Abstract
Objectives:
Migratory birds play a major role in the transmission of pathogens globally, but still their role in the transmission of fungi in Bangladesh is not known. The present study was carried out for the isolation and molecular detection of fungi including Aspergillus from migratory birds traveling to Bangladesh.
Materials and methods:
A total of 50 fecal samples were collected from BaojaniBaor, Magura, and areas close to Jahangirnagar University, Savar. The isolation of fungus was based on culture on Potato Dextrose Agar (PDA), followed by staining, morphology, and molecular detection using polymerase chain reaction (PCR).
Results:
Among 50 samples, 40 showed positive for fungal growth on PDA, of which 30 yield only yeast-like colonies, five only molds, and five yielded both yeast and molds. The isolated molds produced various pigmented colonies, namely, black, whitish, grayish, olive green, and yellow. Among 10 molds, six were confirmed as fungi by PCR using genus-specific primers such as ITS1 and ITS4. Later, of these six fungi, five were confirmed as Aspergillus by PCR with primers such as ASAP1 and ASAP2 specific for Aspergillus genus. Therefore, the overall occurrence of Aspergillus was 10% (5/50). PCR specific for Aspergillus fumigatus and Aspergillus niger failed to produce specific PCR amplicon, suggesting that the isolated Aspergillus belongs to other groups.
Conclusion:
This is the first report describing the isolation and molecular detection of Aspergillus from fecal samples of migratory birds in Bangladesh. The present findings confirm that migratory birds are potential source for Aspergillus and other fungus in Bangladesh.
Keywords: Migratory birds, yeast, mold, transmission, Aspergillus spp, PCR
Introduction
Bangladesh is known as a riverine country. Different types of water bodies including rivers, ponds, and lakes are spread like a net throughout the country, thus making it a suitable habitat for migratory birds [1]. Almost 176 types of migratory birds are known to visit Bangladesh over the year during the winter, summer, and spring [2]. These birds migrate to Bangladesh mainly for food and suitable habitats when they face life-threatening challenges from their original habitats [3,4].
Migratory birds play a vital role in the ecosystem by being excellent referential in circumstance of environment and performing services in the regulation of ecology and distribution of species [5,6]. Migratory birds have the potentiality to act as a carrier and thus transmit pathogens across the international borders, from one location to another [7] and may cross the host-species barrier leading to a notorious impact on the ecosystem [8]. These birds land mainly on water bodies and can deteriorate the water quality [9]. Water contamination depends on the size of the water body, density of birds, types of birds, season, and others [9]. The reports are available suggesting the involvement of migratory birds in the transmission of various types of viral, bacterial, or fungal diseases such as avian influenza virus, Newcastle disease virus, Duck plague virus, Salmonella spp., Mycobacterium avium, Chlamydophila psittaci, Escherichia coli, Aspergillus spp., Candida spp., and enterotoxin and verotoxin producers [10–14]. Among these, fungi transmitted by migratory birds have a tremendous ecological magnificence for their life strategies, morphological varieties, and significant interaction with biotic and abiotic ingredients of the environment [15,16]. In oligotrophic cold environments, fungi have efficiency to grow [17] and produce some enzymes [18] which are cold-active, notwithstanding that the ecological role of them cannot be adequately realized [19].
Aspergillus is ubiquitous and saprophytic [20,21]. Other characteristics of the members of Aspergillus genus encircle pigmented conidia, thermophilic nature, production of aflatoxin or gliotoxin, or ochratoxin or other mycotoxins which make these organisms more successful saprobes, opportunistic pathogens as well as primary pathogens [22]. The disease caused by Aspergillus is known as aspergillosis. It affects a wide range of species such as avian, mammals, a variety of animals from sea fans to elephants, and invertebrate species. Besides, birds show the best susceptibility to this disease leading to an ominous impact on economic sector of a country [23–26]. Among poultry, duck is directly associated with aquatic environment through searching for their food from water bodies. Thus, the existence of Aspergillus into the water bodies can affect the duck, and followed by these, the affected ducks can disseminate Aspergillus to other poultry including humans and animals through their feces and direct contact with them.
