interventions to arrest or reverse this devastating disease.
Disclosure
None declared.
Declaration of Competing Interest
None declared.
Footnotes
please refer to online data supplement for additional references
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105051.
Contributor Information
Suowen Xu, Email: sxu1984@ustc.edu.cn.
Jianping Weng, Email: wengjp@ustc.edu.cn.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References2
- 1.Wu R., Wang L., Kuo H.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.N. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao J., Li C., Zhang K., Kang H., Chen W., Gu B. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020 doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 6.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.