Abstract
Purpose
Menopause is a crucial physiological transition during a woman’s life, and it occurs with growing risks of health issues like osteoporosis. To identify postmenopausal osteoporosis-related genes, we performed transcriptome-wide expression analyses for human peripheral blood monocytes (PBMs) using Affymetrix 1.0 ST arrays in 40 Caucasian postmenopausal women with discordant bone mineral density (BMD) levels.
Methods
We performed multiscale embedded gene coexpression network analysis (MEGENA) to study functionally orchestrating clusters of differentially expressed genes in the form of functional networks. Gene sets net correlations analysis (GSNCA) was applied to assess how the coexpression structure of a predefined gene set differs in high and low BMD groups. Bayesian network (BN) analysis was used to identify important regulation patterns between potential risk genes for osteoporosis. A small interfering ribonucleic acid (siRNA)-based gene silencing in vitro experiment was performed to validate the findings from BN analysis.
Result
MEGENA showed that the “T cell receptor signaling pathway” and the “osteoclast differentiation pathway” were significantly enriched in the identified compact network, which is significantly correlated with BMD variation. GSNCA revealed that the coexpression structure of the “Signaling by TGF-beta receptor complex pathway” is significantly different between the 2 BMD discordant groups; the hub genes in the postmenopausal low and high BMD group are FURIN and SMAD3 respectively. With siRNA in vitro experiments, we confirmed the regulation relationship of TGFBR2–SMAD7 and TGFBR1–SMURF2.
Main Conclusion
The present study suggests that biological signals involved in monocyte recruitment, monocyte/macrophage lineage development, osteoclast formation, and osteoclast differentiation might function together in PBMs that contribute to the pathogenesis of postmenopausal osteoporosis.
Keywords: postmenopausal osteoporosis, coexpression analysis, osteoclast, transcriptomics, siRNA
An imbalance process between bone resorption (by osteoclasts) and bone formation (by osteoblasts) results in osteoporosis, which is a major health problem in postmenopausal women (1). It is well recognized that during the menopausal transition status, depletion of estrogen greatly affects osteoclastic bone resorption and the bone loss process (2, 3). Circulating monocytes can differentiate into a variety of cells (4), and mature peripheral blood monocytes (PBMs) can be further categorized into 3 subpopulations in accordance with the surface expression of CD14/CD16 (5, 6). The classic subset of PBMs (high level of CD14 and lack of CD16) can migrate to bone resorption sites, thus serving as latent osteoclast precursors. PBMs play important roles in osteoclastogenesis by acting as osteoclast precursors and secreting osteoclastogenic factors (7-9). Therefore, PBMs represent a class of cells that are of high functional relevance to osteoporosis development, and have been accepted for pathophysiology studies in the bone field (10).
Transcriptome-wide expression studies are a powerful tool for researchers to comprehensively perceive and quantify complex biological systems. Microarray studies have been generally applied in osteoporosis research in the identification of biomarkers crucial for osteoclasts and of functional pathways having profound effects for bone remodeling (11, 12).
Network-based analyses through expression data have become useful tools for systems biology. Multiscale embedded gene coexpression network analysis (MEGENA) is a method designed for constructing and analyzing large-scale coexpression networks (13). MEGENA first applies the network embedding technique on the topological sphere to construct unweighted coexpression networks and then identifies multiscale organizations of coherently coexpressed gene units (14, 15). The application of this method helped to reveal meaningful multiscale organizations of coexpressed gene clusters, and showed improved performance in identifying more coherent modules over well-established de novo network construction approaches such as weighted gene coexpression network analyses (13). Gene sets net correlations analysis (GSNCA) is a multivariate differential coexpression test for gene sets and it can detect significant changes in the coexpression structure of gene groups between 2 different biological conditions (16). Instead of considering differences in pairwise correlations between genes, the GSNCA method accounts for the global correlation structure of gene sets, and it works well on microarray datasets (17). As a probabilistic graphical model, Bayesian networks (BNs) has been applied in genetic related studies (18). Through a graphical separation process, the main role of BNs is to express conditional dependencies among variables. Recently, a BN generated from gene coexpression modules was used to uncover regulatory driver genes affecting coronary artery disease (19), and it helped to recognize the causal network structures relevant to late-onset Alzheimer’s disease as well (20). The directional relationships between nodes built by BNs could further help us to explore gene expression patterns from a mechanistic point of view.
In this study, we proposed a network-based platform for transcriptome-wide expression study of PBMs from 40 postmenopausal women with high and low bone mineral density (BMD). The first goal was to identify individual genes contributing to postmenopausal osteoporosis pathogenesis. By employing advanced network analyses strategies, the second aim was to detect highly correlated gene groups (cofunctioning genes) and gene sets important for postmenopausal BMD variation. The innovative part of our study was that with a series of bioinformatics analyses, we not only found genes and pathways important for postmenopausal osteoporosis, but also validated regulation patterns between crucial gene pairs in PBMs based on in vitro experiments. In addition, we applied small interfering ribonucleic acid (siRNA)-based gene silencing in vitro experiments to support regulatory relationships found by causal network inference.
Materials and Methods
Subjects for the microarray study
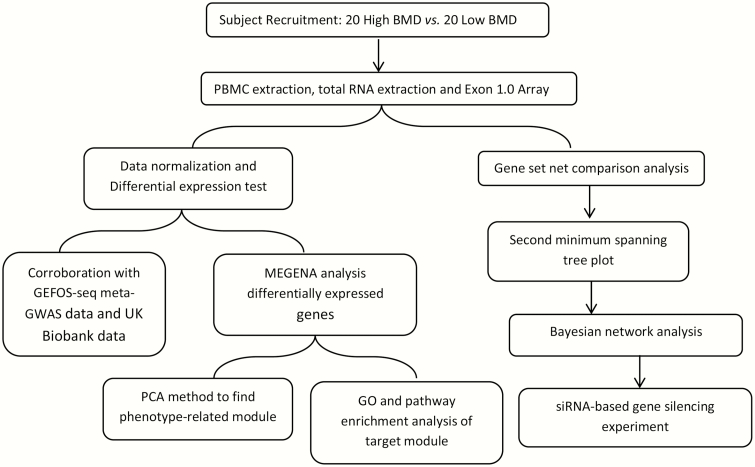
The study was approved by the Institutional Review Board, and signed consent documents were obtained from all study participants before being enrolled in the study. The detailed recruitment criteria and BMD measurement procedures have introduced in our previous studies (21). Hip BMD (g/cm2) was measured using a Hologic dual-energy X-ray absorptiometer scanner (Hologic Corp., Waltham, MA). None of our subjects had any known conditions that might artificially increase BMD values, such as osteophytes and facet sclerosis. The obtained BMD value was transformed into a Z-score, which is the unit of standard deviation above or below the mean of a healthy, ethnic-, gender-, and age-matched reference population. Our discovery cohort includes 40 Caucasian postmenopausal females (GSE56815), 20 with high BMD and 20 with low BMD, and the 2 groups were matched for age and years of postmenopausal status. The workflow of this study is shown in Fig. 1.
Figure 1.
The workflow of the current study.
