Abstract
In December 2019, severe cases of pneumonia of unknown aetiology were reported in Wuhan city, in China. Lately, the pneumonia was related to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the diseases was termed coronavirus disease-2019 (COVID-19). At the end of January 2020, the infection spread all over Italy, but with high infection rates and mortality in the northern part, especially in Lombardy, the most industrialized and polluted region of the country. It is noteworthy that a strong association between severe viral respiratory disease and air pollution has been described. Air pollutant could be solid particles, liquid droplets, or gases and can be of natural origin (such as ash from a volcanic eruption) or released from motor vehicle depletes (carbon monoxide gas) or factories (sulfur dioxide). Volcanic eruptions release large amounts of sulphuric acid, hydrogen sulfide, and hydrochloric acid into the atmosphere. Pulmunary diseases spread by means of small droplets in the breath, also called aerosols, and air pollution may facilitate the outside survival of viruses. We suppose that ash and gases emitted from the Mount Etna contributed to air pollution, potentially favouring the major contagion of COVID-19 in the eastern flank of the mountain, as in Catania city. In fact, ash and gases (with regard to radon) are usually particularly intense in winter, with a reduction of emission of specific metals with warmer weather. This is the first paper that elaborates the hypothesis of a potential role of volcanic gases and heavy metals-related air pollution, combined to specific climatic conditions and regional topography, in favouring severe COVID-19 diffusion in Sicily. Clinical and epidemiological studies are needed to support the hypothesis and plan the due prevention and awareness-raising campaigns.
Background
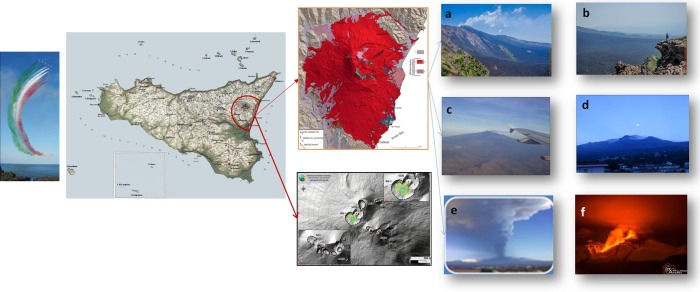
Mount Etna is the highest active volcano in Europe, and it is located on the east coast of Sicily, between the cities of Messina and Catania. The Etna covers an area of 1190 km2 with a basal circumference of 140 km (Fig. 1 ) [1]. During the uprising of the volcanic eruptive plume, soluble ash fraction and solid particles are emitted [2]. These latter include gases trapped in the volcanic rocks, dissolved or dissociated gases in magma and lava or ashes, or gases emanating directly from lava or indirectly through ground water heated by the volcanic action. Volcanoes may discharge flows of ash and gas up to 50 km away, with the generation of cloud columns 1–5 km high and blasting about 5 km-wide chunk off the tip of the volcano [3], [4]. In volcanic areas, the emissions and deposits of volcanogenic elements are key factors for geochemical mobility of trace elements (TEs), and their distribution in the environment might impair animals and human health. Among all the volcano elements, metals represent the main natural source [3], [4]. Fine particulate matter with an aerodynamic diameter of 2.5 m or less (PM2.5), 10 m or less (PM10) are sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO) and ozone (O3) that affect airways through inhalation and exacerbate the susceptibility to and severity of respiratory virus infections [5], [6], [7]. Moreover, trace elements deposit in soil and plants representing a high risk of ingestion of metals by the population with serious toxic effects for public health [8]. When added to other environmental toxicants, such as herbicides, pesticides and industrial emissions, heavy metals are associated with an increased risk of neurodegenerative diseases [9], [10], [11], [12].
Fig. 1.
shows the location of Mount Etna, with images of its eruptions. a, b: Valle Bove 2013; c: Etna view landing to Catania airport; d: Etna view from Giarre, a city in the eastern flank of the mountain; e: Strombolian Etna eruption with high km of ash emission; f: Etna eruption in 2013.
