Abstract
Hemoglobin-vesicles (Hb-V) are being developed as red blood cell (RBC) substitutes. In this study, we report on quantitative and qualitative alterations of hepatic cytochrome P450 (CYPs) and the pharmacokinetics of CYP-metabolizing drugs, with a focus on four CYP isoforms (CYP1A2, CYP2C11, CYP2E1 and CYP3A2), after Hb-V resuscitation from a massive hemorrhage. The results of proteome analysis and western blot data indicate that resuscitation with both Hb-V and packed RBC (PRBC) resulted in a decrease in the protein levels of CYPs. Along with a decrease in the protein expression of CYPs, pharmacokinetic studies showed that the elimination of CYP-metabolizing drugs was prolonged in the Hb-V and PRBC resuscitation groups. It is also noteworthy that the CYP-metabolizing drugs in the Hb-V resuscitation group was retained for a longer period compared to the PRBC resuscitation group, and this is attributed to the CYP isoforms having a lower metabolic activity in the Hb-V resuscitation group than that for the PRBC resuscitation group. These findings suggest that resuscitation with Hb-V after a massive hemorrhage has a slight but not clinically significant effect on drug metabolism via CYPs in the liver due to decreased protein levels and the metabolic activity with respect to the CYPs.
Keywords: Hemoglobin, Artificial blood, Red blood cell, Cytochrome P450, Drug-drug interaction, Proteomics, Hemorrhage
Graphical abstract
1. Introduction
Transfusion of red blood cells (RBC) are the primary intervention in the treatment of patients with massive hemorrhages and this has contributed to saving thousands of human lives world-wide, each year. However, several social and medicinal issues have surfaced concerning such transfusions, such as a decrease in the amounts of donated blood due to low birth rates, viral contamination and short storage life of RBC, make it difficult to assure a stable supply and the safety of RBC preparations. Furthermore, an international and a national pandemic emergency, such as the appearance of a novel virus such as COVID-19, would likely interfere with the current hospital-based transfusion service [1]. To compensate for these problems, attempts have been made to develop artificial RBC for more than a half century [2].
Hemoglobin-vesicles (Hb-V) are a cellular type of artificial RBC, in which human hemoglobin is encapsulated within a lipid bilayer (liposome) the surfaces of which are modified with polyethylene glycol (PEG) [3]. Accumulated preclinical evidence indicate that Hb-V has favorable characteristics that would allow it to be used as an artificial RBC preparation, as follows: (i) there is no risk of viral contamination and no need for blood typing because viruses and blood group antigens are completely removed during the preparation process [3]. (ii) Hb-V can be preserved for over 2 years at room temperature [4,5]. (iii) The oxygen carrying capacity of Hb-V is comparable to that for RBC [[6], [7], [8]]. (iv) Hb-V shows no serious adverse effects and good pharmacokinetic properties (no bioaccumulation) [[9], [10], [11]]. Based on the above evidence, we are now in the preparation to start clinical studies of Hb-V with the goal of realizing a stable supply of an RBC preparation that can be used safely.
Assuming that Hb-V would be administered to patients with massive hemorrhages, they would also be likely to be receiving one or more additional medications, such as narcotic, anti-arrhythmic, analgesic, anti-seizure and steroid medication, in response to their systemic condition. About three-quarts of prescribed drugs that are cleared via metabolism are metabolized by cytochrome P450 (CYP) [12,13], while Hb-V is mainly metabolized by Kupffer cells in the liver [14]. Because of this, risks associated with such drugs interacting with Hb-V have never been a concern. However, it was previously reported that the pharmacokinetics of CYP-metabolizing drugs, such as mephenytoin, chlorzoxazone, dapsone and flurbiprofen, are altered in injured patients who receiving RBC transfusions [15]. Furthermore, our previous studies showed that resuscitation from a massive hemorrhage by RBC was accompanied by a reduction in hepatic CYP protein expression, resulting in an increase in the plasma concentration of CYP-metabolizing drugs [[16], [17], [18]]. These facts lead us to the hypothesis that resuscitation from massive hemorrhage by Hb-V induced an alteration in hepatic CYP protein expression similar to that for a RBC transfusion, resulting in changes in the pharmacokinetics of concomitantly administered CYP-metabolizing drugs. Since an alteration in the plasma concentration of a drug sometimes leads to an insufficient curative effect and adverse events, accumulating meaningful evidence that clarifies the effects of Hb-V transfusion on the pharmacokinetics of co-administered CYP-metabolizing drugs after massive hemorrhage and resuscitation would be highly desirable.
The aim of this study was to investigate the influence of resuscitation from a massive hemorrhage by Hb-V on hepatic CYP and the in vivo pharmacokinetics of CYP-metabolizing drugs. For this purpose, we first quantitatively evaluated the protein expression of four CYP isoforms, CYP1A2, CYP2C11, CYP2E1 and CYP3A2, which are homologized to human CYP1A2, CYP2C9, CYP2E1 and CYP3A4, respectively, in sham rats and hemorrhagic shock model rats resuscitated with Hb-V and packed RBC (PRBC). Changes in the plasma concentrations of the above four CYP-metabolizing drugs were then evaluated in sham rats and hemorrhagic shock model rats that were resuscitated by Hb-V and PRBC. Finally, the metabolic activities of the hepatic CYP isoforms after massive hemorrhage and resuscitation with Hb-V and PRBC were also evaluated.
2. Materials and methods
2.1. Animals and ethics
All Sprague-Dawley rats (male, 8 weeks of age or retired; Japan SLC, Inc) were housed in a conventional room with 12-hour light-dark cycles. All experiments conducted in this study were reviewed and approved by the institutional Animal Care and Use Committee (Approval #: 2015-P-026). The handling and care of the rats were done according to the National Institutes of Health guidelines. All surgical procedures for rats were performed under isoflurane anesthesia.
