Abstract
Context
Per- and polyfluoroalkyl substances (PFAS) exposure may alter glucose homeostasis. Research on PFAS exposure and glucose tolerance during pregnancy is limited.
Objective
The objective of this work is to estimate associations between first-trimester plasma PFAS concentrations and glucose tolerance assessed in late second pregnancy trimester.
Design, Setting, Participants, and Main Outcome Measures
Pregnant women (n = 1540) enrolled in Project Viva in 1999 to 2002 provided first-trimester plasma samples analyzed for 8 PFAS. At approximately 28 weeks’ gestation, women completed 1-hour nonfasting, 50-g oral glucose challenge tests (GCTs); if abnormal, women completed subsequent 3-hour oral glucose tolerance tests (OGTTs) to screen for gestational diabetes mellitus (GDM). We assessed both continuous GCT glucose levels and 4 categories of glucose tolerance (normal glycemia [reference], isolated hyperglycemia, impaired glucose tolerance, GDM). We used multinomial logistic regression to estimate associations of PFAS with glucose tolerance categories. We used multivariable linear regression and Bayesian kernel machine regression (BKMR) to assess individual and joint effects of PFAS on continuous GCT glucose levels, respectively. We evaluated effect modification by maternal age and race/ethnicity.
Results
PFAS were not associated with glucose tolerance categories. In BKMR analyses, we observed a positive association between ln-perfluorooctane sulfonate (PFOS) and glucose levels (Δ25th to 75th percentile: 6.2 mg/dL, 95% CI, 1.1-11.3) and an inverse-U shaped association between 2-(N-perfluorooctane sulfonamide) acetate and glucose levels. Individual linear regression results were similar. We found suggestive evidence that associations varied by age and racial/ethnic group.
Conclusion
Certain PFAS may alter glucose homeostasis during pregnancy, but may not be associated with overt GDM.
Keywords: PFAS, gestational diabetes, pregnancy glucose, impaired glucose tolerance, metabolic disruptors
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications affecting an estimated 7% of pregnancies, although prevalence estimates vary across populations (1-3). GDM is associated with numerous adverse short- and long-term health outcomes both for mother and child, such as preeclampsia, preterm birth, and cesarean delivery in mothers, as well as macrosomia and hypoglycemia in their children (1, 4). In later life, women with gestational diabetes are at increased risk of developing type 2 diabetes (5-7) and cardiovascular disease (8, 9) and their offspring may be at increased risk of altered glucose metabolism and increased adiposity (4, 10, 11). In addition to overt GDM, recent studies suggest that even subclinical hyperglycemia during pregnancy is associated with an increased risk of adverse health outcomes, with no obvious lower threshold for glucose levels (4, 10). Although lifestyle and genetic factors are known risk factors for maternal hyperglycemia, growing evidence suggests that exposure to environmental metabolism-disrupting chemicals (12) that can alter glucose metabolism, such as per- and polyfluoroalkyl substances (PFAS), may also affect maternal glucose regulation during pregnancy (13, 14).
PFAS, a group of highly fluorinated synthetic organic chemicals, are extensively used in commercial and consumer products such as nonstick cookware, food packaging, upholstery and carpeting, clothing, and firefighting foams (15). Owing to their strong carbon-fluorine bonds, PFAS are environmentally persistent and long-chain PFAS are also biologically persistent, with half-lives in humans ranging from 2 to 5 years (16, 17). These characteristics have led to ubiquitous human exposure through contaminated diet, drinking water, indoor environments, and direct contact with PFAS-containing products (13, 15, 18, 19). PFAS exposure is associated with a range of adverse human health effects (15, 20) and may alter metabolic pathways through their ability to bind to peroxisome proliferator-activated receptors (PPARs) (21), which are involved in adipogenesis, lipid handling, and glucose homeostasis (22).
Previous studies on PFAS exposure and GDM or altered glucose levels in pregnancy have been inconsistent, with associations varying by individual PFAS (23-31). Multiple studies reported positive associations between PFAS exposure and increased risk of GDM (23, 24, 28, 29, 31) and increased glucose levels during pregnancy (25, 27, 29), whereas others reported no association between PFAS exposure and GDM (25, 26, 30). However, almost all these studies have assessed effects of PFAS individually (24-26, 31) and have not evaluated the effects of exposure to multiple PFAS on glycemic outcomes. Further, recent studies suggest that certain subgroups of the population, such as women with GDM risk factors including elevated body mass index (BMI) and a family history of diabetes, may also be more susceptible to the effects of PFAS exposure on glucose homeostasis (27, 28). However, few studies have assessed potential effect modification of the associations of PFAS with maternal glucose tolerance.
The primary aim of this study is to evaluate associations between exposure to multiple PFAS during early pregnancy and late-second trimester maternal glucose tolerance in a large cohort of pregnant women. As a secondary aim, we assessed heterogeneous susceptibility to the effects of PFAS exposure by evaluating whether associations with maternal glucose intolerance differed across maternal factors related to GDM risk, specifically advanced maternal age, prepregnancy BMI, and race/ethnicity.
Materials and Methods
Study participants
Study participants were pregnant women enrolled in Project Viva, a longitudinal prebirth cohort in the Boston, Massachusetts, area. Project Viva recruited women at their first prenatal visit (median 9.7 weeks’ gestation) at Atrius Harvard Vanguard Medical Associates facilities between 1999 and 2002. Eligible women spoke English, had a singleton pregnancy, and were at 22 weeks’ gestation or less at the time of enrollment. Details on this cohort can be found elsewhere (32). Of the 2128 eligible live births in Project Viva, 1645 (77%) had PFAS plasma measurements from early pregnancy samples. Of those, 1596 women had data on glucose tolerance status and/or GCT glucose levels from routine GDM screening in the late second trimester. We excluded an additional 56 women for the following reasons: missing covariate data (n = 38), second Viva pregnancy (n = 17), and prior history of type 1 or type 2 diabetes (n = 1). The final analytic sample consisted of 1540 pregnant women.
The institutional review boards of all participating institutions approved the study protocols and all study participants provided informed consent. The Centers for Disease Control and Prevention laboratory’s involvement did not constitute engagement in human-participant research.
Plasma per- and polyfluoroalkyl substances concentrations
We collected participant plasma samples at the first prenatal visit (median 9.7 weeks gestation) and stored them prior to analysis as previously described (33). The Division of Laboratory Sciences at the Centers for Disease Control and Prevention (Atlanta, Georgia) analyzed the samples for concentrations of 8 PFAS (perfluorooctane sulfonate, PFOS; perfluorooctanoate, PFOA; perfluorohexane sulfonate, PFHxS; perfluorononanoate, PFNA; 2-(N-ethyl-perfluorooctane sulfonamide) acetate, EtFOSAA; 2-(N-methyl-perfluorooctane sulfonamide acetate, MeFOSAA; perfluorodecanoate, and perfluorooctane sulfonamide, FOSA) according to previously published methods (34). Limits of detection (LOD) were 0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS. We replaced values below the LOD by the LOD/√2. Perfluorodecanoate and FOSA were detected in less than 50% of samples and were not included in further analyses.