Experiment procedures
PBM isolation, RNA extraction, and array procedure
As described in previous studies by our group, PBMs were isolated from whole blood samples with a monocyte-negative isolation kit (Miltenyi Biotec Inc., Auburn, CA) (22) that best represented the in vivo expression profiles in human subjects. The Qiagen RNeasy Mini kit (Qiagen, Inc., Valencia, CA) was used to extract total RNA from monocytes, and the Agilent Bioanalyzer (Agilent, Santa Clara, CA) was applied to control RNA quality (23, 24). Preparation of complementary deoxyribonucleic acid (cDNA), hybridization, and scanning of the GeneChip Human Exon 1.0 ST array was performed based on the manufacturer’s protocol for the Exon 1.0 array (Affymetrix, Santa Clara, CA).
Differential expression analysis
The robust multiarray average method (25) was applied to normalize the array signals through the Bioconductor Oligo package (26). After data normalization, we performed differential expression analysis through the Bioconductor LIMMA (linear models for microarray data) package (27).
To gain additional support for the differentially expressed genes in our discovery study, we checked the single-nucleotide polymorphisms (SNPs) of the top genes for their association signals in the GEFOS-seq (Genetic Factors for Osteoporosis Consortium) genome-wide association study (GWAS) datasets and UK Biobank GWAS datasets. In the bone field, GEFOS has developed consecutive meta-analyses of GWAS with the largest sample sizes, aiming to identify genetic variants related to BMD (28-30). The GEFOS-seq study meta-analyzed association results for BMD from 9 discovery cohorts (n = 32 965), including a whole-genome sequencing in the UK10K project (n = 2882), a whole-exome sequencing study with 5 cohorts (n =3549), and genotyped samples with deeply imputation using a combined UK10K/1000 Genomes reference panel (n = 26 534) (30). Since GEFOS-seq did not use hip BMD as a phenotype, we checked the genetic association between SNPs with femoral neck BMD (FN-BMD) and lumbar spine BMD. The UK Biobank osteoporosis dataset contains estimated BMD (eBMD) GWAS summary statistics from 426 824 individuals. In this study, BMD was measured by quantitative ultrasound of the heel (a noninvasive eBMD that predicts fracture) (31). With stringent quality control of both eBMD and genome-wide genotypes, the study identified 518 genome-wide significant loci, explaining 20% of its variance (32). Since the UK Biobank osteoporosis study is the representative GWAS study that measures association between SNPs (and thus their annotated genes) with eBMD, it could provide further partial support for our identified differential expressed genes.
MEGENA (with differential expressed genes)
MEGENA is an innovative coexpression network analysis method that can effectively and efficiently construct large-scale coexpression planar filtered networks (13). Parallelization of embedded network construction and multiscale clustering structure identification are the 2 major components of this method. It may uncover biologically meaningful gene orchestrating groups and key regulators of important biological processes. In the current study, we applied this method to construct the coexpression pattern among differential expressed genes.
Fast planar filtered network construction
The first step of MEGENA analysis is fast planar filtered network (PFN) construction, in which respective interaction strength (eg, Pearson’s correlation) was used to rank gene pairs with greater similarity. Then the planarity was iteratively tested to build the embedded network including gene pairs with most similarities (33). Insignificant interactions were removed with FDR > 0.05.
Multiscale clustering analysis
The constructed PFNs were input to multiscale clustering analysis (MCA) to achieve the subsequent analyses. Three criteria, shortest path distances, local path index, and overall modularity, were incorporated in the MCA to identify locally compact clusters (34, 35). A hierarchical divisive approach is adopted by MCA to split complex interactions from PFN into more coherent gene groups through iterating processes (13).
Downstream analysis
Multiscale hub analysis was applied to identify significant hubs within each cluster (14, 35). To relate an established modular structure to phenotypes in our dataset, we calculated Pearson’s correlation between eigengene (first principal component of cluster) and clinical traits; modules with P < .05 were taken as phenotype-related modules. Interacting patterns for all members in the target module were searched through STRING version 9.1 (36). The GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis of compact gene clusters identified by MEGENA was performed with STRING software as well. The Bonferroni-adjusted P value (< .05) was used as a threshold for identifying significant functional clusters.
Gene sets net correlations analysis
GSNCA is a derivative method from the original Gene Sets Comparison Analysis. To address the limitation that traditional gene set comparison methods do not take into account the structure of the gene coexpression pattern, Rahmatallah et al. proposed the GSNCA method, which is based on the impression that biological systems tend to be more affected by changes in the activities of “high weight” genes (16). The GSNCA method introduces a weight (wi) to each gene based on the gene’s cross-correlation with all the other genes to quantify their corresponding regulatory importance, and compares the normalized weight vectors for gene groups between 2 conditions. In this study, gene sets were taken from molecular signature database (MSigDB) C2 curated gene sets (37, 38), including 3272 metabolic, biochemical, and signaling pathways from the Reactome, BioCarta, and KEGG databases. The GSNCA analysis was implemented through the “GSAR” R Package. The detailed network features (average absolute cross-correlation, degree centrality of hub gene, normalized betweenness centrality of hub gene, average normalized betweenness centrality, normalized closeness centrality of hub gene, average normalized closeness centrality) were calculated for coexpression gene sets as well. MST2 (first and second minimum spanning trees) method was applied to present the coexpression network features in the low versus high BMD groups. It is believed that the union of the first and second MST, constructed from correlation distances, generates the minimal set of essential connections among genes (16).
Bayesian network analysis
BNs are graphical based models where nodes (vertex) represent random variables and the corresponding edges (arcs) represent probabilistic dependencies (18). BNs have been applied in numerous kinds of biological data like SNP data and gene expression profiles (39). In this study, we applied BN analysis to differentially coexpressed gene sets identified by the previous GSNCA. The gene expression data in the identified gene sets were discretized using Hartemink’s method (40), which was designed to preserve most pairwise dependencies. Then we performed constraint-based structure learning on the discretized data (39). After repeating the structure-learning procedure 100 times, gene network structures learned in the previous stage were averaged to construct a more optimal one using the model-averaging approach (41). Arcs present in at least 80% of the networks were used to generate the averaged network structure (18). The bootstrap resampling approach was performed to learn a set of 500 network structures for model averaging.
siRNA validation experiment for the identified TGFBR2–SMAD7 and TGFBR1–SMURF2 regulations
To provide general support for our approach and results, we chose to validate the causal regulatory relationships of TGFBR2–SMAD7 and TGFBR1–SMURF2 identified in the BN analysis within subjects with low BMD, by performing siRNA-based gene silencing in vitro experiments.
Cell culture
Since THP-1 (Human acute monocytic leukemia cell line) cells represent a valuable tool for investigating monocyte structure and function in both health and disease conditions (42), we used it in our experiment. THP-1 cells (Origin: Cell Biology Research Laboratory Modern Analytical Testing center, Central South University, Changsha, China) were cultured in RPMI 1640 medium (Gibco BRL, Rockville, MD) containing 10% heat-inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA) and antibiotics (100 U/mL penicillin G and 100 µg/mL streptomycin) under a humidified atmosphere (37°C, 5% CO2).
Transfection
siRNAs were generated against human TGFBR1 (sense strand 5′-GAACAGAAGTTAAGGCCAA-3′ and antisense strand 5′-UUGGCCUUAACUUCUGUUC-3′), and TGFBR2 (sense strand 5′-CCATCATCCTGGAAGATGA-3′ and antisense strand 5′-UCAUCUUCCAGGAUGAUGG-3′) (Ribobio Company, Guangzhou, China). Cells were seeded at 1 × 105 cells/well in 24-well plates. After 24 hours, cells were transfected with siRNA-TGFBR1 or siRNA-TGFBR2 in the presence of riboFECTTM CP transfection (Ribobio Company, Guangzhou, China) for 48 hours. Transfected cells were then collected for subsequent experiments. Flow cytometry was used to detect transfection efficiency.
