Abstract
Background and aims
Interest in corticosteroid therapy in COVID-19 has been rekindled after the results from Randomized Evaluation of COVid-19 thERapY (RECOVERY) Trial. However, the World health Organization has not recommended corticosteroid in the treatment of COVID-19. We sought to conduct a systematic review on the role of corticosteroid in the management of patients of COVID-19.
Methods
A systematic electronic search of PubMed, Cochrane and MedRxiv database using specific keywords was made up till June 17, 2020. Full text of all the original articles with supplementary appendix that fulfilled the inclusion criteria were retrieved and a detailed analysis of results were represented.
Results
Of the 5 studies (4 retrospective studies and 1 quasi-prospective study) conducted for evaluating the role of corticosteroids, 3 studies have shown benefit, while 2 studies shown no benefit and there was a suggestion of significant harm in critical cases in one sub-study. RECOVERY trial is the only randomized controlled trial that has shown a significant reduction of death by 35% in ventilated patients and by 20% amongst patients on supplemental oxygen therapy with the dexamethasone, although no benefit was observed in mild cases.
Conclusions
While the results from retrospective studies are heterogenous and difficult to infer of a definitive protective benefit with corticosteroids, RECOVERY trial found a significantly better outcome with dexamethasone, mostly in severe cases. Nonetheless, more studies are needed to replicate the outcome shown in RECOVERY trial for a substantial conclusion.
Keywords: COVID-19, SARS-CoV-2, Corticosteroids, Dexamethasone, Methylprednisolone, ARDS
Highlights
-
•
Corticosteroids have been tried in COVID-19 to improve the outcomes.
-
•
Retrospective studies have shown inconsistent results.
-
•
Recently conducted randomized RECOVERY trial has shown a significant benefit in severe and critical patients.
1. Introduction
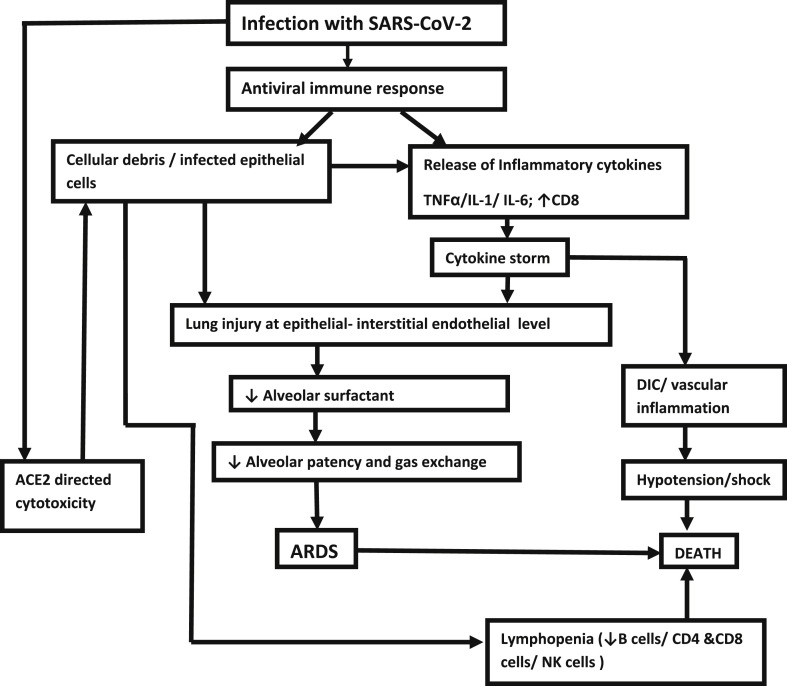
Coronavirus diseases 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Corona virus 2 (SARS-CoV-2), is responsible for the global pandemic that originated from Wuhan in December 2019. Though the majority of patients undergo an uneventful recovery, in around 19% there is a progressive worsening leading to severe pneumonia in 14% and critical pneumonia in 5% of patients [1]. There is a staged progression in the course of events after a median incubation period of 4 days (interquartile range 2–7 days) [2]. The adult respiratory distress syndrome (ARDS) usually develops from the second week onwards. This does not only happen because of uncontrolled viral replication but also because of an explosive immune response from the host. In presence of uncontrolled viral replication, the presence of an increased number of infected epithelial cells and cell debris triggers a massive cytokine release - the so-called ‘cytokine storm’ - with hyperinflammation and immune suppression, characterized by decreased memory CD4 + T helper cells and increased CD8 cytotoxic activity [3].