2.2. Preparation of PRBC and Hb-V solutions
PRBC suspended in saline ([Hemoglobin] = 10 g/dL) was prepared from whole blood collected from retired rats (n = 14) as reported previously [5]. Hb-Vs suspended in saline ([Hemoglobin] = 10 g/dL) were prepared as reported previously [7]. The lipid membrane of the Hb-V was composed with 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, cholesterol and 1,5-bis-O-hexadecyl-N-succinyl-L-glutamate (5/4/0.9 at a molar ratio) with 0.3 mol% of 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-PEG. The particle diameter of Hb-V was regulated to ca. 280 nm. Before the start of the experiments, the Hb-V and PRBC suspensions were mixed with human serum albumin (final concentration: 5 g/dL) to maintain the colloid osmotic pressure of the suspensions at around 20 torr [8].
2.3. Preparation of hemorrhagic shock model rats and resuscitation by PRBC and Hb-V
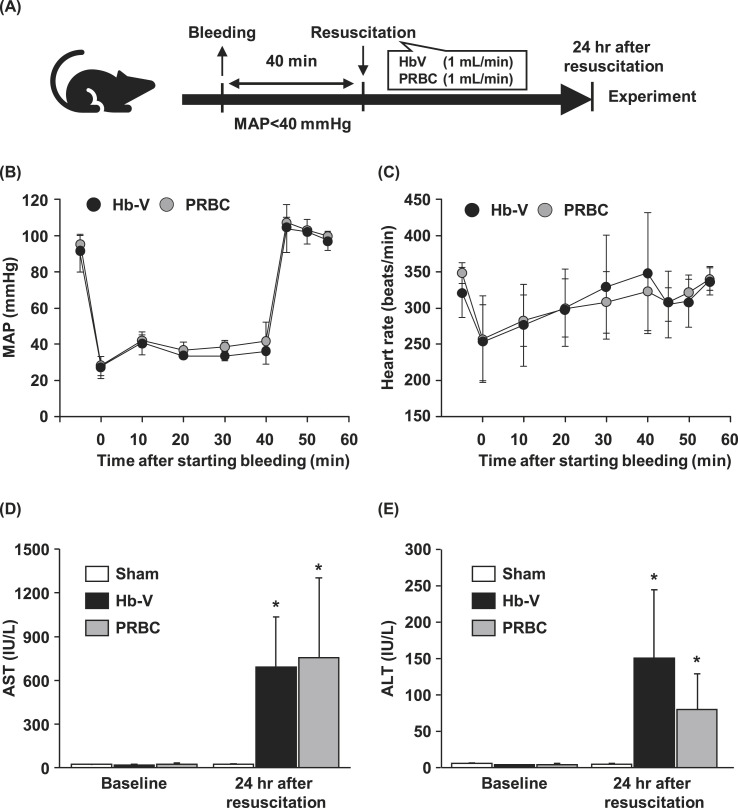
All rats (n = 36) were introduced with a polyethylene catheter (PE-50 tubing) into the right artery for monitoring the mean arterial pressure (MAP) and heart rate (HR) and into the femoral vein for bleeding and transfusion. Whole blood was removed from twenty-four rats at a rate of 1 mL/min using a syringe pump, and MAP was maintained at under 40 mmHg for 40 min (Fig. 1 A). At 40 min after the start of bleeding, the rats were resuscitated with equal amounts of either PRBC (n = 12) or Hb-V (n = 12) (1 mL/min). After resuscitation, MAP and HR values were monitored for 20 min, and the catheters were then removed. Sham rats (n = 12) were subjected to the same surgical procedures but without bleeding and any administration.
Fig. 1.
(A) Scheme showing the experimental procedure. Changes in (B) mean arterial pressure (MAP) and (C) heart rate after bleeding and resuscitation with Hb-V and PRBC. (D) Plasma aspartate aminotransferase (AST) and (E) alanine aminotransferase (ALT) levels in sham and hemorrhagic shock model rats at baseline (before hemorrhage) and at 24 h after resuscitation with Hb-V or PRBC.
Values are means ± SD. (n = 4) ∗p < 0.05 vs. baseline.
2.4. Clinical chemistry
Before bleeding (baseline) and at 24 h after resuscitation, blood samples were collected from the jugular vein, and plasma was then obtained by means of centrifugation (3,000 g, 10 min). Hb-V in the plasma were then removed by means of ultracentrifugation (50,000 g, 30 min) due to interference with some of clinical chemistries [19]. All samples were stored at −80 °C until used in an analysis. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were analyzed using Alanine Aminotransferase Activity Assay Kit (Fujifilm Wako Pure Chemical Corp.).
2.5. Quantitative proteomic analysis of hepatic CYP protein expression
At 24 h after resuscitation, the livers were collected after perfusion with phosphate-buffered saline (PBS) (n = 3/each group), and homogenized on ice in buffer (Tris-HCl (10 mM, pH 7.4), 1.25 mM phenylmethylsulfonyl fluoride, 1.5 mM magnesium chloride, 10 mM sodium chloride, 1 v/v% protease inhibitor cocktail). The homogenized liver preparations were centrifuged (10,000 g, 4 °C) for 10 min and the supernatant was then further ultracentrifuged (100,000 g, 4 °C) for 1 h. After suspending the precipitates in Tris-HCl (10 mM, pH 7.4) containing 250 mM sucrose, the protein content in the samples was measured by a BCA protein assay kit. The samples were analyzed by means of quantitative proteomics, as previously described [20]. The analysis system consisted of a TripleTOF® 5600 system (SCIEX) and a DIONEX Ultimate™ 3000 RSLCnano system (Thermo Scientific). Target peptides were identified by Protein Pilot (SCIEX) with MS/MS data from information-dependent acquisition. Targeted peptide peaks extracted from the SWAHT-MS data by the Peak View Software (SCIEX). Protein peak areas were calculated as the sum of the peak area of unique peptides for each protein.