Glycemic screening and outcome assessment
Study participants underwent standard clinical screening for GDM using the 2-step method in the late second trimester/early third trimester of pregnancy (mean ± SD: 27.7 ± 2.2 weeks’ gestation). First, participants underwent a 1-hour nonfasting, 50-g oral glucose challenge test (GCT). Participants with glucose levels 140 mg/dL or greater after 1 hour underwent a subsequent 3-hour fasting, 100-g oral glucose tolerance test (OGTT) (35). We used the Carpenter and Coustan Criteria thresholds for assessing OGTT values, with abnormal levels defined as 95 mg/dL or greater fasting glucose, 180 mg/dL or greater 1-hour glucose, 155 mg/dL or greater 2-hour glucose, and 140 mg/dL or greater 3-hour glucose (35). As in prior Project Viva analyses, we used a categorical glucose tolerance outcome based on results both from the GCT and OGTT using the following category definitions: 1) GDM defined as failing the GCT and having 2 or more abnormal OGTT values; 2) impaired glucose tolerance (IGT) defined as failing the GCT and having 1 abnormal OGTT value; 3) isolated hyperglycemia (IH) defined as failed GCT and having all normal OGTT values; and 4) normal glucose tolerance (NGT) defined as normal GCT and not requiring additional testing using the OGTT. We used the latter NGT group as the referent category. In addition to categorical glucose tolerance, we also evaluated continuous blood glucose levels from the GCT. Seven women were missing GCT glucose data and were excluded from the continuous glucose analysis.
Covariate assessment
We collected maternal sociodemographic and pregnancy information using detailed in-person interviews and questionnaires at study visits. A priori, we identified the following variables as potential confounders: maternal age at enrollment, race/ethnicity, education, prepregnancy BMI (kg/m2), gestational weight gain (GWG) until the GCT, smoking habits, parity, prior GDM, diet, average total physical activity during pregnancy, family history of diabetes, marital status, and household income. We measured physical activity using a modified version of the leisure-time activity section of the Physical Activity Scale for the Elderly (36, 37), and defined average total physical activity as a sum of hours per week of walking, light to moderate, and vigorous exercise (hours per week) based on participant questionnaire data from midpregnancy. We calculated prepregnancy BMI as self-reported weight (in kilograms)/self-reported height (in meters squared). To assess dietary patterns, we calculated a composite alternative healthy eating index score using data from first-trimester food frequency questionnaires (38). Data on breastfeeding history were not available in Project Viva. Instead, we used a proxy variable of prior breastfeeding history based on parity and breastfeeding data from the current pregnancy/child by assuming that women who breastfed their current child were likely to have breastfed their previous child or children.
Statistical analyses
We natural-log (ln) transformed continuous PFAS concentrations in our analyses to account for their skewed distributions when modeled continuously. Blood glucose (1-hour post-GCT) was relatively normally distributed and was left untransformed for all analyses. We used multinomial logistic regression to assess the individual associations of PFAS concentrations with glucose tolerance categories and used multivariable linear regression models to estimate individual associations of PFAS concentrations with continuous glucose levels, fitting separate models for each individual PFAS. To relax the assumption of linearity of the association of PFAS concentrations and our glycemic outcomes, we categorized PFAS concentrations based on quartiles of their distributions. As a sensitivity analysis, we modeled PFAS concentrations continuously.
We evaluated the effects of exposure to multiple PFAS on continuous glucose 1 hour post–50-g GCT, using Bayesian kernel machine regression (BKMR), a recently developed method for modeling exposure mixtures (39). BKMR uses a kernel function to flexibly model the overall joint effect of an exposure mixture and estimate individual exposure-outcome associations. BKMR allows for potential nonlinear exposure-response relationships and can also be used to visualize and identify potential interactions between exposures. We used BKMR to model the joint and individual effects of exposure to all 6 PFAS on continuous glucose levels. We ln-transformed and standardized PFAS concentrations for the BKMR analyses. Because BKMR is sensitive to extreme values, we excluded 3 observations with PFAS concentrations greater than 5 SDs from the ln-PFAS means. We ran our BKMR analysis including all PFAS observations as a sensitivity analysis.
Based on prior evidence and directed acyclic graphs, we adjusted all statistical models for maternal age (continuous, years), prepregnancy BMI (continuous, kg/m2), race/ethnicity (Asian, non-Hispanic black, Hispanic, non-Hispanic white, other), education (no college degree vs college degree), smoking status (former smoker, pregnancy/current smoker, never smoker), and prior GDM/parity (prior GDM, no prior GDM, nulliparous). We assessed potential confounding by additional covariates based on directed acyclic graphs by further adjusting our multinomial and linear regression models for maternal family history of diabetes (yes, no, don’t know/missing), alternative health eating index (continuous), average total physical activity (hours/week), annual household income (≤ $70 000 vs > $70 000), and prior breastfeeding (yes vs no) individually. Adjusting for the additional covariates did not meaningfully change our results; therefore, we did not include these additional variables in our final models. Based on prior evidence (40-42), we hypothesized that GWG could be on the causal pathway between PFAS exposure and glycemic outcomes during pregnancy, therefore we chose not to control for GWG in our models.
As a secondary analysis, we assessed effect measure modification by maternal characteristics related to GDM risk (ie, advanced maternal age, prepregnancy BMI, and race/ethnicity), to evaluate potential heterogeneity in effects across susceptible population subgroups. Recent studies have found heterogeneity in associations between environmental chemicals, including PFAS, and glycemic outcomes during pregnancy in women with predetermined GDM risk factors compared to women without these risk factors (27, 28, 43, 44). We hypothesized that women of advanced maternal age, higher prepregnancy BMI, and certain racial/ethnic groups (ie, Asian and non-Hispanic black women) may be more susceptible to effects of PFAS on glucose tolerance during pregnancy because of underlying differences in biological and/or environmental factors (45-48). We stratified our linear regression models and estimated cross-product interaction term P values to evaluate the following established GDM risk factors as potential effect modifiers: maternal age (< 35, ≥ 35 years), prepregnancy BMI (< 25, ≥ 25 kg/m2), and race/ethnicity (Asian, non-Hispanic black, Hispanic, non-Hispanic white, other) (49). Given the relatively small number of women with GDM and IGT, we were unable to stratify our multinomial logistic regression models. We used SAS version 9.4 (SAS Institute Inc) to perform all analyses, except for BKMR, for which we used R version 3.5.2 (R Foundation For Statistical Computing).
Results
Table 1 presents baseline population characteristics of the analytic study participants and those excluded from this analysis; characteristics were generally comparable between the 2 groups. Study participants were predominantly non-Hispanic white (69%), never smokers (68%), with a college degree or higher (65%). A total of 101 women (7%) reported a family history of diabetes and of the 773 parous women, 29 (4% of parous, 2% of study population) reported a history of GDM in a prior pregnancy. Based on our screening definitions, 85 (6%) women had GDM, 45 (3%) had IGT, and 126 (8%) had IH.
Table 1.
Characteristics of pregnant women from Project Viva included in the present study (n = 1540)
| Characteristic | Study participants (1540) | Excluded participants (588) | ||
|---|---|---|---|---|
| n (%) | Mean ± SD | n (%) | Mean ± SD | |
| Age at enrollment, y | 1540 | 31.9 ± 5.1 | 588 | 31.7 ± 5.5 |
| GCT blood glucose, mg/dL | 1533 | 114 ± 27 | 525 | 115 ± 28 |
| Alternative Healthy Eating Index Score | 1350 | 60.5 ± 10.2 | 427 | 60.8 ± 10.3 |
| Physical activity, hrs/wk | 1224 | 7.3 ± 8.0 | 399 | 6.9 ± 6.4 |
| Prepregnancy BMI, kg/m2 | 1540 | 25.0 ± 5.5 | 572 | 24.6 ± 5.5 |
| < 25 | 950 (62) | 369 (65) | ||
| ≥ 25 | 590 (38) | 203 (35) | ||
| Glucose tolerance | ||||
| GDM | 85 (6) | 32 (6) | ||
| IGT | 45 (3) | 19 (4) | ||
| IH | 126 (8) | 54 (10) | ||
| Normal | 1284 (83) | 422 (80) | ||
| College degree or higher | 1002 (65) | 358 (63) | ||
| Race/Ethnicity | ||||
| Asian | 69 (5) | 51 (9) | ||
| Black | 235 (15) | 113 (20) | ||
| Hispanic | 109 (7) | 45 (8) | ||
| White | 1068 (69) | 331 (59) | ||
| Other | 59 (4) | 24 (4) | ||
| Smoking | ||||
| Pregnancy | 202 (13) | 64 (11) | ||
| Former | 293 (19) | 105 (19) | ||
| Never | 1045 (68) | 398 (70) | ||
| Prior GDM/parity | ||||
| Yes | 29 (2) | 20 (4) | ||
| No | 744 (48) | 292 (52) | ||
| Nulliparous | 767 (50) | 248 (44) | ||
| Family history of diabetes | ||||
| Yes | 101 (7) | 33 (6) | ||
| No | 1203 (78) | 412 (70) | ||
| Don’t know/missing | 236 (15) | 143 (24) | ||
| History of breastfeeding | 464 (30) | 181 (31) | ||
| Annual household income | ||||
| ≤ $70 000 | 210 (15) | 83 (17) | ||
| > $70 000 | 1176 (85) | 405 (83) | ||
Abbreviations: BMI, body mass index; GCT, glucose challenge test; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; IH, isolated hyperglycemia.