Reverse transcription and real-time quantitative polymerase chain reaction
Total cellular RNA was isolated from the transfected THP-1 cells with Trizol (Invitrogen Corp, Carlsbad, CA) according to the manufacturer’s instructions. The extracted RNA was subjected to reverse transcription using the GoScript™ Reverse Transcription System kit (Promega Corp, Madison, WI). Real-time quantitative PCR (RT-PCR) was used to detect the relative mRNA level of SMAD7 and SMURF2. RT-PCR was conducted with cDNA converted from total RNA using amplification by 40 cycles of 95°C for 300 seconds, 95°C for 10 seconds, and 58°C for 10 seconds, 72°C for 30 seconds. The CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) was used with MonAmp™ ChemoHS qPCR Mix (Monad Biotech Corp, Zhuhai, China). Expression of PCR products was normalized for that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers for RT-PCR are shown in Table 1.
Table 1.
Primers for determining expression levels of TGFBR1, TGFBR2, SMAD7, SMURF2, and GAPDH genes.
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| TGFBR1 | 5′-TCAGCTCTGGTTGGTGTCAG-3′ | 5′-ATGTGAAGATGGGCAAGACC-3′ |
| TGFBR2 | 5′-CAACATCAACCACAACACAGAG-3′ | 5′-CCGTCTTCCGCTCCTCAG-3′ |
| SMAD7 | 5′-TTTGCCTCGGACAGCTCAAT-3′ | 5′-ATGGAGAAACCGGGGAACAC-3′ |
| SMURF2 | 5′-CGCTTGATCCAAAGTGGAAT-3′ | 5′-GGTTGATGGCATTGGAAAGA-3′ |
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
Statistical analysis
All the experiments were repeated 3 times to insure the reproducibility of the results. Differences between control and experimental groups were evaluated by the Kruskal–Wallis test with Steel’s post hoc test. A value of P < .05 was regarded as statistically significant.
Results
Differential expression analysis
Of the 40 samples, 22 283 probes were detected in total, and they could be well annotated to 12 434 genes, among which 716 genes were found significantly differentially expressed with Benjamini and Hochberg adjusted P< .05. Table 2 shows the information for the top 25 differentially expressed genes and their corresponding P values in the GEFOS-seq database and the UK Biobank database.
Table 2.
Top significantly expressed genes in low vs high BMD postmenopausal Caucasians.
| Gene | logFC | adj. P value | GEFOSFN.adjpval | GEFOSLS.adjpval | UKBiobank.adjpval |
|---|---|---|---|---|---|
| ATP5H | –0.24 | 2.27 × 10–4 | NA | 4.29 × 10–3 | 2.58 × 10–2 |
| C1D | –0.55 | 1.71 × 10–4 | NA | 4.24 × 10–2 | NA |
| CD320 | 0.26 | 1.42 × 10–3 | NA | 9.46 × 10–3 | 6.26 × 10–3 |
| DHX35 | 0.17 | 3.82 × 10–3 | NA | 3.44 × 10–2 | 1.73 × 10–2 |
| DICER1 | –0.58 | 2.99 × 10–3 | 1.64 × 10–2 | 7.41 × 10–3 | 2.41 × 10–3 |
| DIDO1 | 0.19 | 5.62 × 10–4 | 1.03 × 10–3 | 7.95 × 10–5 | 1.34 × 10–2 |
| FAM222B | 0.22 | 3.94 × 10–3 | NA | 1.81 × 10–2 | 8.06 × 10–3 |
| HIRA | –0.61 | 1.88 × 10–3 | 9.91 × 10–3 | 4.08 × 10–3 | 2.21 × 10–3 |
| ITM2A | 0.23 | 1.52 × 10–3 | NA | NA | NA |
| MYO9A | 0.26 | 3.94 × 10–3 | 1.17 × 10–2 | 1.31 × 10–2 | 1.51 × 10–2 |
| NDUFC1 | –0.28 | 2.63 × 10–3 | 9.25 × 10–3 | NA | 3.36 × 10–2 |
| OSTF1 | 0.34 | 1.25 × 10–3 | 4.37 × 10–3 | 4.24 × 10–2 | 5.20 × 10–3 |
| PDPK1 | 0.18 | 4.46 × 10–3 | NA | NA | 3.52 × 10–2 |
| PFDN4 | –0.41 | 1.71 × 10–4 | 3.35 × 10–3 | 2.66 × 10–2 | 2.52 × 10–2 |
| RPL3 | 0.21 | 3.10 × 10–4 | 1.06 × 10–2 | 1.11 × 10–2 | 3.03 × 10–2 |
| RPL35A | –0.17 | 3.94 × 10–3 | NA | NA | 1.33 × 10–3 |
| SEC22B | –0.57 | 1.02 × 10–3 | NA | NA | NA |
| SET | –0.63 | 2.27 × 10–4 | 4.92 × 10–2 | NA | 3.68 × 10–2 |
| SLC35D2 | –0.28 | 1.00 × 10–5 | 9.20 × 10–4 | 1.32 × 10–3 | NA |
| SNRPE | –0.25 | 3.94 × 10–3 | 4.16 × 10–2 | 1.12 × 10–2 | 5.32 × 10–4 |
| TBC1D2 | –0.3 | 3.16 × 10–3 | 5.64 × 10–3 | 7.21 × 10–3 | 1.75 × 10–2 |
| TMEM258 | –0.32 | 1.82 × 10–3 | NA | 1.90 × 10–2 | 3.67 × 10–3 |
| UBE2E1 | –0.3 | 1.25 × 10–3 | 1.21 × 10–2 | 2.86 × 10–3 | 4.01 × 10–3 |
| UBE3C | –0.3 | 3.94 × 10–3 | 8.98 × 10–3 | 1.04 × 10–2 | 9.63 × 10–3 |
| XPO7 | 0.28 | 7.87 × 10–4 | 2.56 × 10–2 | 2.58 × 10–2 | 6.20 × 10–4 |
Abbreviations: FN, femoral neck; LS, lumbar spine; LogFC, log of fold changes between high vs low BMD group; NA, the SNP of gene was not detected in the GEFOS-seq dataset or UK Biobank dataset.
Adjusted P value < .05 is used as thresholds for identifying significant genes.
MEGENA analysis
The 716 differentially expressed genes were used in the subsequent MEGENA analysis. After parallelization, early termination and prior quality control steps, PFNs with 150 gene pairs entered into MCA to identify multiscale clusters. With MCA, there were in total 5 distinct scales identified: S1 (0.23 << α << 0.55), S2 (0.56 << α << 0.66), S3 (0.73 << α << 1.04), S4 (1.05 << α << 1.05), and S5 (1.07 << α << 1.61). In total 21 significant module clusters were constructed with module P< .05, with module size varying from 11 to 120.