2.6. Western blot
At 24 h after resuscitation, the livers were collected after perfusion with PBS (n = 3/each group), and homogenized on ice in buffer (Tris-HCl (10 mM, pH 6.8), 1.25 mM phenylmethylsulfonyl fluoride, 1.5 mM magnesium chloride, 10 mM sodium chloride, 1 v/v% protease inhibitor cocktail). The homogenized liver preparations were centrifuged (10,000 g, 4 °C) for 10 min and the supernatant was then further ultracentrifuged (100,000 g, 4 °C) for 1 h. After suspending the precipitates in Tris-HCl (10 mM, pH 7.4) containing 250 mM sucrose, the protein content in the samples was measured by a BCA protein assay kit. Each sample was suspended in loading buffer (Tris-HCl (150 mM, pH 6.8), 6% sodium dodecyl sulfate, 30% glycerol, 300 mM dithiothreitol, 0.03% bromophenol blue) and boiled at 100 °C for 3 min. The protein samples were separated by SDS-PAGE on a 10% polyacrylamide gel and transferred to PVDF membranes. After being blocked with 5% skim milk, the membranes were incubated with primary antibodies specific against each CYP isoform or β-actin (Table S1) in TBS-T (Tris-buffered saline, 0.1% Tween 20). Followed by three washings with TBS-T, the membranes were further incubated with secondary antibodies (Table S1). Finally, the bands on the membrane were visualized, and their intensities were analyzed using ImageJ (http://rsbweb.nih.gov/ij/).
2.7. Pharmacokinetic experiments
The pharmacokinetic experiments of substrates for the CYP isoforms were performed by the administration of a CYP cocktail as previously reported by Ogaki et al. with minor modification [18]. In a typical experiment, at 24 h after resuscitation (n = 4 each time), the CYP cocktail (10 mg/mL caffeine, 5 mg/mL tolbutamide, 10 mg/mL chlorzoxazone and 15 mg/mL midazolam) was intravenously administered to rats via the tail vein at a dose of 2 mL/kg. At 10 time points after the administration of the CYP cocktail (5, 15, 30 and 45 min and 1, 1.5, 2, 3, 5, 8 h), blood samples (150 μL) were collected from the jugular vein, and then centrifuged (3,000 g, 10 min, 4 °C) to obtain plasma. The concentration of each drug was simultaneously measured by high-performance liquid chromatography (HPLC) as previously reported with minor modifications [21]. The HPLC system consisted of a Hitachi L-2300 (set at 40 °C), Hitachi L-2130 (flow rate: 0.8 mL/min), Hitachi L-2400 UV detector (fixed at 230 nm) and YMC-Pack ODS-AM (5 μm particles, 4.6 mm ID × 250 mm) (YMC). The linear gradient elution of the solvents (sodium phosphate (50 mM, pH 3.4) and methanol (MeOH)) was programmed for the quantitation as follows: 0–5 min: 30% MeOH, 5–6 min: 30%–65% MeOH, 6–19 min: 65% MeOH, 19–20 min: 65-30% MeOH, and 20–28 min: 30% MeOH.
2.8. CYP metabolic activity
At 24 h after resuscitation, the livers were collected after perfusion with PBS (n = 5/each group), and homogenized on ice in a buffer (Tris-HCl (10 mM, pH 7.4), 1 mM EDTA and 250 mM sucrose). The homogenized liver preparations were centrifuged (10,000 g, 4 °C) for 10 min, and the supernatant was then further ultracentrifuged (100,000 g, 4 °C) for 1 h. After suspending the precipitates in Tris-HCl (10 mM, pH 7.4) containing 250 mM sucrose, the protein content in the samples was measured by BCA protein assay kit. CYP metabolic activities of each isoform were assessed by the formation of acetaminophen, 4-hydroxytolbutamide, 6-hydroxychlorzoxazone and 6β-hydroxytestosterone from phenacetin (CYP1A2), tolbutamide (CYP2C11), chlorzoxazone (CYP2E1) and testosterone (CYP3A2), respectively, as previously reported by Sun et al. [22] with minor modifications. In a typical experiment, each substrate was mixed with microsomal protein in Tris-HCl (50 mM, pH 7.4) containing a NADPH-regenerating system (NADP+, magnesium chloride, glucose-6-phosphate, glucose-6-phosphate dehydrogenase). After incubation for 30 min (phenacetin, chlorzoxazone and testosterone) or for 60 min (tolbutamide) at 37 °C, the reactions were stopped by the addition of ice-cold acetonitrile (ACN) containing an internal standard (metacetamol for CYP1A2, chlorpropamide for CYP2C11, and phenacetin for CYP2E1 and CYP3A2) and then centrifuged (13,000 g, 12 min). The supernatant was mixed with ethyl acetate using an Intelli-mixer for 30 min. After centrifugation (8,000 g, 8 min, 25 °C), the ethyl acetate layer was collected and evaporated to dryness. The residue was dissolved in each mobile phase for the HPLC analyses. The configuration of the HPLC system was the same as described in “2.7. Pharmacokinetic experiments” section. The linear gradient elution of the solvents (A: sodium phosphate (50 mM, pH 3.4), B: ACN) and flow rate were programmed for the quantitation of acetaminophen, 6-hydroxychlorzoxazone and 6β-hydroxytestosterone as follows: (acetaminophen; 0–5 min: 10% ACN, 5–8 min: 10%–40% ACN, 8–14 min: 40% ACN, 14–15 min: 40%–10% ACN, and 13–21 min: 10% ACN, 1 mL/min), (6-hydroxychlorzoxazone; 0–5 min: 20% ACN, 5–9 min: 20–40% ACN, 9–14 min: 40%–20% ACN, and 14–20 min: 20% ACN, 1 mL/min), and (6β-hydroxytestosterone: 0–2 min: 25% ACN, 2–10 min: 25–50% ACN, 10–15 min: 50% ACN, and 15–16 min: 50–25% ACN, 1 mL/min). For the quantitation of 4-hydroxytolbutamide, the mobile phase was composed of sodium phosphate (50 mM, pH 3.4) and ACN (60:40, vol/vol) with a flow rate of 1 mL/min. The UV detector wavelength for the acetaminophen, 4-hydroxytolbutamide, 6-hydroxychlorzoxazone and 6β-hydroxytestosterone analysis was fixed at 245 nm, 230 nm, 287 nm and 245 nm, respectively.