A recent study evaluating the level of TEs in scalp hair of school-children living around the Mount Etna area, showed a high level of metals and nickels [12]. Moreover, areas of different flank present different susceptibility to neurodegenerative diseases, being eastern to southern flank more exposed than western one [13]. Heavy metals have been dosed in the groundwater of the Etna (used for water plants or to drink), especially in the eastern and southern sectors of the volcano, and they are believed to contribute to intoxication of public health and to pulmonary or neurodegenerative diseases [12], [14], [15].
Moreover, food, especially, fishes are highly concentrate of several metals [16], [17], and this is likely related to gases emitted by the Etna mountain [18], [19], [20], that blow from the eastern to the southern of the volcano, most of the time westerly to north-westerly trade winds, emitting about 16% of the global volcanic heavy metals, including radon [21]. Radon (222Rn) is a noble gas, invisible, odorless, tasteless, and short-lived decay product of uranium (238U), whose high activity concentration in radon emissions at the topographic surface is produced by convective flow of gases that facilitate the transport of radon from greater depth within soils (22–23).
The collapsing flanks are bordered by numerous active faults [24] that cross urban areas, and are likely the locations of high soil degassing and elevated radon activities [25]. In the areas of strong degassing, particularly in the east and south-west flanks of the volcano, due to continuous tectonic deformations and gravitational collapses [26], [27], radon activity was up to 60,000 Bq/m3 [28].
In Sicily radon has been highly related with lung cancer (29–30), and could favour pulmonary infections, as well as other elements do [31].
The hypothesis
In December 2019, severe cases of pneumonia of unknown aetiology were reported in Wuhan city, in China. Lately, the pneumonia was related to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), previously named 2019 novel coronavirus (2019-nCoV), and the diseases was termed coronavirus disease-2019 (COVID-19) [32], [33]. At the end of January 2020, after the first case in Codogno, the infection spread all over Italy, but with high infection rates and mortality in the northern part, especially in Lombardy, the most industrialized and polluted region of the country. It is noteworthy that a strong association between severe viral respiratory disease and air pollution has been described [34]. Air pollutant could be solid particles, liquid droplets, or gases. A pollutant can be of natural origin or man-made and classified as primary or secondary, based on natural origin (such as ash from a volcanic eruption: primary) or released from motor vehicle depletes (carbon monoxide gas) or factories (sulfur dioxide) [35], [36].
The inhalable fraction of particles in the air that can enter the nose or mouth depends on external wind speed and direction, as well as on the particle-size distribution by aerodynamic diameter [37], [38], [39] . Two alternative size-selective criteria, often used in atmospheric monitoring, are PM10 and PM2.5. PM10 is defined by ISO as “particles which pass through a size-selective inlet with a 50% efficiency cut-off at 10 μm aerodynamic diameter (defined as “thoracic convention”), whereas PM2.5 as “particles which pass through a size-selective inlet with a 50% efficiency cut-off at 2.5 μm aerodynamic diameter”; these latter correspond to the “high-risk respirable convention” [40].
Then, particles with a diameter smaller than 10 μm can enter the bronchi, while the ones with an effective diameter smaller than 2.5 μm can enter as far as the gas exchange region in the lungs [41], because of prolonged permanence in the air than heavier particles, bypassing the nose and throat.
High concentrations of PM 2.5 particles characterize the air of Hubei Region in China, as well as the Po Valley (Italy), revealing the possible correlation between the major distribution of COVID-19 and the concentration of pollutants [42]. In fact, pollution insult decreases airway ciliary activity, and increases excessive mucus production, exposing to progressive and chronic inflammation of the respiratory airways with severe respiratory diseases after viral infections.
Several factors contribute to individual reactions to air pollutants: the type of pollutant a person is exposed to, the degree of exposure, and the individual's health status and genetics [43]. To this end, air pollution is a significant risk factor for viral respiratory infections, heart disease, chronic obstructive pulmonary disease, stroke and lung cancer [44], [34]. Origin of pollutant could be volcanoes emissions.
Volcanic eruptions release large amounts of sulphuric acid, hydrogen sulfide, and hydrochloric acid into the atmosphere. These gases react with other atmospheric particles to form aerosols and eventually return to earth as acid rain, having a number of adverse effects on the environment and human life [37]. Pulmunary diseases spread by means of small droplets in the breath, also called aerosols [38], and air pollution may facilitate the outside survival of viruses.