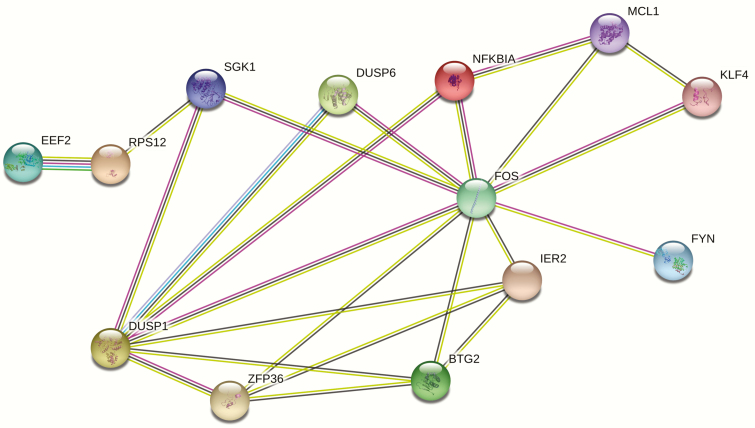
By calculating Pearson’s correlation between eigengenes (first component of the genes in the identified significant gene clusters) and phenotype, the gene cluster including DUSP1, ZFP36, DUSP6, FOS, IER2, NFKBIA, KLF4, EEF2, FYN, MCL1, SGK1, RPS12, and BTG2 is significantly associated with BMD variation with P = .036. Based on GO enrichment analysis, 2 GO terms, “T cell receptor signaling pathway” and “Osteoclast differentiation” were significantly enriched. Detailed information is shown in Table 3. Based on computational predictions by STRING software, interacting relationships between genes were detected, as shown in Fig. 2. Detailed pairwise combined scores for the interacting network are shown in Table 4. The combined prediction score for pairwise association between DUSP1–DUSP6, DUSP1–FYN, FYN–FOS, and EEF2–RPS12 reached the level of high confidence (combined prediction score > 0.7).
Table 3.
Significant GO term in the BMD-related gene cluster by MEGENA analysis.
| Term | P value | P value_fdr | P value_bonferroni |
|---|---|---|---|
| T cell receptor signaling pathway | 2.94 × 10–5 | 8.33 × 10–3 | 8.45 × 10–3 |
| Osteoclast differentiation | 5.81 × 10–5 | 8.33 × 10–3 | 1.67 × 10–2 |
Figure 2.
Interacting network view of genes in target BMD-related module. The network nodes represent genes, and lines represent the existence of evidence used in predicting the associations. Darker connecting lines between nodes represent higher combined score.
Table 4.
Interacting network identified in the BMD-related module by STRING.
| Interaction network | Score | Evidence |
|---|---|---|
| BTG2–FOS | 0.45 | Comentioned in PubMed abstracts, Coexpression |
| DUSP1–BTG2 | 0.40 | Comentioned in PubMed abstracts, Coexpression |
| DUSP1–DUSP6 | 0.96 | Comentioned in PubMed abstracts, Coexpression |
| DUSP1–FOS | 0.65 | Comentioned in PubMed abstracts, Coexpression, Experimental/Biochemical data |
| DUSP1–FYN | 0.85 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| DUSP1–SGK1 | 0.50 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| DUSP1–ZFP36 | 0.46 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| FYN–FOS | 0.91 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| KLF4–FOS | 0.41 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| MCL1–FOS | 0.41 | Comentioned in PubMed abstracts |
| NFKBIA–FOS | 0.50 | Comentioned in PubMed abstracts |
| SGK1–FOS | 0.41 | Comentioned in PubMed abstracts, Experimental/Biochemical data |
| ZFP36–FOS | 0.56 | Comentioned in PubMed abstracts, Coexpression |
| EEF2–RPS12 | 0.900 | Comentioned in PubMed abstracts, Coexpression, Experimental/Biochemical data, Neighborhood in the Genome, Association in Curated Database |
Gene set net correlation analysis
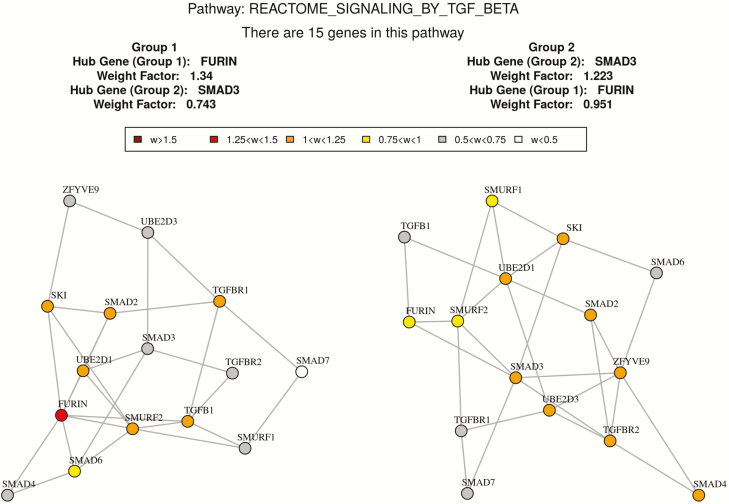
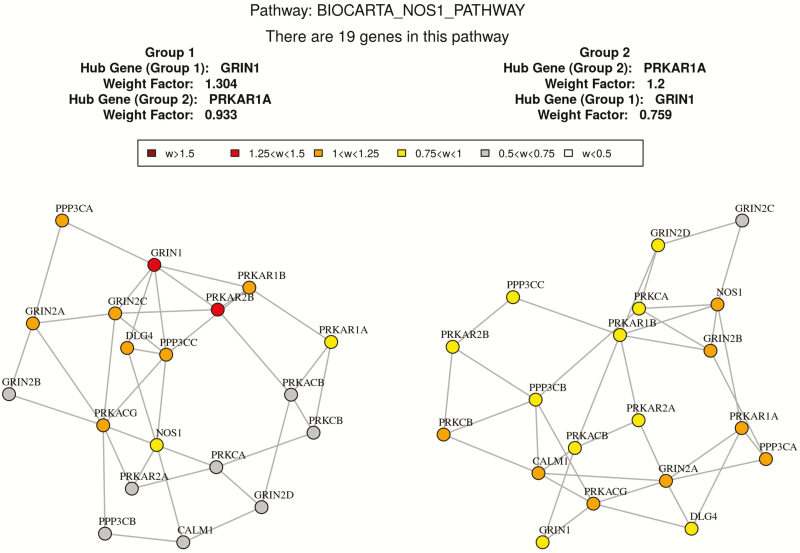
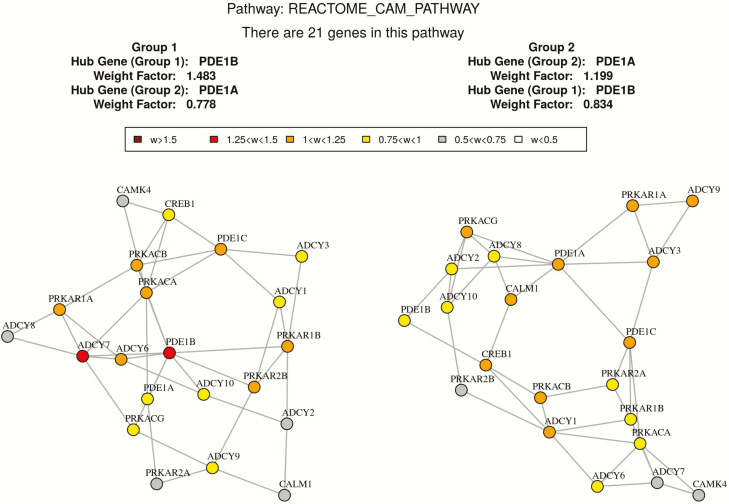
Based on GSNCA, the coexpression structure of 26 predefined pathways were significantly different between high and low BMD groups with P < .05 as shown in Table 5. The coexpression pattern of “Signaling by TGF-beta receptor complex” is significantly different between 2 discordant BMD groups with P= .015. In this pathway, 15 genes (UBE2D3, UBE2D1, TGFB1, TGFBR2, SMAD2, FURIN, SMAD3, SKI, SMAD4, ZFYVE9, TGFBR1, SMURF1, SMAD6, SMURF2, SMAD7) in our expression dataset were identified. The hub gene with largest weight in the high BMD group is SMAD3, while the hub gene in the low BMD group is FURIN. Since TGFB1 plays an important role in this pathway, we compared some network features of TGFB1 in the high versus low BMD groups. In the low BMD group, the degree centrality and the normalized betweenness centrality of TGFB1 is 5 and 0.21, respectively. While in the high BMD group, the degree centrality and the normalized betweenness centrality of TGFB1 is 2 and 0, respectively. It suggests that TGFB1 has more connections and influence with the gene network in the low BMD group. The MST2 plot of correlation patterns in the 2 BMD groups are shown in Fig. 3. And the network feature comparison is shown in Table 6. In addition, another 2 vital gene sets “REACTOME_CAM_PATHWAY” and “BIOCARTA_NOS1_PATHWAY” are found to be significantly different between the high and low BMD groups with P < .05. The MST2 plots of the correlation pattern of these 2 gene sets are shown in Figs. 4 and 5.