In avian host, aspergillosis can cause brooder pneumonia characterized by granulomatous deep nodular form in parenchyma and a pyogranulomatous noncapsulated superficial diffuse form in lungs and serosae [27,28]. It is a major cause of mortality in captive birds and, less frequently, in free-living birds. Predominantly, aspergillosis is a disease of the respiratory tract, and the other organs can be infected, resulting in a variety of illnesses ranging from acute to chronic infections. Poor immunity and inhalation of a considerable number of spores are major contributing factors to aspergillosis. In cattle, it causes bovine alimentary mycosis and mycotoxicosis such as tremorgenic neuromycotoxicosis [22]. Aspergillosis also has public health significance because of its zoonotic nature.
In Bangladesh, the poultry industry is a well-developed sector contributing to the fulfillment of human daily protein requirements through the supply of eggs and meat. However, from time to time, poultry industries suffer an economic loss, resulting from disease outbreak and mortality. Besides bacterial and viral pathogens, fungi are also known to cause production loss, augment mortality rate, and ultimately provoke economic loss [29].
Several studies have shown that people of Bangladesh have been affected by fungal infections such as blastomycosis, mucormycosis, renal aspergillosis, pulmonary aspergilloma, and cryptococcal meningitis [30–35]. Migratory birds could be a potential source for many of these fungi. To the best of authors’ knowledge, there is no report till now on the isolation of fungus such as Aspergillus spp. from migratory birds traveling to Bangladesh in the winter. This study was, therefore, designed to isolate and identify Aspergillus spp. from migratory birds using the cultural- and molecular-based approach for the first time in Bangladesh.
Materials and Methods
Ethical approval
The methodologies and related protocols used in this study were approved by the Institutional Ethical Committee (AWEEC/BAU/2019(14)).
Collection and processing of sample
A total of 50 fecal samples were collected from two locations, namely, areas close to Jahangirnagar University (n = 10), Savar (23.8818° N, 90.2625° E), and BaojaniBaor (n = 40), Magura (23.4855° N, 89.4198° E) during November 2018. All samples were aseptically collected individually in zipper bags using sterilized cotton buds and directly transported to the laboratory using an icebox. The collected feces samples were then taken into Falcon tubes (12 ml), followed by mixing with 10 ml of distilled water through vortexing, and the inoculum was prepared as previously described [36,37].
Culture of fungi
In sterile condition, inoculum prepared from feces samples was spread onto Potato Dextrose Agar (PDA) medium (HiMedia, India) with 0.05 mg/ml of chloramphenicol. Depending on growth rate, all plates were inoculated at 28°C–30°C for 3–7 days [38]. After incubation, both mold and yeast colony were observed. Considering molds, a small lump of fungal mycelia was picked up by a sterile toothpick, directly placed on PDA, and incubated at 28°C–30°C for 3–7 days to obtain pure cultures. The fungal isolates were then identified and diagnosed based on culture characteristics, the morphology of spores and hyphae, and microscopic characteristics [39]. The morphological characterization of fungus was done by slide culture and lactophenol cotton blue staining method as previously described [40].
Molecular detection of Aspergillus spp.
For molecular detection, genomic DNA was extracted from pure cultures using a mini-preparation procedure as previously described [41,42]. In brief, initially, 500 μl of lysis buffer was taken into a 1.5-ml Eppendorf tube (Thomas Scientific, Swedesboro, NJ), and a small lump of mycelia from pure culture was added by a sterilized toothpick followed by adding 150 μl of potassium acetate after keeping at room temperature for 10 min. Then, the tube was vortexed and centrifuged at ≥10,000 × gm for 1 min followed by transferring of supernatant into another Eppendorf tube and centrifuged again as described above. The supernatant was again transferred to another Eppendorf tube, and an equal volume of ice-cool isopropanol alcohol was added. After spinning at ≥10,000 × gm for 2 min, the supernatant was discarded, and the resultant pellet was washed in 300 μl of 70% ethyl alcohol. After the pellet was centrifuged at ≥10,000 × gm for 2 min, the supernatant was discarded, and the DNA pellet was air-dried and dissolved in 50 μl of deionized water. DNA from each tube was used as a template for polymerase chain reaction (PCR). The extracted DNA was stored at −20°C for further use.