On the other hand, heavy metals origin from geologic cycle (e.g. erosion, volcanic activity, wind-blown dust, etc.) and other industrial or artificial activities (industrialization, fuel combustion, roadway traffic, etc.). Indeed, metals accumulate in aquatic and terrestrial systems with biomagnification in food chain, after being conveyed by air further contributing to air pollution.
In the North-west of Beijing, a high urbanised city of China, where an elevated numbers of COVID-19 contagious have been recorded, there is the Datong-Fengzhen volcano group that contains about 80 volcanic vents, including 30 cinder cones and small lava domes [45]. Then, there are three active volcanoes in China locate in the Changbaishan area, Jingbo Lake, Wudalianchi, Tengchong and Yutian. Several of these volcanoes have explosive eruptions of magma with repercussion on global system (for example Millennium eruption of 1000 years BP involved at least 20–30 km3 of magma with important global impact) [46]. Another active and very dangerous Asian volcano is the Taal, on the island of Luzon, Philippines. In December 2019, an alert level to four due to an explosive eruption was raised and a “total evacuation” order to population living within 17-kilometer around the volcano was done for the high risk of toxicity of volcanic ash (that could travel hundreds of kilometres an hour), toxic gases emitted from the eruption, and mud flows caused by ash mixing with water vapour in the atmosphere. A column of ash raised up as it erupts on January 2020 shower down on the south-west sector of the volcano [47]. Then, volcanoes gases and heavy metals could contribute to air pollution of the China.
As well as in China, we suppose that ash and gases emitted from the Mount Etna contributed to air pollution, potentially favouring the major contagion in the eastern flank of the mountain, as in Catania. Moreover, the reduction in number of contagion and virulence in the last month would be justified by the interaction of several factors, as social distances and lockdown, and environmental issues, including a warmer climatic season and concentration of gases emitted. In fact, ash and gases are usually particularly intense in winter, with a reduction of emission of specific metals with warmer weather [48]. In particular, radon concentration is generally lower in summer, when air temperature is higher. A negative correlation between radon concentration and air temperature has been found with consequent lowering of indoor radon concentration. On the other hand, no seasonal cyclicity due to meteorological parameters was found. Then, active faults (40–150 m) and volcanic substrates could be an alternative explanation of radon and other pollution elements variation [48].
Based on all this information, our hypothesis is that the volcano ash/gas together with climatic conditions may have promoted a longer persistence of the viral particles in the air, mleading to a higher prevalence of COVID-19 in the eastern to southern part of Sicily.
Evaluation of hypothesis
Air pollution has been suggested as cofactor of the spread of COVID-19 in industrial cities, in an attempt to explain the major incidence of the virus infection in the North of Italy, especially in the Po valley. The main involved Italian cities are, indeed, Lodi, Cremona and Bergamo, defined as the “Industrial Triangle” which is characterized by a high density of factories, traffic and intensive agriculture with the highest pollution levels [49].
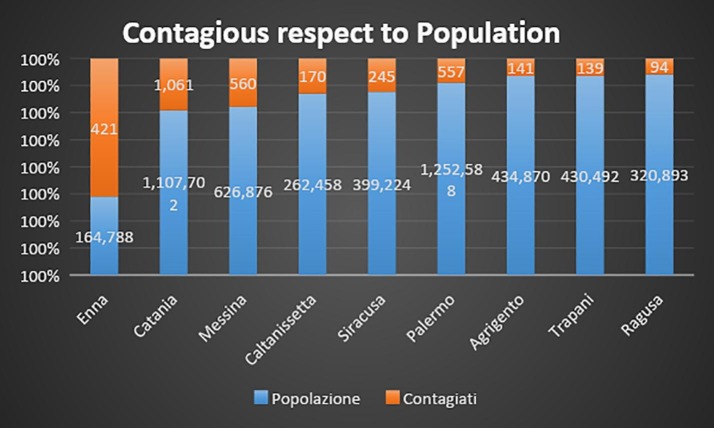
In Sicily, the highest incidence of infection was in Catania, followed by Messina and Palermo, although this latter city is the biggest Sicilian one (see Fig. 3, Fig. 3). Because of its altitude and geographical position of Mount Etna, the volcano displays a role in the exposure of the flanks to the dominant winds: most of the rainfall is on the eastern flank, as the volcano itself induces condensation of wet air masses coming from the Ionian Sea, where Catania is [50].
