Abstract
Context
Not all obese individuals develop cardiovascular disease (CVD). Hyperaldosteronism is suggested to cause inflammation and metabolic dysregulation, and might contribute to CVD development in obese individuals.
Objective
We aimed to investigate the association of aldosterone concentrations with inflammation, metabolic disturbances, and atherosclerosis in overweight and obese individuals. Additionally, we measured renin concentrations to investigate whether the observed effects reflected general activation of the renin-angiotensin-aldosterone system (RAAS).
Design
A cross-sectional cohort study (300-OB study) was conducted. Various inflammatory parameters, traits of the metabolic syndrome, lipidome and metabolome parameters, fat distribution, and carotid atherosclerosis were associated with plasma aldosterone and renin levels.
Setting
The setting of this study was the Radboudumc (i.o. Radboudumc), the Netherlands.
Patients
A total of 302 individuals with a body mass index greater than or equal to 27 kg/m2 participated.
Main Outcome Measures and Results
Aldosterone was associated with various markers of inflammation and metabolic dysregulation, which partly differed from the associations observed for renin. Although both were associated with inflammatory cell numbers, only renin was associated with classical markers of systemic inflammation. Both were associated with the metabolic syndrome and hepatic steatosis. Of the traits that constitute metabolic syndrome, aldosterone, but not renin, was associated with triglyceride concentrations. Accordingly, aldosterone was associated with large very low-density lipoprotein particles; metabolomics studies further associated aldosterone with urate concentrations and derivatives of the linoleic acid metabolism pathway. Neither aldosterone nor renin was associated with atherosclerotic plaque thickness.
Conclusions
Aldosterone is not an important driver of systemic inflammation in the obese, whereas aldosterone concentrations and metabolic dysregulation are strongly intertwined in these individuals. Although prospective studies are necessary to validate these results, the independent effects of aldosterone on carotid atherosclerosis appear modest.
Keywords: aldosterone, obesity, renin-angiotensin-aldosterone system, atherosclerosis, inflammation, metabolomics
Cardiovascular disease (CVD) is the number one cause of morbidity and mortality worldwide. One of its risk factors is obesity, a growing problem with epidemic proportions in Western as well as non-Western countries (1). Interestingly, not all obese individuals develop metabolic or cardiovascular complications, with approximately 20% to 30% of the obese population being “metabolically healthy” (2). This illustrates the complexity of the pathophysiological mechanisms linking obesity to CVDs. Among other factors, obesity-associated activation of the renin-angiotensin-aldosterone system (RAAS), in particular hyperaldosteronism, might contribute to CVD in obese individuals.
The development of obesity and hyperaldosteronism are closely connected. Hyperinsulinemia and high circulating levels of adipocytokines activate the sympathetic nervous system, which in turn activates the RAAS. The proinflammatory adipocytokine leptin further contributes to hyperaldosteronism by directly stimulating aldosterone production by the adrenals (3). Last, the adipose tissue itself produces aldosterone (4). Aldosterone in turn promotes the maturation and dysfunctional differentiation of adipocytes via the mineralocorticoid receptor, leading to local expansion of fat mass, enhanced production of adipocytokines, and insulin resistance (5), resulting in a maladaptive vicious cycle.
In the general population, aldosterone concentrations are associated with type 2 diabetes, statin use, and metabolic syndrome (6), and they predict future cardiovascular events (7). Preclinical models investigating the mechanisms through which aldosterone contributes to cardiometabolic dysfunction show that aldosterone is causally linked to insulin resistance (8), cardiac and vascular fibrosis (9), as well as inflammation and atherosclerosis (10). Importantly, aldosterone is not the only RAAS component that exerts biological actions with disadvantageous immunological and vasculometabolic consequences. Renin as well as angiotensin II have both been reported to promote inflammation and insulin resistance (11). Therefore, different components of the RAAS system could have additive effects on CVD risk in obesity, and the extent of RAAS activation might in part explain the variation in the individual risk of obese individuals to develop metabolic syndrome and CVD.
The 300-Obese (300-OB) cohort, one of the studies of the Human Functional Genomics Project, comprises a group of more than 300 individuals of Western-European ancestry with a body mass index (BMI) greater than or equal to greater than or equal to 27 kg/m2, aged between 55 and 80 years (12). The study was designed to discover novel pathways contributing to CVD in overweight and obese individuals. Using the comprehensive data set of the 300-OB study, we here provide a detailed assessment of the association of circulating aldosterone and renin levels with inflammation, metabolic dysregulation, and atherosclerosis in the obese to address the hypothesis that RAAS activation—in particular aldosterone—contributes to metabolic dysregulation and atherosclerosis development in these patients and unravel the underlying mechanisms.
Methods
Extended methods are available in the Supplementary materials (13).
The 300-Obese cohort
A total of 302 individuals aged 55 to 80 years were enrolled in the 300-OB study at the Radboud University Medical Center, Nijmegen, the Netherlands, between 2014 and 2016. Inclusion criteria consisted of age older than 55 years and a BMI greater than or equal to 27 kg/m2. Exclusion criteria consisted of a recent cardiovascular event (myocardial infarction, transient ischemic attack, and stroke < 6 months), a history of bariatric surgery or bowel resection, inflammatory bowel disease, renal dysfunction, increased bleeding tendency, use of oral or subcutaneous anticoagulant therapy, use of thrombocyte aggregation inhibitors other than acetylsalicylic acid and carbasalate calcium, or a contraindication for magnetic resonance imaging (MRI). Participants who used lipid-lowering therapy temporarily discontinued this medication 4 weeks before the measurements. Each individual provided written informed consent prior to participation in this study. The study was conducted according to the principles of the International Conference on Harmonization–Good Clinical Practice guidelines.
Sample collection
Venous blood was drawn in the morning after an overnight fast.
Self-reported sodium intake
Each individual kept a detailed food diary for 4 days prior to sampling. Participants filled out an online diary on the Dutch Nutrition Centre website (14) or a standardized paper diary that was subsequently entered into the online diary from the Dutch Nutrition Centre by the researcher. Afterward the nutritional content, including sodium intake, could be extracted for each day.
Aldosterone and renin concentrations
Aldosterone and renin concentrations were measured in EDTA plasma and serum, respectively, by radioimmunometric procedures (Aldosterone RIA, IT1664, Demeditec Diagnostics GmbH; renin, Renin III generation, Cisbio Bioassay).
Vascular measurements
We performed carotid ultrasound after an overnight fast or in the afternoon 6 hours after a standardized breakfast, after abstention from caffeine and smoking. The presence of carotid plaque was defined as focal thickening of the wall of at least 1.5× the mean carotid intima-medial thickness (cIMT) or a cIMT greater than 1.5 mm (15).