The isolation of fungus was confirmed by PCR using fungus-specific universal primers (ITS1 and ITS4). This was followed by the detection of Aspergillus at the genus level by PCR primers such as ASAP1 and ASAP2 specific for the Aspergillus genus [43,44]. The list of primers used in this study is shown in Table 1. All the PCR protocols were done in a final 25-μl reaction with 12.5 μl of master mixture 2X (Promega, San Luis Obispo, CA), 2 μl of genomic DNA (around 30 ng), 1 μl (100 pmol) of each primer, and 8.5 μl of nuclease-free water.
Table 1. Primers used for the detection of fungus genus and Aspergillus spp.
At the end of the PCR, agarose gel (1.5%) electrophoresis was used to analyze the amplified PCR products. Electrophoresis was done at 100 V for 25 min in TAE buffer using the Mupid-One Electrophoresis Apparatus (Advance, Japan). Finally, ethidium bromide was used for staining the PCR products and ultraviolet transilluminator (Biometra, Germany) for visualization. DNA ladder of 100 bp (Promega, San Luis Obispo, CA) was used as a molecular weight marker.
Results and Discussion
Migratory birds are a potential reservoir and transmitter of pathogens. Aspergillosis is an important fungal disease of animals and birds. The studies have shown that migratory birds can play an essential role in the dissemination of yeasts and filamentous fungi during their migrations across the Mediterranean [4,45].
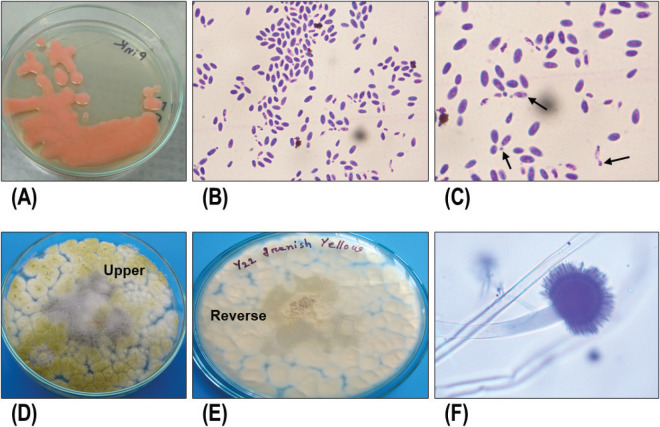
In this study, 50 fecal samples of migratory birds were cultured and screened for fungal growth. Among these, 40 were found positive for fungal growth on PDA. Although we focused on molds, specifically on Aspergillus, many analyzed fecal samples were found positive for yeast. The observed cultural and morphological characteristics of fungi are shown in Figure 1. Among 40 isolated fungi, 10 showed positive for molds, which produced typical fungal colonies and pigmentation, e.g., dark black (2/10), whitish (4/10), grayish (2/10), olive green (1/10), and yellow (1/10) colonies. The abilities of fungi including Aspergillus to produce such colonies and pigmentation have earlier been reported. Aspergillus niger and Aspergillus flavus can produce dark black and olive green colonies [46,47].
Figure 1. Cultural and morphological characteristics of fungi. (A) Growth of yeast on PDA, (B and C) microscopic morphology of yeasts, black arrows are buds, (D–F) cultural and microscopic structures of Aspergillus spp.
On location basis in BaojaniBaor, Magura, 33 out of 40 samples were found positive for fungal growth, of which seven samples showed positive for molds. On the other hand, seven of 10 samples were found positive for fungal growth and three of them showed positive for molds at Jahangirnagar University, Savar. The overall occurrence of fungal growth was 80%, whereas 20% for molds (Table 2). Very few studies had been conducted for the isolation and molecular identification of fungal agents from migratory birds. Abbas et al. [48] found that 88% of fecal samples of migratory birds showed positive for fungal growth. Earlier, Alfonzo et al. [4] and Francesca et al. [45] reported that 33%–61.89% of migratory bird samples showed positive for fungi. The observed variation in the occurrence rate of fungi with this study might be related to the different origins of migratory birds and travel routes that they follow.
