Abstract
Objectives:
Left displaced abomasum (LDA) is a common postparturient condition of high yielding dairy cattle. The diagnosis of LDA is challenging and has historically been based on findings that are not specific to the condition. The objective of the current study was to investigate the diagnostic performance of ultrasonography (USG) in the clinical management of dairy cows identified with left-sided ping sound postpartum.
Materials and methods:
Cows with reduced appetite postpartum and had audible left-sided ping sounds on abdominal auscultation were eligible to be prospectively recruited onto the study. The results of clinical findings and abdominal USG were recorded along with milk β-hydroxybutyrate levels, pH levels of abomaso/rumenocentesis samples, and findings on exploratory laparotomy. The diagnostic performance of USG and other clinical investigations was assessed by calculating the test sensitivity and specificity using exploratory laparotomy as a gold standard test.
Results:
A definitive diagnosis of LDA was made in 23 cows, 8 cows were diagnosed with peritonitis, and 4 cows with frothy tympany. The USG findings that were consistent with LDA were present in all cattle diagnosed with LDA at exploratory laparotomy. The USG findings over the past three intercostal space characteristics of LDA, however, were also present in five cases subsequently diagnosed with peritonitis and in all cases diagnosed with frothy tympany on exploratory laparotomy. The pH of abdomaso/rumenocentesis samples yielded the highest diagnostic accuracy (97.14%) as a single test in the current study.
Conclusions:
USG over the left abdominal wall despite being a highly sensitive test for the diagnosis of LDA has limitations as a diagnostic tool due to suboptimal specificity.
Keywords: Dairy cattle, left displaced abomasum, LDA, postpartum, ultrasonography
Introduction
Left-sided “ping” sounds elicited by concurrent percussion and auscultation over the left abdominal wall of cattle, among other physical examination techniques, have frequently been used to identify cattle with left displaced abomasum (LDA). The condition is usually suspected in postpartum cows with reduced appetite, reduced milk yield, and ketosis [1]. LDA has a reported lactational incidence risk of 1.21%–6% [2,3] and is mostly diagnosed within 6 weeks postpartum [4]. The conservative treatments in the forms of rolling of the cow and correction of systemic electrolyte derangements often fail to resolve the condition [5,6], and cattle are most likely to either undergo surgical treatment or to be electively culled with major economic and welfare consequences [6,7]. Recently, LDA was ranked above mastitis, lameness, metritis, retained placenta, ketosis, and hypocalcaemia as a major cause of economic losses in the US dairy industry [8].
An early diagnosis and treatment of LDA are the major determinants of a successful outcome [9]. Risk factors identified to be associated with an increased risk of LDA included the diagnosis of retained placenta, metritis, ketosis, stillborn calf, twin pregnancy, parturient paresis [10], and high prepartum body condition score [3]. The elevated serum β-hydroxybutyrate (BHBA) [11], a low milk protein/fat ratio during the 1st week after calving [12], and a high plasma non-esterified fatty acid level prepartum [3] have been reported as the potential metabolic predictors for LDA occurring. Knowledge of these risk factors could be used to early identify animals at risk for LDA, which could aid in prompt diagnosis and a better treatment outcome.
Although the diagnosis of LDA is a straightforward procedure in most cases, a definitive diagnosis may not be obvious until the animal has undergone exploratory laparotomy [13].
One of the characteristic clinical examination findings in cattle with LDA is the presence of left-sided high-pitched sounds (pings) when the area between the upper third of the ninth and twelfth ribs is auscultated with simultaneous percussion [4]. The absence of pings does not exclude LDA diagnosis, and similarly, these sounds are audible in cattle suffering from other disease conditions such as ruminal atony, ruminal tympany, and pneumoperitoneum, which may render definitive diagnosis difficult [1]. Ultrasonography (USG) examination over the area of “ping” has become a common practice to confirm LDA diagnosis. The findings of reverberation artifacts dorsally and hypoechogenic fluid ventrally over the area of “ping” have been reported as a consistent finding in all cases with positive LDA diagnosis (100% sensitive) [13,14]. However, this USG picture has also been seen in cattle with ruminal tympany and pneumoperitoneum (not a 100% specific test), and therefore, additional diagnostic techniques are required to confirm the diagnosis. The objectives of the current study were to investigate the performance of USG examination as a diagnostic tool for LDA and to test whether other additional examination techniques such as abomaso/rumenocentesis would improve the diagnostic accuracy.