In the first phase, the antiviral immune response leads to the elimination of the virus at the expense of the immune mediated pulmonary injury. At one end of the spectrum, a balanced immune response keeps the infection under control, but at the other end there is an exaggerated immune response with consequent lung injury. Lung injury initiates at the epithelial-interstitial-endothelial level, with exudation of neutrophils and macrophages, which, in its turn reduces the alveolar surfactant, thereby reducing the alveolar patency and the gas exchange. Infected cellular debris further augments the release of inflammatory cytokines like TNF-α, interleukin-1 (IL-1) and IL-6, further accentuating the ‘cytokine storm” [4].
The second phase begins with uncontrolled viral replication induced angiotensin-converting enzyme 2 (ACE2)-directed cytotoxicity, that triggers a vicious circle of immune activation with consequent worsening of the hyperinflammatory state. At this stage, patients exhibit lymphopenia with reduced B cells, CD4 and CD8 T cells and CD16+ Natural Killer (NK) cells. This probably results because of an increase in extravasations of dysfunctional lymphocytes [5]. The accompanying “cytokine storm” leads to a massive vascular inflammation, disseminated coagulation, shock and hypotension, leading to multi organ failure and death. Fig. 1 briefly summarizes the pathogenesis of ARDS in COVID-19. Studies have shown that any intervention which can prevent this catastrophe can also prevent the lung damage and pulmonary thromboembolism [5,6]. It is with this pathophysiology in mind that intervention with corticosteroids has been thought about in COVID-19.
Fig. 1.
Pathogenesis of ARDS and its consequences in COVID-19.
Since corticosteroids causes immune suppression by impairing the innate immunity, their use has been largely discouraged because of the fear of worsening of viral propagation. However, in patients who are on long term maintenance dose of steroids, there is no increased incidence of development of severe or critical pneumonia in presence of COVID-19 [6]. Interestingly, most of earlier studies done in Severe Acute Respiratory Syndrome 1 (SARS-CoV-1) and the Middle Eastern Respiratory Syndrome (MERS-CoV) showed adverse outcomes with corticosteroid treatment [7,8]. Indeed, two recent commentaries published in the Lancet reported that corticosteroids should be avoided for the treatment of COVID-19 [9,10]. However, these assumptions are mainly based on the experiences in similar viral illness but not on COVID-19 specifically. Both Word Health Organization (WHO) and The Centers for Disease Control and Prevention (CDC), USA also specifically advises against the use of corticosteroids in COVID-19 for the purpose of immune modulation [11,12]. In contrast, the recent multinational Surviving Sepsis Guideline in COVID-19, recommends giving steroids in patients with severe COVID-19 on mechanical ventilation with ARDS, in order to reduce the destructive inflammatory immune response (based on very minimal evidence though), and to treat suspected adrenal insufficiency associated with sepsis, particularly in those with refractory shock, although this guideline advises against the use of corticosteroids in COVID patients in non-ARDS respiratory failure on mechanical ventilation [13]. Nevertheless, the Randomized Evaluation of COVid-19 thERapY (RECOVERY Trial) conducted in patients with COVID-19 has shown a significantly improved the outcome with dexamethasone, a corticosteroid, in the treatment of severe COVID-19 requiring oxygen therapy or on mechanical ventilator [14]. These finding prompted us to conduct a systematic review of studies conducted with corticosteroid in patients with COVID-19 as well as ARDS due to other viral diseases.
2. Methods
A thorough and systematic search from the electronic database of PubMed, MedRxiv, and Cochrane library using specific keywords “Corticosteroids”, “Dexamethasone”, “Methylprednisolone”, “Hydrocortisone”, “Adult Respiratory Distress Syndrome”, “COVID-19”, “SARS-CoV-2”, “Intubation”, “Ventilation”, with a Boolean interposition of “AND” was made up till June 17, 2020. All the original articles fulfilling the inclusion criteria with the supplementary appendix were retrieved. Subsequently, all the articles were meticulously analyzed and presented in the text.
3. Results
The results of treatment with corticosteroids in ARDS are outlined as follows -
3.1. Studies of corticosteroids in ARDS of mixed etiology not due to COVID-19
The role of corticosteroids in non-COVID ARDS remains controversial. In a multicentric randomized controlled trial (RCT) conducted in 2018 involving 17 intensive care units (ICUs) in Spain, the role of dexamethasone in established ARDS of mixed non-COVID etiology was evaluated with 277 patients, of whom 139 were randomly assigned to the dexamethasone group, while the rest served as the controls. Mean number of ventilation free days (at 28 days) was more in the dexamethasone group in comparison with the control group (12.3 days vs. 7.5 days, p < 0.001), with a 15.3% reduction of all-cause mortality on day 60 (21% vs. 36%, p = 0.005), 12.5% reduction of ICU mortality (19% vs. 31%, p = 0.017) and a 12.5% reduction of hospital mortality (24% vs. 36%, p = 0.024). An encouraging fact of this study was that there was no significant increased incidence of hyperglycemia, new onset infections or barotrauma [15]. These results suggest that early therapy with dexamethasone could change the outcome of ARDS by having an effect on the systemic immune responses. The effect on reduced requirement of ventilation is actually a manifestation of better survival. In ARDS, down regulation of the pulmonary and systemic inflammation with long term dexamethasone leads to a substantial improvement in indices of alveolar–capillary membrane permeability and tissue repair [16].