Circulating mediators
Cytokines and circulating mediators were measured in EDTA plasma using enzyme-linked immunosorbent assay following the manufacturer’s instructions (R&D Systems). Interleukin (IL)-6 and IL-18 were measured by Simple Plex cartridges using the ELLA technology (Protein Simple).
Lipidomics and metabolomics
Lipidomics were performed using a high-throughput nuclear magnetic resonance metabolomics platform (Nightingale’s Biomarker Analysis Platform) (16). General Metabolomics performed flow-injection electrospray time-of-flight mass spectrometry to identify metabolic features based on mass to charge ratio (m/z). In total, 1339 m/z signals were assigned to one or more metabolites.
Assessment of fat distribution and hepatic steatosis
Abdominal fat distribution and liver fat content were determined by MRI and proton magnetic resonance spectroscopy (MRS), respectively.
Statistics
Aldosterone and renin as well as outcome parameters were normalized using rank based inverse normal transformation (INT). Aldosterone and renin levels were compared to sets of measurements (eg, metabolomics, circulating cytokine levels). All comparisons were corrected for multiple testing (Benjamini-Hochberg procedure) within each set of measurements; a false discovery rate (FDR) of less than 0.05 was considered significant, and findings with an FDR of less than 0.1 are described. β-Coefficients represent the correlation of the normalized data. The covariates included in the analysis are age, sex, BMI, smoking, systolic and diastolic blood pressure, and season, and are further described in the Supplementary materials (13). Data are plotted as scatterplots with Loess curves. Additional analyses (Tables 1 and 2) were performed on participants not taking antihypertensives (55% of the cohort), dividing them into tertiles based on aldosterone (or renin) levels. Normalized outcome parameters were compared between the lowest and highest tertiles with analysis of covariance correcting for the covariates described above. Benjamini-Hochberg multiple test–corrected P values are reported.
Table 1.
Subgroup analysis in those without antihypertensives, comparing lowest to highest aldosterone tertiles
| Outcome parameter | Lowest tertile (mean [SD]) | Highest tertile (mean [SD]) | P |
|---|---|---|---|
| Peripheral blood cell composition | |||
| Leukocytes, 109/L | 5.38 (1.16) | 6.39 (1.72) | .001 |
| Neutrophils, 109/L | 3.06 (0.99) | 3.53 (1.27) | .032 |
| Lymphocytes, 109/L | 1.65 (0.06) | 2.11 (0.07) | < .001 |
| Monocytes, 109/L | 0.45 (0.12) | 0.52 (0.18) | .062 |
| Circulating markers | |||
| VEGF, pg/mL | 35.93 (20.41) | 62.34 (74.53) | .028 |
| Metabolic syndrome score | 1.96 (0.94) | 2.53 (1.17) | .002 |
| Metabolic markers | |||
| Urate, mmol/L | 0.35 (0.08) | 0.38 (0.09) | .040 |
| Triglycerides, mmol/L | 1.47 (0.58) | 1.74 (0.67) | .034 |
| XL VLDL, mmol/L | 0.069 (0.066) | 0.091 (0.071) | .028 |
| Metabolomics | |||
| Lineoleic acid | 6.97 (0.10) | 7.03 (0.12) | .010 |
| Arachidonic acid | 5.93 (0.11) | 6.01 (0.13) | .010 |
| Adrenic acid | 4.93 (0.14) | 5.03 (0.16) | .010 |
| Docosapentaenoic acid | 5.28 (0.15) | 5.38 (0.20) | .010 |
| Prostaglandin F2A | 4.36 (0.06) | 4.39 (0.07) | .015 |
| Leukotriene B4 | 4.43 (0.07) | 4.47 (0.07) | .015 |
| Liver fat | 0.073 (0.096) | 0.107 (0.128) | .13 |
| Indicators of atherosclerosis | |||
| Plaque presence, % | 44 | 49 | .57 |
| Plaque thickness, cm | 2.25 (1.04) | 2.11 (0.48) | .79 |
| cIMT, µm | 769.5 (133.5) | 788.2 (148.7) | .79 |
| PWV, m/s | 9.28 (1.90) | 8.95 (1.71) | .79 |
Abbreviations: cIMT, carotid intima-medial thickness; PWV, pulse wave velocity; XL VLDL, extra-large very low-density lipoprotein.
Table 2.
Subgroup analysis in those without antihypertensives, comparing lowest to highest renin tertiles
| Outcome parameter | Lowest tertile (mean [SD]) | Highest tertile (mean [SD]) | P |
|---|---|---|---|
| Peripheral blood cell composition | |||
| Leukocytes | 5.68 (1.40) | 6.31 (1.57) | .09 |
| Neutrophils | 3.12 (0.96) | 3.68 (1.17) | .01 |
| Lymphocytes | 1.85 (0.55) | 1.89 (0.57) | .93 |
| Monocytes | 0.48 (0.14) | 0.51 (0.15) | .27 |
| Platelets | 229.5 (51.1) | 238.8 (53.7) | .23 |
| Circulating markers | |||
| IL-6, pg/mLa | 2.13 (1.55-2.87) | 2.45 (1.70-3.42) | .17 |
| IL18BP, ng/mL | 16.77 (5.15) | 17.23 (4.14) | .12 |
| Adiponectin, µg/mL | 5.20 (2.41) | 4.67 (2.38) | .17 |
| Metabolic syndrome score | 2.02 (1.06) | 2.24 (1.19) | .12 |
| Metabolic markers | |||
| Urate | 0.36 (0.08) | 0.35 (0.09) | .37 |
| Triglycerides, mmol/L | 1.53 (0.62) | 1.61 (0.68) | .36 |
| Glucose, mmol/L | 5.25 (0.60) | 5.76 (1.75) | .03 |
| Fat distribution | |||
| Liver fat | 0.079 (0.113) | 0.093 (0.087) | .57 |
| VAT | 92.59 (25.43) | 110.25 (34.56) | .02 |
| Indicators of atherosclerosis | |||
| Plaque presence, % | 47 | 53 | .57 |
| Plaque thickness, cm | 2.32 (1.01) | 2.11 (0.48) | .47 |
| cIMT, µm | 794.8 (175.1) | 775.9 (125.9) | .47 |
| PWV, m/s | 9.43 (1.53) | 9.25 (1.92) | .47 |
Abbreviations: cIMT, carotid intima-medial thickness; IL-6, interleukin 6; IL18BP, interleukin-18-binding protein; VAT, visceral adipose tissue.
aMedian (25th-27th percentile).