We detected PFAS in the following percentage of plasma samples: PFOS = 99.9%, PFOA = 100%, PFHxS = 99.3%, PFNA = 98.6%, EtFOSAA = 99.7%, and MeFOSAA = 100%. PFOS had the highest geometric mean concentration (25.5 ng/mL), followed by PFOA (5.7 ng/mL), PFHxS (2.5 ng/mL), PFNA (1.9 ng/mL), MeFOSAA (1.9 ng/mL), and EtFOSAA (1.2 ng/mL) (Table S1, Supplemental Material [50]). PFAS concentrations were moderately to highly correlated with each other (rs range, 0.2-0.7, Fig. S1, Supplemental Material [50]), with PFOA and PFOS having the highest correlation (rs = 0.7, P < .001). Concentrations in Project Viva were comparable to those reported in a representative sample of women of reproductive age from the U.S. National Health and Nutrition Examination Survey in 1999 to 2000, roughly corresponding to the sampling period of Project Viva (33).
Associations of per- and polyfluoroalkyl substances with glucose tolerance
Table 2 displays the results from multinomial logistic regression evaluating the association between individual PFAS modeled as quartiles and categories of glucose tolerance. We found a suggestive association between higher PFOS concentrations (Q4 vs Q1) and increased odds of GDM vs NGT, but our CI included the null (OR = 1.5; 95% CI, 0.7-3.0); results for PFOA (Q4 vs Q1) were similar (OR = 1.4; 95% CI, 0.7-2.9). Odds of GDM vs NGT were consistently elevated across quartiles of EtFOSAA. For MeFOSAA models, odds of IGT vs NGT were elevated for Q2 (OR = 2.0; 95% CI, 0.9-4.9) and Q3 (OR = 1.9; 95% CI, 0.8-4.4) vs Q1, suggesting a potential nonlinear association. Odds of IH were significantly increased in women with moderate (Q2 vs Q1, OR = 1.6; 95% CI, 1.0-2.7) but not high MeFOSAA concentrations (Q3 or Q4 vs Q1). Results from models of continuous ln-PFAS concentrations were similar, with the exception of the associations with MeFOSAA, which were null (Table S2, Supplemental Material [50]).
Table 2.
Adjusteda odds ratios (95% CIs) for categories of pregnancy glucose tolerance assessed at approximately 28 weeks’ gestation by first-trimester plasma per- and polyfluoroalkyl substances quartiles (n = 1540)
| PFAS, ng/mL | Normal (n = 1284) | IH (n = 126) | IGT (n = 45) | GDM (n = 85) | |
|---|---|---|---|---|---|
| PFOS | Q1 (0.1-18.8) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (18.9-25.7) | 1 (Reference) | 1.1 (0.7-1.9) | 1.2 (0.5-3.0) | 1.1 (0.5-2.4) | |
| Q3 (25.8-34.9) | 1 (Reference) | 1.3 (0.7-2.1) | 1.9 (0.8-4.4) | 1.0 (0.5-2.2) | |
| Q4 (35.0-185.0) | 1 (Reference) | 1.0 (0.6-1.7) | 0.9 (0.3-2.4) | 1.5 (0.7-3.0) | |
| PFOA | Q1 (0.3-4.2) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (4.3-5.9) | 1 (Reference) | 0.8 (0.5-1.4) | 0.6 (0.3-1.4) | 1.1 (0.5-2.3) | |
| Q3 (6.0-7.9) | 1 (Reference) | 0.8 (0.5-1.4) | 0.6 (0.2-1.5) | 1.3 (0.6-2.8) | |
| Q4 (8.0-36.7) | 1 (Reference) | 0.9 (0.5-1.6) | 0.8 (0.3-1.8) | 1.4 (0.7-2.9) | |
| PFHxS | Q1 (0.1-1.6) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (1.7-2.5) | 1 (Reference) | 1.0 (0.6-1.7) | 0.7 (0.3-1.8) | 1.1 (0.6-2.3) | |
| Q3 (2.6-3.8) | 1 (Reference) | 1.2 (0.7-2.0) | 1.3 (0.6-2.8) | 1.2 (0.6-2.4) | |
| Q4 (3.9-74.5) | 1 (Reference) | 1.4 (0.8-2.5) | 1.0 (0.4-2.5) | 1.0 (0.5-2.2) | |
| PFNA | Q1 (0.1-0.5) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (0.6-0.7) | 1 (Reference) | 0.9 (0.5-1.4) | 0.6 (0.3-1.3) | 1.4 (0.8-2.6) | |
| Q3 (0.8-0.9) | 1 (Reference) | 1.0 (0.6-1.7) | 0.5 (0.2-1.5) | 0.8 (0.4-1.9) | |
| Q4 (1.0-6) | 1 (Reference) | 1.1 (0.6-1.8) | 0.9 (0.4-2.0) | 1.0 (0.5-2.0) | |
| EtFOSAA | Q1 (0.1-0.7) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (0.8-1.2) | 1 (Reference) | 0.7 (0.4-1.2) | 0.9 (0.4-2.2) | 1.4 (0.7-2.8) | |
| Q3 (1.3-1.9) | 1 (Reference) | 1.0 (0.6-1.6) | 1.2 (0.5-2.8) | 1.4 (0.7-3.0) | |
| Q4 (1.9-33.6) | 1 (Reference) | 0.9 (0.5-1.5) | 1.1 (0.4-2.5) | 1.5 (0.7-3.2) | |
| MeFOSAA | Q1 (0.1-1.3) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 (1.4-1.9) | 1 (Reference) | 1.6 (1.0-2.7) | 2.0 (0.9-4.9) | 1.1 (0.6-2.2) | |
| Q3 (2.0-3.2) | 1 (Reference) | 0.9 (0.5-1.6) | 1.9 (0.8-4.4) | 1.3 (0.7-2.4) | |
| Q4 (3.3-29.7) | 1 (Reference) | 1.2 (0.7-2.0) | 1.0 (0.4-2.8) | 0.9 (0.4-1.9) | |
Abbreviations: EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamide) acetate; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; IH, isolated hyperglycemia; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
aAdjusted for maternal age (continuous), prepregnancy body mass index (continuous), prior history of GDM/parity, race/ethnicity, smoking, and education.
Associations of per- and polyfluoroalkyl substances with continuous glucose challenge test glucose levels
Individual per- and polyfluoroalkyl substances: linear models.