3.2. Studies with corticosteroid treatment in previous corona virus infections
Corticosteroids had been tried in a few studies with SARS-CoV-1 and MERS-CoV infections. Two studies of patients with SARS-CoV-1 and influenza A (H1N1) viral pneumonia showed that the use of systemic corticosteroids was associated with reduced mortality in critical patients [6,17]. However, a meta-analysis showed a considerable higher mortality (Risk ratio [RR] 1.75; 95% confidence interval [CI], 1.30–2.36; p = 0.0002) and higher rate of secondary infection (RR 1.98; 95% CI, 1.04–3.78; p = 0.04) in steroid treated influenza patients [18]. Methylprednisolone was used in a significant number of studies followed by hydrocortisone, prednisolone and dexamethasone. In MERS-CoV, steroid treatment was associated with an adverse outcome with higher 90-day crude mortality (adjusted odds ratio [OR] 1.87; 95% CI, 1.02–3.44; P = 0.04), higher likelihood of invasive ventilation (93.4 vs. 76.6%; p < 0.0001) and delayed viral clearance (adjusted hazard ratio [HR] 0.35; 95% CI 0.17–0.72; p = 0.005), though it has to be borne in mind that the steroid used was mostly hydrocortisone in 68% [8]. Notwithstanding a lack of uniform protocol in SARS-CoV-1, methylprednisolone, particularly when used in high dosage (up to 1000 mg/day) did show some survival benefit and some degree of reduction in incidence of ARDS and early discharge from the hospital [17,19,20]. Altogether systemic corticosteroids were used in 79.6% of SARS-CoV-1 and 48.9% of MERS-CoV critical cases [8,17]. However, most of these studies had minimal follow up and do not record about the long-term consequences of high dose steroid including the prevalence of relative adrenal insufficiency or unmasking of diabetes [17]. One study showed incidence of avascular necrosis of hip in China as 3.2% (4 of 124 cases) [20], much lower than other studies [21,22], which again was presumably secondary to the lower dose of steroids used in former. Another issue was the timing of the steroids and other treatments used. Early use of steroids in some studies along with antiviral and broad-spectrum antibiotics showed the best result in SARS-CoV-1 but not in MERS-CoV infections [17,20]. Table 1 summarizes the results of studies conducted in patients with previous coronavirus infections.
Table 1.
Studies with corticosteroids in previous Coronavirus infections (SARS-CoV-1 and MERS-CoV).
| Author, Year | Median Age (years) | Acute Lung Injury/ARDS | N | Type of study, center (S/M) | Type of steroid and dose and no (n) | Median day of initiation of steroid after admission | Associated medical treatment | Treatment outcome |
|---|---|---|---|---|---|---|---|---|
| Arabi et al. [8], 2017 | 58 | NR | 309 | Retro, M | HC (68.2%) MP (40.4%) P(13.2%) Dexa (6%) (n = 151) |
3 | Ribavirin, Oseltamivir, INF-α |
Higher 90-day crude mortality (adjusted OR 1.87; 95% CI, 1.02–3.44; P = 0.04), higher likelihood of invasive ventilation (93.4% vs. 76.6%; p < 0.0001) and delayed MERS-CoV viral clearance (adjusted HR 0.35; 95% CI 0.17–0.72; p = 0.005) |
| Lew et al. [19], 2003 | 51 | 45 | 199 | Retro, S |
MP IV pulse for 3 days (n = 32) | 1 | Ribavirin, Oseltamivir, Levofloxacin |
No survival benefit in the steroid treated group |
| Zhao et al. [20], 2003 | 29 | 36 | 190 | Retro, M | a) MP IV pulse 2–3 days if patients failed to improve (n = 60) b) MP IV pulse for 5–14 days (n = 60) |
variable | Ribavirin, INF –α, BS antibiotics |
Early use of high-dose steroids in combination with a quinolone and AZ gave the best outcome: improved clinical features, reduced ARDS, MV and mortality. |
| Chen et al. [17], 2003 | 40 | 121 | 401 | Retro, M | MP in most [n = 147 of non-critical (59%); 121 critical (79.6%) patients] | 5.01 ± 3.48 | Ribavirin IFN-α BS antibiotics |
No overall difference. However, in critical cases steroid therapy significantly reduced mortality after adjustment of death related variables like age, secondary RTI and rigor at onset (OR 0.083; 95% CI 0.07-0.956 p = 0.046), increased early discharge (OR 1.74; 95% CI 1.025–2.964) |