Table 5.
Significant gene sets identified with gene set net comparison analysis.
| Name | Adjusted P value |
|---|---|
| BIOCARTA_SODD_PATHWAY | .0060 |
| KEGG_VIBRIO_CHOLERAE_INFECTION | .0080 |
| REACTOME_GTP_HYDROLYSIS_AND_JOINING_OF_THE_60S_RIBOSOMAL_SUBUNIT | .0110 |
| REACTOME_JNK_PHOSPHORYLATION_AND_ACTIVATION_MEDIATED_BY_ACTIVATED_HUMAN_TAK1 | .0120 |
| KEGG_RIBOSOME | .0130 |
| BIOCARTA_ALK_PATHWAY | .0130 |
| REACTOME_SIGNALING_BY_TGF_BETA | .0150 |
| REACTOME_TRANSLATION | .0160 |
| REACTOME_RNA_POLYMERASE_III_CHAIN_ELONGATION | .0160 |
| KEGG_TAURINE_AND_HYPOTAURINE_METABOLISM | .0200 |
| BIOCARTA_PLCE_PATHWAY | .0230 |
| REACTOME_HORMONE_SENSITIVE_LIPASE_HSL_MEDIATED_TRIACYLGLYCEROL_HYDROLYSIS | .0280 |
| KEGG_LYSOSOME | .0290 |
| KEGG_RNA_POLYMERASE | .0340 |
| REACTOME_PLC_BETA_MEDIATED_EVENTS | .0340 |
| KEGG_BASE_EXCISION_REPAIR | .0380 |
| REACTOME_INFLUENZA_VIRAL_RNA_TRANSCRIPTION_AND_REPLICATION | .0390 |
| REACTOME_BASE_EXCISION_REPAIR | .0400 |
| REACTOME_DOPAMINE_NEUROTRANSMITTER_RELEASE_CYCLE | .0440 |
| REACTOME_FORMATION_OF_A_POOL_OF_FREE_40S_SUBUNITS | .0450 |
| REACTOME_CAM_PATHWAY | .0460 |
| BIOCARTA_NOS1_PATHWAY | .0460 |
| KEGG_GLYOXYLATE_AND_DICARBOXYLATE_METABOLISM | .0500 |
| REACTOME_POST_TRANSLATIONAL_PROTEIN_MODIFICATION | .0500 |
| REACTOME_COMPLEMENT_CASCADE | .0500 |
| REACTOME_ACTIVATION_OF_KAINATE_RECEPTORS_UPON_GLUTAMATE_BINDING | .0500 |
Figure 3.
Co-expression network comparison between high and low BMD subjects in REACTOME_SIGNALING_BY_TGF_BETA by GSNCA.
Table 6.
Coexpression network comparison between high and low BMD subjects in “REACTOME_SIGNALING_BY_TGF_BETA.”
| Low_BMD group | High_BMD group | |
|---|---|---|
| Average absolute cross correlation | 0.25 | 0.28 |
| Degree centrality of hub gene | FURTIN (7) | SMAD3 (6) |
| Degree centrality of TGFB1 | 5 | 2 |
| Normalized betweenness centrality of hub gene | FURIN (0.26) | SMAD3 (0.29) |
| Normalized betweenness centrality of TGFB1 | 0.21 | 0.00 |
| Normalized closeness centrality of hub gene | FURTIN (1.35) | SMAD (1.35) |
| Average normalized closeness centrality | 1.0 | 1.1 |
FURTIN is a hub gene in the low BMD group and it connected to 7 genes in the coexpression structure; SMAD is a hub gene in the high BMD group and it has 6 neighbors. The connections of TGFB1 in 2 BMD groups are 5 and 2 respectively. The normalized shortest paths that pass through FURIN in the low BMD group is 0.26; the normalized shortest paths that pass through SMAD3 in low the BMD group is 0.29. The normalized shortest paths that pass through TGFB1 in 2 BMD groups are 0.21 and 0 respectively. The normalized mean distance from FURIN to other vertices (genes) is 1.35 in the low BMD group, and the normalized mean distance from SMAD3 to other vertices (genes) is 1.35 as well in the high BMD group.
Figure 4.
Co-expression network comparison between high and low BMD subjects in “BIOCARTA_NOS1_PATHWAY” by GSNCA.
Figure 5.
Co-expression network comparison between high and low BMD subjects in “REACTOME_CAM_PATHWAY” by GSNCA.
Bayesian network analysis
BN analysis was applied to the differentially coexpressed gene sets identified by the previous GSNCA. As shown in Table 7, in the “Signaling by TGF-beta” gene set, 4 significant directional gene pairs (strength >0.80, direction >0.5) were identified in the high BMD group, while only 2 significant directional gene pairs were identified in the low BMD group. Strength measures the significance of arcs between nodes (genes). Strength >0.80 means that the arc appears in at least 80% of the networks with 500 times bootstrap resampling to Bayesian network structures used for model averaging; direction >0.5 indicates that the path flow of the 2 nodes appears most frequently. The arc with highest strength in the high BMD group is from UBE2D3 to UBE2D1 (0.98), while the highest strength in the low BMD group is from TGFBR2 to SMAD7 (0.82). It also worth noticing that the second highest strength in low BMD group is from TGFBR1 to SMURF2 (0.80).
Table 7.