Fig. 3.
Number of positives for COVID-19 per province, as compared to the general population.
Gases blow from eastern to the southern due to the westerly to north-westerly trade winds [50]. In fact, quantitative of heavy metals is more representative in the ground waters in the eastern and southern sectors of the volcano. The presence of TEs in the water is the results of degassing of magmatic volatiles, such as radon, by the known faults or faults that are not clearly visible at the surface (26,53). Due to direction of gas emissions, we should expect (how it is actually) a lower incidence of infectious in the western flank of the volcano (Palermo, Trapani, etc) than the eastern or southern (Messina and Catania), as demonstrated by prevalence data in Fig. 2, Fig. 3 .
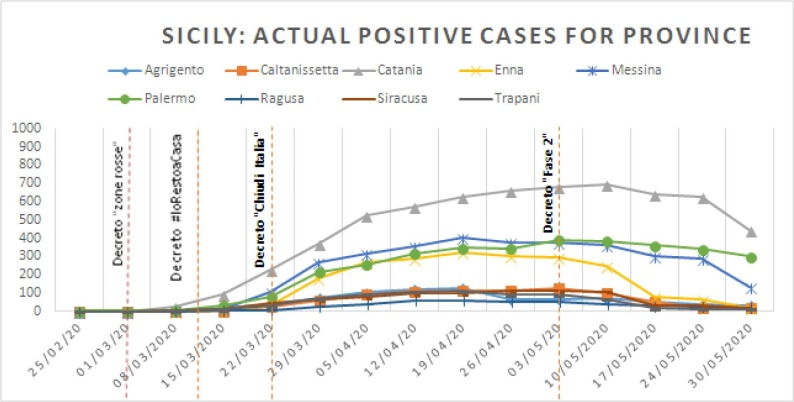
Fig. 2.
Weekly reported numbers of positive cases for Covid-19 in Sicily, evaluated per single province. decreto “zone rosse”: National decree where “red zones” where closed without any possibility to go to or to travel from all these individualized areas (Lumbardy); Decreto #IoRestoaCasa“: national decree with any limitations to go out, unless strictly necessary and to keep social distances (quarantine effort); decreto “chiudi Italia”: all commercial activities were suspended; Decreto “Fase 2”: gradually reopening of all activities. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On the other hand, the reduction of specific and dangerous gases emitted with warmer weather, reveals a potential correlation between the distribution of severe COVID-19 in Sicily and the metals diffusion resulting from a combination of volcano emissions, locally climatic conditions, population genetic predispositions and regional topography.
Consequence of the hypothesis and discussion
This is the first paper that elaborates the hypothesis of a potential role of volcanic gases and heavy metals-related air pollution, combined to specific climatic conditions and regional topography, in favouring severe COVID-19 diffusion in Sicily. The idea stems from the fact that TEs likely lead to a major susceptibility of respiratory system to infection. Based on the Frontera hypothesis, heavy metal air pollutants combined to climatic conditions prolonged the permanence of the virus in the air, as well as the susceptibility to pulmonary virus infection. In Biancavilla, town located in in eastern Sicily, a relation between a higher risk of mesothelioma, as well as chronic obstructive pulmonary disease, and asbestiform fiber used in the local building industry (fluoro-edenite) was found. Then, preventive manoeuvres were done, such as covering with asphalt of roads previously paved with local soil materials, and removal of sources of dust in the urban area [52], [53].