Materials and Methods
Ethical approval
The study was approved by the Zagazig University, Institutional Animal Care and Use Committee (ZU-IACCUC/2/F/22/2018).
Animals
The inclusion criterion in the current study was cows presented with postpartum reduced appetite and milk yield and identified with left-sided “ping” sounds on clinical examination regardless of other clinical examination findings. A total of 35 Holstein Friesian cows met the inclusion criterion and were prospectively recruited onto the study. The cows were recruited from three commercial dairy herds located in the Ismailia Province in Egypt, who consented to participate in the study. A thorough clinical examination was performed on all animals, which included simultaneous percussion and auscultation over the area of “ping,” recording of rectal temperature, pulse and respiratory rates, and rectal examination findings.
USG examination
The ultrasonography examination was performed using a portable real-time B-mode ultrasound machine supplied with a 3.5–5 MHz convex probe (SonoScape A5V Ultrasound Machine, China). The examination was performed according to Braun et al. [13]. The animals were examined unsedated in a standing position. The area over the 9th–12th intercostal spaces was clipped, and a coupling gel was applied. The USG probe was held parallel to the ribs, and the area was screened ventrally to dorsally to identify the characteristic USG findings of LDA, which were previously reported [13]. Besides, the USG examination was extended to include the ventral abdominal region caudal to the xiphoid process as an attempt to visualize the abomasum in its normal position. This should scan as a heterogeneous, moderately echogenic structure according to Braun et al. [15].
Quantifying BHBA in milk samples
A semi-quantitative cow-side milk strip test (PortaBHBTM, PortaCheck, Moorestown, NJ) was used to quantify BHBA in composite milk samples collected from each animal in the current study. Each sample was examined by dipping one PortaBHBTM milk strip into the sample, which was then read after 1 min against the color chart available on the test bottle. The readings of the color chart denoted the values of 0, 50, 100, 200, and 500 μmol/l based on the color density. This test was previously evaluated and found reasonable accuracy to diagnose hyperketonemia in cattle [16].
Abomaso/rumenocentesis and pH evaluation
Percutaneous ultrasound-guided abomaso/rumenocentesis was performed in all animals. Following antiseptic preparation of an area of approximately 10 cm below the distal limit of the audible “ping” sounds, a 15-cm 16-gauge needle was inserted through the skin, layers of abdominal muscles, and the abomasal/ruminal wall, and a 5-ml fluid sample was aspirated [17]. The pH of aspirated fluid was semi-quantitatively evaluated using litmus indicator test strips.
Exploratory laparotomy
Animals underwent a left paralumbar fossa exploratory laparotomy. Surgery was performed while the animal was restrained in a standing position. The left flank region was aseptically prepared for surgery, and lidocaine hydrochloride 2% (Debociane, DBK Pharmaceutical, Cairo, Egypt) was linearly infiltrated to anesthetize the incision line. An incision (approximately 20 cm) was made through the skin and the abdominal muscles, and once the abdomen was entered, it was thoroughly explored for adhesions, abscesses, or displacements. If a diagnosis of LDA was confirmed, the abomasum was deflated, repositioned, and fixed to the ventral abdominal wall (left paralumbar fossa abomasopexy) [18]. If an animal was identified with frothy tympany, rumenotomy was performed, and the contents of the rumen were evacuated. The laparotomy incision was closed in three layers. The peritoneum and the transverse abdominal muscle were closed together in one layer with a size 2 braided polyglycolic acid suture (Egysorb, Taisier-Med, Obour, Egypt) using a simple continuous suture pattern. The internal and external abdominal oblique muscles were closed separately with a size 2 braided polyglycolic acid suture (Egysorb, Taisier-Med SAE, Obour, Egypt) in simple continuous patterns. Finally, the skin was sutured with a size 2 black braided silk (Egysilk, Taisier-Med, Obour, Egypt) using ford-interlocking stitches [18].