Results
Baseline parameters
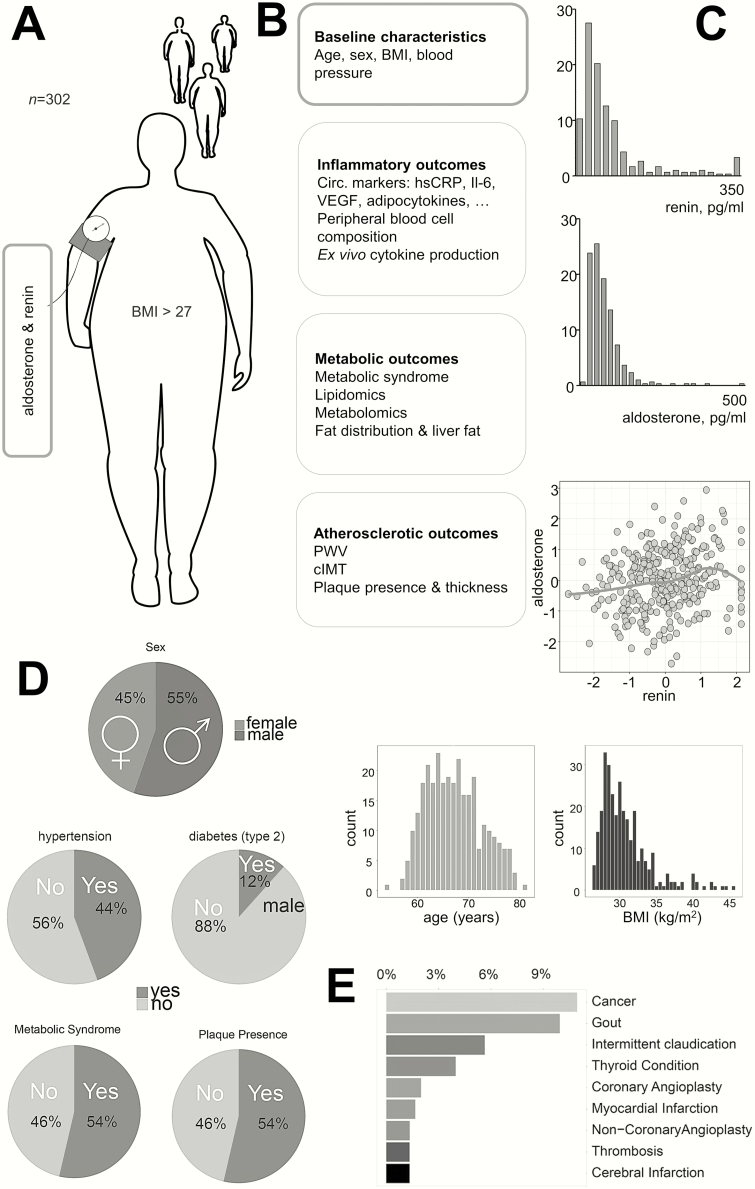
The study measurements and baseline characteristics of the study participants are described in Fig. 1A and 1B, respectively. Aldosterone concentrations (median 50.0 pg/mL, interquartile range 31.0-78.9 pg/mL) were comparable to previously reported values (6, 17). Renin concentrations showed a similar distribution (median 39.2 pg/mL, interquartile range 17.3-76.3 pg/mL), and were significantly correlated to aldosterone levels (r = 0.20, P < 1*10–3) (Fig. 1C). Data on comorbidity are presented in Fig. 1D. Forty-five percent of the population used antihypertensive medication: calcium antagonists (10%), β-blockers (22%), diuretic antihypertensives (23%), or renin-angiotensin system inhibitors (29%) (mineralocorticoid receptor antagonists were used by 1% of the population). Self-reported sodium intake showed a (weak) association with aldosterone levels (σ = –0.103, P = .08), but not with renin levels (σ = –0.002, P = .97).
Figure 1.
Overview of the 300-Obese cohort. A, We measured circulating aldosterone and renin in 302 individuals with a body mass index of 27 or greater. B, All individuals were extensively profiled. C, Histogram showing aldosterone and renin levels. D, Graphical representation of baseline characteristics of the cohort. E, Bar chart showing the most prevalent comorbidities in the cohort.
Renin, but not aldosterone, associates with classical circulating markers of inflammation
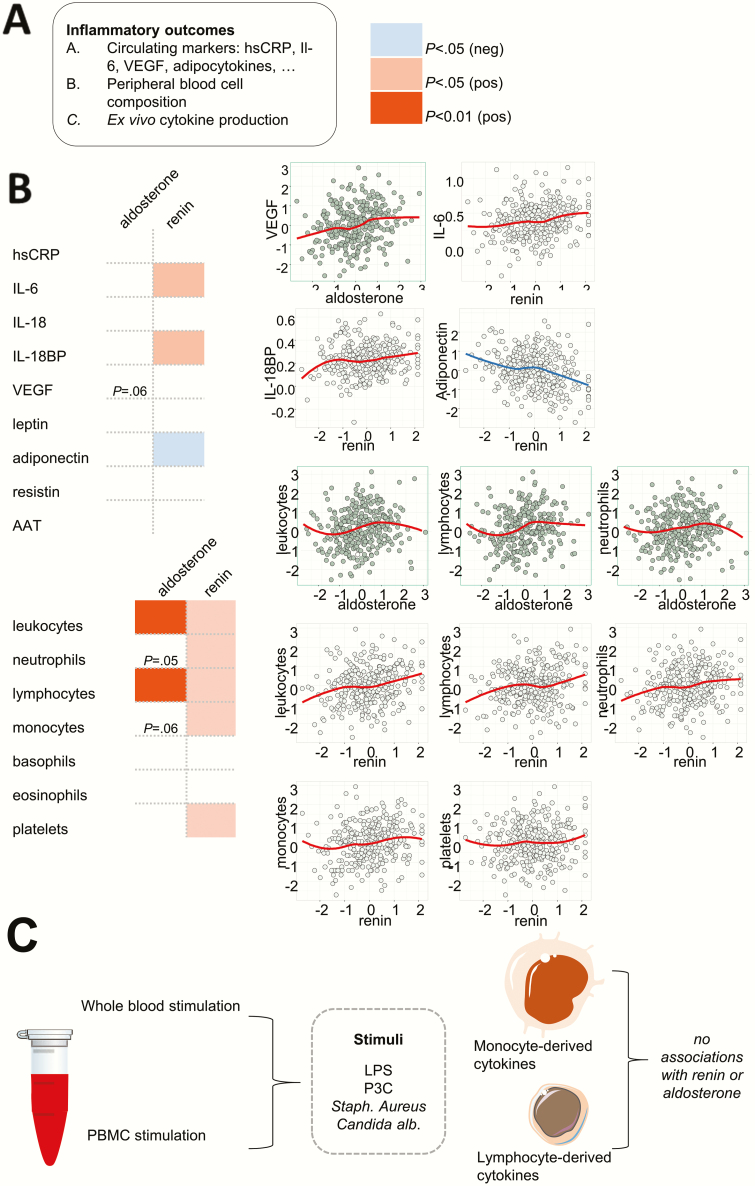
Aldosterone did not correlate significantly with any of the circulating markers. However, it displayed a trend to correlate with vascular endothelial growth factor A (VEGF-A) concentrations (β = .14, P = .06). Renin was significantly associated with the classical inflammatory markers interleukin (IL)-6 (β = .16) and IL-18 binding protein (IL18BP) (β = .17) and negatively with adiponectin (β = –.16). Neither aldosterone nor renin were associated with leptin or resistin levels. This is depicted in Fig. 2A.