Moreover, a recent environmental survey showed the presence of both C. neoformans and C. gattii species complexes in the environment. In particular, these species were previously recovered from Messina (Northeast Sicily) in several samples of bird excreta, as well as in Eucalyptus camaldulensis, Prunus dulcis (almond), and Ceratonia siliqua (carob) [54], [55], [56]. In 2019, Trovato et al found that C. neoformans and C. gattii species colonize olive trees, samples from 124 olive trees collected from 14 different sites in Eastern Sicily around Mount Etna, as well as carob trees [57], [58]. Assays showed that volcanic soil is a suitable substrate for the growth of C. neoformans and C. gattii species for the characteristics of the soil rich in iron and copper, potassium, phosphorus and magnesium, but poor of nitrogen and calcium [59].
Therefore, the blastospores and basidiospores producted by cryptococcal yeasts represent a potential source of infection since soil aerosols could transfer small cells and spores (and potentially virus particles) in pulmonary alveoli of humans and animals causing the onset of the infection [57].
Pulmunary diseases caused by TEs were also demonstrated by Censi et al. [60]. It is noteworthy that two rare pulmonary diseases, i.e. dendriform pulmonary ossification and pulmonary microlithiasis were related to inhalation of atmospheric particles released by industrial practices or hydrocarbon combustion [61]. One possible cause of these pathologies is the disposal of metal dust with large affinity to phosphate precipitation, such as lanthanides (YLn), produced during the manufacture of mirrors, optical lenses, and certain electronic [62], [63]. Lanthanides can crystallize in interstitial lung spaces and it could be diagnosed by broncho-alveolar lavage fluid dosing YLn content. Pulmunary characteristics are phosphatic microcrysts in intraaveolar areas of the lungs [64]. The microcrysts may precipitate with YLn-phosphates and progress to pulmonary fibrosis due to dissolution of atmospheric particles [65]. Due to the increasing utilization of YLn for agricultural and industrial applications, the measurement of YLn fractionation in lung fluid has the potential to be a viable tracer of human exposure to heavy fluxes of fine particulates enriched in heavy metals pollutants.
Based on these information, we could hypothesize that volcanic trace elements might play an important role in the predisposition and development of viral infection, as Covid-19, and thus prevention of respiratory diseases due to pollutions is fundamental. Because of the underestimation of volcanic gases emitted, we would like to encourage an accurate surveillance of the level of heavy metals and air pollution in the predisposed areas. Our hypothesis may imply a higher level of attention to the risk of infection spread in Sicily. It would be very important to carry out a clinical and epidemiological survey to identify heavy metals in soils and water, especially in the exposed flank areas of the volcanoes, reducing the usage and consumption of products with the higher levels of TEs. Further epidemiological and ecological surveys to confirm our hypothesis should be carried out.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Del Negro C, Cappello A, Neri M, Bilotta G, Hérault A, Ganci G. Lava flow hazards at Mount Etna: constraints imposed by eruptive history and numerical simulations. Sci Rep 2013;3:3493. Published 2013 Dec 13. doi:10.1038/srep03493. [DOI] [PMC free article] [PubMed]
- 2.Scollo S, Delcarlo P, Coltelli M. Tephra fallout of 2001 Etna flank eruption: analysis of the deposit and plume dispersion, J. Volcanol. Geotherm. Res. 160; 2007: 147–164.
- 3.Allard P. Endogenous magma degassing and storage at Mount Etna. Geophys Res Lett - Geophys Res Lett. 1997;24:2219–2222. doi: 10.1029/97GL02101. [DOI] [Google Scholar]
- 4.Allard P., Carbonelle D., Dajlevic J. Eruptive and diffuse emissions of carbon dioxide from Etna volcano. Nature. 1991;351:387–391. [Google Scholar]
- 5.Ciencewicki J. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 6.Bruno N., Caltabiano T., Romano R. SO 2 emissions at Mt. Etna with particular reference to the period 1993-1995. Bull Volcanol. 1999;60(6):405–411. doi: 10.1007/s004450050240. [DOI] [Google Scholar]
- 7.Caltabiano T, Romano R, and Budetta G. SO2 flux measurements at Mount Etna (Sicily). J Geophys Res (1994): 99, 12,809–12,819.