| MP- Methylprednisolone; HC- Hydrocortisone; P- Prednisolone; Dexa- Dexamethasone; ARDS- Adult Respiratory Distress Syndrome; OR- Odds ratio, HR- Hazard ratio, CI- Confidence Interval, RTI- Respiratory tract infection; NR- Not reported; AZ- Azithromycin; MV- Mechanical ventilation; BS- Broad spectrum, IF- Interferon; Retro- Retrospective; M-Multicentric, S-Single center | ||||||||
3.3. Studies with corticosteroids in COVID-19
3.3.1. Studies in COVID-19 where corticosteroid was incidentally used
Corticosteroids have been used in quite a few studies with COVID-19 without any pre-adjudication to look at the outcomes dedicated to the steroid therapy. Most of these studies had a small cohort size and had a high degree of heterogeneity regarding the choice of steroids, the dose and timing of the steroids, and had a co-prescription of broad-spectrum antibiotics and antivirals [2,[23], [24], [25], [26], [27]]. Majority of these studies, owing to their design were not adequately powered to give the answer as to whether steroids produced any outcome benefits. Barring one study [24], others did not find any benefit in patients with acute lung injury/ARDS, irrespective of the timing of administration of steroids. Corticosteroid therapy was also shown to increase the time of viral excretion from the body in one study [26]. Table 2 summarizes the outcomes with corticosteroid in these studies.
Table 2.
Studies in COVID-19 where corticosteroid was incidentally used and evaluated.
| Author, Year | Median Age (years) | Acute Lung Injury/ARDS | N | Type of study, center (S/M) | Type of steroid and dose and no (n) | Median day of steroid initiation after admission | Associated medical treatment | Treatment outcome |
|---|---|---|---|---|---|---|---|---|
| Guan et al. [2], 2020 | 47 | 67 | 1099 | Retro, M | Systemic glucocorticoid (details unknown) (n = 204) |
Unknown | IV antibiotic (58%) Oseltamivir (35.8%) |
NR |
| Zhou et al. [23], 2020 | 56 | 59 | 191 | Retro, M | Systemic corticosteroids (details unknown) (n = 57) |
Unknown | Antibiotics (95%) L/R (21%) IV Ig |
Though use of steroids was higher amongst survivors (48% vs. 23%, p < 0.005), no definitive benefit was documented |
| Wu et al. [24], 2020 | 51 | 84 | 201 | Retro, S | MP (n = 62) | Unknown | Empirical antibiotics, Oseltamivir (66.7%) Ganciclovir (40.3%) L/R (14.9%) Recombinant IFN-α (10.9%) Antioxidants Immunomodulators (34.8%) |
Administration of MP reduced the risk of death in ARDS by 62% (HR 0.38; 95% CI, 0.20–0.72; p = 0.003). |
| Wang et al. [25], 2020 | 56 | 27 | 138 | Retro, S | Systemic corticosteroids, variable dosage (details unknown) (n = 62) |
Unknown | Oseltamivir (89.9%), Moxifloxacin (64.4%) Ceftriaxone (24.6%) AZ (18.1%) |
No outcome benefit |
| Ling et al. [26], 2020 | 44 | NR | 66a | Retro, S | Prednisolone and dexamethasone (dosage and duration not specified) (n = 6) |
Unknown | Details unknown | Duration of viral RNA detection more prolonged in the glucocorticoid group than the non-glucocorticoid group in throat swabs (15 days vs. 8 days, p = 0.013) and fecal samples (20 days vs. 11 days, p < 0.001). |
| Liu et al. [27], 2020 | 57 | NRb | 137 | Retro, M | IV MP (30–80 mg/day) (n = 40) |
Variable | BS antibiotics (86.9%) Antivirals (76.6%) Human Ig (32.1%) |
Irrespective of the timing corticosteroids did not lead to any differences in outcome. |
Convalescent patients.
Bilateral lung involvement as per CT scan imaging criteria was noted in 116 patients (84.7%) of whom 36 (31%) were in early stage, 55 (47.4%) were in the middle stage and 25 (21.6%) were in the advanced stage; IFN-α- Interferon α; MP- Methylprednisolone; NR- Not reported specifically, BS- Broad spectrum, L/R- Lopinavir/Ritonavir, Ig- Immunoglobulin; AZ- Azithromycin; IV- Intravenous; Retro-Retrospective; S- Single center; M- Multicentric.