2.9. Statistics analysis
Data are expressed as the mean ± standard deviation (SD). Differences compared among the groups were determined by ANOVA followed by the Bonferroni method. Comparisons of two measures between before and after procedures were performed by paired t-test. Differences were considered to be significant when the value of p < 0.05. Pharmacokinetic parameters were calculated using the moment analysis program developed by Tabata et al. [23].
3. Results
3.1. Hemodynamics and hepatic injury after PRBC and Hb-V resuscitation
The MAP baseline was around 95 mmHg. MAP decreased to around 30 mmHg immediately after the start of bleeding and was maintained under 40 mmHg during the removal and infusing of blood (Fig. 1B). HR was decreased from 330 beats/min (baseline) to 250 beats/min by bleeding, but gradually increased to the baseline level during hypotension (Fig. 1C). After resuscitation by PRBC and Hb-V, the MAP value recovered to the baseline, while the HR was temporary decreased. All hemorrhagic shock model rats resuscitated by PRBC and Hb-V survived until they were used for further experiments. There was no significant difference in total bleeding volume between the PRBC and Hb-V resuscitation groups (PRBC group: 34.7 ± 2.1 mL/kg, Hb-V group: 34.2 ± 1.8 mL/kg). The AST and ALT levels were significantly increased by massive hemorrhage and resuscitation with Hb-V and PRBC compared to baseline (Fig. 1D and E). No significant difference in any of the hemodynamics and clinical chemistries were found between the Hb-V and PRBC resuscitation group.
3.2. Hepatic CYP protein expression after PRBC and Hb-V resuscitation
As a result of the quantitative evaluation of the protein expression of the hepatic CYP1A2, CYP2C11, CYP2E1, and CYP3A2 by quantitative proteomics, the hepatic protein expressions of CYP1A2 and CYP2E1 in both the PRBC and Hb-V resuscitation groups were decreased by 2-fold, while that for CYP2C11 and CYP3A2 were decreased slightly (<1.25-fold) compared to that in the sham group (Table 1 ). Furthermore, the protein expressions of these four CYP isoforms were also quantitatively evaluated by means of Western blotting. The results showed that all target CYP isoforms in the Hb-V and PRBC resuscitation groups were decreased compared to those in the sham group (Fig. 2).
Table 1.
Fold changes in protein expression levels of hepatic CYP isoforms (CYP1A2, CYP2C11, CYP2E1, CYP3A2) in hemorrhagic model rats at 24 h after resuscitation with Hb-V or PRBC in comparison with sham rats, as determined by quantitative proteomics.
| PRBC/sham | Hb-V/sham | |
|---|---|---|
| CYP1A2 | 0.47 ± 0.07 | 0.53 ± 0.16 |
| CYP2C11 | 0.77 ± 0.23 | 0.88 ± 0.19 |
| CYP2E1 | 0.44 ± 0.33 | 0.40 ± 0.05 |
| CYP3A2 | 0.89 ± 0.25 | 0.81 ± 0.23 |
The values are means ± SD; n = 3 each group.
Fig. 2.
Levels of protein expression of hepatic CYP isoforms ((A) CYP1A2, (B) CYP2C11, (C) CYP2E1 and (D) CYP3A2) in sham and hemorrhagic shock rats at 24 h after resuscitation with Hb-V and PRBC. β-actin was used as a loading control. Each CYP isoform/β-actin in sham-treated group is arbitrarily set at 100% and data are expressed as the percentage of that. The values are means ± SD (n = 3). ∗p < 0.05, ∗∗p < 0.01 vs. sham.
3.3. Pharmacokinetics of CYP-metabolizing drugs at 24 h after PRBC and Hb-V resuscitation
As a result of administration of substrates for CYP isoforms (CYP1A2, CYP2C11, CYP2E1 and CYP3A2) at 24 h after Hb-V resuscitation, the plasma retentions of all four substrates were increased in the Hb-V resuscitation group compared to the sham group (Fig. 3 ). Accompanied with an increase in plasma concentration, pharmacokinetic parameters, such as the area under the blood concentration-time curve (AUCinf) and clearance (CLtot), of caffeine (CYP1A2), chlorzoxazone (CYP2E1) and midazolam (CYP3A2) in the Hb-V resuscitation group were significantly increased/decreased compared to the sham group (Table 2 ). On the other hand, the pharmacokinetic parameters for tolbutamide (CYP2C11) were not significantly changed but were slightly increased/decreased compared to the sham group. In the case of the PRBC resuscitation group, the plasma concentrations of the four substrates for the CYP isoforms were increased compared to that for the sham group, but these changes were not increased as much as those for Hb-V resuscitation group (Fig. 3). In addition, most of the pharmacokinetic parameters, except for midazolam, in the PRBC resuscitation group were significantly changed compared to those for the Hb-V resuscitation group (Table 2).