Figure 2.
Associations of aldosterone and renin with markers of inflammation. A, Aldosterone and renin associated with different circulating markers of inflammation. B, The association of aldosterone and renin with inflammatory cell subtypes largely overlapped. C, Whole blood and peripheral blood mononuclear cells were isolated from all individuals and stimulated ex vivo with various stimuli, but no association of ex vivo cytokine production and aldosterone concentrations (or renin) was observed.
Aldosterone and renin associate with leukocyte counts
Aldosterone and renin were both associated with total leukocyte (β = .18 and β = .17, respectively), and lymphocyte counts (β = .18 and β = .14, respectively). Renin also was significantly associated with neutrophil and monocyte counts (β = .13 and β = .15); aldosterone showed a strong trend to correlate with these cell types (β = .13 and β = .11, respectively). Renin was also associated with thrombocyte counts (β = .19) (Fig. 2B).
Neither aldosterone nor renin associate with ex vivo cytokine or lactate production
Whole blood and peripheral blood mononuclear cells from all individuals were stimulated ex vivo with various stimuli (Fig. 2C). Neither aldosterone nor renin were associated with cytokine production. Accordingly, lactate production on ex vivo stimulation, an indicator of glycolytic activity of immune cells, was not associated with aldosterone or renin concentrations (data not shown).
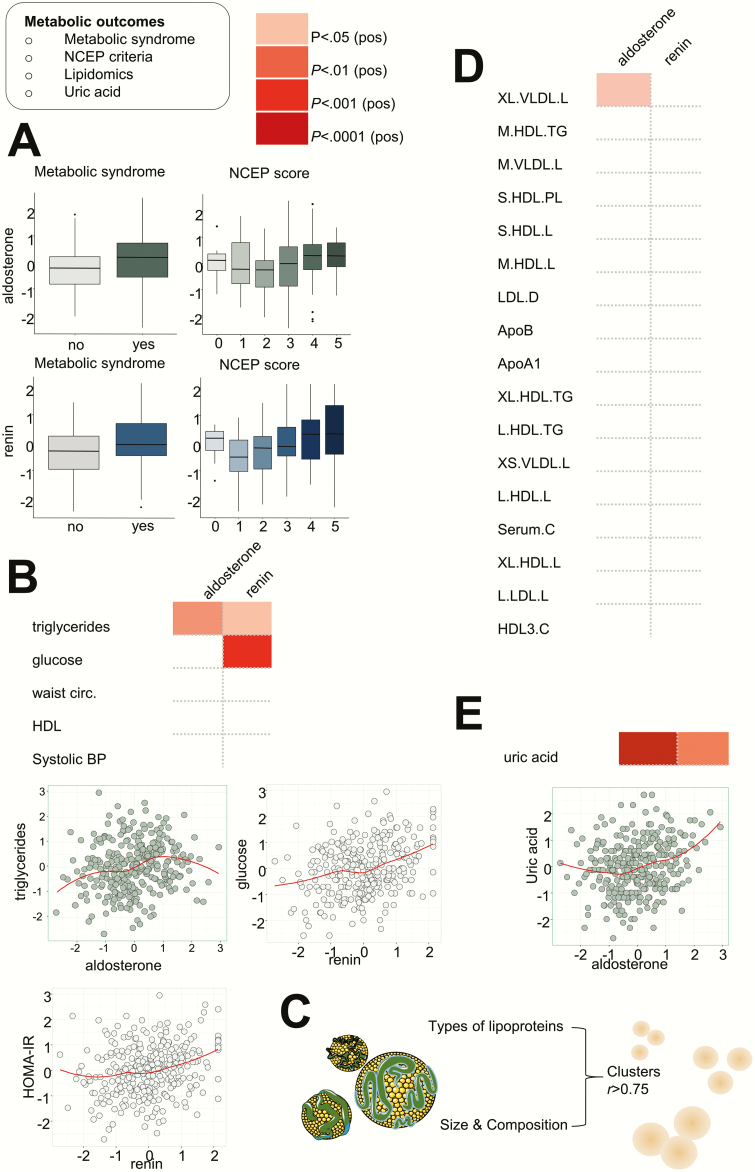
Aldosterone and renin both are associated with markers of metabolic dysregulation
Aldosterone and renin are both strongly associated with the presence of metabolic syndrome, and patients with metabolic syndrome displayed higher aldosterone and renin concentrations. In line with this, aldosterone and renin associated with metabolic syndrome scores based on the National Cholesterol Education Program (NCEP) criteria (Fig. 3A). Interestingly, the association with metabolic syndrome was established through different traits for aldosterone and renin. Aldosterone was associated with triglyceride levels (β = .16), whereas renin most strongly associated with glucose levels (β = .24) (Fig. 3B). Renin, but not aldosterone, was associated with the Homeostatic Model Assessment for Insulin Resistance (β = .15), indicating insulin resistance (Fig. 3B). Extensive lipidomic analysis provided information on lipid particle size and composition (Fig. 3C). Because of the strong correlations between lipid particles, they were grouped into associated clusters (clusters include particles with an r > 0.75). Using selected representative particles per cluster, we found that aldosterone was associated with large and extra-large very low-density lipoprotein (VLDL) particles (β = .17), whereas renin was not associated with lipid clusters (Fig. 3D). Last, aldosterone showed a significant association with urate concentrations (β = .24) (Fig. 3E); the association of renin with this metabolite was less strong (β = .19).
Figure 3.
Associations of aldosterone and renin with metabolic syndrome and metabolic derangements. A, Aldosterone was associated with the presence of metabolic syndrome, in line, patients with the metabolic syndrome had higher circulating aldosterone levels. Similar associations were observed for renin. Both aldosterone and renin associated with the number of positive NCEP criteria for metabolic syndrome. B, Aldosterone was associated with triglyceride levels, whereas renin associated with glucose levels. In line, renin associated with the HOMA-IR. C, Lipidomics was performed, and lipid particles clustered. D, Aldosterone was associated with the cluster of large to extra-large VLDL particles. E, Last, we observed a striking association of aldosterone with uric acid. HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; NCEP, National Cholesterol Education Program, VLDL, very low-density lipoprotein.