Table 2. Occurrence of fungi isolated from fecal samples of migratory birds in BaojaniBaor, Magura, and Jahangirnagar University Jhill, Savar.
| Location | No. of samples | No. of fungus culture-positive sample | Types of fungus | Occurrence | ||
|---|---|---|---|---|---|---|
| Yeast | Mold | Both yeast and mold | ||||
| BaojaniBaor, Magura | 40 | 33 | 26 (65%) | 4 (10%) | 3 (7.5%) | 82.5% |
| Jahangirnagar University Jhill, Savar | 10 | 7 | 4 (40%) | 1 (10%) | 2 (20%) | 70% |
| Total | 50 | 40 | 30 | 5 | 5 | 80% |
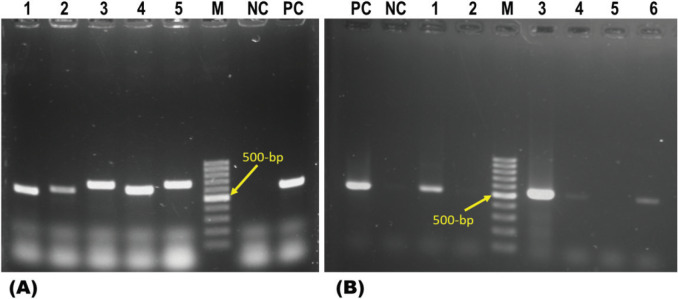
DNA extracted from these fungal cultures was PCR screened using fungal genus-specific primers such as ITS1 and ITS4 as recommended by Sugita et al. [44]. These fungus-specific universal primer pairs (ITS1 and ITS4) have also earlier been successfully used by others for the detection of fungi [43]. Among 10 samples, six were found positive for fungi by PCR (Fig. 2). All these six samples were further PCR screened using Aspergillus genus-specific primers such as ASAP1 and ASAP2. Five of these six samples were found positive, confirming them as Aspergillus spp. (Fig. 2 and Table 3). Attempts were made to detect Aspergillus at species level using primers such as FLA-1 and FLA-2 specific for A. flavus and primers such as Af3r and Nilr specific for A. niger, as previously used by Nazir et al. [42]. However, none of the DNA samples were found positive, suggesting that these were probably the other species of Aspergillus spp.
Figure 2. PCR-based detection of fungi and Aspergillus spp. (A) PCR-based detection of fungi using the primers such as ITS1 and ITS4. Lanes 1–5: representative fungus isolates, M = 100-bp DNA ladder (Promega, San Luis Obispo, CA), lane NC: negative control, lane PC: positive control. (B) PCR-based detection of Aspergillus spp. using the primers ASAP1 and ASAP2. Lanes 1–6: representative isolates from migratory birds, M = 100-bp DNA ladder (Promega, San Luis Obispo, CA), lane NC: negative control, lane PC: positive control.
Table 3. Occurrence of molds and Aspergillus using PCR in fecal samples of migratory birds in BaojaniBaor, Magura, and Jahangirnagar University Jhill, Savar.
| Sample locations | No. of samples | No. of positive samples for mold culture | No. of positive samples for mold by PCR | No. of positive samples for Aspergillus spp. by PCR | Overall occurrence (%) |
|---|---|---|---|---|---|
| BaojaniBaor, Magura | 40 | 7 | 4 | 3 | 10 |
| Jahangirnagar University Jhill, Savar | 10 | 3 | 2 | 2 | |
| Total | 50 | 10 | 6 | 5 |
Based on molecular detection, the overall occurrence of Aspergillus spp. was 10% (5/50) (Table 3). This occurrence rate is much lower than the available previous reports. Abbas et al. [48] detected that 15% of bird dropping were positive for Aspergillus spp., whereas Al-Temimay and Hasan [36] reported 28.24% of Aspergillus spp. These observed differences could be linked with different species of birds involved in these studies, geographic locations, and environmental parameters such as temperature and humidity.
Although Aspergillus and other fungus were isolated from migratory birds, this study has some limitations. First, only 50 samples were analyzed. It was simply due to difficulty to get access to samples from migratory birds without interfering with their natural habitat. Besides, the isolates were not identified at species level using primers other than primers FLA-1 and FLA-2. The pathogenicity and zoonotic potentiality of the isolates were not evaluated in this study, which need to be addressed in the future.