3.3.2. Studies in COVID-19 where corticosteroid was used to evaluate its outcome
Prior to the announcement of the preliminary results from the RECOVERY trial, notwithstanding the WHO and CDC contraindications, corticosteroids had been used globally up to as much as 50% of patients affected by COVID-19, particularly in China [14,25]. All these trials included patients with severe and critical COVID-19 patients with pulmonary involvement. Of the 5 studies (4 retrospective and 1 quasi-prospective study) conducted with corticosteroids, 3 studies have shown a benefit, while 2 studies shown no benefit, and there was a suggestion of significant harm especially in the critical cases in one sub-study (propensity-matched adjusted hazard ratio [HR] 2.90; 95% CI, 1.17–7.16; p = 0.021) [[28], [29], [30], [31], [32]]. A common pattern evolving from these retrospective trials suggests more benefit with low dose steroids compared to the high dose steroids. Moreover, judicious use of corticosteroids has been shown to improve several parameters of severe and critical COVID-19, including reduction of duration of hospital stay, prevention of worsening of the ventilator parameters, progression to ARDS, and death [30], quicker normalization of pyrexia and improvement in the status of oxygenation [31], reduced incidence of intubation and subsequent ventilation [32]. It should be noted that none of these retrospective and quasi-prospective trials evaluated the data in patients with mild COVID-19 and majority of the patients were receiving other repurposed drugs beside the absence of controlled arm.
RECOVERY is the only prospective study, conducted in 176 NHS hospitals, UK where 2104 patients were randomized to dexamethasone 6 mg per day (oral or intravenous) for 10 days and compared to the 4321 patients receiving usual care. 24% of the patients had diabetes, 27% had heart disease, 21% had chronic lung disease and at least 56% had one major comorbidity. 54% patients were younger than 70 years, 22% were between 70 and 80 years and 24% were more than 80 years of age. Significantly lesser number of patients of the dexamethasone cohort died within 28 days (primary outcome) compared to the standard of care cohort (21.6% vs. 24.6%; age-adjusted rate ratio [RR] 0.83; 95% CI, 0.74–0.92; p < 0.001). Evaluation at 28-days found a significant reduction in mortality by 35% amongst the invasive mechanical ventilation patients (29.0% vs. 40.7%; RR 0.65; 95% CI, 0.48–0.88; p = 0.003) and by 20% among the patients on supplemental oxygen therapy with or without noninvasive ventilation (21.5% vs. 25.0%; RR 0.80; 95% CI, 0.67–0.96; p = 0.0021), although no benefit was observed in mild or moderate cases, not requiring oxygen support (17.0% vs.13.2%; RR 1.22; 95% CI, 0.93–1.61; p = 0.14). This is significant in view of the fact that the overall COVID-19 mortality in UK is >26% and >37% patients require invasive ventilation. Secondary outcome of the study showed shorter duration of hospitalization with dexamethasone compared to standard of care (median 12 days vs. 13 days) and a 11% higher probability of discharge within 28 days (RR 1.11; 95% CI, 1.04–1.19; p = 0.002). Patients showing positive response to dexamethasone therapy started improving within the first 7 days of dexamethasone therapy [14].
Table 3 outlines the detailed results from studies conducted with corticosteroid in patients with COVID-19.
Table 3.
Studies in COVID -19 where corticosteroid was used to evaluated outcome.
| Author/Study, Year | Median Age (years) | N | Type of study (S/M) | Type of steroid and dose, (n) | Median day/time of steroid initiation after admission | Associated medical treatment | Treatment outcome |
|---|---|---|---|---|---|---|---|
| Wu et al. [28], 2020 | 61 | 1514 (severe) | Retro, M | Equivalent to 40 mg MP daily (n = 531, 35.1%) | Median Time 2.2 h, In 359 (67.6%) patients within first-24 h |
Not specified |
|
| 68 | 249 (critical) | Retro, M | Equivalent to 40 mg MP daily (n = 159, 63.1%) | Median time 0.6 h, In 127 (79.9%) patients within first-24 h |
Not specified |
|
|
| Lu et al. [29], 2020 | 62 | 244 | Retro, S | MP, Dexa and HC (dosage equivalent 100–800 mg of HC) (n = 151, 62%) |
Not specified | Oseltamivir, Arbidol, Ganciclovir, L/R, IFN α |
|
| Fadel et al. [30], 2020 | 61 | 213 (MP-132, SOC-81) |
Quasi-P, M | 0.5–1 mg/kg/day of MP IV in 2 divided dosage X 3–7 days (ICU), X 3 days (non -ICU), (n = 132, 62%) |
Median time to steroid initiation 2 days. Within first-48 h in majority (n = 65, 30.5%). Greater initiation of steroid in the post corticosteroid group within 48 h (12.4% vs. 41.7%, p < 0.001) |
L/R, Remdesivir, Ribavirin, HCQ |
|
| Wang et al. [31], 2020 | 54 | 46 | Retro, S | 1–2 mg/kg/day IV MP X 5–7 days (n = 26) | Within first-24 h | L/R, IFN-α, Thymosin |
|
| Choroboczek et al. [32], 2020 | 61 (mean) | 70 | Retro, S | Corticosteroids unspecified (n = 21, 30%) | At least 7 days after onset of symptoms | AZ (41%) HCQ (17%) L/R (7.5%) |