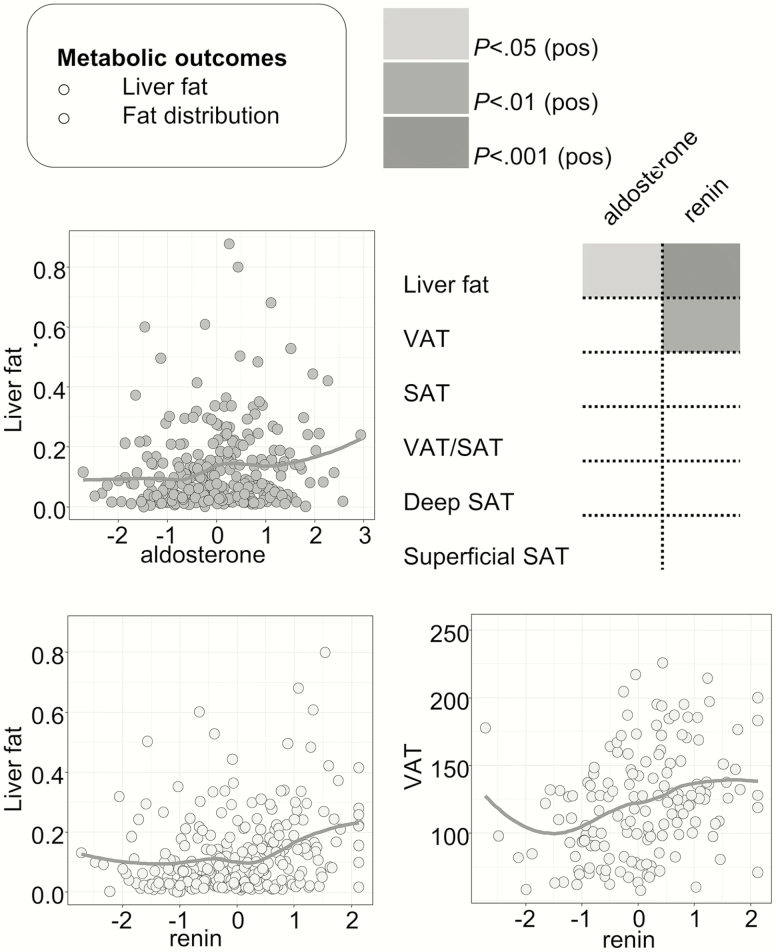
Both aldosterone and renin associate with liver fat on magnetic resonance spectroscopy
Aldosterone, and to a greater extent renin, was associated with the amount of liver fat on MRS. Renin concentrations also associated with the total amount of visceral adipose tissue on MRI (Fig. 4).
Figure 4.
Associations of aldosterone and renin with abdominal fat distribution and liver fat. Aldosterone, and more strongly renin, both were associated with the amount of liver fat on magnetic resonance spectroscopy. Renin was also associated with the amount of visceral adipose tissue on magnetic resonance imaging.
Metabolome profiling shows a strong association of aldosterone with various fatty acids in the α-linolenic and linoleic acid metabolism pathway.
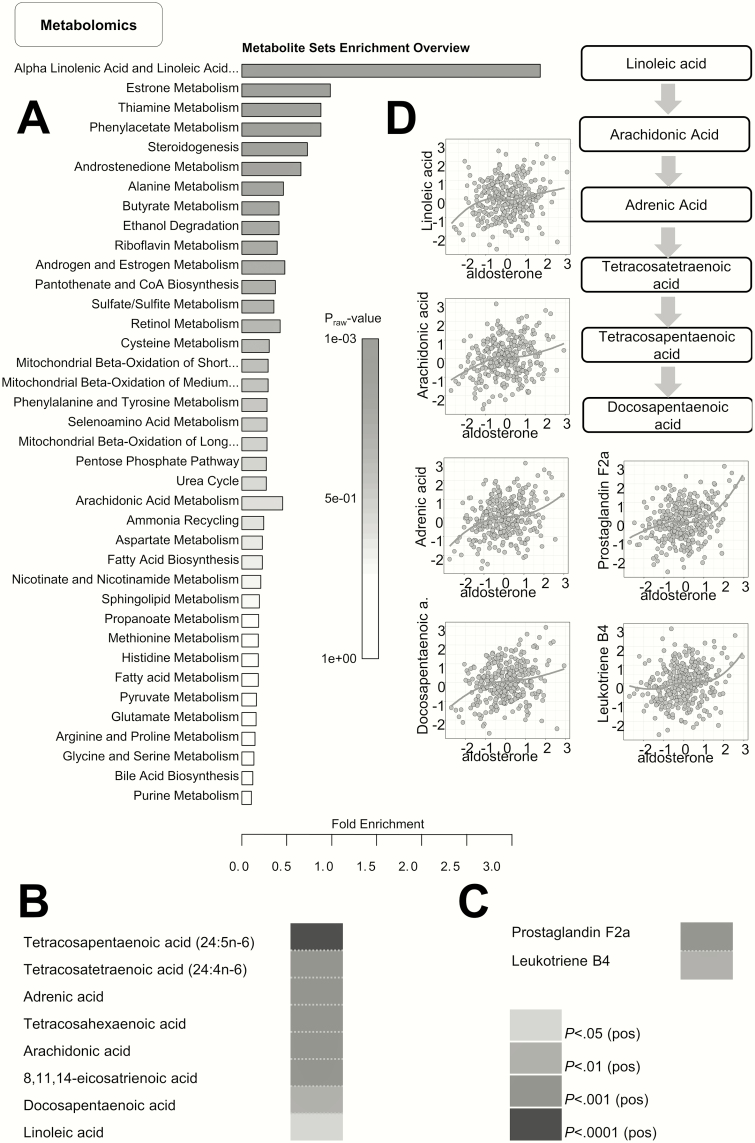
Metabolome assessment revealed that out of 1394 plasma metabolites, 165 were positively associated with aldosterone after multiple test correction, but none with renin. A list of these 165 metabolites is presented in Supplementary Table 2 (13). As expected, various steroids and steroid precursors were positively associated with aldosterone levels, among which were androstenedione, androsterone glucuronide, and pregnenolone. Furthermore, we identified several fatty acids of medium to very long chain length and saturated as well as unsaturated nature, in addition to phospholipids and eicosanoids. Subsequently, we performed a pathway enrichment analysis of the 165 metabolites. Although no pathways were significantly enriched, there was a trend toward enrichment of α-linolenic and linoleic acid metabolism pathway (raw P value = .001, FDR = .118), shown in Fig 5A. Metabolites contributing to enrichment of this pathway are depicted in Fig 5B, and mainly clustered in the linoleic acid, rather than the α-linolenic acid metabolism pathway (Fig. 5C)
Figure 5.