- 8.Vocaturo G., Colombo F., Zanoni M., Rodi F., Sabbioni E., Pietra R. Human exposure to heavy metals. Rare earth pneumoconiosis in occupational workers. Chest. 1983;83:780–783. doi: 10.1378/chest.83.5.780. [DOI] [PubMed] [Google Scholar]
- 9.Cannon J.R., Greenamyre J.T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124:225–250. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marras C., Goldman S.M. Genetics meets environment: Evaluating gene-environment interactions in neurologic diseases. Semin Neurol. 2011;31:553–561. doi: 10.1055/s-0031-1299793. [DOI] [PubMed] [Google Scholar]
- 11.Giacoppo S., Galuppo M., Calabrò R.S. Heavy metals and neurodegenerative diseases: an observational study. Biol Trace Elem Res. 2014;161(2):151–160. doi: 10.1007/s12011-014-0094-5. [DOI] [PubMed] [Google Scholar]
- 12.Nicoletti A., Vasta R., Venti V. The epidemiology of amyotrophic lateral sclerosis in the Mount Etna region: a possible pathogenic role of volcanogenic metals. Eur J Neurol. 2016;23(5):964–972. doi: 10.1111/ene.12973. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti A, Bruno E, Nania M, et al. Multiple Sclerosis in the Mount Etna region: possible role of volcanogenic trace elements [published correction appears in PLoS One 2014;9(6):e100942]. PLoS One. 2013;8(12):e74259. Published 2013 Dec 11. doi:10.1371/journal.pone.0074259. [DOI] [PMC free article] [PubMed]
- 14.Gellein K., Skogholt J.H., Aaseth J., Thoresen G.B., Lierhagen S. Trace elements in cerebrospinal fluid and blood from patients with a rare progressive central and peripheral demyelinating disease. J Neurol Sci. 2008;266:70–78. doi: 10.1016/j.jns.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Schwenk M, Templeton DM. Cytokine Profiles In Human Exposure To Metals (Iupac Technical Report). Pure Appl Chem (2006): 78 (11):2155–2168].
- 16.Lo Turco V., Di Bella G., Furci P., Cicero N., Pollicino G., Dugo G. Heavy metals content by ICP-OES in Sarda sarda, Sardinella aurita and Lepidopus caudatus from the Strait of Messina (Sicily, Italy) Nat Prod Res. 2013;27:518–523. doi: 10.1080/14786419.2012.673611. [DOI] [PubMed] [Google Scholar]
- 17.Kommalapati, Raghava R.; Valsaraj, Kalliat T. Atmospheric aerosols: Characterization, chemistry, modeling, and climate. 1005. Washington, DC: American Chemical Society. (2009). pp. 1–10. doi:10.1021/bk-2009-1005.ch001. ISBN 9780841224827).
- 18.Oskarsson N, The interaction between volcanic gases and tephra: fluorine adhering to tephra of the 1970 Hekla eruption, J Volcanol Geotherm Res. 8; 1980. 16–23.
- 19.Frogner P., Gislason S.R., Oskarsson N. Fertilizing potential of volcanic ash in ocean surface water. Geology. 2001;29:487–490. [Google Scholar]
- 20.elmelle P, Lambert M, Dufrêne Y, Gerin P, Óskarsson N, Gas/aerosol-ash interaction in volcanic plumes: new insights from surface analyses of fine ash particles, Earth Planet Sci Lett 259 (2007) 159–170.