Table 3 presents covariate-adjusted differences for individual first trimester PFAS concentrations in quartiles and second-trimester glucose levels 1-hour post–nonfasting 50-g GCT for the overall cohort. In the overall cohort, PFOS concentrations were positively associated with glucose levels (Q2 vs Q1, β = 2.4 mg/dL, 95% CI, –1.2 to 6.0; Q3 vs Q1, β = 3.7 mg/dL, 95% CI: 0.0, 7.4; Q4 vs Q1, β = 4.3 mg/dL, 95% CI, 0.5-8.0). Additionally, we observed a potential nonlinear effect of MeFOSAA on glucose levels in which moderate MeFOSAA concentrations (Q2 vs Q1) were associated with a significant increase in glucose levels (β = 5.8 mg/dL, 95% CI, 2.1-9.4) but high MeFOSAA concentrations (Q3 or Q4 vs Q1) were associated with smaller increases in glucose levels (Q3 vs Q1, β = 2.4 mg/dL, 95% CI, –1.1 to 5.9; Q4 vs Q1, β = 2.7 mg/dL, 95% CI, –1.0 to 6.4).
Table 3.
Adjusteda difference in blood glucose levels (mg/dL) 1-hour post 50-g nonfasting glucose challenge test assessed at approximately 28 weeks’ gestation by first-trimester plasma per- and polyfluoroalkyl substances quartiles, overall and stratified by age at enrollment (< 35 years, ≥ 35 years)
| PFAS, ng/mL | All (n = 1533)b | < 35 y (n = 1105) | ≥ 35 y (n = 428) | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||
| PFOS | Q1 (0.1-18.8) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (18.9-25.7) | 2.4 (–1.2 to 6.0) | 5.2 (0.8 to 9.6) | –4.0 (–10.8 to 2.8) | |
| Q3 (25.8-34.9) | 3.7 (0.0 to 7.4) | 5.2 (0.8 to 9.7) | 0.3 (–6.7 to 7.3) | |
| Q4 (35.0-185.0) | 4.3 (0.5 to 8.0) | 6.5 (2.1 to 10.9) | –0.7 (–8.0 to 6.7) | |
| PFOA | Q1 (0.3-4.2) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (4.3-5.9) | –0.4 (–4.0 to 3.3) | –0.5 (–5.0 to 4.0) | 0.4 (–6.1 to 7.0) | |
| Q3 (6.0-7.9) | 1.0 (–2.8 to 4.8) | 2.5 (–2.1 to 7.1) | –2.4 (–9.5 to 4.7) | |
| Q4 (8.0-36.7) | 1.5 (–2.4 to 5.3) | 2.6 (–1.9 to 7.2) | –1.4 (–9.0 to 6.2) | |
| PFHxS | Q1 (0.1-1.6) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (1.7-2.5) | –0.7 (–4.3 to 3.0) | 0.8 (–3.7 to 5.3) | –1.8 (–8.7 to 5.1) | |
| Q3 (2.6-3.8) | 1.9 (–1.8 to 5.6) | 3.5 (–0.9 to 8.0) | –1.1 (–7.9 to 5.7) | |
| Q4 (3.9-74.5) | 1.2 (–2.6 to 5.0) | 3.3 (–1.1 to 7.7) | –5.6 (–13.3 to 2.1) | |
| PFNA | Q1 (0.1-0.5) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (0.6-0.7) | –0.9 (–4.2 to 2.5) | –0.8 (–4.7 to 3.1) | 0.6 (–6.1 to 7.4) | |
| Q3 (0.8-0.9) | 1.1 (–2.8 to 5.1) | 3.0 (–1.7 to 7.7) | –0.4 (–8.1 to 7.2) | |
| Q4 (1.0-6) | 0.8 (–2.8 to 4.4) | 4.2 (–0.2 to 8.6) | –3.4 (–10.0 to 3.3) | |
| EtFOSAA | Q1 (0.1-0.7) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (0.8-1.2) | –1.9 (–5.4 to 1.7) | –0.8 (–5.1 to 3.4) | –6.2 (–13 to 0.6) | |
| Q3 (1.3-1.9) | 1.7 (–2.0 to 5.4) | 2.1 (–2.3 to 6.5) | –2.2 (–9.4 to 5.0) | |
| Q4 (1.9-33.6) | 0.4 (–3.4 to 4.1) | 0.0 (–4.5 to 4.4) | –1.5 (–8.8 to 5.9) | |
| MeFOSAA | Q1 (0.1-1.3) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (1.4-1.9) | 5.8 (2.1 to 9.4) | 5.5 (1.0 to 10.0) | 4.5 (–2.2 to 11.2) | |
| Q3 (2.0-3.2) | 2.4 (–1.1 to 5.9) | 2.1 (–2.1 to 6.3) | 1.1 (–5.5 to 7.8) | |
| Q4 (3.3-29.7) | 2.7 (–1.0 to 6.4) | 2.3 (–2.0 to 6.6) | –1.4 (–8.9 to 6.0) | |
Cross-product interaction term P values: PFOS = .13, PFOA = .46, PFHxS = .005, PFNA = .06, EtFOSAA = .70, MeFOSAA = .93.
Abbreviations: EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamide) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
aAdjusted for prepregnancy body mass index (continuous), prior history of gestational diabetes mellitus/parity, race/ethnicity, smoking, and education.
bAdditionally adjusted for maternal age (continuous).
Per- and polyfluoroalkyl substances mixture: Bayesian kernel machine regression.
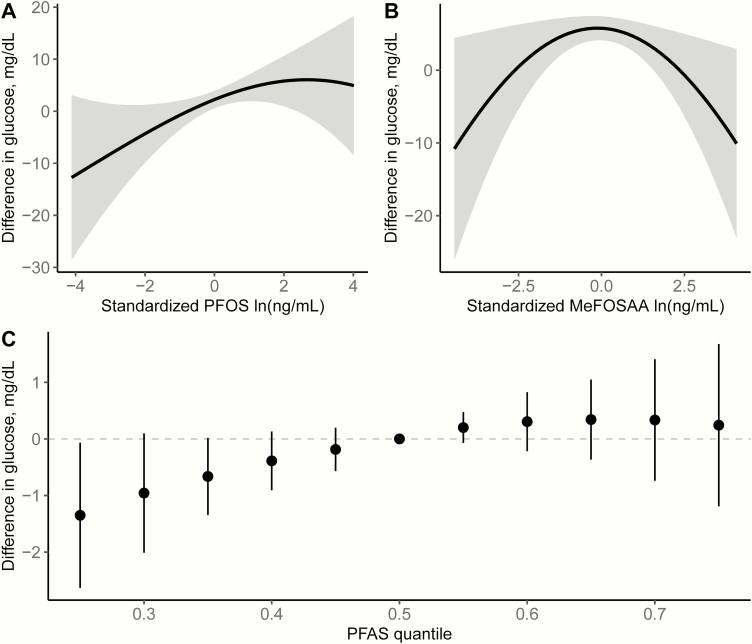
Fig. 1 shows the results of BKMR analyses for continuous glucose levels 1-hour post–50-g GCT modeling PFAS as a mixture. Fig. 1A and 1B show the univariate exposure-response functions and 95% confidence bands for PFOS (Fig. 1A) and MeFOSAA (Fig. 1B) in the full cohort, holding all other PFAS at their median concentrations. As suggested in our individual linear models in which PFAS were modeled in quartiles, we observed an independent positive linear association between plasma ln-PFOS concentrations and glucose levels 1-hour post–50-g GCT and a nonlinear association between plasma ln-MeFOSAA concentrations and GCT glucose levels. Holding all other PFAS at their median concentrations, the difference in glucose levels between the 25th and 75th percentiles of ln-PFOS concentrations was 6.2 mg/dL (95% credible interval, 1.1-11.3). We did not find evidence for associations between plasma concentrations of the remaining PFAS and glucose levels post-GCT (Table S3, Supplemental Material [50]). Fig. 1C represents the overall joint effect of the PFAS mixture as the estimated difference in glucose levels (95% credible intervals) comparing concentrations of all PFAS together at different percentiles of their distributions (eg, 20th, 40th, 60th, 80th) to all PFAS at their median concentrations. Exposure to all 6 PFAS was positively associated with GCT glucose levels, but the association leveled off at higher concentrations. Based on the estimated posterior inclusion probabilities (PIP), BKMR identified PFOS (PIP = 0.37) and MeFOSAA (PIP = 0.31) as being important contributors to the overall association, but not PFOA (PIP = 0.01), PFHxS (PIP = 0.001), PFNA (PIP = 0.004), or EtFOSAA (PIP = 0.005). We did not observe any evidence for nonadditive interactions among the PFAS (data not shown). In our sensitivity analysis, including the extreme PFAS values (n = 3) did not change the interpretation of our results (Fig. S2, Supplemental Material [50]).