Metabolomics reveal that aldosterone levels associate with various metabolites in the linoleic acid metabolism pathway. A. Metabolite set enrichment analysis of all metabolites showing a significant positive association revealed linoleic acid metabolism as one of the most enriched pathways. B, Correlation of aldosterone with various intermediates of the linoleic acid metabolism, that is, C, arachidonic acid and its derivatives. D, Graphical representation of the linoleic acid pathway showing scatterplots of correlation of aldosterone with its derivatives.
Renin and aldosterone do not associate with carotid atherosclerosis
Neither aldosterone nor renin associated with pulse wave velocity or cIMT. Aldosterone and renin concentrations did not predict the presence of an atherosclerotic plaque. In patients with atherosclerotic plaques, neither aldosterone nor renin was associated with plaque thickness (data not shown).
Confirmation of findings in individuals without antihypertensives
Forty-five percent of the study population used antihypertensives. Of those, patients using diuretics displayed significantly higher aldosterone concentrations than those not using these drugs (aldosterone 78 ± 63 pg/mL vs 48 ± 57 pg/mL). Therefore, we validated all significant and trending associations (P < .1) described in previous paragraphs in those without antihypertensive drugs (n = 166), dividing this subgroup into tertiles based on plasma aldosterone levels (Table 1) and plasma renin levels (Table 2) and comparing outcomes in lowest to highest aldosterone/renin tertiles. Self-reported sodium intake did not differ significantly between the lowest and highest aldosterone tertiles (2.5 ± 1.2 vs 2.2 ± 1.1 g/24 hours, P = .15), or renin tertiles (2.5 ± 1.2 vs 2.4 ± 1.2 g/24 hours, P = .81). Comparable to the association in the complete study cohort, we observed a significant, albeit weak, association between renin and aldosterone levels (Spearman rho 0.194, P = .012) in this subgroup. We confirm associations of aldosterone with white blood cell counts, VEGF, metabolic syndrome scores, triglycerides, and extra-large VLDL, urate, and linoleic acid and its derivatives (Table 1). Moreover, similar to our observations in the complete cohort, we did not observe an association with classical markers of inflammation (not shown) or indicators of atherosclerosis (Table 1). Last, we provide coefficients and P values for the linear associations between aldosterone/renin and outcome parameters in those without antihypertensives and specifically diuretic antihypertensives in Supplementary Table 1 (13).
Discussion
This study provides a comprehensive assessment of the association of aldosterone concentration with inflammation, metabolic dysregulation, and atherosclerosis in a well-characterized cohort of more than 300 overweight and obese individuals. Based on previous preclinical research (10), we hypothesized that aldosterone—partly independent from renin—induces systemic inflammation and activation of circulating immune cells and thereby contributes to the development of vasculometabolic dysregulation in the obese. Although aldosterone showed a strong association with leukocyte numbers, it was not associated with systemic inflammatory markers, nor with cytokine production capacity of circulating immune cells. Therefore, we conclude that in obese individuals, aldosterone is not a major driver of systemic inflammation and immune system activation. We did however reveal specific associations of aldosterone, and not renin, with VLDL particles, linoleic acid metabolism, and urate, which provide novel clues about the pathogenic effects of aldosterone in obesity.
Obesity is characterized by low-grade inflammation with a multifaceted pathophysiology (18). Landmark trials have provided irrefutable evidence that inflammation is causally related to atherosclerotic CVD (19), underscoring the importance of the identification of common pathways driving inflammation in those at high CVD risk. Although murine models of hyperaldosteronism suggest proinflammatory effects of aldosterone (10, 20), and we previously showed long-term activation of human monocyte–derived macrophages after aldosterone exposure (21), we did not find evidence of proinflammatory effects of aldosterone in this cohort of obese individuals. These findings are in line with our recent findings that there are no significant differences in circulating IL-6 and high-sensitivity C-reactive protein levels or ex vivo cytokine production in a cohort of patients with primary aldosteronism compared to matched hypertensive controls (22). Moreover, no association of aldosterone with high-sensitivity C-reactive protein was found in a large population-based study (17). There are several potential explanations for these discrepant results. First, in preclinical hyperaldosteronism models the aldosterone concentration is often higher that the in vivo concentration in humans. Second, many of the conclusions on the effects of mineralocorticoid excess are extrapolated from MR knockout models. It is to be debated whether the magnitude of the biological effect of the absence of mineralocorticoid signaling would compare to—relatively subtle—effects of MR overstimulation. Third, many of these models are confounded by the effects of mineralocorticoids on blood pressure, which in itself affects cardiovascular health. Last, our present study is unique in that it explores the effect of aldosterone on inflammation in the context of obesity, which in itself promotes inflammation. Our findings provide evidence that in this context, aldosterone does not impose major additional proinflammatory effects.
Surprisingly, we could not confirm the previously reported association of aldosterone with the proinflammatory adipocytokine leptin (3), which is probably because in our cohort of obese individuals the variation in BMI and therefore leptin levels was small. Renin, however, was associated with the proinflammatory cytokine IL-6, a cytokine causally related to atherogenesis and CVD (23-25), and showed a negative association with the anti-inflammatory adiponectin, which has been described before (26). Of interest, we observed a trending association between aldosterone concentrations and VEGF-A, a pivotal regulator of angiogenesis (27). Although its role in CVD is inconclusive, VEGF-A has been suggested to increase monocyte chemotaxis and atherosclerotic plaque neovascularization (28). Recently, VEGF-A was shown to directly induce aldosterone synthase activity in adrenal cells (29). Alternatively, aldosterone could affect VEGF-A production because aldosterone induced VEGF-A expression in neutrophils (30) and the expression of VEGF family members in vascular smooth muscle cells (31).
Next, we investigated the association of aldosterone with metabolic derangements. We confirmed its association with metabolic syndrome (6). Surprisingly, aldosterone and renin were associated with metabolic syndrome via different traits. Aldosterone was associated with triglyceride levels, in line with previous reports (6). Renin concentrations, on the other hand, were associated most strongly with glucose levels and insulin resistance, confirming previous reports (32). The observation that renin, but not aldosterone, negatively associates with adiponectin—a potent insulin sensitizer (33), might explain part of this association. For aldosterone, a lipidomics approach pinpointed that large and extra-large VLDL particles most significantly contributed to the observed association with triglycerides. VLDL, a triglyceride-rich remnant particle, has been convincingly linked to CVD. In a large mendelian randomization trial, elevated nonfasting remnant cholesterol was associated with inflammation and ischemic heart disease (34). Moreover, VLDL-associated apolipoproteins were the strongest predictors of cardiovascular events in a prospective population-based study (35). Interestingly, an increasing amount of in vitro data suggest that VLDL can activate aldosterone production in the adrenals (36), suggesting that hyperaldosteronism results from, rather than causes, hypertriglyceridemia. This fits with our observation that renin is not associated with VLDL, excluding an effect of general RAAS system activation on VLDL concentrations. Further supporting this hypothesis is the clinical observation that statin use lowers aldosterone production independently of changes in renin or potassium (37).