The present study revealed the association of aquatic environments and Aspergillus spp. disseminated by migratory birds. The organisms can be transmitted to humans, animals, and poultry from contaminated water in a diversified way. As people from Bangladesh reared ducks near such water bodies in free-ranging methods, they are directly linked with aquatic environments. Thus, they can be affected easily by Aspergillus. Some previous reports revealed the existence of Aspergillus infection in duck and duck-like birds worldwide [49–51]. Unfortunately, not enough data are available in the presence of Aspergillus spp. in ducks in Bangladesh. The robust study needs to reveal the exact association of ducks to disseminate Aspergillus spp.
Environmental sources – fecal materials can play a vital role in the dissemination of fungi. The occurrence of fungi, especially Aspergillus, found in the present study, suggesting that migratory birds could be the carriers and transmitters of fungal pathogens. Therefore, humans and poultry may get exposure to Aspergillus from these birds. As far we know, no published literature is available describing migratory birds as a source for the dissemination of fungal pathogens, especially Aspergillus, in Bangladesh. To the best of authors’ knowledge, this is the first study describing the molecular detection of Aspergillus from fecal materials of migratory birds traveling to Bangladesh.
Conclusions
Aspergillosis is an economically important disease in poultry. Aspergillosis is also zoonosis in nature. The detection of fungal pathogens, especially Aspergillus, from fecal samples of migratory birds traveling to Bangladesh in the winter has a significant impact on the poultry sector as well as on the ecosystem. In the context of this research, migratory birds need to be put under active surveillance for monitoring fungi and other pathogens that they are carrying from a far distance to ensure better health of animals, birds, and humans.
Acknowledgment
The authors sincerely acknowledge the Bangladesh Agricultural University Research System for funding (Project No. 2019/8/BAU) and also the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh, for providing NST fellowship to MA.
Conflict of interest
The authors declare that there is no conflict of interest in the publication of this article.
Authors’ contribution
MTR, SR, and KHMNHN designed the experiment; MSI, MAI and MSJ collected samples; MA, MAI, MSI, and MAS analyzed samples; MA, MSI, and MAS made the initial draft of the manuscript; and MTR and KHMNHN critically reviewed and updated the manuscript to this version. The final version of the manuscript was approved by all authors.
References
- [1].Claassen AH. World Wide Fund (WWF) for Nature. Phnom Penh, Cambodia: 2004. Abundance, distribution, and reproductive success of sandbar nesting birds below the Yali Falls hydropower dam on the Sesan River, Northeastern Cambodia; pp. 1–45. [Google Scholar]
- [2].Siddiqui KU, Islam MA, Kabir SM, Ahmad M, Ahmed AT, Rahman AK, et al. Vol. 26 Birds. Dhaka, Bangladesh: Asiatic Society of Bangladesh; 2008. Encyclopedia of flora and fauna of Bangladesh; pp. 1–662. [Google Scholar]
- [3].Zurell D, Graham CH, Gallien L, Thuiller W, Zimmermann NE. Long-distance migratory birds threatened by multiple independent risks from global change. Nat Clim Chang. 2018;8(11):992–6. doi: 10.1038/s41558-018-0312-9. https://doi.org/10.1038/s41558-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alfonzo A, Francesca N, Sannino C, Settanni L, Moschetti G. Filamentous fungi transported by birds during migration across the Mediterranean sea. Curr Microbiol. 2013;66(3):236–42. doi: 10.1007/s00284-012-0262-9. https://doi.org/10.1007/s00284-012-0262-9. [DOI] [PubMed] [Google Scholar]
- [5].Somveille M, Rodrigues AS, Manica A. Why do birds migrate? A macroecological perspective. Glob Ecol Biogeogr. 2015;24(6):664–74. https://doi.org/10.1111/geb.12298. [Google Scholar]
- [6].Mahmud S. Birds, people and ecosystem. [Mar 30;2020 ]; Available via https://www.thedailystar.net/news-detail-233757 .