Bayesian network analysis between high and low BMD subjects in “REACTOME_SIGNALING_BY_TGF_BETA.”
| From–To (high BMD) | Strength | Direction | From–To (low BMD) | Strength | Direction |
|---|---|---|---|---|---|
| UBE2D3–UBE2D1 | 0.98 | 0.70 | TGFBR2–SMAD7 | 0.82 | 0.73 |
| UBE2D3–SMAD2 | 0.92 | 0.77 | TGFBR1–SMURF2 | 0.80 | 0.55 |
| UBE2D1–SMAD2 | 0.82 | 0.68 | |||
| FURIN–SMURF2 | 0.88 | 0.80 |
Strength measures the significance of arcs between nodes (genes). Arcs are considered significant if they appear in at least 80% of the networks with bootstrap resampling to Bayesian network structures use for model averaging. Direction measures the direction of arcs learned by Bayesian network. Direction that appears most frequently (>0.5) is kept in the table.
siRNA gene silencing experiment
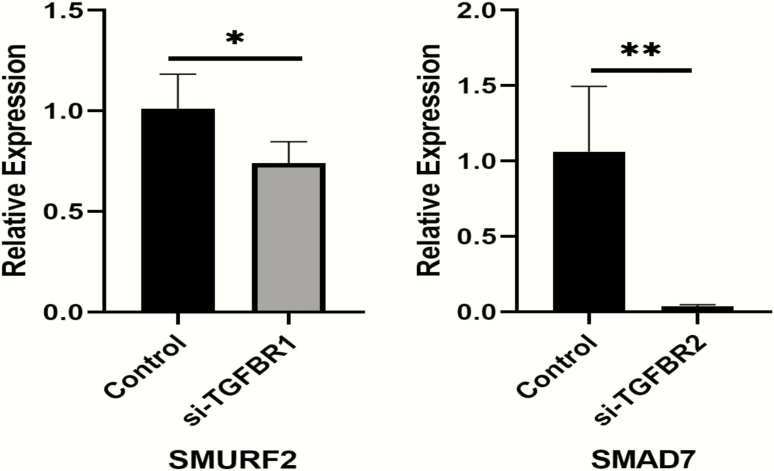
RT-PCR experiments showed that transfection with siRNA-TGFBR1 significantly decreases SMURF2 mRNA expression levels in THP1 cells (P = .044) when the silencing efficiency reaches 80% compared with siRNA control (Fig. 6). Transfection with siRNA-TGFBR2 significantly decreases SMAD7 mRNA expression levels in THP1 cells (P = .018) when the silencing efficiency reaches 70% to 80% compared with the siRNA control (Fig. 6).
Figure 6.
siRNA gene silencing effect of TGFBR2-SMAD7 and TGFBR1-SMURF2 in THP-1 cells.
Discussion
The present study applied both traditional differential expression analysis and state-of-the-art network analyses to transcriptome-wide gene expression data. We applied comprehensive network-based system biology analyses (de novo multiscale network construction, gene set comparison with predefined pathway information, and Bayesian network construction). The MEGENA method helps us build a coexpression network based on data expression features, while the coexpression network constructed by GSNCA incorporates more biological pathway information. Since MEGENA and GSNCA do not provide causal relationship between genes, BNs are a great complement, helping us explore regulatory function between genes. These 3 network analysis approaches together helped us discover transcriptome data more comprehensively. With a series of bioinformatics analyses, we not only found genes and pathways important for postmenopausal osteoporosis, but also validated regulation patterns between crucial gene pairs in PBMs based on in vitro experiments.
Based on differential expression analysis, 716 genes were significantly differentially expressed (Padjust <.05) in subjects with extremely low versus high BMD. We checked the SNPs of the top 25 genes (Padjust < .005) for their association signals in the GEFOS dataset and the UK Biobank dataset. Since GEFOS study is a large-scale meta-analysis performed in the bone field and the UK Biobank osteoporosis study is the largest GWAS study that measures the association between SNPs with eBMD (crucial for bone fracture), the consistent results make our cohort study much more convincing.
Much previous research has provided further evidence for our findings. DICER1 (Pval = 2.99 × 10–3) is a conserved RNAse III endonuclease that is essential for the processing of several classes of small RNAs. Downregulation of DICER1 expression inhibits osteogenic differentiation of several cells (43), and Dicer1 processed microRNAs may have an important role in the regulation of skeletal homeostasis (44). HIRA (Pval = 1.88× 10–3) has been recorded downregulated in osteoporosis blood samples, and plays a significant role in histone modification in bone development in osteoporosis (45). OSTF1 (Osteoclast stimulation factor) (Pval = 1.25 × 10–3) was initially identified as a factor involved in the indirect activation of osteoclasts, and it could indirectly enhance osteoclast formation and bone resorption activity in cell culture assays (46). TBC1D2 (Pval = 3.16 × 10–3) is a protein-coding gene from the TBC1 domain family. It participates in the GTPase activator process, and regulation of TBC1D2-dependent activities plays a critical role in osteoclast function (47). In addition, we also found a novel gene ITM2A which was not detected in either of the meta-analyses. From previous research, ITM2A had been shown to be involved in bone growth, mineralization, and bone morphogenic protein metabolism (48). The significant change of ITM2A in our gene expression data suggests the importance of this gene in regulating the bone formation/resorption process. The differential expression analysis helps detection of target genes associated with the pathology of osteoporosis, providing us with some new genes that may serve as diagnostic biomarkers of postmenopausal BMD variation.
In MENEGA analysis, the 2 most significantly enriched pathways in the constructed BMD-associated gene cluster were T Cell Receptor signaling and aggregation and Osteoclast differentiation. T Cell Receptor (TCR) signaling could promote plenty of signaling cascades that ultimately determine cell fate, and TCR signaling provokes rapid increases in integrin function that facilitate T-cell activation (49). Integrin is central to physiological bone resorption, and it acts as a candidate antibone resorptive therapeutic target (50). Under certain pathological circumstances such as estrogen deficiency, the activation of T cells may disturb bone homeostasis and bring about bone destruction process (51). T cells work closely with RANK ligand or RANKL (receptor activator of nuclear factor-κB ligand), essential stimulating signals for osteoclastogenesis, which is involved in activating mature osteoclasts (52). Moreover, T cells could secret various cytokines such as interleukin (IL)-1, IL-6, interferon-γ, or IL-4 that mediating osteoclastogenic signals in bone turnover (53). Osteoclast differentiation signaling has formerly been revealed to cause postmenopausal osteoporosis. Estrogen deficiency stimulates osteoclast formation/differentiation by increasing hematopoietic progenitors and provides a larger recruited osteoclast progenitor pool (54), as well as increase the life span of osteoclasts (55). Furthermore, osteoclast differentiation signaling is controlled by 2 essential cytokines: macrophage colony-stimulating factor (M-CSF) and RANKL. M-CSF and RANKL bind to their respective receptors c-Fms/RANK to stimulate osteoclast differentiation through regulation of delicate signaling systems (55). Thus, the detected enriched “Osteoclast differentiation” pathway could provide further evidence that the activities of M-CSF-c-Fms signaling and RANKL–RANK signaling might contribute to the pathogenesis of postmenopausal osteoporosis through their specific actions on osteoclastogenesis. Furthermore, based on the enrichment analysis in the target model, the combined prediction score for pairwise association between DUSP1–DUSP6 and DUSP1–FYN reached the level of high confidence, which gives us a novel insight that the DUSP (Dual-specificity phosphatase) gene family is very active and important for osteoclastogenesis in PBMs.
In GSNCA, the gene coexpression pattern of REACTOME_SIGNALING_BY_TGF_BETA is significantly different in high versus low BMD subjects with P < .05. Transforming growth factor (TGF)-β signaling is involved in a variety of cellular processes and plays an significant role in a wide range of biological systems (56). This signaling pathway incorporates various TGF-β/superfamily ligands and receptors as well as the receptor-regulated SMADs. TGF-β is known to regulate RANKL-induced osteoclast formation and bone resorbing activity and it has been demonstrated that in the presence of M-CSF, it could induce mononuclear phagocytes to differentiate into osteoclastic cells. Fox et al. suggested that the osteoclast/macrophage commitment could be efficiently induced by TGF-β-related cytokine signaling (57). In addition, Yan et al. have demonstrated that TGF-β-induced upregulation of RANK expression facilitated RANKL-induced osteoclastogenesis (58). From our analysis, the hub genes of REACTOME_SIGNALING_BY_TGF_BETA in the low BMD group and high BMD group are FURIN and SMAD3 respectively, and their structure features are quite different. These have not been detected in any previous postmenopausal osteoporosis analysis. The result not only discloses different gene interacting patterns in PBMs between high and low BMD groups, but also suggests the importance of FURIN and SMAD3 genes in PBMs during bone turnover.