Two other findings in this study are of particular interest for CVD. First, we observed an association of aldosterone with urate levels, a metabolite that is linked to inflammation and cardiovascular morbidity and mortality (38). Although data from mechanistic and population-based studies are conflicting (39, 40), ours suggest that in an obese population, aldosterone levels are associated with urate levels independently of classical CVD risk factors. Second, a metabolomics approach revealed that aldosterone was associated with fatty acids in the linoleic acid metabolism pathway. Linoleic acid, an ω-6 essential fatty acid, is the most abundant polyunsaturated fatty acid in humans. In CVD, its role appears mainly protective (41). Interestingly, oxidative derivatives of linoleic acid were previously shown to stimulate aldosterone production in vitro, and to be associated with aldosterone levels in a small cohort of healthy adults (42). Because linoleic acid is an essential amino acid, these findings also raise the question whether its dietary intake could directly affect aldosterone levels. Linoleic acid has several important downstream derivatives. One of the most well known is arachidonic acid, with which aldosterone showed a clear association (43). Although the direct inflammatory activity of arachidonic acid seems limited, it is the precursor of inflammatory eicosanoids. Among these is leukotriene B4, which we identified to be associated with aldosterone. Leukotriene B4, a potent chemoattractant mainly recruiting neutrophils, is associated with various inflammatory processes and mechanistic studies established its role in atherosclerosis (44, 45).
Last, we assessed the interaction of aldosterone and renin with carotid artery atherosclerosis. In animal models, RAAS activation, in particular induction of angiotensin II (46) and aldosterone (10), has been associated with accelerated atherogenesis. In patients with essential hypertension, aldosterone levels correlate to markers of preclinical atherosclerosis (40). Accordingly, we found that patients with primary hyperaldosteronism display enhanced arterial wall inflammation compared to hypertensive controls, suggestive of active (pre)atherosclerotic disease (22). In the present study, neither aldosterone nor renin was associated with markers of atherosclerosis in obese individuals. However, it is important to realize that our cohort differs significantly from the models and studies mentioned. Most important, the prevalence of risk factors for atherosclerosis, for example, insulin resistance and dyslipidemia, is significantly higher in obese individuals, which is likely to reduce the relative impact of renin and aldosterone on atherosclerosis development. Moreover, the design of our model and the age of our participants do not enable us to prospectively investigate the relation between RAAS activation and atherosclerosis development. Importantly, most participants in this cohort had no clinical symptoms of atherosclerotic CVD. Because symptomatic atherosclerosis is more strongly associated with inflammation than asymptomatic atherosclerosis, and inflammation is particularly important in destabilization of atherosclerotic plaques (47, 48), it is possible that RAAS activation induces inflammatory changes in atherosclerotic plaques, rather than the development of asymptomatic atherosclerosis per se.
Our study exemplifies the complex interaction of aldosterone, the RAAS, and obesity. Our findings result in 4 important hypotheses. First, because the studied associations differ between aldosterone and renin, our findings underscore the hypothesis that aldosterone in obese individuals is regulated by factors that are in part renin independent. Second, it has recently been hypothesized that aldosterone synthesis by the adrenals can be induced by inflammatory (ie, VEGF-A) and metabolic compounds (ie, VLDL), which increases the complexity of studying the independent effects of aldosterone on CVD in individuals with inflammatory and vasculometabolic derangements (such as obese individuals). However, numerous preclinical studies, with limited impact of confounding factors, described the immunologic and metabolic effects of aldosterone. Therefore, we thirdly hypothesize that part of the established associations are bidirectional, with aldosterone and common inflammometabolic indices intertwined in a vicious cycle. Fourth, based on our findings we hypothesize that different RAAS components could have different, additive effects on vasculometabolic health. Further elucidation of these mechanisms could contribute to the development of individualized pharmacological strategies for obese patients with increased cardiovascular risk.
Several limitations of our study require consideration. Almost half of our studied participants used antihypertensive medication, which affects the RAAS. Also, levels of RAAS hormones are dependent on sodium intake, diurnal rhythms, circulating volume, and exercise (49). As expected, self-reported sodium intake correlated negatively (albeit weakly) with aldosterone levels in our cohort. Although we aimed to reduce the influence of diurnal rhythms on hormone levels by obtaining all biomaterial in the morning, we cannot fully exclude variation due to the timing of sampling. However, because mineralocorticoid receptor antagonists were used in only 1% of our cohort, the height of circulating aldosterone levels reflects bioactivity at the MR, which is independent from the factors that determine these aldosterone levels. Importantly, we confirmed our findings in a subanalysis of the 55% of our cohort not using antihypertensives and comparable in salt intake, further underscoring the robustness of the associations of aldosterone and the reported outcome parameters. A second limitation of our study is the cross-sectional nature of our measurements, which does not enable us to determine the directionality of the observed effects. Mendelian randomization approaches would help to unravel whether RAAS hormones are causally related to the outcome parameters measured, whereas prospective studies would be the gold standard. Last, we did not measure angiotensin II levels in our cohort. Angiotensin II is a potent effector of renal and extrarenal RAAS effects, and therefore some associations—especially those of renin—might be exerted via angiotensin II.
In conclusion, our study revealed that aldosterone is associated with inflammatory cell counts and metabolic dysregulation in obese individuals, in part independently of renin. The increasing scale of the worldwide obesity problem warrants understanding of all the mechanisms that affect CVD risk in the obese. Importantly, because medication blocking the specific targets of aldosterone and other RAAS hormones are readily available, the potential therapeutic consequences of our findings are discernible. Although future studies are needed to investigate the directionality of the effects, our data reveal several novel pathways that could link aldosterone and RAAS activation to development of CVD in the obese.
Acknowledgments
We thank the staff of 300-OB for the data acquisition.
Financial Support: This work was supported by the European Union Horizon 2020 Research and Innovation Program REPROGRAM (under grant agreement No. 667837 to N.P.R., L.A.B.J., and M.G.N.); an IN-CONTROL CVON grant from the Dutch Heart Foundation (CVON2012-03 and CVON2018-27 to N.P.R., L.A.B.J., and M.G.N.); a Dutch Organization for Scientific Research Spinoza grant (NWO SPI 94–212 to M.G.N.); a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (HINT, ID P_37_762; MySMIS 103587 to L.A.B.J.); a grant of the ERA-CVD Joint Transnational Call 2018, which is supported by the Dutch Heart Foundation (JTC2018, project MEMORY; 2018T093 to N.P.R.); and the Deutsche Forschungsgemeinschaft (CRC/TRR 205 to J.D.).