- [7].Reed KD, Meece JK, Henkel JS, Shukla SK. Birds, migration and emerging zoonoses: west nile virus, lyme disease, influenza a and enteropathogens. Clin Med Res. 2003;1(1):5–12. doi: 10.3121/cmr.1.1.5. https://doi.org/10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hussong D, Damare JM, Limpert RJ, Sladen WJ, Weiner RM, Colwell RR. Microbial impact of Canada geese (Branta canadensis) and whistling swans (Cygnus columbianus) on aquatic ecosystems. Appl Environ Microbiol. 1979;37(1):14–20. doi: 10.1128/aem.37.1.14-20.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fleming R, Fraser PE. Guelph, Canada: University of Guelph; 2001. The impact of waterfowl on water quality; pp. 1–14. [Google Scholar]
- [10].Moschetti G, Alfonzo A, Francesca N. Cham, Switzerland: Springer; 2017. Yeasts in birds. Yeasts in natural ecosystems: diversity; pp. 435–54. Available via https://doi.org/10.1007/978-3-319-62683-3_14. [Google Scholar]
- [11].Viana DS, Santamaría L, Figuerola J. Migratory birds as global dispersal vectors. Trends Ecol Evol. 2016;31(10):763–75. doi: 10.1016/j.tree.2016.07.005. https://doi.org/10.1016/j.tree.2016.07.005. [DOI] [PubMed] [Google Scholar]
- [12].Foti M, Rinaldo D, Guercio A, Giacopello C, Aleo A, De Leo F, et al. Pathogenic microorganisms carried by migratory birds passing through the territory of the Island of Ustica, Sicily (Italy) Avian Pathol. 2011;40(4):405–9. doi: 10.1080/03079457.2011.588940. https://doi.org/10.1080/03079457.2011.588940. [DOI] [PubMed] [Google Scholar]
- [13].Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331(6015):296–302. doi: 10.1126/science.1194694. https://doi.org/10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- [14].Hubálek Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis. 2004;40(4):639–59. doi: 10.7589/0090-3558-40.4.639. https://doi.org/10.7589/0090-3558-40.4.639. [DOI] [PubMed] [Google Scholar]
- [15].Singh SM, Tsuji M, Gawas-Sakhalker P, Loonen MJ, Hoshino T. Bird feather fungi from Svalbard Arctic. Polar Biol. 2016;39(3):523–32. https://doi.org/10.1007/s00300-015-1804-y. [Google Scholar]
- [16].Peay KG, Kennedy PG, Bruns TD. Fungal community ecology: a hybrid beast with a molecular master. Biosci. 2008;58(9):799–810. https://doi.org/10.1641/B580907. [Google Scholar]
- [17].Connell L, Redman R, Craig S, Scorzetti G, Iszard M, Rodriguez R. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb Ecol. 2008;56(3):448–59. doi: 10.1007/s00248-008-9363-1. https://doi.org/10.1007/s00248-008-9363-1. [DOI] [PubMed] [Google Scholar]
- [18].Buzzini P, Branda E, Goretti M, Turchetti B. Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol Ecol. 2012;82(2):217–41. doi: 10.1111/j.1574-6941.2012.01348.x. https://doi.org/10.1111/j.1574-6941.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- [19].Dynowska M, Biedunkiewicz A, Kisicka I, Ejdys E, Kubiak D, Sucharzewska E. Epidemiological importance of yeasts isolated from the beak and cloaca of healthy Charadriiformes. Bull Vet Inst Pulawy. 2015;59(1):65–9. https://doi.org/10.1515/bvip-2015-0010. [Google Scholar]
- [20].Satlin MJ, Jacobs SE, Walsh TJ. Aspergillosis. In: Safdar A, editor. Principles and practice of transplant infectious diseases. New York, NY: Springer; 2019. pp. 559–76. Available via https://doi.org/10.1007/978-1-4939-9034-4_33. [Google Scholar]
- [21].Raper KB, Fennell DI. The genus Aspergillus. In: Edinburgh E, Livingstone S, editors. The genus Aspergillus. Baltimore, MD: Williams & Wilkins; 1965. pp. 238–68. [Google Scholar]