Statistical analyses
A definitive final diagnosis of all cases was based on the exploratory laparotomy findings, which was considered the gold standard test in the current study. Positive USG findings indicating LDA diagnosis was described as the presence of a gas cap with reverberation artifacts dorsally and hypoechogenic fluid with occasional echogenic, sickle-shaped structures (abomasal folds) ventrally over the area of “ping” [13]. Positive rectal examination findings of LDA were described as the emptiness of the upper right abdomen with a rumen smaller than expected [4]. Animals with a pH value of 2–4 of aspirated fluid were considered to be LDA positive [4], whereas, for the milk BHBA level, a cutoff value of 100 μmol/l was evaluated [16]. The ability of each of these diagnostic techniques to identify cases with LDA was evaluated by the calculation of sensitivity, specificity, and positive and negative predictive values [19]. The Kruskal–Wallis equality-of-populations rank test was used to compare age, days in milk (DIM), heart rate, respiratory rate, and rectal temperature between different diagnosis categories identified during surgery. Pairwise comparisons were performed using Dunn’s test with Bonferroni adjustment [20].
Results
LDA-positive cattle
According to the exploratory laparotomy findings, 23 cows were diagnosed with LDA, 8 cows with peritonitis, and 4 cows with frothy tympany. Cows identified with LDA had a median age of 4.93 years (interquartile range [IQR]: 4.27–5.91 years). Ten of these animals were in their third lactation season, five in their second lactation season, and four, three, and one animals were in their fourth, fifth, and ninth lactation seasons, respectively (median 2 seasons, IQR 3–4 seasons). The number of DIM before LDA diagnosis had a median of 25 days (IQR 17.5–30.5 days). These animals had a mean pulse rate of 73.41 beats/min (median 70, IQR 65–80), a mean respiratory rate of 34.13 breaths/min (median 35, IQR 30–40), and a mean rectal temperature of 38.5°C (median 38.5, IQR 38.45–39).
The characteristic USG findings of LDA (Fig. 1) were consistent in all cattle diagnosed with LDA on exploratory laparotomy with the absence of the abomasum in its normal location on USG examination from the ventral abdominal wall. The abomasocentesis fluid had a pH value of 2–4 in all animals with LDA diagnosis. These findings indicated that USG over the area of “ping,” USG over the ventral abdomen, and abomasocentesis each had a 100% sensitivity to identify the cattle with LDA. The positive rectal examination findings consistent with an LDA diagnosis were present in 19 (82.61%) cattle. A milk BHBA value of 100 μmol/l was present in 20 (86.97%) cattle with LDA-positive diagnosis.
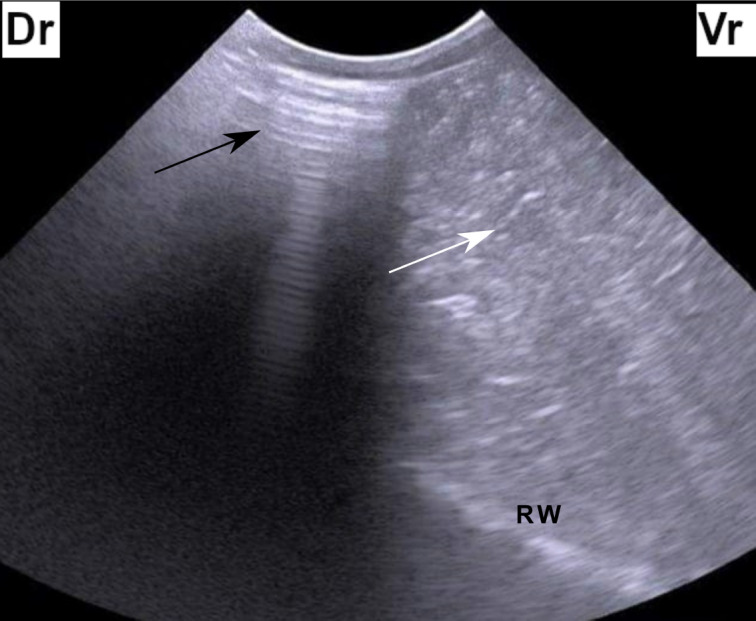
Figure 1. Ultrasonogram of the left abdomen at the level of the upper third of the 11th intercostal space in a cow identified with left-sided ping sounds. The figure shows reverberation artifact dorsally (black arrow) which represents a gas cap and hypoechoic fluid ventrally (white arrow). Dr = dorsal, Vr = Ventral, RW = Ruminal wall.