- 21.Neri M, Giammanco S and Leonardi A. Preliminary Indoor Radon Measurements Near Faults Crossing Urban Areas of Mt. Etna Volcano (Italy). Public Health, 03 May 2019 | https://doi.org/10.3389/fpubh.2019.00105. [DOI] [PMC free article] [PubMed]
- 24.Barreca G., Bonforte A., Neri M. A pilot GIS database of active faults of Mt. Etna (Sicily): A tool for integrated hazard evaluation. J Volcanol Geotherm Res. 2013;251:170–186. doi: 10.1016/j.jvolgeores.2012.08.013. [DOI] [Google Scholar]
- 25.Ajari T.R., Adepelumi A.A. Reconnaissance soil-gas Radon survey over faulted crystalline area of ile-Ife. Nigeria Environ Geol. 2002;41:608–613. doi: 10.1007/s002540100428. [DOI] [Google Scholar]
- 26.Neri M., Giammanco S., Ferrera E., Patanè G. Zanon V. Spatial distribution of soil radon as a tool to recognize active faulting on an active volcano: the example of Mt. Etna (Italy) J Environ Radioact. 2011;102:863–870. doi: 10.1016/j.jenvrad.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Urlaub M., Petersen F., Gross F., Bonforte A., Puglisi G., Guglielmino F. Gravitational collapse of Mount Etna’s southeastern flank. Sci Adv. 2018;4:9700. doi: 10.1126/sciadv.aat9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BurtonM NeriM, Condarelli D. High spatial resolution radon measurements reveal hidden active faults on Mt. Etna. Geophys Res Lett. 2004;31:L07618. doi: 10.1029/2003GL019181). [DOI] [Google Scholar]
- 31.1 Trovato L, Oliveri S, Esposto MC, Prigitano A, Romanò L, Cogliati M. Cryptococcus neoformans and Cryptococcus gattii Species Complex Isolates on the Slopes of Mount Etna, SICILY, Italy. Front Microbiol 2019;10:2390. Published 2019 Oct 18. doi:10.3389/fmicb.2019.02390). [DOI] [PMC free article] [PubMed]
- 32.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P., Yang X.L., Wang Y. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S. Global seasonal influenza-associated mortality collaborator network. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brimblecombe, Peter. “History of air pollution.” in Composition, Chemistry and Climate of the Atmosphere Van Nostrand Reinhold (1995): 1–18.
- 36.Brimblecombe, Peter; Makra, László “Selections from the history of environmental pollution, with special attention to air pollution. Part 2*: From medieval times to the 19th century”. Int J Environ Pollut (2005): 23 (4): 351–67. doi:10.1504/ijep.2005.007599).
- 37.Allen, Bob. “Atmospheric Aerosols: What Are They, and Why Are They So Important?”. NASA. NASA. Retrieved 8 July 2014.
- 38.Fuller Joanna Kotcher (2017-01-31). Surgical Technology – E-Book: Principles and Practice. Elsevier Health Sciences. ISBN 978-0-323-43056-2.
- 39.Hinds, William C. (1999). Aerosol Technology (2nd ed.). Wiley - Interscience. ISBN 978-0-471-19410-1.
- 40.Particulate pollution – PM10 and PM2.5“. Recognition, Evaluation, Control. News and views from Diamond Environmental Limited. 2010-12-10. Retrieved23 September 2012).
- 41.Grainger, Don. “Volcanic Emissions”. Earth Observation Data Group, Department of Physics, University of Oxford. University of Oxford. Retrieved 8 July 2014.
- 42.Arpa Lombardia: air pollutants monitoring; www.arpalombardia.it.
- 43.Daniel A. Vallero. “Fundamentals of Air Pollution”. Elsevier Academic Press.
- 44.7 million premature deaths annually linked to air pollution. WHO. 25 March 2014. [PubMed]
- 45.Wei Three active volcanoes in China and their hazards. J Asian Earth Sci. 2003;21(5):515–526. [Google Scholar]
- 46.Weia H., Sparksb R.S.J., Liua R., Fana Q., Wanga Y., Honga H. Three active volcanoes in China and their hazards. J Asian Earth Sci. 2003;21:515–526. [Google Scholar]
- 47.Dost-PHIVOLCS, https://www.phivolcs.dost.gov.