Another two important signaling pathways detected in GSNCA analysis are BIOCARTA_NOS1_PATHWAY and REACTOME_CAM_PATHWAY. In the NOS1 pathway, glutamate stimulates NMDA-mediated calcium influx, promoting nitric oxide (NO) synthesis, thus activating guanylate cyclase. It has been found that 17β-estradiol protects osteocytes against apoptosis via the NO/cGMP signaling pathway (59), and NO is capable of inducing inflammatory cell apoptosis including direct downregulation of leukocyte functions. In addition, it has been shown that the exogenous NO could stimulate apoptosis in cell types such as monocytes, monocyte-derived macrophages, neutrophils, and eosinophils (60). The significant difference of NOS1 signaling between high and low BMD group may suggest different apoptosis activities of monocytes, and our analysis provides further support that NOS1 signaling plays an important role in postmenopausal osteoporosis. Ca2+/CAM-mediated cell signaling is ubiquitous and functions at all levels of the cell life cycle including cell genesis, cell function/activity and cell death. It has been reported that CAM pathways participates in the regulation of both osteoclasts differentiation and function (61). Therefore, our gene set comparison analysis of the transcriptome data not only helped to identify several vital genes that may be ignored by traditional differential expression analyses, but also suggested several important signaling process in postmenopausal osteoporosis.
In the BN analysis, the highest strength between gene pairs in the low BMD group of the SIGNALING BY TGF-BETA pathway is from TGFBR2 to SMAD7 (strength = 0.82, direction = 0.73). The result indicates the arc between TGFBR2 and SMAD7 appears in at least 82% of the networks with 500 times bootstrap resampling to BN structures used for model averaging, and the most frequent path flow direction is from TGFBR2 to SMAD7. Through functional validations, when the silencing efficiency reaches 70% to 80%, the expression of SMAD7 mRNA was significantly downregulated (P = .018). As transmembrane kinase, TGFBR2 could induce apoptosis and differentiation processes activated by TGF-β (62). In previous studies, it has been demonstrated that the binding process between TGF-β and TGFBR2 could initiate phosphorylation of SMAD families and regulate the transcription of several well-known TGFBR2 target genes like SMAD7 (63). Additionally, SMAD7 is an essential factor for the regulation loop of the TGF-β/Smad signaling pathway (64). Our functional validation confirms the regulatory information flow from TGFBR2 to SMAD7 in PBM that we derived in vivo in humans, and their regulation pattern might have some further influence on those signaling pathways that are important for postmenopausal osteoclastogenesis. The gene pair with second highest strength in the BN analysis within the low BMD group is TGFBR1–SMURF2 (strength = 0.79, direction = 0.55). The arc between TGFBR1 and SMURF2 appears in at least 79% of the networks with 500 times bootstrap resampling to BNs structures used for model averaging, and the probability of direction between these 2 genes is 0.55. The result indicates interaction between TGFBR1 and SMURF2 in TGF-β signaling is very active in the PBM of low BMD group. Through functional validations, we found that when the silencing efficiency reaches 80%, the expression of SMURF2 mRNA was significantly downregulated (P = .044). TGFBR1 is an important transmembrane protein for TGF-β signals, and it could be regulated by SMAD ubiquitination regulatory factors (Smurf) including SMURF2 (65). In addition, TGFBR1 could interact with SMURF2 to affect ubiquitin-dependent degradation (66). Most of previous studies indicated the regulation pattern of SMURF2 to TGFBR1 (67), but our BN analysis provides an innovative insight that the feedback signal from TGFBR1 to SMURF2 is predominant for monocyte activities within low BMD group. Since SMURF2 could act as an essential regulator of the molecular communication between osteoblasts and osteoclasts (67). Our result support a new hypothesis that in TGF-β pathway, the regulation effect of TGFBR1 to SMURF2 may influence the bone resorption activities in PBMs within low BMD group, and the information flow from TGFBR1 to SMURF2 is predominant.
Integrative network-based analysis with transcriptome-wide expression data holds great promise for illuminating the genetic basis of complex diseases. The present study demonstrated that MEGENA, GSNCA, and BN analyses were useful for identifying postmenopausal osteoporosis-related biologically meaningful gene groups and signaling pathways. Since genes do not usually work independently, the significant coexpression pattern could reveal how genes function within PBMs. This integrative analysis strategy not only helps to reveal disease-associated genes through transcriptomics data, but also shed light on potential therapeutic strategies for postmenopausal osteoporosis.
Acknowledgments
Financial Support: This study was partially supported or benefited by grants from the National Institutes of Health [R01AR059781, P20GM109036, R01MH107354, R01MH104680, R01GM109068, R01AR069055, and U19AG055373].
Glossary
Abbreviations
- BMD
bone mineral density
- BN
Bayesian network
- cDNA
complementary deoxyribonucleic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GEFOS
Genetic Factors for Osteoporosis Consortium
- GO
Gene Ontology
- GSNCA
gene sets net correlations analysis
- GWAS
genome-wide association study
- IL
interleukin
- MCA
multiscale clustering analysis
- M-CSF
macrophage colony-stimulating factor
- MEGENA
multiscale embedded gene coexpression network analysis
- MST
minimum spanning tree
- PBM
peripheral blood monocyte
- PFN
planar filtered network
- siRNA
small interfering ribonucleic acid
- SNP
single-nucleotide polymorphism
- TCR
T Cell Receptor
- TGF
transforming growth factor
Additional Information
Disclosure Summary: All authors approved the final content of the manuscript and declared no conflict of interest related to this manuscript.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Lerner UH. Bone remodeling in post-menopausal osteoporosis. J Dent Res. 2006;85(7):584-595. [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal N, Raveendran A, Khandelwal N, et al. Prevalence and related risk factors of osteoporosis in peri- and postmenopausal Indian women. J Midlife Health. 2011;2(2):81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sioka C, Fotopoulos A, Georgiou A, Xourgia X, Papadopoulos A, Kalef-Ezra JA. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric. 2010;13(1):63-71. [DOI] [PubMed] [Google Scholar]
- 4. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37(3):549-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669-692. [DOI] [PubMed] [Google Scholar]
- 7. Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018;18(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zupan J, Jeras M, Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb). 2013;23(1):43-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y, Deng HW, Shen H. Circulating monocytes: an appropriate model for bone-related study. Osteoporos Int. 2015;26(11):2561-2572. [DOI] [PubMed] [Google Scholar]
- 11. Grundberg E, Brändström H, Lam KC, et al. Systematic assessment of the human osteoblast transcriptome in resting and induced primary cells. Physiol Genomics. 2008;33(3):301-311. [DOI] [PubMed] [Google Scholar]
- 12. Liu YZ, Zhou Y, Zhang L, et al. Attenuated monocyte apoptosis, a new mechanism for osteoporosis suggested by a transcriptome-wide expression study of monocytes. PLoS One. 2015;10(2):e0116792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song WM, Zhang B. Multiscale embedded gene co-expression network analysis. PLoS Comput Biol. 2015;11(11):e1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song WM, Di Matteo T, Aste T. Building complex networks with Platonic solids. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;85(4 Pt 2):046115. [DOI] [PubMed] [Google Scholar]