Figure 1.
Combined effects of the per- and polyfluoroalkyl substances (PFAS) mixture on blood glucose levels 1-hour post–50-g nonfasting glucose challenge test (n = 1530) estimated by Bayesian kernel machine regression, adjusting for maternal age (continuous), prepregnancy body mass index (continuous), prior history of gestational diabetes mellitus/parity, race/ethnicity, smoking, and education. Univariate exposure-response function and 95% confidence bands for A, PFOS and B, MeFOSAA holding all other PFAS at the median. C, Overall effect of the PFAS mixture. This plot shows the estimated difference in blood glucose levels and 95% credible intervals when all PFAS concentrations are held at a certain percentile compared to when PFAS concentrations are held at the median. Abbreviations: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctane sulfonate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate.
Secondary effect measure modification analyses
The association between PFOS and glucose was modified by maternal age (Table 3). In stratified models, we observed significant positive associations between PFOS concentrations and glucose levels in younger women (< 35 years at enrollment; Q4 vs Q1, β = 6.5 mg/dL; 95% CI, 2.1-10.9) but not in older women (≥ 35 years; Q4 vs Q1, β = –0.7 mg/dL; 95% CI, –8.0 to 6.7); interaction P value = .13). Additionally, in younger women, PFNA concentrations were suggestively associated with higher glucose levels (Q3 vs Q1, β = 3.0 mg/dL, 95% CI, –1.7 to 7.7; Q4 vs Q1, β = 4.2 mg/dL, 95% CI, –0.2 to 8.6; interaction P value = .06), with similar patterns present for PFHxS (interaction P value = .005) and PFOA (interaction P value = .46).
We also observed evidence of potential effect modification by race/ethnicity (Table 4). When stratified by maternal race/ethnicity (Table 4), we observed large estimated differences in glucose levels for certain PFAS among Asian and Hispanic women; however, CIs were wide likely because of the small sample sizes. In Asian women, high PFOS and PFNA concentrations (Q4 vs Q1) were significantly associated with a 22.2 mg/dL (95% CI, 9.4-34.9; interaction P value = .30) and 19.2 mg/dL (95% CI, 4.9-33.6; interaction P value = .73) increase in glucose levels, respectively. In Hispanic women, high PFOS concentrations (Q4 vs Q1) were suggestively associated with a 13.0 mg/dL (95% CI, –3.0 to 28.9; interaction P value = .30) increase in glucose levels. Associations of PFOS and PFNA with glucose levels in white and black women were relatively null (Table 4). We did not observe evidence of effect modification by prepregnancy BMI (< 25, ≥ 25 kg/m2) (Table S4, Supplemental Material [50]).
Table 4.
Adjusteda difference in blood glucose levels (mg/dL) 1-hour post 50-g nonfasting glucose challenge test assessed at approximately 28 weeks’ gestation by first-trimester plasma per- and polyfluoroalkyl substances quartiles, overall and stratified by race/ethnicity
| All (n = 1533)b | Asian (n = 68) | Black (n = 234) | Hispanic (n = 108) | White (n = 1064) | Other (n = 59) | ||
|---|---|---|---|---|---|---|---|
| PFAS, ng/mL | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| PFOS | Q1 (0.1-18.8) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (18.9-25.7) | 2.4 (–1.2 to 6.0) | –1.0 (–15.8 to 13.7) | -0.6 (–11 to 9.7) | 0.0 (–13.7 to 13.6) | 2.0 (–2.3 to 6.3) | 15.2 (–6.9 to 37.3) | |
| Q3 (25.8-34.9) | 3.7 (0.0 to 7.4) | 6.2 (–7.4 to 19.8) | 7.4 (–2.5 to 17.3) | 0.3 (–14.5 to 15) | 2.6 (–1.9 to 7.1) | 12.0 (–9.1 to 33.2) | |
| Q4 (35.0-185.0) | 4.3 (0.5 to 8.0) | 22.2 (9.4 to 34.9) | 1.5 (–8.4 to 11.3) | 13.0 (–3.0 to 28.9) | 2.3 (–2.2 to 6.8) | 13.1 (–9.8 to 35.9) | |
| PFOA | Q1 (0.3-4.2) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (4.3-5.9) | –0.4 (–4.0 to 3.3) | –3.6 (–17.2 to 10) | 0.5 (–8.4 to 9.5) | 1.4 (–14.4 to 17.2) | –0.1 (–4.6 to 4.5) | 5.7 (–13.8 to 25.3) | |
| Q3 (6.0-7.9) | 1.0 (–2.8 to 4.8) | 8.4 (–6.8 to 23.6) | –0.5 (–10.3 to 9.2) | 8.5 (–8.0 to 25.0) | 1.1 (–3.6 to 5.8) | –1.7 (–23.6 to 20.1) | |
| Q4 (8.0-36.7) | 1.5 (–2.4 to 5.3) | 9.6 (–6.4 to 25.7) | –1.3 (–10.9 to 8.2) | 5.7 (–10.6 to 22.1) | 1.9 (–2.8 to 6.7) | –0.1 (–22.6 to 22.4) | |
| PFHxS | Q1 (0.1-1.6) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (1.7-2.5) | –0.7 (–4.3 to 3.0) | 4.4 (–9.5 to 18.3) | 1.0 (–7.8 to 9.9) | –7.9 (–20.9 to 5.1) | 0.2 (–4.4 to 4.9) | –2.4 (–27 to 22.3) | |
| Q3 (2.6-3.8) | 1.9 (–1.8 to 5.6) | 8.4 (–7.1 to 23.8) | 0.8 (–8.4 to 10.0) | 0.6 (–14.2 to 15.4) | 2.6 (–1.9 to 7.2) | –4.5 (–24.6 to 15.5) | |
| Q4 (3.9-74.5) | 1.2 (–2.6 to 5.0) | 9.3 (–6.5 to 25.1) | –0.8 (–11.3 to 9.7) | –8.0 (–25.4 to 9.4) | 1.8 (–2.8 to 6.4) | 12.2 (-8.8 to 33.1) | |
| PFNA | Q1 (0.1-0.5) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (0.6-0.7) | –0.9 (–4.2 to 2.5) | 0.3 (–19.8 to 20.4) | –0.4 (–9.3 to 8.4) | –0.9 (–14 to 12.2) | –1.2 (–5.2 to 2.8) | –1.0 (–20.6 to 18.6) | |
| Q3 (0.8-0.9) | 1.1 (–2.8 to 5.1) | 10.6 (–9.0 to 30.2) | –1.7 (-12.1 to 8.7) | 7.3 (–10.4 to 25.0) | 1.3 (–3.4 to 6.0) | 1.3 (–23.4 to 26.1) | |
| Q4 (1.0-6) | 0.8 (–2.8 to 4.4) | 19.2 (4.9 to 33.6) | 2.1 (–7.2 to 11.4) | 5.8 (–9.7 to 21.2) | –1.6 (–6.1 to 2.8) | 6.3 (–17.8 to 30.5) | |
| EtFOSAA | Q1 (0.1-0.7) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (0.8-1.2) | –1.9 (–5.4 to 1.7) | 2.1 (–14.1 to 18.4) | –1.9 (–11.9 to 8.1) | 7.6 (–7.2 to 22.4) | -3.7 (–7.9 to 0.5) | –1.6 (–20.0 to 16.8) | |
| Q3 (1.3-1.9) | 1.7 (–2.0 to 5.4) | –4.1 (–19.7 to 11.4) | 2.6 (–7.3 to 12.5) | 5.9 (–9.7 to 21.6) | 0.5 (–3.9 to 5.0) | 16.7 (–2.2 to 35.6) | |
| Q4 (1.9-33.6) | 0.4 (–3.4 to 4.1) | 6.5 (–9.8 to 22.8) | –4.8 (–15 to 5.4) | 10.0 (–6.4 to 26.3) | 0.6 (–3.8 to 5.1) | –13.6 (–33 to 5.8) | |
| MeFOSAA | Q1 (0.1-1.3) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Q2 (1.4-1.9) | 5.8 (2.1 to 9.4) | 5.5 (–9.7 to 20.8) | –3 (–13.6 to 7.5) | 4.0 (–10.8 to 18.8) | 7.5 (3.1 to 11.8) | 10.5 (–14.1 to 35.2) | |
| Q3 (2.0-3.2) | 2.4 (–1.1 to 5.9) | 8.8 (–6.8 to 24.4) | 6.2 (–3.5 to 15.8) | –5.5 (–20.9 to 9.9) | 1.5 (–2.7 to 5.7) | 4.6 (–14.2 to 23.3) | |
| Q4 (3.3-29.7) | 2.7 (–1.0 to 6.4) | 2.8 (–12.5 to 18.1) | 0.4 (–9.2 to 10.0) | 1.0 (–15.2 to 17.1) | 3.0 (–1.5 to 7.5) | 12.4 (–7.3 to 32.0) |
Cross-product interaction term P values: PFOS = .30, PFOA = .83, PFHxS = .83, PFNA = .73, EtFOSAA = .25, MeFOSAA = .42.
Abbreviations: EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamide) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
aAdjusted for maternal age (continuous), prepregnancy body mass index (continuous), prior history of GDM/parity, smoking, and education.
bAdditionally adjusted for maternal race/ethnicity.
Discussion