Two animals diagnosed with LDA deteriorated following surgery and were culled from the farm. The other 21 animals improved after surgery and remained in the dairy production. These animals produced an average daily milk of 27.57 kg (medium 30 kg, IQR 25–40 kg). Seven animals were later culled due to poor milk yield (n = 3) or conception failure following multiple insemination attempts (n = 4). Fourteen animals remained in the production in the following season and produced an average daily milk of 32.5 kg (median 32, IQR 30–35 kg).
LDA-negative cattle
Eight animals were diagnosed with peritonitis during surgery. Of these, two had traumatic reticuloperitonitis, two had ruptured uterus, three had perforated abomasal ulcers, and one animal had intestinal volvulus with ischemic necrosis. All animals diagnosed with peritonitis were culled directly after surgery. The typical USG picture of LDA was evident over the area of “ping” in five animals that were further confirmed as having peritonitis during surgery. The abomasum could not be seen in its normal location from the ventral abdominal wall in two animals with peritonitis, and the pH value of centesis fluid was above four in all of them. Rectal examination findings similar to those observed in LDA-positive cases were evident in three animals, and two animals had a milk BHBA value of 100 μmol/l. These findings indicated that abomaso/rumenocentesis was 100% sensitive to identify animals with pneumoperitoneum and was 100% specific to differentiate them from those with LDA in the current study. Cattle diagnosed with peritonitis had 43.25 mean DIM (median 27, IQR 18.75–32.75) and were in their first to fourth lactation seasons. They had a mean rectal temperature of 39.05°C (median 39, IQR 38.9, 39.2), a mean heart rate of 74 beats/min (median 75, IQR 67.25–81.25), and a mean respiratory rate of 35 breaths/min (median 36, IQR 33.25–40).
Four animals were identified with frothy tympany during surgery. The characteristic of USG findings of LDA was evident in all of them; however, the abomasum could be viewed in its normal location when examined from the ventral abdominal wall in all. Furthermore, they had neither positive rectal findings of LDA nor elevated milk BHBA. One of these animals had a pH value of the ruminal fluid sample aspirated via rumenocentesis of four. Three animals diagnosed with frothy tympany remained in the production, whereas one animal was culled due to a diagnosis of a diaphragmatic hernia at the surgery. These animals were in their first to fourth lactation seasons and were 85 mean DIM (medina 95, IQR 45–135). They had a mean rectal temperature of 38.65°C (median 38.6, IQR 38.58–38.67), a mean pulse rate of 72.5 beats/min (median 72.5, IQR 68.75–76.25) and a mean respiratory rate of 40 breaths/min (median 38.75, IQR 32.5, 46.25).
The diagnostic performance of each of the diagnostic procedures used to differentiate between LDA positive and LDA negative cases is shown in Table 1. Three diagnostic techniques (USG over the area of “ping,” USG over the ventral abdominal wall, and a pH between 2 and 4 of abdominocentesis fluid aspirate) had 100% sensitivity to identify LDA-positive animals. Diagnostic accuracy was the greatest for the abomaso/rumenocentesis procedure, which also had the highest test specificity. Animals diagnosed with LDA were significantly older than those diagnosed with peritonitis or frothy tympany. The respiratory and pulse rates did not vary significantly between groups, whereas animals diagnosed with peritonitis had significantly higher rectal temperature compared with those diagnosed with LDA.