- [22].Tell LA, Burco JD, Woods L, Clemons KV. Aspergillosis in birds and mammals: considerations for veterinary medicine. In: Gupta A, Singh N, editors. Recent developments in fungal diseases of laboratory animals. Fungal biology. Cham, Switzerland: Springer; 2019. pp. 49–72. Available via https://doi.org/10.1007/978-3-030-18586-2_4. [Google Scholar]
- [23].Savelieff MG, Pappalardo L, Azmanis P. The current status of avian aspergillosis diagnoses: veterinary practice to novel research avenues. Vet Clin Pathol. 2018;47(3):342–62. doi: 10.1111/vcp.12644. https://doi.org/10.1111/vcp.12644. [DOI] [PubMed] [Google Scholar]
- [24].Seyedmousavi S, Guillot J, Arné P, De Hoog GS, Mouton JW, Melchers WJ, et al. Aspergillus and aspergilloses in wild and domestic animals: a global health concern with parallels to human disease. Med Mycol. 2015;53(8):765–97. doi: 10.1093/mmy/myv067. https://doi.org/10.1093/mmy/myv067. [DOI] [PubMed] [Google Scholar]
- [25].Dhama K, Chakraborty S, Verma AK, Tiwari R, Barathidasan R, Kumar A, et al. Fungal/mycotic diseases of poultry-diagnosis, treatment and control: a review. Pak J Biol Sci. 2013;16(23):1626–40. doi: 10.3923/pjbs.2013.1626.1640. https://doi.org/10.3923/pjbs.2013.1626.1640. [DOI] [PubMed] [Google Scholar]
- [26].Nazir KN, Ichinose H, Wariishi H. Construction and application of a functional library of cytochrome P450 monooxygenases from the filamentous fungus Aspergillusoryzae. Appl Environ Microbiol. 2011;77(9):3147–50. doi: 10.1128/AEM.02491-10. https://doi.org/10.1128/AEM.02491-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Talbot JJ, Thompson P, Vogelnest L, Barrs VR. Identification of pathogenic Aspergillus isolates from captive birds in Australia. Med Mycol. 2018;56(8):1038–41. doi: 10.1093/mmy/myx137. https://doi.org/10.1093/mmy/myx137. [DOI] [PubMed] [Google Scholar]
- [28].Ganguly S, Paul I, Mukhopadhayay SK. Systemic impact of aspergillosis or brooder’s pneumonia in birds. Poult Line. 2011;11(2):49. [Google Scholar]
- [29].Redig P. The North American Veterinary Conference Proceedings. Gainesville, FL: Eastern States Veterinary Association; 2005. Mycotic infections in birds I: aspergillosis; pp. 1192–4. [Google Scholar]
- [30].Gugnani HC, Denning DW, Rahim R, Sadat A, Belal M, Mahbub MS. Burden of serious fungal infections in Bangladesh. Eur J Clin Microbiol Infect Dis. 2017;36(6):993–7. doi: 10.1007/s10096-017-2921-z. https://doi.org/10.1007/s10096-017-2921-z. [DOI] [PubMed] [Google Scholar]
- [31].Habib R, Islam R, Rahman A, Bhowmik N, Haque A. Rhino-orbito-cerebral-mucormycosis with osteomyelitis in a patient with diabetes mellitus: a case report and literature review. BIRDEM Med J. 2012;2(2):124–8. https://doi.org/10.3329/birdem.v2i2.12331. [Google Scholar]
- [32].Rahim MR, Saleh AA, Miah MR, Anwar S, Rahman MM. Pattern of dermatophyte in Bangabandhu Sheikh Mujib Medical University. Bangladesh J Med Microbiol. 2012;6(2):11–4. https://doi.org/10.3329/bjmm.v6i2.19370. [Google Scholar]
- [33].Rahman MH, Hadiuzzaman M, Bhuiyan MK, Islam N, Ansari NP, Mumu SA, Chowdhury IJ. Prevalence of superficial fungal infections in the rural areas of Bangladesh. Iran J Dermatol. 2011;14(3):86–91. [Google Scholar]
- [34].Parvin R, Amin R, Mahbub MS, Hasnain M, Arif KM, Miah MT, et al. Deep fungal infection-an emerging problem in Bangladesh. J Med. 2010;11(2):170–5. https://doi.org/10.3329/jom.v11i2.5466. [Google Scholar]
- [35].Hossain A, Islam Q, Siddiqui M, Tamanna N, Sina H, Rahman M, et al. Pulmonary aspergilloma. J Med. 2009;10(2):149–51. https://doi.org/10.3329/jom.v10i2.2836. [Google Scholar]
- [36].Al-Temimay IA, Hasan AM. Isolation and identification of fungi from the droppings of some poultry and some detergents effect on some of them. Iraqi J Sci. 2016;57(4B):2634–40. [Google Scholar]