Fig. 3.
Plasma concentration profile of a CYP cocktail consisting of (A) caffeine, (B) tolbutamide, (C) chlorzoxazone and (D) midazolam in sham rats (open circle) and hemorrhagic shock model rats at 24 h after resuscitation with Hb-V (closed circle) and PRBC (gray circle).
The values are the means ± SD (n = 4).
Table 2.
Pharmacokinetic parameters of four CYP substrates in sham rats and hemorrhagic shock model rats at 24 h after resuscitation with PRBC and Hb-V.
| Sham | PRBC | Hb-V | |
|---|---|---|---|
| Caffeine (CYP 1A2) | |||
| t1/2 (hr) | 1.5 ± 0.2 | 2.1 ± 0.2# | 4.7 ± 1.7∗∗ |
| AUCinf (μg × hr/mL) | 14.1 ± 1.0 | 20.0 ± 2.1# | 37.0 ± 11.6∗∗ |
| MRT (hr) | 2.1 ± 0.3 | 3.0 ± 0.4# | 6.7 ± 2.4∗∗ |
| CLtot (mL/hr) | 355.8 ± 24.6 | 252.2 ± 28.4∗∗## | 146.2 ± 48.9∗∗ |
| Vd (mL) | 734.5 ± 68.5 | 756.4 ± 22.9# | 902.0 ± 70.0∗∗ |
| Tolbutamide (CYP 2C11) | |||
| t1/2 (hr) | 4.4 ± 0.2 | 5.0 ± 0.7# | 6.0 ± 0.3∗∗ |
| AUCinf (μg × hr/mL) | 173.8 ± 4.3 | 195.7 ± 30.8 | 184.3 ± 21.7 |
| MRT (hr) | 6.3 ± 0.2 | 7.2 ± 0.9# | 8.7 ± 0.5∗∗ |
| CLtot (mL/hr) | 14.4 ± 0.4 | 13 ± 2.2 | 13.7 ± 1.7 |
| Vd (mL) | 90.7 ± 5.1 | 92.9 ± 5.4## | 118.5 ± 9.1∗∗ |
| Chlorzoxazone (CYP 2E1) | |||
| t1/2 (hr) | 1.0 ± 0.2 | 1.3 ± 0.2# | 2.0 ± 0.3∗∗ |
| AUCinf (μg × hr/mL) | 28.5 ± 1.8 | 36.5 ± 6.2 | 41.4 ± 4.0∗∗ |
| MRT (hr) | 1.5 ± 0.3 | 1.9 ± 0.3# | 2.8 ± 0.5∗∗ |
| CLtot (mL/hr) | 175.8 ± 11.4 | 140.2 ± 23.9∗ | 121.5 ± 12.1∗∗ |
| Vd (mL) | 258 ± 32.9 | 255.3 ± 8.5## | 341.6 ± 37.6∗∗ |
| Midazolam (CYP 3A2) | |||
| t1/2 (hr) | 0.19 ± 0.04 | 0.18 ± 0.02 | 0.23 ± 0.03 |
| AUCinf (μg × hr/mL) | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.3∗ |
| MRT (hr) | 0.25 ± 0.04 | 0.23 ± 0.03 | 0.28 ± 0.05 |
| CLtot (L/hr) | 7.7 ± 0.4 | 5.8 ± 0.5∗ | 4.9 ± 1.2∗∗ |
| Vd (L) | 1.9 ± 0.4 | 1.3 ± 0.1∗ | 1.4 ± 0.2∗ |
t1/2, the half-life; AUCinf, area under the concentration-time curve; MRT, mean residence time; CLtot, clearance; Vd, distribution volume.
The values are means ± SD; n = 4 each group.
∗p < 0.05, ∗∗p < 0.01 vs. sham group, #p < 0.05, ##p < 0.01 vs. Hb-V group.
3.4. Hepatic CYP metabolic activity at 24 h after resuscitation with PRBC and Hb-V
The metabolic activities of hepatic CYP1A2, CYP2C11, CYP2E1 and CYP3A2 were evaluated using hepatic microsomal protein samples collected from sham rats or hemorrhagic shock rats that had been resuscitated with PRBC or Hb-V. At 24 h after resuscitation, the metabolic activity of all target CYP isoforms in the PRBC and Hb-V resuscitation groups were significantly decreased compared to that in the sham group (Fig. 4 ). Of note, the Hb-V resuscitation group showed much less metabolic activity than the PRBC group in all four CYP isoforms (Fig. 4).
Fig. 4.
Metabolic activities of (A) CYP1A2, (B) CYP2C11, (C) CYP2E1 and (D) CYP3A2 in the livers of sham rats and hemorrhagic shock rats at 24 h after resuscitation with Hb-V or PRBC.
The activity of each CYP isoform in the sham group is arbitrarily set at 100% and data are expressed as the percentage of that value. The values are means ± SD. (n = 5) ∗p < 0.05, ∗∗p < 0.01 vs. sham, #p < 0.05, ##p < 0.01 vs. PRBC.