Table 1. Diagnostic performance of five diagnostic procedures used to differentiate between LDA positive or negative cattle identified with left-sided “ping” sounds.
| Diagnostic test | True +ve | False +ve | True −ve | False −ve | Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | Diagnostic accuracy(%) |
|---|---|---|---|---|---|---|---|---|---|
| USG over the area of “ping” | 23 | 9 | 3 | 0 | 100 | 25 | 71.88 | 100 | 74.3 |
| USG over the ventral abdominal wall | 23 | 2 | 10 | 0 | 100 | 83.33 | 92 | 100 | 94.3 |
| Rectal findings | 19 | 3 | 9 | 4 | 82.6 | 75 | 86.36 | 69.23 | 80 |
| pH value of 2–4 of aspirated fluid | 23 | 1 | 11 | 0 | 100 | 91.67 | 95.83 | 100 | 97.14 |
| Milk BHBA of 100 μmol/l | 20 | 2 | 10 | 3 | 86.96 | 83.33 | 90.9 | 76.92 | 85.71 |
The figures are presented in numbers (true +ve, false +ve, true −ve, and false −ve) and in percentages (sensitivity, specificity, PPV, NPV, diagnostic accuracy).
PPV = positive predictive value; NPP = negative predictive value.
Discussion
The current study has investigated diagnosis, treatment, and outcome of 35 Holstein Friesian cows identified with left-sided “ping” sounds on clinical examination. The goal of this research was to evaluate the diagnostic performance of ultrasound examination and other diagnostic procedures to successfully differentiate between animals that truly had LDA and other conditions, which may have a similar ultrasound picture such as ruminal atony/tympany and peritonitis. The study found that although USG examination over the area of “ping” had 100% sensitivity to identify animals that later confirmed with LDA diagnosis on exploratory laparotomy, it had only 25% specificity to rule in/confirm a diagnosis of LDA (a large number of false-positive cases).
The diagnostic test sensitivity is defined as the ability of the test to correctly identify diseased animals, whereas the test specificity refers to the diagnostic test ability to accurately identify healthy animals. A negative test result from a sensitive test would accurately rule out a diagnosis, whereas a positive test result from a specific test would accurately rule in a diagnosis [19]. Given a myriad of different disease conditions that might affect cattle postpartum with each having its consequences in terms of treatment options, costs, and culling chances, highly accurate diagnostic test/tests are required. LDA often requires a costly surgery to treat [21] with a treatment outcome that is dependent on the duration of the disease [9], and therefore, a rapid and accurate diagnosis by a sensitive and specific test is required.
Ultrasonography over the last three intercostal spaces has been advocated as a decision-making tool in animals with suspected LDA as whether to operate on the animal or not [1]. However, the findings of the current study indicated that this should be complemented with other diagnostic techniques to reduce the number of false-positive diagnoses. The gas–liquid interface, which is responsible for the typical USG picture in animals diagnosed with LDA, is also present if there is an accumulation of gas in the rumen or in animals with peritonitis resulting in similar USG picture. Examination of the pH of fluid aspirates from below the area of “ping” can differentiate ruminal fluid from abomasal contents, resulting in improving the diagnostic accuracy. This was confirmed in the current study, where all cases with a true LDA diagnosis had a pH value of fluid aspirates below four.
Extending the USG examination to include the ventral abdominal wall was used to visualize the abomasum in its normal location in LDA-negative cases. The abomasum could not be seen in its normal location in two animals with peritonitis in the current study. Peritonitis results in fluid and fibrin deposition and gas production if bacteria are involved, which may have resulted in the reflection of the USG beam before reaching the abomasum [22]. The diagnosis of these two cases could have been further improved if abdominocentesis was performed and the peritoneal fluid was evaluated for cellular content and biochemical parameters. A study that investigated the clinicopathological characteristics of peritoneal fluid in cattle with LDA found normal levels of total protein (TP), albumin, cholesterol, fibrinogen, glucose, alkaline phosphatase, lactate dehydrogenase (LDH), L-lactate, creatine phosphokinase (CPK), and leukocyte count [23]. Cattle with peritonitis, conversely, were reported to have elevated peritoneal fluid TP, albumen, D-dimer, CPK, LDH, and leukocyte count [24]. Fibrin deposition and adhesion formation in animals with peritonitis might have been responsible for a number of false-positive diagnoses on rectal palpation in the current study. This should also be considered when investigating an animal with left-sided “ping” sounds.