In the present study of pregnant women from a large prospective cohort in the Boston, Massachusetts, area, we observed a positive association between early-pregnancy plasma PFOS concentrations and late-second trimester nonfasting glucose levels from the GCT, as well as a nonlinear association between MeFOSAA concentrations and glucose levels. These associations persisted even after accounting for exposure to other PFAS in our mixtures analysis. Exposure to multiple PFAS, driven by PFOS and MeFOSAA, was positively associated with GCT glucose levels, mainly when PFAS concentrations were below the median, likely due to the nonlinear association with MeFOSAA. However, we found no associations between any of the PFAS with categories of glucose intolerance when chemicals were assessed individually in the overall population.
Prior studies assessing associations between PFAS concentrations and GDM have reported inconsistent results. Three studies reported no association between PFAS concentrations and GDM, including a Faroese cohort (n = 604; PFAS measured in the third trimester) (30), a Chinese case-control study (n = 84 cases, n = 168 controls; PFAS measured near delivery) (26), and a Chinese cohort study (n = 385; PFAS measured in early pregnancy) (25). Conversely, 5 other studies reported increased odds or risk of GDM and/or IGT associated with preconception or early pregnancy PFAS concentrations, including, among others, PFOA, PFOS, PFHxS, and PFNA (23, 24, 28, 29, 31). However, the specific PFAS measured and those identified as being associated with GDM and/or IGT varied across studies. Inconsistent study findings may potentially be due to substantial variability both in exposure and outcome assessment (23-31). With respect to exposure, studies have varied in individual PFAS measured, timing of PFAS measurement during pregnancy, and year sampled. For outcome assessment, studies differ by GDM screening and diagnostic criteria used, which could affect not only GDM prevalence estimates but also the detection of associations between PFAS and GDM across studies (51, 52).
Fewer studies have evaluated associations of PFAS exposure with continuous glucose levels during pregnancy. However, results from these studies were fairly consistent in terms of direction of effect (25, 27, 29); all these studies reported positive associations between various PFAS (ie, PFOA, PFOS, PFHxS, summed short-chain perfluorocarboxylic acids (PFCAs), summed long-chain PFCAs, summed total PFCAs) with increased glucose levels from 75-g fasting OGTTs (25, 27, 29). In our study, we found a positive association of PFOS concentrations and an inverted U-shaped association of MeFOSAA concentrations with nonfasting 50-g GCT glucose levels, but did not see associations with PFOA, PFNA, PFHxS, or EtFOSAA in the full cohort. Although we are unaware of a potential biological mechanism for the nonlinear association between MeFOSAA concentrations and glucose levels, the observed nonlinearity could also be a result of statistical imprecision due to our limited MeFOSAA concentration range and sparse observations at the upper concentrations. Further research is needed to understand whether there is a biological mechanism behind the observed nonlinear association. Similar to our PFOS findings, Liu et al reported a suggestive positive association between linear PFOS and 2-hour glucose levels from a fasting 75-g OGTT, as well as positive associations between linear PFOA and short- and long-chain PFCA summary measures, with 1-hour and/or 2-hour glucose levels (29). Conversely, PFOS concentrations were inversely associated with pregnancy OGTT glucose levels in a Chinese cohort study. However, median PFOS concentrations (5.4 ng/mL) were significantly lower in this study (25) compared to the Project Viva population (25.7 ng/mL).
Associations between PFAS exposure and glycemic outcomes during pregnancy may be modified by maternal factors. In a large US cohort study, Rahman and colleagues observed evidence of a heterogeneous effect of PFAS exposure on GDM risk, in which higher first-trimester PFNA, PFOA, perfluoroheptanoate, and perfluorododecanoate concentrations were associated with increased risk of GDM only in women with a family history of type 2 diabetes, a known risk factor for GDM (28). Similarly, in a Danish cohort, investigators saw no association with PFAS (including PFOA and PFOS) and OGTT glucose levels in the full population, but did find a positive association between PFHxS concentrations and fasting glucose in women categorized as “high risk” for GDM based on predefined risk factors: BMI greater than or equal to 27 kg/m2, family history of diabetes, glucosuria during pregnancy, previous GDM or delivery of a macrosomic child (27).
In our study, we examined potential effect modification by established GDM risk factors. Owing to the small number of women in our study with a family history of diabetes, we did not assess potential effect measure modification by family history of diabetes, as observed in Rahman et al (28). We found suggestive evidence that these associations varied across racial/ethnic groups. High PFOS and PFNA concentrations were associated with large increases in glucose levels in Asian women (PFOS and PFNA) and Hispanic women (PFOS), whereas associations with PFOS and PFNA were relatively null in white and black women. However, these estimates were imprecise with large CIs, likely because of the relatively small numbers of Asian (n = 68) and Hispanic (n = 108) women, and should be evaluated further in larger, more diverse populations. Studies have reported higher risk of GDM in Asian and Hispanic women compared to other racial/ethnic groups (2, 3). Although race is a social construct, differences in underlying susceptibility due to variations of genetic heritage across ethnic groups based on geographic origin could potential lead to underlying physiological (48) and/or genetic differences (45, 53), which may make certain ethnic groups more susceptible to additional effects of PFAS exposure on altered glucose tolerance during pregnancy. Other studies have observed similar patterns of stronger associations between other environmental exposures and increased risk of GDM or higher glucose levels in Asian women (43, 44). Another potential explanation for the heterogeneous effects seen across racial/ethnic groups could be underlying differences in lifestyle or demographic factors and/or concentrations patterns. However, although PFAS concentrations varied across racial/ethnic groups in Project Viva (33), there were not clear concentrations patterns that would explain our findings. Asian women tended to have lower concentrations of PFOA, PFHxS, EtFOSAA, and MeFOSAA compared to other groups, but had comparable PFOS concentrations and slightly higher PFNA concentrations, although the range of PFNA concentrations was rather small (33).