4. Discussion
A hepatic ischemia-reperfusion injury is typically accompanied by reductions in quantitative (protein expression) and qualitative (metabolic activity) changes of hepatic CYPs, which occur after massive hemorrhage and transfusion with RBC in human and animal studies [15,18]. In this study, the results of quantitative proteomic analyses and Western blot showed that the protein expression of the CYP isoforms (CYP1A2, CYP2C11, CYP2E1 and CYP3A2) were decreased in hemorrhagic shock rats that were resuscitated by PRBC and Hb-V compared to sham rats (Fig. 2 and Table 1). In addition to these four CYP isoforms, proteomic analyses showed that 10 other CYP isoforms (CYP2A2, CYP2B3, CYP2C23, CYP2C6, CYP2C7, CYP2D1, CYP2D4, CYP2D26, CYP4F1 and CYP4F4) in the liver were also decreased in the PRBC and Hb-V resuscitation groups compared to the sham group (Table S2). It should be noted that the magnitudes of these reductions were not significantly different between the PRBC and Hb-V resuscitation groups. It was previously reported that a reduction of hepatic CYP occurred after a massive hemorrhage and transfusion with RBC in the case of an hepatic ischemia-reperfusion injury induced by reactive oxygen species (ROS) generated by oxygen from the RBC [16,24]. In this study, the AST and ALT levels were observed to be elevated after transfusion with PRBC and Hb-V (Fig. 1D and E), leading to the induction of a hepatic ischemia-reperfusion injury. Furthermore, the hepatic accumulation of nitrotyrosine, a type of oxidative product, was reported in hemorrhagic shock model rats that had been resuscitated with both Hb-V and PRBC by virtue of the fact that the oxygen supply capacity of Hb-V was comparable to that for RBC [3,25]. Therefore, hepatic ischemia-reperfusion injury induced by ROS that were generated by oxygen supplied from Hb-V to hypoxic livers would be attributed to a reduction in the protein expression of hepatic CYPs. In addition, it is also known that inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, cause a decrease in CYP expression resulting in the suppression of CYP genes [26]. Since the levels of some systemic cytokines, including tumor necrosis factor-α, were elevated at 1 h after the Hb-V resuscitation from hemorrhagic shock [8], such inflammatory cytokines may be associated with the reduction in the protein expression of CYPs after massive hemorrhage and Hb-V transfusion.
Members of the CYP family play a crucial role in drug metabolism, especially the first phase reaction of drug metabolism, and changes in drug metabolism via these enzymes for assorted reasons, such as degradation, inhibition and induction, has sometimes caused approved drugs to be removed from the market due to the induction of severe adverse effects [13,27]. Hence, the influence of the reduction in the protein expression of CYPs after Hb-V and PRBC transfusion on the pharmacokinetic alteration in CYPs-metabolizing drugs is a serious concern. Accompanied with the decrease in the protein expression of CYPs after Hb-V and PRBC resuscitation, the blood retention of the substrates for four CYP isoforms (CYP1A2, CYP2C11, CYP2E1 and CYP3A2) were increased in hemorrhagic shock model rats at 24 h after Hb-V and PRBC resuscitation compared to sham rats (Fig. 3, Table 2). It was previously reported that a hepatic ischemia-reperfusion injury induced a decrease in hepatic CYP metabolic activity [28,29]. Since Hb-V and PRBC transfusion induced a hepatic ischemia-reperfusion injury in this study (Fig. 1C and D), a decrease in CYP metabolic activity would have pathophysiologically occurred as the result of the Hb-V transfusion as well as the PRBC transfusion (Fig. 4). Interestingly, the Hb-V transfusion showed a higher blood retention of CYP-metabolizing drugs with the CYP isoforms having a lower metabolic activity than the PRBC transfusion (Fig. 3, Fig. 4 and Table 2). This is thought to be causally related to the lipid composition of the Hb-V preparation. In a previous study, it was reported that the physicochemical properties of lipids, such as the degree of saturation of the lipid fatty acyl chain and non-bilayer forming lipids, regulated CYP metabolic activity [30,31]. A study of CYP-based drug-drug interactions with Hb-V in healthy rats also showed that Hb-V administration, but not PRBC administration, prolonged the blood retention of CYP-metabolized drugs, resulting from the inhibition of the metabolic activity of CYPs [32]. Taking these facts into consideration, Hb-V resuscitation from massive hemorrhage would be predicted to decrease CYP metabolic activity resulting from either a synergic or additive inhibition by the pathophysiological effects and lipid components of Hb-V.
It should be noted that the Hb-V has an inhibitory effect on the pharmacology of the concomitantly administered CYP-metabolizing drugs. In this study, the increase in AUCinf for caffeine (CYP1A2), tolbutamide (CYP2C11), chlorzoxazone (CYP2E1) and midazolam (CYP3A2) after Hb-V transfusion in hemorrhagic shock model rats were 2.64-, 1.06-, 1.45- and 1.6-fold, respectively, compared to sham rats (Table 2). According to the guidelines on drug interactions for drug development and the appropriate provision of information published by the Pharmaceutical and Medical Devices Agency (PMDA), inhibitors that increase the AUC of substitutes by more than 5-fold, ≥2-fold but <5-fold, and ≥1.25-fold but <2-fold after oral administration are considered to be a “strong inhibitor”, “moderate inhibitor”, and “weak inhibitor”, respectively [33]. It therefore appears that the Hb-V transfusion had only moderate effects on the inhibition of the drug metabolism of CYP1A2, and weakly or rarely inhibited the metabolism of CYP2C11, CYP2E1 and CYP3A2. These inhibitory rates for the drug metabolism of CYP isoforms were not significant difference between the Hb-V and PRBC transfusion, indicating that the same modern medication in the transfusion of RBC could also be applied to Hb-V transfusion.