In the current study, most animals (20/23) with LDA were ketotic. Blood BHBA level has been used as a prognostic indicator for survival following surgical correction of LDA, where animals that were not ketotic (blood BHBA <1.2 mmol/l) before surgery were at greater risk of being culled within 60 days of surgery [25,26]. The authors of the latter studies explained this as that nonketotic cows may have had reduced ability to return to normal milk production following surgery. In the current study, of the three-nonketotic cows identified, one was culled because of poor milk production following surgical correction of LDA. An early study reported non-significant differences in the level of blood ketone bodies between cattle with primary ketosis and those diagnosed with LDA [27], and therefore, milk BHBA measurement cannot be used as a standalone test to diagnose animals with LDA. However, if milk BHBA testing was interpreted in light of the results of other diagnostic techniques described in the current study, the diagnostic accuracy could be improved
In the current study, semi-quantitative tests were used which might have introduced bias. Furthermore, the findings of the current study were largely dependent on the clinical experience of people involved, which might reduce the generalisability of these results. However, this study was designed to pinpoint the importance of deploying multiple diagnostic procedures when evaluating cattle with left-sided “ping” sounds to avoid unnecessary surgical intervention, which would lead to an unnecessary increase in treatment costs and compromising animal welfare.
Conclusions
The findings of the current study highlighted the importance of detailed physical examination and diagnostic evaluation using multiple diagnostic modalities in cattle presented with left-sided “ping” sounds to improve diagnostic accuracy and aid clinical decision-making. Abomaso/rumenocentesis and examination of pH of fluid aspirate combined with detailed abdominal USG examination are associated with a higher diagnostic accuracy of LDA in cattle presented with left-sided ping sounds.
Acknowledgment
The authors are grateful to the owners of the dairy farms, from which the animals investigated in the current study were recruited.
Conflict of interest
The authors declare that they have no conflict of interest with any other people or organizations in any financial or personal relationship.
Authors’ contribution
Gouda, S. M. participated in the study design and interpretation of the results and revised the manuscript. Abdelaal, A. M. participated in study design, execution, and interpretation of the results and revised the manuscript. Gomaa, M. participated in study design and interpretation of the results and revised the manuscript. Elgioushy, M. M. participated in study design and execution and revised the manuscript. Refaai, W. participated in the study design and interpretation of the results and revised the manuscript. Mouncey, R. R. participated in the interpretation of the results and writing of the manuscript. Salem S. E. participated in the study design, analyzed the data, and drafted the manuscript. All authors approved the final manuscript.