- [37].Sattar A, ZunitaZakaria JA, Aziz SA, Gabriel RP. Evaluation of six decontamination procedures for isolation of Mycobacterium avium complex from avian feces. PloS One. 2018;13(8):e0202034. doi: 10.1371/journal.pone.0202034. https://doi.org/10.1371/journal.pone.0202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Imran ZK, Ali RI. The risk of several fungi associated with bird waste. Int J Med Sci Clin Inventions. 2014;1(10):558–62. [Google Scholar]
- [39].Washington WJ, Stephan A, William J, Elmer K, Gail W. Koneman’s color atlas and textbook of diagnostic microbiology. 6th. Philadelphia, USA: Lippincott Williams and Wilkins; 2006. Laboratory approach to the presumptive identification of fungal isolates; pp. 1166–237. [Google Scholar]
- [40].Fakruddin M, Chowdhury A, Hossain MN, Ahmed MM. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springerplus. 2015;4(1):159. doi: 10.1186/s40064-015-0947-1. https://doi.org/10.1186/s40064-015-0947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu D, Coloe S, Baird R, Pedersen J. Rapid mini-preparation of fungal DNA for PCR. J Clin Microbiol. 2000;38(1):471. doi: 10.1128/jcm.38.1.471-471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nazir KHMNH, Hassan J, Durairaj P, Yun H. Isolation and identification of Aspergillus flavus from poultry feed samples using combined traditional molecular approach and expression of cyp64a1 at mRNA level. Pak J Agric Sci. 2014;51(2):287–91. [Google Scholar]
- [43].Abd El Tawab AA, Maarouf AA, El-Hofy FI, Ahmed K. Molecular characterization of some fungi isolated from broiler chicken farms. Benha Vet Med J. 2015;29(2):106–18. https://doi.org/10.21608/bvmj.2015.31682. [Google Scholar]
- [44].Sugita C, Makimura K, Uchida K, Yamaguchi H, Nagai A. PCR identification system for the genus Aspergillus and three major pathogenic species: Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger. Med Mycol. 2004;42(5):433–7. doi: 10.1080/13693780310001656786. https://doi.org/10.1080/13693780310001656786. [DOI] [PubMed] [Google Scholar]
- [45].Francesca N, Carvalho C, Almeida PM, Sannino C, Settanni L, Sampaio JP, et al. Wickerhamomycessylviaef.a., sp. nov., an ascomycetous yeast species isolated from migratory birds. Int J Syst Evol Microbiol. 2013;63(12):4824–30. doi: 10.1099/ijs.0.056382-0. https://doi.org/10.1099/ijs.0.056382-0. [DOI] [PubMed] [Google Scholar]
- [46].Sultana S, Rashid SM, Islam MN, Ali MH, Islam MM, Azam MG. Pathological investigation of avian aspergillosis in commercial broiler chicken at Chittagong district. Int J Innov Appl Stud. 2015;10(1):366–76. [Google Scholar]
- [47].Gautam AK, Bhadauria R. Characterization of Aspergillus species associated with commercially stored triphala powder. Afr J Biotechnol. 2012;11(104):16814–23. [Google Scholar]
- [48].Abbas MS, Yassein SN, Khalaf JM. Isolation and identification of some important mycological isolates from dropping of birds in Baghdad. J Entomol Zool Stud. 2017;5(53):671–3. [Google Scholar]
- [49].Kaboudi K, Rejeb A, Bouzouaia M, Munir MT, Umar S. Outbreak of respiratory aspergillosis in backyard duck flock in Tunisia. Int J Livest Res. 2017;8(7):361–8. http://dx.doi.org/10.5455/ijlr.20170717090407. [Google Scholar]
- [50].Arné P, Thierry S, Wang D, Deville M, Loc’h L, et al. Aspergillus fumigatus in poultry. Int J Microbiol. 2011;2011:746356. doi: 10.1155/2011/746356. https://doi.org/10.1155/2011/746356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abdel-Gawad KM, Moharram AM. Keratinophilic fungi from the duck nails in Egypt. J Basic Microbiol. 1989;29(5):259–63. doi: 10.1002/jobm.3620290502. https://doi.org/10.1002/jobm.3620290502. [DOI] [PubMed] [Google Scholar]