Glossary
Abbreviations
- 300-OB
300-Obese
- BMI
body mass index
- cIMT
carotid intima-medial thickness
- CVD
cardiovascular disease
- FDR
false discovery rate
- MRS
magnetic resonance spectroscopy
- NCEP
National Cholesterol Education Program
- RAAS
renin-angiotensin-aldosterone system
- VEGF
vascular endothelial growth factor
- VLDL
very low-density lipoprotein
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep. 2016;18(5):21. [DOI] [PubMed] [Google Scholar]
- 2. Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372(9646):1281-1283. [DOI] [PubMed] [Google Scholar]
- 3. Huby AC, Otvos L Jr, Belin de Chantemèle EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67(5):1020-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briones AM, Nguyen Dinh Cat A, Callera GE, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59(5):1069-1078. [DOI] [PubMed] [Google Scholar]
- 5. Jia G, Aroor AR, Sowers JR. The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall. Cardiovasc Res. 2017;113(9):1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannemann A, Meisinger C, Bidlingmaier M, et al. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol. 2011;164(5):751-758. [DOI] [PubMed] [Google Scholar]
- 7. Buglioni A, Cannone V, Sangaralingham SJ, et al. Aldosterone predicts cardiovascular, renal, and metabolic disease in the general community: a 4-year follow-up. J Am Heart Assoc. 2015;4(12):e002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia G, Aroor AR, Hill MA, Sowers JR. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. 2018;72(3):537-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Heijden CDCC, Deinum J, Joosten LAB, Netea MG, Riksen NP. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc Res. 2018;114(7):944-953. [DOI] [PubMed] [Google Scholar]
- 11. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Netea MG, Joosten LA, Li Y, et al. Understanding human immune function using the resources from the Human Functional Genomics Project. Nat Med. 2016;22(8):831-833. [DOI] [PubMed] [Google Scholar]
- 13. van der Heijden C. Aldosterone in obesity: supplementary data Figshare; 2020. Deposited February 7, 2020. https://figshare.com/articles/Aldosterone_in_obesity_supplementary_data/11822160/1
- 14.Voedingscentrum. www.voedingscentrum.nl. Accessed April 17, 2020.
- 15. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soininen P, Kangas AJ, Würtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134(9):1781-1785. [DOI] [PubMed] [Google Scholar]
- 17. Grotevendt A, Wallaschofski H, Reincke M, et al. Associations of aldosterone and renin concentrations with inflammation—the Study of Health in Pomerania and the German Conn’s Registry. Endocrine. 2017;57(2):298-307. [DOI] [PubMed] [Google Scholar]
- 18. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177-185. [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. [DOI] [PubMed] [Google Scholar]
- 20. Marzolla V, Armani A, Feraco A, et al. Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids. 2014;91:46-53. [DOI] [PubMed] [Google Scholar]
- 21. van der Heijden CDCC, Keating ST, Groh L, Joosten LAB, Netea MG, Riksen NP. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc Res. 2020;116(2):317-328. [DOI] [PubMed] [Google Scholar]
- 22. van der Heijden C, Smeets EMM, Aarntzen E, et al. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;105(5):e1967–e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium; Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39(38):3499-3507. [DOI] [PubMed] [Google Scholar]
- 26. Hasan AU, Ohmori K, Hashimoto T, et al. Valsartan ameliorates the constitutive adipokine expression pattern in mature adipocytes: a role for inverse agonism of the angiotensin II type 1 receptor in obesity. Hypertens Res. 2014;37(7):621-628. [DOI] [PubMed] [Google Scholar]
- 27. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549-580. [DOI] [PubMed] [Google Scholar]
- 28. Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98(20):2108-2116. [DOI] [PubMed] [Google Scholar]
- 29. Gennari-Moser C, Khankin EV, Escher G, et al. Vascular endothelial growth factor-A and aldosterone: relevance to normal pregnancy and preeclampsia. Hypertension. 2013;61(5):1111-1117. [DOI] [PubMed] [Google Scholar]
- 30. Walczak C, Gaignier F, Gilet A, Zou F, Thornton SN, Ropars A. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim Biophys Acta. 2011;1813(12):2125-2132. [DOI] [PubMed] [Google Scholar]
- 31. McGraw AP, Bagley J, Chen WS, et al. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc. 2013;2(1):e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226-228. [DOI] [PubMed] [Google Scholar]
- 34. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298-1309. [DOI] [PubMed] [Google Scholar]
- 35. Pechlaner R, Tsimikas S, Yin X, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol. 2017;69(7):789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai YY, Rainey WE, Bollag WB. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J Endocrinol. 2017;232(2):R115-R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baudrand R, Pojoga LH, Vaidya A, et al. Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation. 2015;132(19):1825-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Hu X, Fan Y, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. 2016;6:19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulè G, Castiglia A, Morreale M, et al. Serum uric acid is not independently associated with plasma renin activity and plasma aldosterone in hypertensive adults. Nutr Metab Cardiovasc Dis. 2017;27(4):350-359. [DOI] [PubMed] [Google Scholar]
- 40. Concistrè A, Petramala L, Bisogni V, et al. Subclinical atherosclerosis due to increase of plasma aldosterone concentrations in essential hypertensive individuals. J Hypertens. 2019;37(11):2232-2239. [DOI] [PubMed] [Google Scholar]
- 41. Wu JHY, Marklund M, Imamura F, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43(2):358-363. [DOI] [PubMed] [Google Scholar]
- 43. Kawashima H. Intake of arachidonic acid-containing lipids in adult humans: dietary surveys and clinical trials. Lipids Health Dis. 2019;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mawhin MA, Tilly P, Zirka G, et al. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc Res. 2018;114(12):1656-1666. [DOI] [PubMed] [Google Scholar]
- 45. Heller EA, Liu E, Tager AM, et al. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation. 2005;112(4):578-586. [DOI] [PubMed] [Google Scholar]
- 46. Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103(3):448-454. [DOI] [PubMed] [Google Scholar]
- 47. Skagen K, Johnsrud K, Evensen K, et al. Carotid plaque inflammation assessed with (18)F-FDG PET/CT is higher in symptomatic compared with asymptomatic patients. Int J Stroke. 2015;10(5):730-736. [DOI] [PubMed] [Google Scholar]
- 48. Poredos P, Spirkoska A, Lezaic L, Mijovski MB, Jezovnik MK. Patients with an inflamed atherosclerotic plaque have increased levels of circulating inflammatory markers. J Atheroscler Thromb. 2017;24(1):39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hurwitz S, Cohen RJ, Williams GH. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol (1985). 2004;96(4):1406-1414. [DOI] [PubMed] [Google Scholar]