We hypothesized that women of advanced maternal age would be more susceptible to the effects of PFAS on glucose tolerance during pregnancy because of their increased risk of GDM (46, 54), partially attributed to their potential decreased insulin sensitivity due to deterioration of β-cell function associated with aging (46, 47). Surprisingly, when stratifying by advanced maternal age, a risk factor for GDM (49), we observed positive associations of PFOS, PFNA, and a nonlinear association with MeFOSAA and continuous GCT glucose levels in younger women (< 35 years) but not older women (≥ 35 years). It is possible that these differences are driven by other differences in lifestyle and demographic factors or PFAS concentration profiles rather than underlying physiological differences. For example, in Project Viva, younger women had higher PFOS and MeFOSAA concentrations, but lower PFNA concentrations, although the range of PFNA concentrations was small (33). Our findings suggest that maternal GDM risk factors may modify the associations between PFAS exposure and glycemic outcomes during pregnancy, but these findings should be further explored in larger, more diverse populations.
The need to assess the effects of chemical mixtures is increasingly important, given that individuals are exposed to many different chemicals (55, 56). Almost all previous studies evaluating associations between PFAS concentrations and pregnancy glucose levels have modeled PFAS individually and have not evaluated potential combined effects of multiple PFAS. Liu and colleagues modeled associations of PFAS summed by structural properties with glucose levels during pregnancy (29). However, this approach does not account for potential correlations among summed exposure variables and does not assess contributions/effects of individual exposures. In this study, we used BKMR to examine effects of exposure to 6 PFAS both individually and combined, while accounting for their moderate to high correlations. This allowed us to evaluate the joint effect of the PFAS mixture on glucose levels, while identifying the primary contributors to the effect, PFOS and MeFOSAA. Interestingly, the estimated individual effects of PFOS and MeFOSAA on glucose levels, accounting for all other PFAS, were similar to those observed in our single-PFAS linear models, potentially indicating a lack of confounding or issues of multicollinearity among PFAS in our individual analyses. Although there are a growing number of potential statistical methods for assessing the effects of exposure to environmental mixtures (55), BKMR’s ability to assess both individual and joint effects of exposure mixtures, nonlinear exposure-response functions, and potential interactions among exposures was well suited for our data and research questions.
There are several proposed mechanisms to explain the observed impact of exposure to certain PFAS on pregnancy glucose levels, including oxidative stress and activation of PPARs (21, 57); both pathways could lead to insulin resistance and altered glucose metabolism. In addition, there is evidence that the effects of PFAS may vary by their individual structural properties, such as chain length and functional group, potentially explaining the differences seen in associations among the 6 PFAS measured in our study. For example, Wolf et al demonstrated that PPARα activity in mice and humans is positively correlated with carbon chain length, but PPARα activity is higher in response to carboxylates (eg, PFOA) compared to sulfonates (eg, PFOS) (21). Further research is needed to understand how individual PFAS with detected associations in our study (ie, PFOS and MeFOSAA) might differentially affect glucose metabolism and what these impacts may mean for risk of related maternal and child health effects.
Our study has several limitations. A relatively small number of women in this population had GDM or IGT. As such, we may have been underpowered to evaluate subtle associations of PFAS with these categorical outcomes, as evidenced with the wider CIs for these analyses. Similarly, we were unable to assess effect modification when looking at PFAS and categorical glucose tolerance because of small numbers in certain population subgroups. Another limitation is the use of 1-hour glucose levels post–nonfasting 50-g GCTs rather than fasting glucose values. Although nonfasting glucose levels have been used in previous studies to investigate the effect of environmental exposures on glucose homeostasis during pregnancy (58, 59), they are more likely to be affected by dietary consumption prior to glucose screening compared to fasting glucose levels. This could result in greater variability in glucose levels, potentially contributing to increased noise and decreased precision of our model estimates. To account for the potential confounding of the association between PFAS and nonfasting glucose levels, we adjusted for a range of maternal sociodemographic covariates potentially affecting both nonfasting glucose levels and PFAS concentrations, and additionally assessed potential confounding by dietary patterns. Additionally, we had single measures both of PFAS and glucose levels for our analysis. However, the majority of PFAS in our study, except for EtFOSAA and MeFOSAA, have relatively long biological half-lives and a single measurement of these chemicals during early pregnancy may adequately represent an individual’s exposure over a longer time period (16, 17, 25). Project Viva was conducted in the late 1990s to early 2000s and consisted of mostly high socioeconomic status and non-Hispanic white women living in the United States, and results may not be generalizable to other study populations. However, PFAS concentrations in Project Viva are comparable to concentrations measured in the U.S. National Health and Nutrition Examination Survey during the corresponding sampling period (33). PFOS and PFOA concentrations have decreased dramatically following the general phase-out of long-chain PFAS (33), but capturing the relatively high concentrations of long-chain PFAS may have increased our ability to detect subtle associations between these PFAS and glucose levels. Additionally, we cannot rule out potential residual confounding from uncontrolled factors such as coexposures to other metabolism-disrupting chemicals.
Despite these limitations, our study has several strengths. First, we used a prospective cohort study design to evaluate the associations between early-pregnancy PFAS and pregnancy glucose tolerance assessed in later pregnancy from the large, multiracial/ethnic US-based Project Viva study population. We evaluated multiple categories of pregnancy glucose tolerance, including diagnosis of GDM in addition to IGT and IH, using data from the 2-step approach for clinical GDM assessment. Additionally, we evaluated continuous glucose levels to determine more subtle changes in glucose levels during pregnancy with respect to PFAS concentrations, modeling PFAS individually and as a mixture. To our knowledge, this is the first study to model effects of multiple PFAS on glucose levels during pregnancy using BKMR, a novel statistical method that accounts for correlations among PFAS while estimating both individual and joint effects of PFAS exposures. Finally, we were able to explore potential effect modification by maternal characteristics and observed differences in the associations between PFAS and glucose levels by maternal age, family history of diabetes, and race/ethnicity, including markedly stronger associations between select PFAS and higher glucose levels in Asian women.
Conclusion
We did not find evidence of associations between prenatal exposure to PFAS and odds of GDM or IGT. However, we observed a positive association between plasma PFOS concentrations and a nonlinear association between MeFOSAA concentrations measured in early pregnancy and 1-hour post-GCT glucose levels from late-second trimester GDM screening, when PFAS were modeled both individually and as a mixture. Additionally, we found suggestive evidence that these associations may vary across different population subgroups. These findings suggest that exposure to certain PFAS may affect glucose homeostasis in pregnant women, with potential implications for subsequent maternal and child cardiometabolic health outcomes associated with elevated glucose in pregnancy. Given the ubiquitous nature of PFAS exposures and the rising prevalence of GDM, future studies should further the understanding of potential effects of PFAS exposure on glucose homeostasis during pregnancy, especially in potentially vulnerable population subgroups.