|
| RECOVERY Trial [14], 2020 |
<70 : 54% 70-80 : 22% >80 : 24% |
6425 | RCT, M |
Dexa 6 mg/day X 10 days (n = 2104) vs. no Dexa (n = 4321), Median duration of treatment 6 days. |
Within first-24 h | AZ (23%) (AZ 24% in control arm), Very few received HCQ, L/R |
|
Increased corticosteroids dosage associated with elevated mortality risk (P = 0.003) in matched cases after adjustment for duration of therapy; every 10 mg increase in HC dosage associated with 4% increase in mortality risk (adjusted HR: 1.04,95% CI: 1.01–1.07), MP- Methyl Prednisolone, Dexa- Dexamethasone, HC- Hydrocortisone, IFN-α- Interferon α, HCQ- Hydroxychloroquine, HR- Hazard ratio, RR- Rate ratio, aOR-adjusted Odd’s ratio, AZ- Azithromycin, SOC- Standard of care, O2- Oxygen therapy, L/R- Lopinavir/Ritonavir, Retro- Retrospective, S- Single center, M- Multicentric, P- Prospective, RCT- Randomized control trial.
4. Discussion
No specific treatment, so far, had been shown to definitely alter the mortality outcome in patients with COVID-19 barring dexamethasone. Multiple agents such as antivirals, hydroxychloroquine and azithromycin have been tried, but without any definitive outcome benefit [[33], [34], [35], [36], [37], [38]]. Studies with immunomodulatory agents like tocilizumab and other interleukin-6 (IL-6) blockers for countering the “cytokine storm” has not able to show any significant outcome benefit and has mostly resulted in adverse outcome [39,40]. Other agents like IV immunoglobulins have been found to be less useful in critically unwell patients and the recent multinational guideline by Surviving Sepsis Campaign has specifically advised against its use in critically ill patients [13]. It is in this context that corticosteroids have been tried in COVID-19.
Corticosteroids have primarily been thought about in COVID-19 as a means to stave off the ‘cytokine storm” and its consequences like ARDS, disseminated intravascular coagulation, hypotension, shock and death. As this usually happens in the first 5–7 days, ideally, steroid therapy should be tried in this period, particularly at the onset of dyspnea or even earlier to prevent the progression of the “cytokine storm” [2,30]. The anti-inflammatory properties of corticosteroids reduce systemic inflammation, exudative fluid in the lung tissue and also prevents the further diffuse alveolar damage, thereby improving the hypoxia and minimizes the risk of respiratory failure [41]. In most of the studies, independent risk factors associated with the risk of progression of ARDS to the death include older age, organ dysfunction and coagulopathy as manifested by the higher levels of lactate dehydrogenase (LDH) and d-dimer [24]. Most of the studies on use of corticosteroids in COVID-19 have shown variable results but that is principally because of marked heterogeneity in the methodology of studies. Some studies had evaluated the role of corticosteroids but not as per a definitive pre-adjudicated protocol. In the others, prospective evaluation of the corticosteroids was done as per protocol. The studies have also shown considerable variation in the timing of initiation of steroid treatment, type of the steroid and dosage of steroid.
The principal corticosteroids used in most of these studies and other ongoing trials have been methylprednisolone and dexamethasone because of their high bioavailability in the lungs. Methylprednisolone has least mineralocorticoid activity while dexamethasone possesses highest glucocorticoid activity. Theoretically, methylprednisolone has the advantage of parenteral administration, a quicker onset of action and a shorter duration of action compared to the dexamethasone [42]. This makes the risk of long-term side effects like fluid retention, hypokalemia, hypercortisolism and dysglycemia less likely with methylprednisolone. Though none of the studies make a head to head comparison in the outcome between the different types of corticosteroid preparations, the studies done with methylprednisolone, particularly in the low dose (0.5–2 μg/kg/day) had shown a good mortality and morbidity outcomes [24,30,31]. The limitations of most of the methylprednisolone studies is the small cohort size and the lack of follow up data, so far as the resolution of lung injury is concerned. However, the most robust data amongst corticosteroids came with dexamethasone in the RECOVERY trial, which showed the most significant mortality benefit with low dose dexamethasone. RECOVERY trial shown an impressive 35% mortality reduction among the sickest patients on invasive mechanical ventilation and a 20% reduction of mortality amongst patients on oxygen therapy (with or without noninvasive ventilation). In addition, patients on dexamethasone had a statistically significant reduction of hospital stay and an earlier likelihood of discharge [14].