- 48.Neri M, Giammanco S, Leonardi A. Preliminary Indoor Radon Measurements Near Faults Crossing Urban Areas of Mt. Etna Volcano (Italy). Front Public Health. 2019;7:105. Published 2019 May 3. doi:10.3389/fpubh.2019.00105). [DOI] [PMC free article] [PubMed]
- 49.Frontera Antonio, Martin Claire, Vlachos Kostantinos, Sgubin Giovanni. Regional air pollution persistence links to COVID-19 infection zoning. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calabrese S., Aiuppa A., Allard P., Bagnato E., Bellomo S. Atmospheric sources ad sinks of volcanogenic elements in a basaltic volcano. (Etna, Italy). Geochi et Cosmoch Acta. 2011;75:7401–7425. [Google Scholar]
- 52.Bruno C., Comba P., Zona A. Adverse health effects of fluoro-edenitic fibers: epidemiological evidence and public health priorities. Ann N Y Acad Sci. 2006;1076:778–783. doi: 10.1196/annals.1371.020. [DOI] [PubMed] [Google Scholar]
- 53.Biggeri A, Pasetto R, Belli S, Bruno C, Di Maria G, Mastrantonio M, Trinca S, Uccelli R, Comba P. Mortality from chronic obstructive pulmonary disease and pleural mesothelioma in an area contaminated by natural fiber (fluoro-edenite). Scand J Work Environ Health 2004;30(3):249–252.). [DOI] [PubMed]
- 54.Criseo G., Gallo M. Serotyping of Cryptococcus neoformans isolates from environmental and clinical sources in extreme southern Italy (Calabria and Sicily, central Mediterranean area) Mycoses. 1997;40:95–100. doi: 10.1111/j.1439-0507.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 55.Pernice I., Lo Passo C., Criseo G., Pernice A., Todaro-Luck F. Molecular subtyping of clinical and environmental strains of Cryptococcus neoformans variety neoformans serotype A isolated from southern Italy. Mycoses. 1998;41:117–124. doi: 10.1111/j.1439-0507.1998.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 56.Romeo O., Scordino F., Chillemi V., Criseo G. Cryptococcus neoformans/ Cryptococcus gattii species complex in southern Italy: an overview on the environmental di?usion of serotypes, genotypes and mating-types. Mycopathologia. 2012;174:283–291. doi: 10.1007/s11046-012-9547-6). [DOI] [PubMed] [Google Scholar]
- 57.Trovato L., Oliveri S., Esposto M.C., Prigitano A., Romanò L., Cogliati M. Cryptococcus neoformans and Cryptococcus gattii Species Complex Isolates on the Slopes of Mount Etna, SICILY, Italy. Front Microbiol. 2019;10:2390. doi: 10.3389/fmicb.2019.02390). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cogliati M., D’Amicis R., Zani A. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016;16:fow045. doi: 10.1093/femsyr/fow045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Antone C., Punturo R., Vaccaro C. Rare earth elements distribution in grapevine varieties grown on volcanic soils: an example from Mount Etna (Sicily, Italy) Environ Assess. 2017;189:160. doi: 10.1007/s10661-017-5878-6). [DOI] [PubMed] [Google Scholar]
- 60.Censi P., Tamburo E., Speziale S. Yttrium and lanthanides in human lung fluids, probing the exposure to atmospheric fallout. J Hazard Mater. 2011;186(2–3):1103–1110. doi: 10.1016/j.jhazmat.2010.11.113. [DOI] [PubMed] [Google Scholar]
- 61.Baddini Martinez J.A. Ramos SG Inhalation of hydrocarbon combustion products as a cause of dendriform pulmonary ossification. Med Hypotheses. 2008;71:981–982. doi: 10.1016/j.mehy.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 62.Sabbioni E, Pietra R, Gaglione P, Vocaturo G, Colombo F, Zanoni M, Rodi F. Long-term occupational risk of rare-earth pneumoconiosis. A case report as investigated by neutron activation analysis, Sci Total Environ 26 (1982) 19–32. –11. [DOI] [PubMed]
- 63.Takaya M., Shinohara Y., Serita F., Ono-Ogasawara M., Otaki N., Toya T. Dissolution of functional materials and rare earth oxides into pseudo alveolar fluid. Ind Health. 2006;44:639–644. doi: 10.2486/indhealth.44.639. [DOI] [PubMed] [Google Scholar]
- 64.Yoon H.K., Moon H.S., Park S.H., Song J.S., Lim Y., Kohyama N. Dendriform pulmonary ossification in patient with rare earth pneumoconiosis. Thorax. 2005;60:701–703. doi: 10.1136/thx.2003.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joines R.W., Roggli V.L. Dendriform pulmonary ossification. Report of two cases with unique findings. Am J Clin Pathol. 1989;91:398–402. doi: 10.1093/ajcp/91.4.398. [DOI] [PubMed] [Google Scholar]