In conclusion, resuscitation from massive hemorrhage by Hb-V transfusion caused quantitative (protein expression) and qualitative (metabolic activity) changes in the characteristics of each CYP isoform (CYP1A2, CYP2C11, CYP2E1 and CYP3A2) due to hepatic ischemia-reperfusion injury after resuscitation with Hb-V from a massive hemorrhage, leading to the prolonged retention of concomitantly administered CYP-metabolizing drugs in the blood. However, the clinical relevance of the extents of inhibition would not be expected to be clinically relevant. This is a first report to clarify the effect of Hb-V resuscitation from hemorrhagic shock on in vivo pharmacokinetics of concomitant drugs with a focus on CYPs. The data obtained in present study provides useful information regarding the risk of drug-drug interactions in developing Hb-V as an artificial RBC for clinical use.
Authorship contributions
Participated in research design: Tokuno, Taguchi, and Otagiri.
Hb-V and EV preparation: Sakai.
Conducted experiments: Tokuno, Taguchi, Ohtsuki and Yamasaki.
Performed data analysis: Tokuno.
Wrote or contributed to the writing of the manuscript: Taguchi and Otagiri.
Supervision: Otagiri.
Declaration of competing interest
All of authors have disclosed no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dmpk.2020.06.004.
Funding
This work was supported by the Japan Agency for Medical Research and Development (17lk0201034h0003) [to S, H and M, O.] and by grants-in-aid from the Nakatomi Foundation [to K, T.].
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Pagano M.B., Hess J.R., Tsang H.C., Staley E., Gernsheimer T., Sen N., et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 Novel Coronavirus (COVID-19) pandemic affecting Washington State. Transfusion. 2020;60:908–911. doi: 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 2.Jahr J.S., Guinn N.R., Lowery D.R., Shore-Lesserson L., Shander A. Blood substitutes and oxygen therapeutics: a review. Anesth Analg. 2019 doi: 10.1213/ANE.0000000000003957. [DOI] [PubMed] [Google Scholar]
- 3.Sakai H. Overview of potential clinical applications of hemoglobin vesicles (HbV) as artificial red cells, evidenced by preclinical studies of the academic research consortium. J Funct Biomater. 2017;8:10. doi: 10.3390/jfb8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai H., Tomiyama K.I., Sou K., Takeoka S., Tsuchida E. Poly(ethylene glycol)-conjugation and deoxygenation enable long-term preservation of hemoglobin-vesicles as oxygen carriers in a liquid state. Bioconjugate Chem. 2000;11:425–432. doi: 10.1021/bc990173h. [DOI] [PubMed] [Google Scholar]
- 5.Tokuno M., Taguchi K., Yamasaki K., Sakai H., Otagiri M. Long-term stored hemoglobin-vesicles, a cellular type of hemoglobin-based oxygen carrier, has resuscitative effects comparable to that for fresh red blood cells in a rat model with massive hemorrhage without post-transfusion lung injury. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seishi Y., Horinouchi H., Sakai H., Kobayashi K. Effect of the cellular-type artificial oxygen carrier hemoglobin vesicle as a resuscitative fluid for prehospital treatment: experiments in a rat uncontrolled hemorrhagic shock model. Shock. 2012;38:153–158. doi: 10.1097/SHK.0b013e31825ad7cf. [DOI] [PubMed] [Google Scholar]
- 7.Hagisawa K., Kinoshita M., Takikawa M., Takeoka S., Saitoh D., Seki S., et al. Combination therapy using fibrinogen γ-chain peptide-coated, ADP-encapsulated liposomes and hemoglobin vesicles for trauma-induced massive hemorrhage in thrombocytopenic rabbits. Transfusion. 2019;59:3186–3196. doi: 10.1111/trf.15427. [DOI] [PubMed] [Google Scholar]
- 8.Sakai H., Seishi Y., Obata Y., Takeoka S., Horinouichi H., Tsuchida E., et al. Fluid resuscitation with artificial oxygen carriers in hemorrhaged rats: profiles of hemoglobin-vesicle degradation and hematopoiesis for 14 days. Shock. 2009;31:192–200. doi: 10.1097/SHK.0b013e31817d4066. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi K., Maruyama T., Otagiri M. Pharmacokinetic properties of hemoglobin vesicles as a substitute for red blood cells. Drug Metab Rev. 2011;43:362–373. doi: 10.3109/03602532.2011.558094. [DOI] [PubMed] [Google Scholar]
- 10.Taguchi K., Miyasato M., Ujihira H., Watanabe H., Kadowaki D., Sakai H., et al. Hepatically-metabolized and -excreted artificial oxygen carrier, hemoglobin vesicles, can be safely used under conditions of hepatic impairment. Toxicol Appl Pharmacol. 2010;248:234–241. doi: 10.1016/j.taap.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi K., Nagao S., Yamasaki K., Sakai H., Seo H., Maruyama T., et al. Biological responsiveness and metabolic performance of liposome-encapsulated hemoglobin (hemoglobin-vesicles) in apolipoprotein E-deficient mice after massive intravenous injection. Biol Pharm Bull. 2015;38:1606–1616. doi: 10.1248/bpb.b15-00420. [DOI] [PubMed] [Google Scholar]
- 12.Williams J.A., Hyland R., Jones B.C., Smith D.A., Hurst S., Goosen T.C., et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUC 1/AUC) ratios. Drug Metab Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 13.Wienkers L.C., Heath T.G. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi K., Urata Y., Anraku M., Maruyama T., Watanabe H., Sakai H., et al. Pharmacokinetic study of enclosed hemoglobin and outer lipid component after the administration of hemoglobin vesicles as an artificial oxygen carrier. Drug Metab Dispos. 2009;37:1456–1463. doi: 10.1124/dmd.109.027094. [DOI] [PubMed] [Google Scholar]