Acknowledgments
The authors thank the Project Viva participants and staff, and gratefully acknowledge Kayoko Kato, Ayesha Patel, Tao Jia, and the late Xiaoyun Ye (Centers for Disease Control and Prevention, CDC) for PFAS measurements.
Financial Support: This work was supported by the National Institutes of Health (Grants T32ES007069, R01ES026166, R01ES021447, R01HD034568, and UH3OD023286). The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Glossary
Abbreviations
- BKMR
Bayesian kernel machine regression
- BMI
body mass index
- EtFOSAA
2-(N-ethyl-perfluorooctane sulfonamide) acetate
- GCT
glucose challenge test
- GDM
gestational diabetes mellitus
- GWG
gestational weight gain
- IGT
impaired glucose tolerance
- IH
isolated hyperglycemia
- LOD
limits of detection
- MeFOSAA
2-(N-methyl-perfluorooctane sulfonamide) acetate
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- PFAS
per- and polyfluoroalkyl substances
- PFCA
perfluorocarboxylic acid
- PFHxS
perfluorohexane sulfonate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
- PIP
posterior inclusion probabilities
- PPAR
peroxisome proliferator-activated receptor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium:International Diabetes Federation; 2017. https://www.diabetesatlas.org. [Google Scholar]
- 2. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2018;67(43):1201–1207. doi:10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 5. Lowe WL Jr, Scholtens DM, Lowe LP, et al. ; HAPO Follow-up Study Cooperative Research Group Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862-1868. [DOI] [PubMed] [Google Scholar]
- 7. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Song C, Li C, Liu P, Sun Z, Yang X. Increased risk of cardiovascular disease in women with prior gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:324-338. [DOI] [PubMed] [Google Scholar]
- 9. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905-914. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S103-S105. [DOI] [PubMed] [Google Scholar]
- 11. Lowe WL Jr, Scholtens DM, Kuang A, et al. ; HAPO Follow-up Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sargis RM, Heindel JJ, Padmanabhan V. Interventions to address environmental metabolism-disrupting chemicals: changing the narrative to empower action to restore metabolic health. Front Endocrinol (Lausanne). 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varshavsky J, Smith A, Wang A, et al. Heightened susceptibility: a review of how pregnancy and chemical exposures influence maternal health. [Published online ahead of print May 2, 2019.] Reprod Toxicol. doi: 10.1016/j.reprotox.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho SM. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol. 2016;33(13):1313-1318. [DOI] [PubMed] [Google Scholar]
- 15. Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29(2):131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118(2):222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Environ Sci Technol. 2011;45(19):8037-8045. [DOI] [PubMed] [Google Scholar]
- 19. Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res. 2019;177:108648. [DOI] [PubMed] [Google Scholar]
- 20. Rappazzo KM, Coffman E, Hines EP. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health. 2017;14(7):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci. 2008;106(1):162-171. [DOI] [PubMed] [Google Scholar]
- 22. Janani C, Ranjitha Kumari BD. PPAR gamma gene—a review. Diabetes Metab Syndr. 2015;9(1):46-50. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro GD, Dodds L, Arbuckle TE, et al. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC Study. Environ Res. 2016;147:71-81. [DOI] [PubMed] [Google Scholar]
- 24. Matilla-Santander N, Valvi D, Lopez-Espinosa MJ, et al. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: evidence from the Spanish INMA birth cohorts. Environ Health Perspect. 2017;125(11):117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Yang J, Du H, et al. Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a repeat measurement-based prospective study. Environ Int. 2018;114:12-20. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Zhang L, Teng Y, et al. Association of serum levels of perfluoroalkyl substances with gestational diabetes mellitus and postpartum blood glucose. J Environ Sci (China). 2018;69:5-11. [DOI] [PubMed] [Google Scholar]
- 27. Jensen RC, Glintborg D, Timmermann CAG, et al. Perfluoroalkyl substances and glycemic status in pregnant Danish women: the Odense Child Cohort. Environ Int. 2018;116:101-107. [DOI] [PubMed] [Google Scholar]
- 28. Rahman ML, Zhang C, Smarr MM, et al. Persistent organic pollutants and gestational diabetes: a multi-center prospective cohort study of healthy US women. Environ Int. 2019;124:249-258. [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Zhang L, Chen L, et al. Structure-based investigation on the association between perfluoroalkyl acids exposure and both gestational diabetes mellitus and glucose homeostasis in pregnant women. Environ Int. 2019;127:85-93. [DOI] [PubMed] [Google Scholar]
- 30. Valvi D, Oulhote Y, Weihe P, et al. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int. 2017;107:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril. 2015;103(1):184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol. 2015;49(19):11849-11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218(15):2133-2137. [DOI] [PubMed] [Google Scholar]
- 35. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12-S54. [DOI] [PubMed] [Google Scholar]
- 36. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153-162. [DOI] [PubMed] [Google Scholar]
- 37. Kong KL, Gillman MW, Rifas-Shiman SL, Wen X. Leisure time physical activity before and during mid-pregnancy and offspring adiposity in mid-childhood. Pediatr Obes. 2016;11(2):81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109(6):1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashley-Martin J, Dodds L, Arbuckle TE, et al. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int J Environ Res Public Health. 2016;13(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacDonald SC, Bodnar LM, Himes KP, Hutcheon JA. Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology. 2017;28(3):419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201(1):61.e1-61.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams AD, Grantz KL, Zhang C, Nobles C, Sherman S, Mendola P. Ambient volatile organic compounds and racial/ethnic disparities in gestational diabetes mellitus: are Asian/Pacific Islander women at greater risk? Am J Epidemiol. 2019;188(2):389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaffer RM, Ferguson KK, Sheppard L, et al. ; TIDES Study Team Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. 2019;123:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu L, Cui L, Tam WH, Ma RC, Wang CC. Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci Rep. 2016;6:30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kahveci B, Melekoglu R, Evruke IC, Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. 2018;18(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helman A, Avrahami D, Klochendler A, et al. Effects of ageing and senescence on pancreatic β-cell function. Diabetes Obes Metab. 2016;18(Suppl 1:58-62. [DOI] [PubMed] [Google Scholar]
- 48. Hasson BR, Apovian C, Istfan N. Racial/Ethnic differences in insulin resistance and beta cell function: relationship to racial disparities in type 2 diabetes among African Americans versus Caucasians. Curr Obes Rep. 2015;4(2):241-249. [DOI] [PubMed] [Google Scholar]
- 49. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. [DOI] [PubMed] [Google Scholar]
- 50. Preston EV, Rifas-Shiman SL, Hivert MF, et al. Supplemental Material for: Associations of per- and polyfluoroalkyl substances (PFAS) with glucose tolerance during pregnancy in the Project Viva cohort Deposited 15 May 2020. https://github.com/evpreston/PFAS-and-Glucose-Tolerance--Project-Viva/blob/master/Preston_Viva_PFAS_Glucose_Supplemental%20Material.pdf.
- 51. Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pedersen M, Olsen SF, Halldorsson TI, et al. Gestational diabetes mellitus and exposure to ambient air pollution and road traffic noise: a cohort study. Environ Int. 2017;108:253-260. [DOI] [PubMed] [Google Scholar]
- 53. Franks PW. Gene × environment interactions in type 2 diabetes. Curr Diab Rep. 2011;11(6):552-561. [DOI] [PubMed] [Google Scholar]
- 54. Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. Plos One. 2017;12(10):e0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taylor KW, Joubert BR, Braun JM, et al. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ Health Perspect. 2016;124(12):A227-A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124(1):A6-A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366-394. [DOI] [PubMed] [Google Scholar]
- 58. Bellavia A, Cantonwine DE, Meeker JD, et al. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environ Int. 2018;113:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. James-Todd TM, Chiu YH, Messerlian C, et al. ; EARTH Study Team Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health. 2018;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]