References
- [1].Braun U. Ultrasound as a decision-making tool in abdominal surgery in cows. Vet Clin North Am Food Anim Pract. 2005;21(1):33–53. doi: 10.1016/j.cvfa.2004.11.001. https://doi.org/10.1016/j.cvfa.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [2].Hamann H, Wolf V, Scholz H, Distl O. Relationships between lactational incidence of displaced abomasum and milk production traits in German Holstein cows. J Vet Med A Physiol Pathol Clin Med. 2004;51(4):203–8. doi: 10.1111/j.1439-0442.2004.00626.x. https://doi.org/10.1111/j.1439-0442.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- [3].Cameron RE, Dyk PB, Herdt TH, Kaneene JB, Miller R, Bucholtz HF, et al. Dry cow diet, management, and energy balance as risk factors for displaced abomasum in high producing dairy herds. J Dairy Sci. 1998;81(1):132–9. doi: 10.3168/jds.S0022-0302(98)75560-2. https://doi.org/10.3168/jds.S0022-0302(98)75560-2. [DOI] [PubMed] [Google Scholar]
- [4].Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine A textbook of the diseases of cattle, sheep goats, pigs and horses. 10th. Oxford, UK: Elservier; 2011. Diseases of the alimentary tract - I I; pp. 354–60. [Google Scholar]
- [5].Oman RE, Streeter RN, Reppert EJ, Chako CZ. Left displacement of the abomasum in 4 beef calves. J Vet Intern Med. 2016;30(4):1376–80. doi: 10.1111/jvim.14353. https://doi.org/10.1111/jvim.14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Melendez P, Romero C, Pithua P, Marin MP, Pinedo P, Duchens M. Retrospective evaluation of milk production and culling risk following either surgical, toggle-pin suture or conservative treatment of left displaced abomasum in Chilean dairy cows. N Z Vet J. 2017;65(6):292–6. doi: 10.1080/00480169.2017.1360162. https://doi.org/10.1080/00480169.2017.1360162. [DOI] [PubMed] [Google Scholar]
- [7].Haine D, Delgado H, Cue R, Sewalem A, Wade K, Lacroix R, et al. Contextual herd factors associated with cow culling risk in Quebec dairy herds: a multilevel analysis. Prev Vet Med. 2017;144:7–12. doi: 10.1016/j.prevetmed.2017.05.014. https://doi.org/10.1016/j.prevetmed.2017.05.014. [DOI] [PubMed] [Google Scholar]
- [8].Liang D, Arnold LM, Stowe CJ, Harmon RJ, Bewley JM. Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci. 2017;100(2):1472–86. doi: 10.3168/jds.2016-11565. https://doi.org/10.3168/jds.2016-11565. [DOI] [PubMed] [Google Scholar]
- [9].Rohn M, Tenhagen BA, Hofmann W. Survival of dairy cows after surgery to correct abomasal displacement: 2. Association of clinical and laboratory parameters with survival in cows with left abomasal displacement. J Vet Med A Physiol Pathol Clin Med. 2004;51(6):300–5. doi: 10.1111/j.1439-0442.2004.00650.x. https://doi.org/10.1111/j.1439-0442.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- [10].Rohrbach BW, Cannedy AL, Freeman K, Slenning BD. Risk factors for abomasal displacement in dairy cows. J Am Vet Med A. 1999;214(11):1660–3. [PubMed] [Google Scholar]
- [11].Seifi HA, Leblanc SJ, Leslie KE, Duffield TF. Metabolic predictors of post-partum disease and culling risk in dairy cattle. Vet J. 2011;188(2):216–20. doi: 10.1016/j.tvjl.2010.04.007. https://doi.org/10.1016/j.tvjl.2010.04.007. [DOI] [PubMed] [Google Scholar]
- [12].Mecitoglu Z, Demir G, Senturk S, Uzabaci E, Darici R. Milk protein/fat ratio on day 7 postpartum as a predictor of left displacement of abomasum. Vet Rec. 2012;171(8):197. doi: 10.1136/vr.100641. https://doi.org/10.1136/vr.100641. [DOI] [PubMed] [Google Scholar]
- [13].Braun U, Pusterla N, Schonmann M. Ultrasonographic findings in cows with left displacement of the abomasum. Vet Rec. 1997;141(13):331–5. doi: 10.1136/vr.141.13.331. https://doi.org/10.1136/vr.141.13.331. [DOI] [PubMed] [Google Scholar]