- 15. Song WM, Di Matteo T, Aste T. Hierarchical information clustering by means of topologically embedded graphs. PLoS One. 2012;7(3):e31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahmatallah Y, Emmert-Streib F, Glazko G. Gene Sets Net Correlations Analysis (GSNCA): a multivariate differential coexpression test for gene sets. Bioinformatics. 2014;30(3):360-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Dam S, Võsa U, van der Graaf A, Franke L, de Magalhães JP. Gene co-expression analysis for functional classification and gene-disease predictions. Brief Bioinform. 2018;19(4):575-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman N, Linial M, Nachman I, Pe’er D. Using Bayesian networks to analyze expression data. J Comput Biol. 2000;7(3-4):601-620. [DOI] [PubMed] [Google Scholar]
- 19. Mäkinen VP, Civelek M, Meng Q, et al. ; Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Consortium Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet. 2014;10(7):e1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng FY, Lei SF, Zhang Y, et al. Peripheral blood monocyte-expressed ANXA2 gene is involved in pathogenesis of osteoporosis in humans. Mol Cell Proteomics. 2011;10(11):M111.011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82(2):244-252. [DOI] [PubMed] [Google Scholar]
- 23. Schroeder A, Mueller O, Stocker S, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiewe P, Gueller S, Komor M, Stroux A, Thiel E, Hofmann WK. Prediction of qualitative outcome of oligonucleotide microarray hybridization by measurement of RNA integrity using the 2100 Bioanalyzer capillary electrophoresis system. Ann Hematol. 2009;88(12):1177-1183. [DOI] [PubMed] [Google Scholar]
- 25. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rivadeneira F, Styrkársdottir U, Estrada K, et al. ; Genetic Factors for Osteoporosis (GEFOS) Consortium Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng HF, Forgetta V, Hsu YH, et al. ; AOGC Consortium; UK10K Consortium Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bauer DC, Ewing SK, Cauley JA, Ensrud KE, Cummings SR, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Group Quantitative ultrasound predicts hip and non-spine fracture in men: the MrOS study. Osteoporos Int. 2007;18(6):771-777. [DOI] [PubMed] [Google Scholar]
- 32. Morris JA, Kemp JP, Youlten SE, et al. ; 23andMe Research Team An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tumminello M, Aste T, Di Matteo T, Mantegna RN. A tool for filtering information in complex systems. Proc Natl Acad Sci U S A. 2005;102(30):10421-10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert R, Barabasi AL. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74(1):47–97. [Google Scholar]
- 35. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103(23):8577-8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Snel B, Lehmann G, Bork P, Huynen MA. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000;28(18):3442-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dawson JA, Kendziorski C. An empirical Bayesian approach for identifying differential coexpression in high-throughput experiments. Biometrics. 2012;68(2):455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chu T, Glymour C, Scheines R, Spirtes P. A statistical problem for inference to regulatory structure from associations of gene expression measurements with microarrays. Bioinformatics. 2003;19(9):1147-1152. [DOI] [PubMed] [Google Scholar]
- 41. Claeskens G, Hjort NL.. Model Selection and Model Averaging. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 42. Bosshart H, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4(21):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim DS, Lee SY, Lee JH, Bae YC, Jung JS. MicroRNA-103a-3p controls proliferation and osteogenic differentiation of human adipose tissue-derived stromal cells. Exp Mol Med. 2015;47(7):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bendre A, Moritz N, Väänänen V, Määttä JA. Dicer1 ablation in osterix positive bone forming cells affects cortical bone homeostasis. Bone. 2018;106:139-147. [DOI] [PubMed] [Google Scholar]
- 45. Xia B, Li Y, Zhou J, Tian B, Feng L. Identification of potential pathogenic genes associated with osteoporosis. Bone Joint Res. 2017;6(12):640-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vermeren M, Lyraki R, Wani S, et al. Osteoclast stimulation factor 1 (Ostf1) KNOCKOUT increases trabecular bone mass in mice. Mamm Genome. 2017;28(11-12):498-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xing WR, Goodluck H, Zeng C, Mohan S. Role and mechanism of action of leucine-rich repeat kinase 1 in bone. Bone Res. 2017;5(1):17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Camirand A, Goltzman D, Gupta A, Kaouass M, Panda D, Karaplis A. The role of parathyroid hormone-related protein (PTHrP) in osteoblast response to microgravity: mechanistic implications for osteoporosis development. PLoS One. 2016;11(7):e0160034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218(1):65-81. [DOI] [PubMed] [Google Scholar]
- 50. Teitelbaum SL. Osteoporosis and integrins. J Clin Endocrinol Metab. 2005;90(4):2466-2468. [DOI] [PubMed] [Google Scholar]
- 51. Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao W, Liu Y, Cahill CM, Yang W, Rogers JT, Huang X. The role of T cells in osteoporosis, an update. Int J Clin Exp Pathol. 2009;2(6):544-552. [PMC free article] [PubMed] [Google Scholar]
- 53. D’Amico L, Roato I. Cross-talk between T cells and osteoclasts in bone resorption. Bonekey Rep. 2012;1:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88-91. [DOI] [PubMed] [Google Scholar]
- 55. Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci. 2012;9(9):825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19(1):71-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fox SW, Evans KE, Lovibond AC. Transforming growth factor-beta enables NFATc1 expression during osteoclastogenesis. Biochem Biophys Res Commun. 2008;366(1):123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan T, Riggs BL, Boyle WJ, Khosla S. Regulation of osteoclastogenesis and RANK expression by TGF-beta1. J Cell Biochem. 2001;83(2):320-325. [DOI] [PubMed] [Google Scholar]
- 59. Joshua J, Kalyanaraman H, Marathe N, Pilz RB. Nitric oxide as a mediator of estrogen effects in osteocytes. Vitam Horm. 2014;96:247-263. [DOI] [PubMed] [Google Scholar]
- 60. Taylor EL, Megson IL, Haslett C, Rossi AG. Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ. 2003;10(4):418-430. [DOI] [PubMed] [Google Scholar]
- 61. Wu X, Ahn EY, McKenna MA, Yeo H, McDonald JM. Fas binding to calmodulin regulates apoptosis in osteoclasts. J Biol Chem. 2005;280(33):29964-29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim SJ, Im YH, Markowitz SD, Bang YJ. Molecular mechanisms of inactivation of TGF-beta receptors during carcinogenesis. Cytokine Growth Factor Rev. 2000;11(1-2):159-168. [DOI] [PubMed] [Google Scholar]
- 63. Lee J, Ballikaya S, Schönig K, et al. Transforming growth factor beta receptor 2 (TGFBR2) changes sialylation in the microsatellite unstable (MSI) Colorectal cancer cell line HCT116. PLoS One. 2013;8(2):e57074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang H, Ta N. Effect of isopsoralen on Smad7 in osteoblastic MC3T3-E1 cells. Exp Ther Med. 2017;14(2):1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bizet AA, Tran-Khanh N, Saksena A, Liu K, Buschmann MD, Philip A. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J Cell Biochem. 2012;113(1): 238-246. [DOI] [PubMed] [Google Scholar]
- 66. Cai Y, Zhou CH, Fu D, Shen XZ. Overexpression of Smad ubiquitin regulatory factor 2 suppresses transforming growth factor-β mediated liver fibrosis. J Dig Dis. 2012;13(6):327-334. [DOI] [PubMed] [Google Scholar]
- 67. Xu Z, Greenblatt MB, Yan G, et al. SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts. Nat Commun. 2017;8(1):14570. [DOI] [PMC free article] [PubMed] [Google Scholar]