It should be noted that majority of the studies with corticosteroid intervention were done in patients with serious or critically unwell patients, particularly those who required high flow nasal oxygen or ventilation. Studies on patients with mild COVID 19 did not show any significant benefit [14,43].
Another issue of considerable importance of using corticosteroids in COVID-19 is prolongation of the viral excretion from the body. Systemic corticosteroids by its immunosuppressant effect has been hypothesized to aggravate the viral load and prolong the viral excretion. However recent studies suggest that corticosteroids may have both a stimulatory as well as inhibitory effects on the immune response depending upon their timing and blood levels [44]. Corticosteroid use in previous viral respiratory illnesses have also demonstrated a delayed viral clearance [9,10,17]. Similar results have been shown in convalescent COVID-19 patients as well [26]. However, short course of corticosteroids in some reports have been shown to be beneficial and safe in critically ill patients with SARS-CoV-2 and not predispose to prolonged viral RNA shedding [11,45]. These discordant findings may be explained by the observational nature of the studies, variable patient acuity, timing of initiation and duration of therapy and variable dosing regimens. It is also true that unlike in SARS-CoV-1, where viral replication peaks in the second week of the disease, the peak viral shedding in COVID-19 appears to be quicker in onset and declines thereafter [[46], [47], [48]]. Steroid therapy has also been shown to increase the risk of secondary bacterial infection, for which adequate broad-spectrum antibiotics cover should be given.
4.1. Corticosteroid therapy in COVID 19- its sequalae
A potential sequelae of corticosteroid therapy is the worsening of dysglycemia/unmasking of latent diabetes. Corticosteroids produce increased lipolysis, increased hepatic glucose output and can increase the insulin resistance by up to 60–80% by directly interfering with the signaling cascade of the GLUT-4 receptors. This leads to a 30–50% reduction of insulin stimulated glucose uptake of the skeletal muscle cells, contributing to a postprandial hyperglycemia, as well as a 50–70% reduction of hepatic glycogenesis. Corticosteroids also produce breakdown of proteins and the resultant surge in amino acids also interferes with the insulin signaling of the muscle cells. Corticosteroids also have a direct inhibitory action on β cells. Lipotoxicity from the lipolysis can also have a similar effect on the β cells [49,50]. The effects of steroids are usually transient and reversible with the stoppage of the steroids. Of the corticosteroids used in COVID-19, methylprednisolone is a short/intermediate acting glucocorticoid of 4–6 h duration, while dexamethasone is a long acting steroid with steroid induced hyperglycemia lasting for more than 24 h after the last dose, with a minimal fall after an overnight fast. Recent data suggests that the impact is maximum when steroid is administered acutely, but a spontaneous remission usually happens [51]. With a short course of steroids in most of the COVID-19 trials (10-days of dexamethasone in RECOVERY, 3–7 days by other trials with methylprednisolone), it is less likely that steroid induced hyperglycemia or worsening of glycemic control in preexisting diabetes contributed in any significant way given the fact that in RECOVERY trial both dexamethasone and usual care arm had equal percentage (nearly 25%) of patients with diabetes. Nevertheless, all treating physician must be careful about the expected worsening of hyperglycemia with the use of corticosteroids in patients with COVID-19 and should take all corrective measures to tackle it.
In view of the shorter follow-up experience with the survivors of acute lung injury/ARDS patients, other side effects of steroid therapy namely bone changes like avascular necrosis of the femoral head and suppression of the hypothalamic –pituitary-adrenal (HPA) axis have not been evaluated extensively in COVID-19. As with other critical illness, COVID-19 has also been shown to worsen adrenal insufficiency, particularly in those with pre-existing adrenal hypofunction [6]. HPA axis suppression is also expected in critical COVID-19 patients and corticosteroid replacement is expected to be beneficial in this situation [13].
5. Conclusion
Although the results from retrospective studies are not strongly supportive of corticosteroid use in COVID-19 despite the signals for some benefits, the dedicated RECOVERY trial found a significant reduction in death with dexamethasone only in severe case on ventilator or moderate case on supplemental oxygen therapy but no benefit observed in mild to moderate case requiring no oxygen. More studies are still necessary to substantiate conclusive benefit with corticosteroid in COVID-19.
Contribution of authors
AKS conceptualized; AKS and RS did a systematic review; SM prepared the first draft; SM and AKS drafted and edited the tables and figures; AKS, RS, SM and AM revised the manuscript.
Funding
None.