- 15.Harbrecht B.G., Frye R.F., Zenati M.S., Branch R.A., Peitzman A.B. Cytochrome P-450 activity is differentially altered in severely injured patients. Crit Care Med. 2005;33:541–546. doi: 10.1097/01.ccm.0000155989.54344.e0. [DOI] [PubMed] [Google Scholar]
- 16.Ogaki S., Taguchi K., Watanabe H., Ishima Y., Otagiri M., Maruyama T. Carbon monoxide-bound red blood cell resuscitation ameliorates hepatic injury induced by massive hemorrhage and red blood cell resuscitation via hepatic cytochrome P450 protection in hemorrhagic shock rats. J Pharmaceut Sci. 2014;103:2199–2206. doi: 10.1002/jps.24029. [DOI] [PubMed] [Google Scholar]
- 17.Ogaki S., Taguchi K., Maeda H., Watanabe H., Ishima Y., Otagiri M., et al. Kupffer cell inactivation by carbon monoxide bound to red blood cells preserves hepatic cytochrome P450 via anti-oxidant and anti-inflammatory effects exerted through the HMGB1/TLR-4 pathway during resuscitation from hemorrhagic shock. Biochem Pharmacol. 2015;97:310–319. doi: 10.1016/j.bcp.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Ogaki S., Taguchi K., Watanabe H., Otagiri M., Maruyama T. Carbon monoxide-bound red blood cells protect red blood cell transfusion-induced hepatic cytochrome P450 impairment in hemorrhagic-shock rats. Drug Metab Dispos. 2013;41:141–148. doi: 10.1124/dmd.112.048744. [DOI] [PubMed] [Google Scholar]
- 19.Sakai H., Tomiyama K., Masada Y., Takeoka S., Horinouchi H., Kobayashi K., et al. Pretreatment of serum containing hemoglobin vesicles (oxygen carriers) to prevent their interference in laboratory tests. Clin Chem Lab Med. 2003;41:222–231. doi: 10.1515/CCLM.2003.036. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K., Hirayama-Kurogi M., Ito S., Kuno T., Yoneyama T., Obuchi W., et al. Large-scale multiplex absolute protein quantification of drug-metabolizing enzymes and transporters in human intestine, liver, and kidney microsomes by SWATH-MS: comparison with MRM/SRM and HR-MRM/PRM. Proteomics. 2016;16:2106–2117. doi: 10.1002/pmic.201500433. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Jiao J., Zhang C., Lou J. A simplified method to determine five cytochrome P450 probe drugs by HPLC in a single run. Biol Pharm Bull. 2009;32:717–720. doi: 10.1248/bpb.32.717. [DOI] [PubMed] [Google Scholar]
- 22.Sun M., Tang Y., Ding T., Liu M., Wang X. Inhibitory effects of celastrol on rat liver cytochrome P450 1A2, 2C11, 2D6, 2E1 and 3A2 activity. Fitoterapia. 2014;92:1–8. doi: 10.1016/j.fitote.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Tabata K., Yamaoka K., Kaibara A., Suzuki S., Terakawa M., Hata T. Moment analysis program available on Microsoft Excel. Drug Metabol Pharmacokinet. 1999;14:286–293. doi: 10.2133/dmpk.14.286. [DOI] [Google Scholar]
- 24.Lehnert M., Arteel G.E., Smutney O.M., Conzelmann L.O., Zhong Z., Thurman R.G., et al. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Sakai H., Horinouchi H., Tsuchida E., Kobayashi K. Hemoglobin vesicles and red blood cells as carriers of carbon monoxide prior to oxygen for resuscitation after hemorrhagic shock in a rat model. Shock. 2009;31:507–514. doi: 10.1097/SHK.0b013e318188f83d. [DOI] [PubMed] [Google Scholar]
- 26.Aitken A.E., Richardson T.A., Morgan E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 27.Lasser K.E., Allen P.D., Woolhandler S.J., Himmelstein D.U., Wolfe S.M., Bor D.H. Timing of new black box warnings and withdrawals for prescription medications. J Am Med Assoc. 2002;287:2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 28.Izuishi K., Wakabayashi H., Maeba T., Ryu M., Maeta H. Lidocaine-metabolizing activity after warm ischemia and reperfusion of the rat liver in vivo. World J Surg. 2000;24:49–52. doi: 10.1007/s002689910010. discussion 53. [DOI] [PubMed] [Google Scholar]
- 29.Eum H.-A., Lee S.-M. Effects of Trolox on the activity and gene expression of cytochrome P450 in hepatic ischemia/reperfusion. Br J Pharmacol. 2004;142:35–42. doi: 10.1038/sj.bjp.0705758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn T., Kim M., Yun C.-H., Chae H.-J. Functional regulation of hepatic cytochrome P450 enzymes by physicochemical properties of phospholipids in biological membranes. Curr Protein Pept Sci. 2007;8:496–505. doi: 10.2174/138920307782411392. [DOI] [PubMed] [Google Scholar]
- 31.Shinkyo R., Guengerich F.P. Inhibition of human cytochrome P450 3A4 by cholesterol. J Biol Chem. 2011;286:18426–18433. doi: 10.1074/jbc.M111.240457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuno M, Taguchi K, Sakai H, Ohtsuki S, Yamasaki K, Otagiri M. Assessing cytochrome P450-based drug-drug interactions with Hemoglobin-vesicles, an artificial red blood cell preparation, in healthy rats. Drug Metabol Pharmacokinet. 2020 doi: 10.1016/j.dmpk.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Pharmaceutical and Medical Devices Agency. Guideline on drug interaction for drug development and appropriate provision of information. [n.d]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.