- [14].Li XW, Xu QS, Zhang RH, Yang W, Li Y, Zhang YM, et al. Ultrasonographic findings in cows with left displacement of abomasum, before and after reposition surgery. BMC Vet Res. 2018;14(1):44. doi: 10.1186/s12917-018-1358-7. https://doi.org/10.1186/s12917-018-1358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Braun U, Wild K, Guscetti F. Ultrasonographic examination of the abomasum of 50 cows. Vet Rec. 1997;140(4):93–8. doi: 10.1136/vr.140.4.93. https://doi.org/10.1136/vr.140.4.93. [DOI] [PubMed] [Google Scholar]
- [16].Denis-Robichaud J, DesCoteaux L, Dubuc J. Accuracy of a new milk strip cow-side test for diagnosis of hyperketonemia. Bovine Pr. 2011;45(2):97–101. [Google Scholar]
- [17].Braun U, Wild K, Merz M, Hertzberg H. Percutaneous ultrasound-guided abomasocentesis in cows. Vet Rec. 1997;140(23):599–602. doi: 10.1136/vr.140.23.599. https://doi.org/10.1136/vr.140.23.599. [DOI] [PubMed] [Google Scholar]
- [18].Trent AM. Surgery of the abomasum. In: Fubini S, Ducharme N, editors. Farm Animal Surgery. Amsterdam, Netherlands: Elsevier; 2004. pp. 218–9. [Google Scholar]
- [19].Kumar R. Evaluation of diagnostic tests. Clin Epidemiol Global Health. 2016;4(2):76–9. https://doi.org/10.1016/j.cegh.2015.12.001. [Google Scholar]
- [20].Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–52. https://doi.org/10.1080/00401706.1964.10490181. [Google Scholar]
- [21].Fubini SL, Ducharme NG, Erb HN, Sheils RL. A comparison in 101 dairy cows of right paralumbar fossa omentopexy and right paramedian abomasopexy for treatment of left displacement of the abomasum. Canadian Vet J. 1992;33(5):318–24. [PMC free article] [PubMed] [Google Scholar]
- [22].Abdelaal AM, Floeck M, El Maghawry S, Baumgartner W. Clinical and ultrasonographic differences between cattle and buffaloes with various sequelae of traumatic reticuloperitonitis. Vet Med. 2009;54(9):399–406. https://doi.org/10.17221/128/2009-VETMED. [Google Scholar]
- [23].Grosche A, Furll M, Wittek T. Peritoneal fluid analysis in dairy cows with left displaced abomasum and abomasal volvulus. Vet Re. 2012;170(16):413. doi: 10.1136/vr.100381. https://doi.org/10.1136/vr.100381. [DOI] [PubMed] [Google Scholar]
- [24].Wittek T, Grosche A, Locher LF, Furll M. Diagnostic accuracy of d-dimer and other peritoneal fluid analysis measurements in dairy cows with peritonitis. J Vet Intern Med. 2010;24(5):1211–7. doi: 10.1111/j.1939-1676.2010.0548.x. https://doi.org/10.1111/j.1939-1676.2010.0548.x. [DOI] [PubMed] [Google Scholar]
- [25].Reynen JL, Kelton DF, LeBlanc SJ, Newby NC, Duffield TF. Factors associated with survival in the herd for dairy cows following surgery to correct left displaced abomasum. J Dairy Sci. 2015;98(6):3806–13. doi: 10.3168/jds.2014-9017. https://doi.org/10.3168/jds.2014-9017. [DOI] [PubMed] [Google Scholar]
- [26].Croushore WS, Jr., Ospina PA, Welch DC, Zawisza DJ, Nydam DV. Association between beta-hydroxybutyrate concentration at surgery for correction of left-displaced abomasum in dairy cows and removal from the herd after surgery. J Am Vet Med Assoc. 2013;243(9):1329–33. doi: 10.2460/javma.243.9.1329. https://doi.org/10.2460/javma.243.9.1329. [DOI] [PubMed] [Google Scholar]
- [27].Itoh N, Koiwa M, Hatsugaya A, Yokota H, Taniyama H, Okada H, et al. Comparative analysis of blood chemical values in primary ketosis and abomasal displacement in cows. Zentralblatt für Veterinärmedizin. Zentralbl Veterinarmed A. 1998;45(5):293–8. doi: 10.1111/j.1439-0442.1998.tb00830.x. https://doi.org/10.1111/j.1439-0442.1998.tb00830.x. [DOI] [PubMed] [Google Scholar]