Declaration of competing interest
We hereby declare that we have no conflict of interest, related to this article.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the corona virus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [Google Scholar]
- 2.Guan W., Ni Z., Wu H., Liang W. Clinical characteristics of corona virus disease 2019 in China. N Engl J Med. March 2020 doi: 10.1056/NEJMoa2002032. [DOI] [Google Scholar]
- 3.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney R.M., McAuley D.F. Acute respiratory distress syndrome. Lancet. 2016;388(10058):2416–2430. doi: 10.1016/s0140-6736(16)00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isidori A.M., Arnaldi G., Boscaro M., Falorni A., Giordano R. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest. April 2020 doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 9.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected.
- 12.The Centers for Diseases Control and Prevention, CDC, USA Covid-19 treatment guidelines. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf9
- 13.Alhazzani W., Moller M., Arabi Y.M., Loeb M., Gong M.N., Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Landary M.J. Effect of dexamethasone in hospitalized patients with COVID-19 – preliminary report. medRxiv. June 22,2020 doi: 10.1101/2020.06.22.20137273. preprint. [DOI] [Google Scholar]
- 15.Villar J., Ferrando C., Martinez D. On behalf of Dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomized controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 16.Gu Meduri, Annane D., Chrousos G.P., Marik P.E., Sinclair S.E. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 17.Chen R., Tang X., Tan S. Treatment of severe acute respiratory syndrome with glucosteroids. The Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew T.W.K., Kwek T., Tai D., Earnest A., Loo S. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. J Am Med Assoc. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z., Zhang F., Xu M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- 21.Griffith J.F., Antonio G.E., Kumta S.M. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235:168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.M., Wang S.X., Gao H.S. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence. Natl Med J China (Peking) 2004;84:1348–1353. [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. March 13, 2020 doi: 10.1001/jamainternmed.2020.0994. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel corona virus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling Y., Xu S., Lin Y., Zhu Z. Persistence and clearance of viral RNA in 2019 novel corona virus disease rehabilitation patients Chinese. Med J. 2020;133(9) doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Fang Y., Liu W., Wang M.F. Clinical characteristics of novel corona virus cases in tertiary hospitals in Hubei Province. Chinese Med J. 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Huang J, Zhu G, Liu Y et al. Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study, medRxiv preprint doi: 10.1101/2020.05.11.20097709. [DOI]
- 29.Lu X, Chen T, Wang Y, Wang J, et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19, medRxiv preprint doi: 10.1101/2020.04.07.20056390[not certified by peer review]. [DOI] [PMC free article] [PubMed]
- 30.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P et al. Early short course corticosteroids in hospitalized patients with COVID-19. medRxiv preprint doi: 10.1101/2020.05.04.20074609(not certified by peer review). [DOI] [PMC free article] [PubMed]
- 31.Wang Y, Jiang W, He Q, Liu B, Dong N, et al. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China, medRxiv preprint doi: 10.1101/2020.03.06.20032342. [DOI]
- 32.Chorobozcek T, Lacoste M, Wackenheim C, Challan Belval T, et al., Beneficial effect of corticosteroids in severe COVID-19 pneumonia: a propensity score matching analysis. medRxiv preprint doi: 10.1101/2020.05.08.20094755[not certified by peer review]. [DOI]
- 33.No benefit with hydroxychloroquine in RECOVERY trial. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19 (Accessed on June 20, 2020)
- 34.NIH halts clinical trial of hydroxychloroquine. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine
- 35.Hydroxychloroquine arm of Solidarity trial stopped. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- 36.Singh A.K., Singh A., Singh R., Misra A. Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020 May 12;14(4):589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report. Chest. 2020 Apr 25;S0012–3692(20):30764–30769. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roumier M., Paule R., Groh M., Vallee A., Ackermann F. Interleukin-6 blockade for severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.04.20.20061861. [DOI] [Google Scholar]
- 41.Rhen T., Cidlowski J.A. Anti-inflammatory action of corticosteroid--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 42.Zaroob J., Cender D. A different look at corticosteroids. Am Fam Physician. 1998 Aug 1;58(2):443–450. [PubMed] [Google Scholar]
- 43.Zha L., Li S., Pan L. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrousos G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/nejm199505183322008. [DOI] [PubMed] [Google Scholar]
- 45.Xu K., Chen Y., Yhan J. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020:ciaa351. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng P.K., Wong D.A., Tong L.K. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 48.To K.K., Tsang O.T., Leung W.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez A., Jansen-Chaparro S., Saigi I., Bernal-Lopez M.R., Miñambres I., Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6:9–20. doi: 10.1111/1753-0407.12090. [DOI] [PubMed] [Google Scholar]
- 50.Tames-Perez H.E., Quintannila-Flores D.L., Rodríguez-Gutiérrez R., González-González J.G., Tamez-Peña A.L. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015 July 25;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukins M.B., Manninen P.H. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100:1129–1133. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]